Abstract
In the present paper, the interaction between metal oxide nanoparticles and carbon materials was studied, and the results showed a synergetic effect, leading to an improvement in the properties of the obtained hybrid composites. The In2O3 NPs were prepared by the precipitation method and thermal treatment at 550 °C. The composites were obtained using an ex situ method, by mixing the In2O3 NPs with reduced oxide graphene (rGO) in a ratio of 10:1. The structural, morphological, and chemical composition studies of the In2O3 NPs and In2O3-rGO composites were investigates by FTIR and EDX spectroscopy, SEM microscopy, and XRD analysis. These techniques have highlighted the obtaining of In2O3 of high purity, and crystallinity, with the mean particle size in the range of 8–25 nm, but also, the dispersion of In2O3 NPs onto rGO sheets. We examined the influence of the In2O3 nanostructure morphology and In2O3-rGO composites on the electrochemical properties using cyclic voltammetry. The surface properties of the In2O3 and composite films were studied by contact angles, which indicate the maintenance of the hydrophilic nature. The obtained results establish the synergy between the main components to form In2O3-rGO, which can be used for the development of biosensors to enhance the device performance.
1. Introduction
Composites that combine metal oxides with different carbonaceous materials are the subject of intense research to develop high-performance materials. The synergistic effect between the main components is oriented to obtain hybrid materials with physical, chemical, mechanical, optical, and thermal properties superior to their individual properties, and one can be open to the possibility of identifying new opportunities for use in various industrial and technological applications [1,2].
Indium oxide (In2O3) is an amphoteric oxide of indium, and appears as yellowish green odorless crystals. It has become one of the most intensively studied semiconductor materials, being a transparent n-type functional material, with direct band gap energy of 3.6 eV at room temperature, high values of optical constants (i.e., extinction coefficient and refractive index), low resistivity, chemical stability, low toxicity, high thermal stability, good redox behavior, high electrical conductivity, as well as high electron mobility, and metal-like electrical conductivity. Also, it is known to have cubic bixbyte-type (c-In2O3), hexagonal corundum-type (h-In2O3), and orthorhombic-type (rh-In2O3) structures, all of which are related to each other under different pressures and temperature conditions. Due to these characteristics, In2O3 particles offer new opportunities to be involved in a multitude of applications, such as gas sensors, optoelectronic and photovoltaic devices, antireflection coatings for silicon solar cells, photodiodes, liquid crystal display, ultraviolet lasers, field-effect transistors, infrared reflective devices, and electrochromic windows [3,4,5,6,7,8].
Generally, the properties of In2O3 depend on the synthesis method used, with the possibility of being obtained in nanostructured powders, and as thin films. Until now, different physical methods have been known to obtain nanostructured In2O3, among which the most intensively used are pulsed laser deposition (PLD), spray pyrolysis, RF magnetron sputtering, physical vapor deposition (PVD), electrochemical deposition, atomic layer de-position (ALD), e-beam evaporation (EBE), Successive Ionic Layer Adsorption and Reaction (SILAR) method, or sol–gel spin coating; each of these methods is being developed to fulfill specific applications. In addition, by controlling the process parameters (e.g., temperature, film thickness, precursor concentration, volume, sputtering speed) and the type of substrate (i.e., glass, silicon, alumina, quartz), In2O3 films with specific properties have been obtained with good control of the structure and morphology. It has been found that the transparency of an In2O3 film can vary from 75% to 95%, being influenced by the precursor type (i.e., InCl3, In(C2H3O2)3, In(NO3)2, [In(NH3)4]3+ etc.), substrate temperature (300–550 °C), and subsequent thermal treatment. The sintering temperature and film thickness influence the shape and size of In2O3 nanostructures, suggesting an increase in the defect density with increasing temperature, but also with effect on the crystallite size [9,10,11,12,13,14,15,16].
Chemical methods (i.e., solid state, sol–gel, hydrothermal, co-precipitation, solvothermal, sonochemical), allow control of the size, crystallinity, and the obtaining of different morphologies (i.e., nanoparticles, rods, wires, needles, belts, tubes) of In2O3 NPs. These techniques raise issues related to agglomeration tendency, homogeneity, monitoring of process parameters, use of surfactants (e.g., SDS, CTAB, CTAC, PDDA, Span-60), the need to use relatively expensive equipment, and the presence of intermediates formed during the synthesis, etc. [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. Modification of In2O3 by doping with Co, Ti, Zn, Cu, Cr, etc., or by forming composites using carbon materials (graphene, graphene oxide, reduced graphene oxide, fullerene, carbon nanotube, carbon black, etc.) leads to improvement in photocatalytic performance, storage capacity, detection limit, charge transport, sensitivity, repeatability, and stability [35,36,37,38].
According to the literature data, nanostructured metal oxides and graphene can be obtained by in situ and ex situ methods, with different particularities and applicative interests. Carbon materials (i.e., graphite, graphene, CQDs, MWCNT) by combining with different nanostructures lead to hybrid composites that have demonstrated their application properties by developing emission diodes and reliable UV photodetection devices (e.g., ZnO-rGO, In2O3-rGO) [39,40], electrochemical sensors for the sensitive detection of epinephrine, oxytetracycline, acetylcholine (e.g., TiO2-rGO, Co-ZnO/rGO, ZnO-rGO) [40,41,42], membrane for removal of heavy metal ions from wastewater (e.g., ZnO-GO-NiO,) [43,44], removing the organic and pollutants dyes from water (ZnO-GO, ZnO-doped TiO2/rGO) [45,46], and gas sensors (e.g., In2O3-rGO, SnO-rGO, ZnO-rGO, Co3O4-rGO, Pt-rGO) [47,48,49], in energy storage, and high-performance optoelectronic devices (e.g., ZnO-rGO, Cu2O-rGO, TiO2-rGO) [50,51,52].
Reduced graphene oxide (rGO) is considered a carbon-based nanometric material, having a greater surface area, remarkable thermal, and mechanical resilience, and outstanding electrical conductivity, with potential applications. Generally, the rGO synthesis process involves graphite powder oxidation to form graphene oxide (GO), followed by reduction by well-established methods (i.e., chemical, electrochemical, thermal). Most reduction reactions use reducing agents such as hydrazine hydrate, L-ascorbic acid, sodium borohydride, glucose, and formaldehyde. The properties of rGO vary not only depending on the method selected, but also on the functional groups containing oxygen, such as hydroxyl, carboxyl, and epoxy [52,53,54,55]. It is known that rGO exhibits a large surface-to-volume ratio, small concentration of oxygenated groups bonded to its surface, strong ion exchange ability, efficient electrocatalytic activity, and high electrical and thermal conductivity. Therefore, anchoring metal or metal oxide nanoparticles to graphene oxide sheets have proven to be an efficient method to enhance the adsorption capacity, photocatalytic behavior, electrochemical performance, mechanical, and thermal properties [56,57,58,59,60]. Detailed characterization of various nanostructures using advanced techniques enables the correlation between synthesis parameters, and functional performance, with a direct impact on their potential application domains [61,62].
The present paper aims to establish the synergic effect between the components for obtaining hybrid composites based on In2O3 NPs and rGO, as materials for electrochemical applications. We report the methods for the synthesis of In2O3 using two types of precursors, and suitable thermal treatment of the synthesized precursors, and the ex situ method for In2O3-rGO hybrid composites. Further, we report the results of the structural analysis (using FTIR spectroscopy, X-ray diffraction and EDX spectroscopy), morphological evaluation (using SEM microscopy), electrochemical properties (using cyclic voltammetry), and wetting and percolation capacity (using contact angle measurements). Based on the accumulated knowledge, future directions in the synthesis of nanomaterials and their applicability in the development of electrochemical biosensors can be outlined.
2. Experiment and Methods
2.1. Synthesis of In2O3 Nanoparticles
Indium oxide (In2O3) nanoparticles were synthesized using the chemical precipitation method. The following raw materials were used: indium acetate [In(CH3COO)3, 99.99%], indium chloride trihydrate [InCl3·3H2O, 99.9%], aqueous ammonia solution [NH3, 25%], ethanol [C2H6O], and deionized water [H2O, DIW]. The precursors were provided by Carl Roth and Merck, and used as received without any further purification.
In the first step of the process, the two different indium precursors were dissolved in deionized water [DIW] to obtain aqueous solutions of 0.01 M [In(CH3COO)3], and 0.01 M [InCl3·3H2O], under continuous stirring using a magnetic stirrer at room temperature. Subsequently, the aqueous ammonia solution [NH3] was slowly dropped under stirring, until the formation of yellow precipitates. Once the pH value of the solutions was controlled at a constant value of 10, the precursors were kept under magnetic stirring for 2 h, and left overnight for the aging process. The resulting precipitates were centrifuged and washed several times with a mixture of C2H6O and DIW (1:1) to remove the secondary reaction products and unreacted raw materials. Finally, the samples were dried at 100 °C, followed by thermal treatment at 550 °C, for 3 h, with an oven heating speed of 5 °C/min, to obtain yellow powders.
2.2. Synthesis of In2O3-rGO Composites
For the synthesis of rGO, the method described in detail in previous work was used [56,63]. Briefly, the synthesis method used is the modified Hummer method, which involves a graphite oxidation step using a sulfuric acid solution (H2SO4) in the presence of sodium nitrate (NaNO3), and potassium permanganate (KMnO4). At the time of the process, the hydrogen peroxide (H2O2) solution was used to neutralize the KMnO4. For the removal of the reaction by-products, the graphene oxide (GO) washing step with hydrochloride acid solution (HCl) was performed, followed by several washing steps with deionized water (DIW). The GO suspension was redispersed in deionized water and ultrasonicated. Reduction GO was achieved by adding an N-Methyl-2-pyrrolidone (NMP, C5H9NO) co-solvent, and hydrazine hydrate (N2H4), keeping the mixture under magnetic stirring. The purification of rGO involves a succession of centrifugation–decantation–washing with ethanol–redispersing steps.
For the synthesis of the In2O3-rGO composites, the ex situ method was used, where rGO was dispersed in ethanol and ultrasonicated for 60 min. Over the obtained mixture, In2O3 nanoparticles were added in a ratio of 10:1, and ultrasonication was continued for another 120 min. at 45 kHz. Based on the characterization data, we opted to continue the synthesis process to obtain In2O3-rGO composites, using the In2O3 nanoparticles obtained from indium acetate.
2.3. Characterization Methods
The FTIR analysis was performed at room temperature using Tensor 27 spectrometer (Bruker Optics, Ettlingen, Germany) to investigate the chemical bond configuration for the In2O3 and In2O3-rGO composite samples. All measurements were carried out in the range of 4000–400 cm−1, at a resolution of 4 cm−1, and averaging 64 scans using an ATR Platinum holder. The spectra were plotted using OPUS 6.0 software.
The FEI Nova NanoSEM 630 (FEI) is a Field Emission Scanning Electron Microscope (FEI Company, Hillsboro, OR, USA) equipped with an Energy Dispersive X-Ray Analyzer (EDX, Smart Insight AMETEK, Inc., Berwyn, PA, USA) used for surface morphology and elemental composition analysis, performed at an acceleration voltage of 10 kV.
The size distribution samples were obtained from the SEM (FEI Company, Hillsboro, OR, USA) images by measuring around 200 nanoparticles. The program “Image J” (software Version IJ 1.46r) was used to extract the necessary data, and the results were compiled into histograms.
The X-ray diffraction (XRD) measurements were carried out using a SmartLab diffraction system (Rigaku Corporation, Tokyo, Japan), equipped with a CuKα source that provides a monochromatic X-ray beam with a wavelength of 1.5406 Å, over a 2θ range of 10–90° at an accelerating voltage of 40 kV and current strength of 75 mA.
With the help of the Theta Optical Tensiometer (KSV Instruments, Helsinki, Finland) equipped with the CAM 101 camera, light source, lens, and the 1394 firewire interface for fast image acquisition, the contact angles were determined to ascertain the wetting and percolation capacity. Subsequently, various volumes (between 1 and 2 μL) of water were deposited in drops on the analyzed surfaces, and the contact angles were calculated using the drop shape method. For each sample, an average value of three measurements was used to calculate the final result and to establish the hydrophilic and/or hydrophobic character of the In2O3, rGO and In2O3-rGO composite samples.
The electrochemical behavior was determined by recording cyclic voltammograms using a VoltaLab PGZ100 (Radiometer Analytical SAS, Neuilly-Plaisance, France). The experiments were carried out in a conventional three-electrode cell consisting of working electrode formed from Au/Si substrates coated with In2O3 and In2O3-rGO samples deposited by the drop-casting method, the platinum wire used as the counter electrode, and the reference electrode was the silver electrode/silver chloride (3 M KCl). All potentials indicated in this study are relative to the Ag/AgCl (3 M KCl) reference electrode.
3. Results
3.1. Structural Analysis
3.1.1. Functional Group Analysis (FTIR)
To establish the corresponding functional groups of the In2O3 NPs, the samples were characterized using FTIR spectroscopy. The spectroscopic data and possible spectral band assignments for the In2O3 NPs obtained from In(III) acetate and In(III) chloride precursors, rGO control sample, and the In2O3-rGO composite are shown in Figure 1.
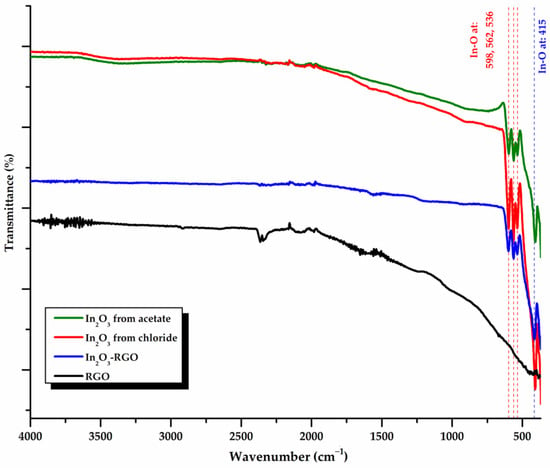
Figure 1.
FTIR spectra for In2O3 samples obtained from In(III) acetate, In(III) chloride, rGO control sample, and In2O3-rGO composite.
In2O3 is a polymorphic oxide, for which the characteristic peaks of In-O are found in approximately the same areas, with slight variations in intensity or wavenumber [64]. It has been observed that regardless of the synthesis method and precursors used, the peaks can be attributed to the vibrational mode of the In-O occurring in the same region. According to the literature, due to its large mass, the absorption bands of In-O stretching vibrations were found in the range 600–400 cm−1, which can be associated with the cubic structure of In2O3 [17,65]. Generally, due to its five atoms, the molecule exhibits nine normal vibrational modes: ΓVib = 5A1 + 1A2 + 1B1 + 2B2 [66]. For our samples, the symmetric and asymmetric vibrational mode of the In-O bonds can be observed by the appearance of bands with maxima at 562, 536, and 409 cm−1, while the deformation of the cubic In2O3 bonds is supported by the 598 cm−1 band [67]. The optimization of the synthesis conditions is sustained by the fact that no peaks attributable to secondary phases or untransformed compounds were observed.
Reduced graphene oxide (rGO) is a material that does not show absorption bands in the MIR range. The spectrum of rGO is defined by low-intensity bands due to the high adsorption capacity of the carboneous material, water, and carbon dioxide during handling [56,68].
The spectrum of the In2O3-rGO composite is mainly characterized by bands that can be associated with the vibrational mode of the In-O bonds, with a slight shift compared to the oxide spectrum, which may be due to the interaction between the nanoparticles and the carbon material. The formation of the composite is also supported by the appearance in the composite spectra of low-intensity bands due to the adsorption of water and carbon dioxide by the rGO. The spectrum of the composite obtained with indium chloride is similar to that from acetate, which is why, in order not to clutter the images in Figure 1, only the spectrum for In2O3-rGO is shown, with In2O3 obtained from acetate.
The main peaks and the possible spectral band assignments for the In2O3 nanoparticles obtained from different precursors, rGO control sample, and In2O3-rGO composite are detailed in Table 1.

Table 1.
The spectral bands position of the In2O3 obtained from acetate and chloride precursors, the rGO control sample, and the In2O3-rGO composite [17,56,64,65,66,67,68,69,70,71].
3.1.2. Compositional Analysis (EDX)
EDX analysis was used to determine the elemental composition at the atomic level of the nanoparticles. Figure 2 shows the characteristic spectra of the In2O3 samples from different precursors. The results obtained from the EDX elemental analysis clearly show the presence of the characteristic indium and oxygen atom peaks. The absence of other types of peaks indicates the lack of contamination during the processing of the synthesized samples, the only peak identified being that of the substrate (Si(K)), used to allow the samples to be analyzed. It was estimated to obtain some finished products with a structural formula appropriate to the stoichiometry of 2:3, as follows: In2.26O2.74 from acetate, and In1.99O3.01 from chloride.
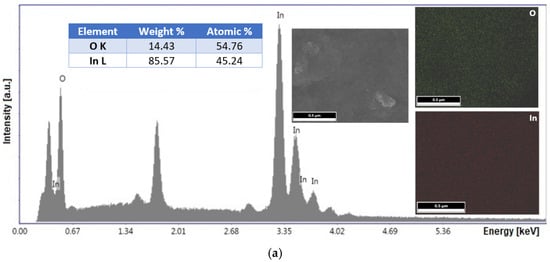
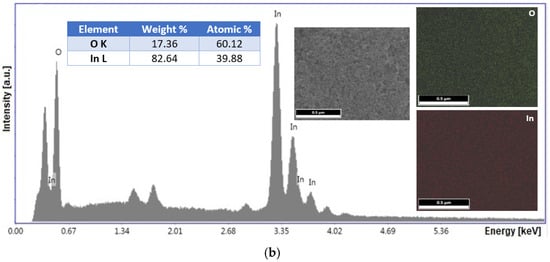
Figure 2.
EDX spectra with percentage of elements (insert table), and elements distribution maps for In2O3 samples obtained from In(III) acetate (a), and In(III) chloride (b).
3.1.3. Structural Studies (XRD)
XRD analysis was used to determine the crystal structure type, presence of the impurities, mean crystallite size, and unit cell parameters. Figure 3 shows XRD experimental profile (black curve) and fitted curve (red curve) with the corresponding fitting parameters determined using least squares fitting, for In2O3 samples synthesized using the related precursors, rGO, and In2O3-rGO composite.
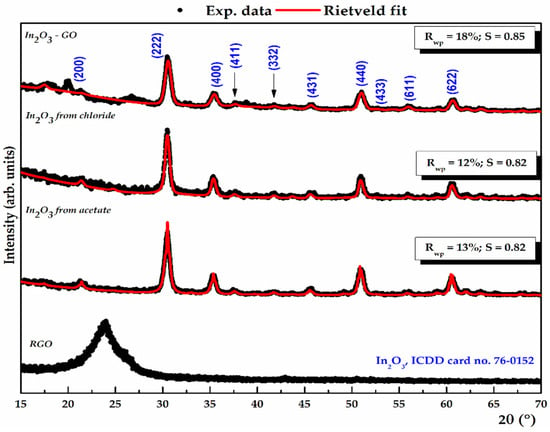
Figure 3.
XRD diffraction patterns for In2O3 samples obtained from In(III) acetate, In(III) chloride, rGO control sample, and In2O3-rGO composite are showed with black curve, and the theoretical profile obtained in the Rietveld refinement framework is presented with red line.
For all samples, the diffraction peaks could be indexed in agreement with the International Center for Diffraction Data (ICDD) database with card no. 76-0152, but also with the existing results in the literature, and attributed to a cubic structure, belonging to the 206:Ia3 space group. In the X-ray diffractograms, the main diffraction peaks at angles of ~21°, 30°, 35°, 45°, 50°, 60°, and 62° correspond to the crystallographic planes characterized by the Miller indices (211), (222), (400), (431), (440), (622), and (631). The XRD technique supports the purity of the samples by the absence of other diffraction peaks that could be associated with the secondary or polymorphic phases of In2O3. By comparing the diffraction peaks of the In2O3 samples, it can be observed that the sample synthesized from indium acetate shows higher intensities and narrower widths compared to the other precursors used, as a result of a higher degree of crystallinity [22,72,73,74].
The XRD plot showed a diffraction peak at 2θ = 24.16°, corresponding to the (002) index from rGO, possibly due to the partial elimination of oxygen functionalities from the stacked Sp2 layers. The inset in Figure 3 for In2O3-rGO composite shows an increase in the intensity of the diffraction peaks assigned to the cubic structure of In2O3, and a displacement of the peak attributed to the carbon material from 24.16° to 25.39°, indicating the insertion of oxide nanoparticles between the graphene sheets [37,56,75].
The X-ray diffractogram for the samples is dominated by a maximum at about 30°, which can be assigned to (222) Miller plane in the In2O3 structure. The average crystallite sizes were calculated using the Debye–Scherrer formula, being estimated at 13.3 ± 2 nm and 11.85 ± 2 nm. Nevertheless, this method considers only the effect of crystallite size on the diffraction peak broadening and does not provide information about the lattice strain. Thus, information regarding the mean crystallite size and average lattice strain was obtained through Rietveld refinement of grazing incidence XRD data, based on the least squares fitting of the theoretical profile (red line) to the experimental data (black line) [76,77,78]. Using the least squares method, fitted profiles were obtained with S (scale factor) values ranging from 0.82 to 0.85, while the Rwp (weighted parameter) varied from 12% to 18%.
Table 2 presents the indexing of the diffraction peaks assigned to the corresponding Miller indices, along with mean crystallite size, lattice strain, as well the lattice constants for the samples. According to the Rietveld refinement results, it can be observed that the unit cell parameters show slight variations, possibly due to differences in crystallite size, and the interaction between In2O3 and rGO.

Table 2.
Structural parameters of In2O3, rGO, and In2O3-rGO samples.
3.2. Morphological Analysis
The particle size, shape, and surface morphology of the nanostructured materials were determined using SEM analysis. Figure 4 presents SEM images with higher magnification to better define the morphology and size distribution of the In2O3 samples synthesized from the different precursors, rGO, and In2O3-rGO composite.
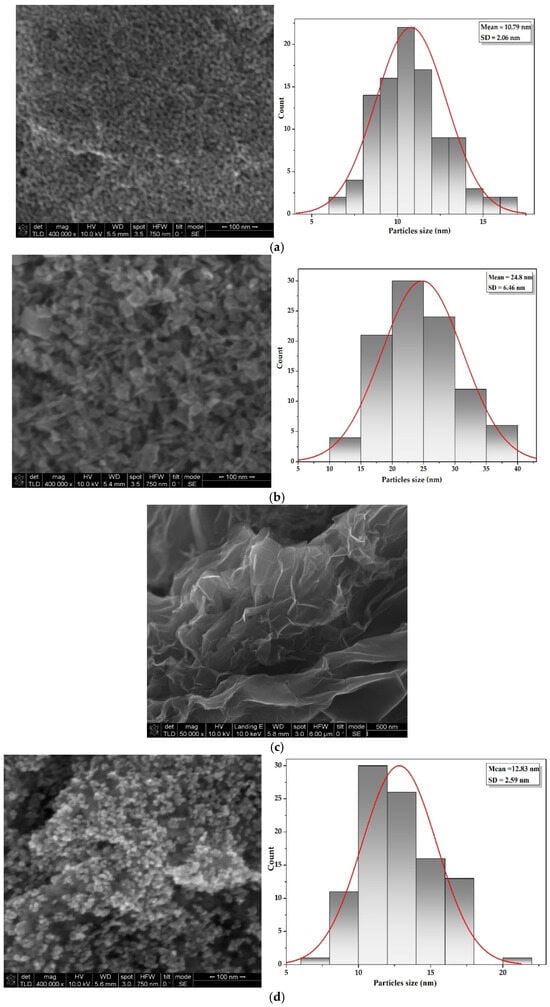
Figure 4.
SEM images and frequency histogram for In2O3 samples obtained from In(III) acetate (a), In(III) chloride (b), rGO control sample (c), and In2O3-rGO composite (d).
Figure 4a reveals that the In2O3 NPs synthesized from indium acetate are predominantly spherical with a tendency to agglomerate, and an average particle size of up to 10 nm. When indium chloride is used (Figure 4b), the In2O3 particles are predominantly cubic in shape, with irregular sizes larger than 10 nm. It can be deduced that, due to the agglomeration tendency, the particles conglomerate into asymmetric formations, but remain in the nanometer range. Figure 4c presents the image of the carbon material very thin and wrinkled in appearance, with a random arrangement and bent surfaces with the exact features of rGO sheets. Finally, Figure 4d for In2O3-rGO composite displays a homogenous distribution of the In2O3 NPs at the surface and into the rGO sheets, indicating the linking between nanoparticles and carbon material. A slight agglomeration of In2O3 on the rGO surface may be due to the nanometric nature of the particles.
From the histograms, it was identified that the main particle sizes of the In2O3 NPs obtained from different precursors varied between 8–35 nm, and In2O in composites In2O3-rGO had sizes in the range of 8–16 nm. The decrease in spherical In2O3 particle size in the composite may be associated with the dispersion and good interaction between the oxide particles and the carbon material. The data obtained are in agreement with the XRD analysis and the measurements obtained from the literature [21,33,79].
3.3. Wetting Capacity (Contact Angle)
Contact angle (CA) measurements were performed to investigate the surface wetting capacity of the In2O3 nanostructures, rGO, and In2O3-rGO composite deposited as films on the silicon substrate using water as the reference liquid. Depending on the surface behavior, the angle between 0–90° indicates a hydrophilic character with a high wetting capacity, while large angles above 90° correspond to a low wetting level, leading to a hydrophobic character [80,81].
The wetting capacity measured by the contact angle (CA) for the In2O3 nanoparticles, rGO, and In2O3-rGO composite is shown in Figure 5. The In2O3 NPs indicate a strong hydrophilic character, with a contact angle value varying in the range of 26–35°. The measurements showed that reduced graphene oxide has hydrophobic character with an angle at 107°, as a result of a very low concentration of oxygen, and the addition of In2O3 nanoparticles into rGO determines a hydrophilic character of the In2O3-rGO composite, with a contact angle value of 22° [82].
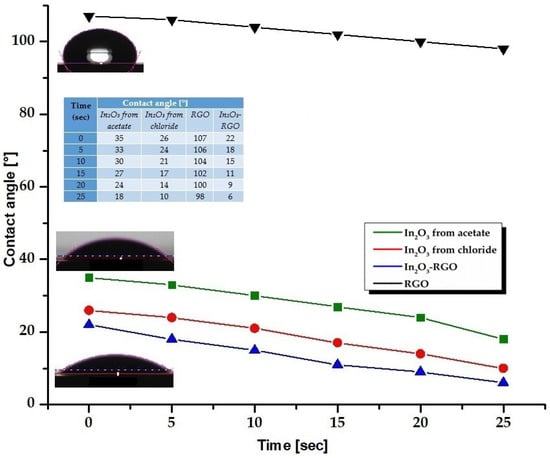
Figure 5.
The evolution of the contact angle as a function of the retention time of the water drop on the surface of In2O3 samples obtained from In(III) acetate, In(III) chloride, rGO control sample, and In2O3-rGO composites.
To demonstrate the applicability in the electrochemical field, the percolation capacity of the In2O3 samples was studied, following the evolution in time of the contact angle values at the interaction between the liquid and the surface of the samples. The values of the contact angle decrease with the increase in the retention time of the water droplet on the surface, revealing a surface with a strong hydrophilic character, with an angle reaching up to ~6°. In this context, the surface properties of the In2O3 samples indicate good wettability and high percolation capacity, which may be due to the morphology and very small size of the particles.
3.4. Electrochemical Analysis (CV)
Cyclic voltammetry was used to investigate the electrochemical performance of the In2O3 nanostructures deposited on the Au/Cr/Si substrate, which was used as the working electrode. Figure 6 shows the cyclic voltammograms recorded at a potential between −0.2 and 0.8 V, depending on the Ag/AgCl electrode with a scan rate of 25 mV/s. The redox couple of the [Fe(CN)6]3−/4− electrolyte solution in PBS (pH 7.4) is characterized by the electron transfer mechanism in the inner sphere, depending on the electrode homogeneity and the applied modifications, providing information about the subtle kinetic modifications of the charge transfer [83].
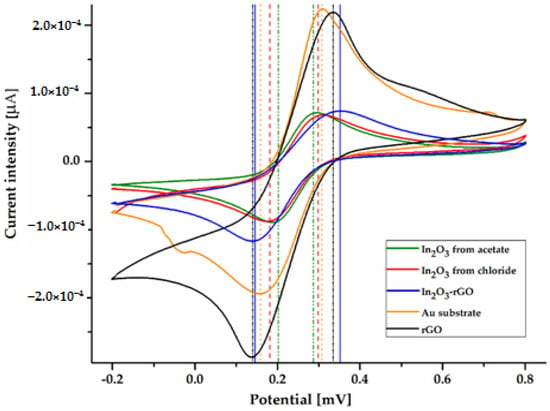
Figure 6.
CV curves for In2O3 samples obtained from In(III) acetate, In(III) chloride, and In2O3-rGO composite.
From the evaluation of the cyclic voltammetry plots corresponding to the surfaces with nanostructured In2O3, and In2O3-rGO composite, the presence of two distinct peaks can be observed, the oxidation peaks and the reduction peaks, regardless of the type of precursor used. Table 3 shows the oxidation peaks (anodic, Epa), the reduction peaks (cathodic, Epc), the related currents, the formal redox potential of the reversible couple (E0’), and the peak separation potential (ΔEp). According to the CV data, the shift of the potential’s upper limit towards positive values and the low value of ΔEp shows that the indium oxide layer’s reversibility and reduction are increasing, with the type of precursor being a key factor in this process [84].

Table 3.
Results of cyclic voltammetry of In2O3 nanoparticles obtained from different indium precursors, and In2O3-RGO composite based on CV studies (at 25 mV/s scan rate).
It is also found that this reversibility is not affected by the type of precursor used and the thickness of the deposited films, which indicates an increase in the electron transfer rates in the electrochemical reaction. However, it seems that the In2O3 obtained from the acetate precursor among the samples showed the lowest peak separation, which evidences higher redox properties, due to smaller crystallite and particle sizes that ensure more surface availability for charge transfer in contact with the electrolyte. The CV response of rGO displays higher peak intensities, and more distinct redox signals compared to In2O3, due to its superior electrical conductivity and large active surface area. The voltammogram of the In2O3-rGO composite exhibits well-defined peaks and high peak current intensity, confirming SEM observations regarding to the uniform dispersion of In2O3 nanoparticles into the rGO sheets, and shows the possibility of improving the electrocatalytic activity.
4. Conclusions
In2O3 nanostructures were obtained by a chemical precipitation method, while the In2O3-rGO composite was prepared using an ex situ method, ensuring intimate contact between the main components.
FTIR spectroscopy confirmed the appearance of bands, which can be assigned to the In-O characteristic of cubic In2O3 bonds. The spectrum of the In2O3-RGO composite is characterized by bands associated with the vibrational mode of In-O bonds, with a slight shift relative to the oxide spectrum, due to the interaction between the nanoparticles and the carbon material. The FTIR observations were complemented and supported by elemental analysis at atomic level by EDX spectroscopy.
XRD results show that, regardless of the type of precursor used to obtain In2O3, the synthesized powders exhibit a cubic crystalline structure, without other characteristic impurity peaks. The morphological analysis revealed the influence of the cation precursor, resulting in spherical particles using indium acetate, and cubic particles were formed from chloride, with a tendency to agglomerate and without surface defects. Histogram analysis showed that the average particle size increased from 8 to 25 nm for In2O3 powders, while for the In2O3-rGO composite, the average size remained around 13 nm.
Surface property analysis confirmed the hydrophilic character of the oxide films and the composite, indicating a good wettability and the liquid percolation across their surfaces. Cyclic voltammetry measurements revealed well-defined redox peaks, with current intensity varying significantly between the samples. Additionally, the potential difference suggests an enhancement of the electrochemical performance of the In2O3-rGO composite. The obtained results highlight the synergy between In2O3 nanoparticles with rGO sheets in the composite, considering their potential as materials for biosensor development. Furthermore, their applicability could extend to photocatalysis for treatment and remediation, gas sensing, supercapacitors, and other related applications.
Author Contributions
Conceptualization, A.M.; validation, A.M., C.O. and V.Ț.; investigation, A.M., CO., C.R., O.B., M.S. and V.Ț.; writing—original draft preparation, A.M.; writing—review and editing, A.M., C.O., C.R., M.S. and V.Ț.; visualization, A.M., C.O., C.R., O.B., M.S. and V.Ț. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded from a grant of the Ministry of Research, Innovation and Digitization, CNCS-UEFISCDI, project number PN-IV-P2-2.1-TE-2023-0417, within PNCDI IV. Also, this research was supported from the Core Program within the National Research Development and Innovation Plan 2022–2027, carried out with the support of MCID, project no. 2307 (µNanoEl).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors give thanks to Gabriel Craciun for his technical support in the samples characterization.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DIW | Deionized water |
| SDS | Sodium dodecyl sulfate |
| CTAB | Cetyltrimethylammonium bromide |
| CTAC | Cetyltrimethylammonium chloride |
| PDDA | Poly(diallyldimethylammonium chloride) |
| rGO | Reduced oxide graphene |
| GO | Graphene oxide |
| FTIR | Fourier Transform Infrared spectroscopy |
| SEM | Scanning Electron Microscopy |
| EDX | Element energy dispersive spectroscopy |
| XRD | X-ray diffraction |
| CA | Contact angle |
| CV | Cyclic voltammetry |
| PBS | Phosphate Buffered Saline |
References
- Channabasavana Hundi Puttaningaiah, K.P. Innovative Carbonauceous Materials and Metal/Metal Oxide Nanoparticles for Electrochemical Biosensor Applications. Nanomater 2024, 14, 1890. [Google Scholar] [CrossRef]
- Dawood, A.; Ahmad, J.; Ali, S.; Ullah, S.; Asghar, Z.; Shah, M. Metal oxide-carbon composites and their applications in optoelectronics and electrochemical energy devices. In Metal Oxides, Metal Oxide-Carbon Hybrid Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 309–339. [Google Scholar]
- Chavall, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Harinath Babu, S.; Kaleemulla, S.; Madhusudhana Rao, N.; Krishnamoorthi, C. Indium oxide: A transparent, conducting ferromagnetic semiconductor for spintronic applications. J. Magn. Magn. Mat. 2016, 416, 66–74. [Google Scholar] [CrossRef]
- Bhardwaj, S.K.; Bhardwaj, N.; Kukkar, M.; Sharma, A.L.; Kim, K.H.; Deep, A. Formation of high-purity indium oxide nanoparticles and their application to sensitive detection of ammonia. Sensors 2015, 15, 31930–31938. [Google Scholar] [CrossRef]
- Yang, W.; Huo, Y.; Wang, T.; Liu, X.; Li, D.; Yu, H.; Dong, X.; Yang, Y. RGO@In2O3 based flexible gas sensor: Efficient monitoring of trace NO2 gas at room temperature. Sens. Actuators B Chem. 2025, 430, 137359. [Google Scholar] [CrossRef]
- Liang, C.; Cao, Z.; Hao, J.; Zhao, S.; Yu, Y.; Dong, Y.; Liu, H.; Huang, C.; Gao, C.; Zhou, Y.; et al. Gas Sensing Properties of Indium–Oxide–Based Field–Effect Transistor: A Review. Sensors 2024, 24, 6150. [Google Scholar] [CrossRef]
- Liu, D.; Lei, W.; Qin, S.; Hou, L.; Liu, Z.; Cui, Q.; Chen, Y. Large-scale synthesis of hexagonal corundum-type In2O3 by ball milling with enhanced lithium storage capabilities. J. Mater. Chem. A 2013, 1, 5274. [Google Scholar] [CrossRef]
- Qurashi, A.; Irfan, M.F.; Alam, M.W. In2O3 nanostructures and their chemical and biosensor applications. Arab. J. Sci. Eng. 2010, 35, 125–145. [Google Scholar]
- Yahia, A.; Attafa, A.; Saidi, H.; Dahnoun, M.; Khelifi, C.; Bouhdjer, A.; Saadi, A.; Ezzaoui, H. Structural, optical, morphological and electrical properties of indium oxide thin films prepared by sol gel spin coating process. Surf. Interfaces 2019, 14, 158–165. [Google Scholar] [CrossRef]
- Horoz, B.; Tuna Yıldırım, S.; Soltabayev, B.; Ates, A. Effect of SILAR cycle on gas sensing properties of In2O3 thin films for CO gas sensor. J. Mater. Sci. Mater. Electron. 2024, 35, 163. [Google Scholar] [CrossRef]
- Yang, B.; Li, P.; Chen, Z.; Xu, H.; Fu, C.; Ding, X.; Zhang, J. Effect of Titanium Cation Doping on the Performance of In2O3 Thin Film Transistors Grown via Atomic Layer Deposition. Coatings 2023, 13, 605. [Google Scholar] [CrossRef]
- Fakhri, M.A. Effect of substrate temperature on optical and structural properties of indium oxide thin films prepared by reactive PLD method. Eng. Technol. J. 2014, 32, 1323–1330. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhu, X.; Wang, X.; Cai, X.; Zhang, B.; Qiu, D.; Wu, H. Annealing effects of In2O3 thin films on electrical properties and application in thin film transistors. Thin Solid Film. 2011, 519, 3254–3258. [Google Scholar] [CrossRef]
- Veeraswamy, Y.; Vijayakumar, Y.; Ramana Reddy, M.V. Effect of substrate on structural and optical properties of In2O3 thin films prepared by electron beam evaporation. Asian J. Appl. Sci. 2014, 7, 737–744. [Google Scholar] [CrossRef]
- Jothibas, M.; Manoharan, C.; Dhanapandian, S. Effect of precursor concentration on the preparation of In2O3 thin films prepared by spray pyrolysis. Int. J. Curr. Res. 2013, 5, 3268–3275. [Google Scholar]
- Goh, K.W.; Johan, M.R.; Wong, Y.H. Enhanced structural properties of In2O3 nanoparticles at lower calcination temperature synthesised by co-precipitation method. Micro Nano Lett. 2018, 13, 270–275. [Google Scholar] [CrossRef]
- Lin, L.T.; Tang, L.; Zhang, R.; Deng, C.; Chen, D.J.; Cao, L.W.; Meng, J.X. Monodisperse In2O3 nanoparticles synthesized by a novel solvothermal method with In(OH)3 as precursors. Micro Nano Lett. 2018, 13, 270–275. [Google Scholar] [CrossRef]
- Askarinejad, A.; Askarinejad, M.; Bahramifar, N.; Morsali, A. Synthesis and characterisation of In(OH)3 and In2O3 nanoparticles by sol-gel and solvothermal methods. J. Exp. Nanosci. 2010, 5, 294–301. [Google Scholar] [CrossRef]
- Sabry, S.R.; Ibrahim, R.; Agool, R.; Asaad Abbas, M. Calcination temperature dependent of hydrothermal indium oxide nanostructures. Aust. J. Basic Appl. Sci. 2014, 8, 165–168. [Google Scholar]
- Nirmala, E.I.; Kartharinal Punithavathy, I.; Johnson Jeyakumar, S.; Jothibas, M. Annealing temperature effect on hydrothermally prepared indium oxide spherical nano particles. J. Nano. Adv. Mat. 2017, 5, 11–16. [Google Scholar]
- Jothibas, M.; Manoharan, C.; Johnson Jeyakumar, S.; Praveen, P. Study on structural and optical behaviors of In2O3 nanocrystals as potential candidate for optoelectronic devices. J. Mater. Sci. Mater. Electron. 2015, 26, 9600–9606. [Google Scholar] [CrossRef]
- Nguyen, T.T.D.; Choi, H.N.; Ahemad, M.J.; Van Dao, D.; Lee, I.H.; Yu, Y.T. Hydrothermal synthesis of In2O3 nanocubes for highly responsive and selective ethanol gas sensing. J. Alloy. Compd. 2020, 820, 153133. [Google Scholar] [CrossRef]
- Ullah, H.; Yamani, Z.H.; Qurashi, A.; Iqbal, J.; Kashif, S. Study of the optical and gas sensing properties of In2O3 nanoparticles synthesized by rapid sonochemical method. J. Mater. Sci. Mater Electron. 2020, 31, 17474–17481. [Google Scholar] [CrossRef]
- Zhang, S.; Song, P.; Yang, Z.; Wang, Q. Facile hydrothermal synthesis of mesoporous In2O3 nanoparticles with superior formaldehyde-sensing properties. Phys. E Low Dimens. Syst. Nanostruct. 2018, 97, 38–44. [Google Scholar] [CrossRef]
- Husain, Z.A.; Majeed, A.A.; Rasheed, R.T.; Mansoor, H.S.; Hussein, N.N. Antibacterial activity of In2O3 nanopowders prepared by hydrothermal method. Mater. Today Proc. 2021, 42, 1816–1821. [Google Scholar] [CrossRef]
- Shao, M.; Chen, H.; Shen, M.; Chen, W. Synthesis and photocatalytic properties of In2O3 micro/nanostructures with different morphologies. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 503–507. [Google Scholar] [CrossRef]
- Dodd, A. Synthesis of indium oxide nanoparticles by solid state reaction. J. Nanopart. Res. 2009, 11, 2171–2177. [Google Scholar] [CrossRef]
- Song, P.; Han, D.; Zhang, H.; Li, J.; Yang, Z.; Wang, Q. Hydrothermal synthesis of porous In2O3 nanospheres with superior ethanol sensing properties. Sens. Actuators B 2014, 196, 434–439. [Google Scholar] [CrossRef]
- Han, B.; Wang, J.; Yang, W.; Chen, X.; Wang, H.; Chen, J.; Zhang, C.; Sun, J.; Wei, X. Hydrothermal synthesis of flower-like In2O3 as a chemiresistive isoprene sensor for breath analysis. Sens. Actuators B Chem. 2009, 309, 127788. [Google Scholar] [CrossRef]
- Li, P.; Fan, H. Porous In2O3 microstructures: Hydrothermal synthesis and enhanced Cl2 sensing performance. Mater. Sci. Semicond. Process. 2015, 29, 83–89. [Google Scholar] [CrossRef]
- Latha, C.K.; Raghasudha, M.; Aparna, Y.; Ramchander, M.; Ravinder, D.; Jaipal, K.; Veerasomaiah, P.; Shridhar, D. Effect of capping agent on the morphology, size and optical properties of In2O3 nanoparticles. Mater. Res. 2017, 20, 256–263. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, J.M.; Kim, K.N.; Kim, C.H.; Jang, C.H. Synthesis of In2O3 nano-materials with various shapes. Curr. Appl. Phys. 2006, 6, e198–e201. [Google Scholar] [CrossRef]
- Li, P.; Cai, C.; Cheng, T.; Huang, Y. Hydrothermal synthesis and Cl2 sensing performance of porous-sheets-like In2O3 structures with phase transformation. RSC Adv. 2017, 7, 50760–50771. [Google Scholar] [CrossRef]
- Hong, X.; Wang, X.; Li, Y.; Fu, J.; Liang, B. Progress in Graphene/Metal Oxide Composite Photocatalysts for Degradation of Organic Pollutants. Catalysts 2020, 10, 921. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, P.; Li, J.; Shao, T.; Jin, L. Synthesis of In2O3-graphene composites and their photocatalytic performance towards perfluorooctanoic acid decomposition. J. Photochem. Photobiol. A Chem. 2013, 271, 111–116. [Google Scholar] [CrossRef]
- An, D.; Wang, Q.; Tong, X.; Lian, X.; Zou, Y.; Li, Y. ZnO-enhanced In2O3-based sensors for n-butanol gas. Ceram. Int. 2019, 45, 6869–6874. [Google Scholar] [CrossRef]
- Yaragani, V.; Kamatam, H.P.; Deva Arun Kumar, K.; Mele, P.; Christy, A.J.; Gunavathy, K.V.; Alomairy, S.; Al-Buriahi, M.S. Structural, Magnetic and Gas Sensing Activity of Pure and Cr Doped In2O3 Thin Films Grown by Pulsed Laser Deposition. Coatings 2021, 11, 588. [Google Scholar] [CrossRef]
- Boukhoubza, I.; Derkaoui, I.; Basyooni, M.A.; Achehboune, M.; Khenfouch, M.; Belaid, W.; Enculescu, M.; Matei, E. Reduced graphene oxide-functionalized zinc oxide nanorods as promising nanocomposites for white light emitting diodes and reliable UV photodetection devices. Mater. Chem. Phys. 2023, 306, 128063. [Google Scholar] [CrossRef]
- Joseph, T.; Thomas, N. A facile electrochemical sensor based on titanium oxide (TiO2)/reduced graphene oxide (RGO) nano composite modified carbon paste electrode for sensitive detection of epinephrine (EP) from ternary mixture. Mater. Today Proc. 2021, 41, 606–609. [Google Scholar] [CrossRef]
- Mliki, H.; Echabaane, M.; Rouis, A.; El Ghoul, J.M.; Bessueille, F.; Ayed, D.; Jaffrezic-Renault, N. Highly electroactive Co–ZnO/GO nanocomposite: Electrochemical sensing platform for oxytetracycline determination. Helyion 2024, 10, e30265. [Google Scholar] [CrossRef]
- Pitiphattharabun, S.; Auewattanapun, K.; Htet, T.L.; Thu, M.M.; Panomsuwan, G.; Techapiesancharoenkij, R.; Ohta, J.; Jongprateep, O. Reduced graphene oxide/zinc oxide composite as an electrochemical sensor for acetylcholine detection. Sci. Rep. 2024, 14, 14224. [Google Scholar] [CrossRef]
- Maqbool, A.; Shahid, A.; Jahan, Z.; Khan Niazi, M.B.; Inam, M.A.; Tawfeek, M.A.; Kamel, E.M.; Akhtar, M.A. Development of ZnO-GO-NiO membrane for removal of lead and cadmium heavy metal ions from wastewater. Chemosphere 2023, 338, 139622. [Google Scholar] [CrossRef] [PubMed]
- Motitswe, M.G.; Badmus, K.O.; Khotseng, L. Application of Reduced Graphene Oxide-Zinc Oxide Nanocomposite in the Removal of Pb(II) and Cd(II) Contaminated Wastewater. Appl. Nano 2024, 5, 162–189. [Google Scholar] [CrossRef]
- Pruna, A.; Poliac, I.; Busquets Mataix, D.; Ruotolo, A. Synergistic effects in ZnO nanorod films by pulsed electrodeposition on graphene oxide towards enhanced photocatalytic degradation. Ceram. Int. 2024, 50, 4622–4631. [Google Scholar] [CrossRef]
- Bao, H.V.; Dat, N.M.; Giang, N.T.H.; Thinh, D.B.; Tai, L.T.; Trinh, D.N.; Hai, N.D.; Khoa, N.A.D.; Huong, L.M.; Nam, H.M.; et al. Behavior of ZnO-doped TiO2/rGO nanocomposite for water treatment enhancement. Surf. Interface 2021, 23, 100950. [Google Scholar] [CrossRef]
- Khan, M.; Ferlazzo, A.; Crispi, S.; Hussain, M.; Neri, G. Easy preparation of cobalt oxide/copper oxide composites for gas sensing application. Phys. Scr. 2023, 98, 125927. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, B.; Cheng, M.; Zhao, L.; Lu, G. Gas Sensors Based on Metal Oxides Decorated by Reduced Graphene Oxide with Enhanced Gas Sensing Properties. In Proceedings of the 17th International Meeting on Chemical Sensors, Vienna, Austria, 15–19 June 2018. [Google Scholar]
- Mirzaei, A.; Bang, J.H.; Choi, M.S.; Han, S.; Lee, H.Y.; Kim, S.S.; Kim, H.W. Changes in characteristics of Pt-functionalized RGO nanocomposites by electron beam irradiation for room temperature NO2 sensing. Ceram. Int. 2020, 46, 21638–21646. [Google Scholar] [CrossRef]
- Kant, R.; Panwar, R.S. Synergistic effect of rGO with Co-doped ZnO in tuning dielectric and optical properties synthesized by two step chemical method. J. Mol. Struct. 2025, 1327, 141177. [Google Scholar] [CrossRef]
- Tuan, P.V.; Hung, N.D.; Ha, L.T. Effect of rGO on the microstructure, electrical, optical, and photocatalytic properties of ZnO/rGO nanorods. J. Mol. Struct. 2025, 1330, 141515. [Google Scholar] [CrossRef]
- Khamkhash, L.; Em, S.; Molkenova, A.; Hwang, Y.H.; Atabaev, T.S. Crack-Free and Thickness-Controllable Deposition of TiO2–rGO Thin Films for Solar Harnessing Devices. Coatings 2022, 12, 218. [Google Scholar] [CrossRef]
- Rehman, N.; Pandey, A.; Pandey, A. Preparation of a label-free and prompt immuno sensing of Salmonella enterica via electrochemical techniques. Sens. Biosens. Res. 2025, 48, 100789. [Google Scholar]
- Liu, Z.; Navik, R.; Tan, H.; Xiang, Q.; Wahyudiono; Goto, M.; Ibarra, R.M.; Zhao, Y. Graphene-based materials prepared by supercritical fluid technology and its application in energy storage. J. Supercrit. Fluids. 2022, 188, 105672. [Google Scholar] [CrossRef]
- Das, P.; Ibrahim, S.; Chakraborty, K.; Ghosh, S.; Pal, T. Stepwise reduction of graphene oxide and studies on defect-controlled physical properties. Sci. Rep. 2024, 14, 294. [Google Scholar] [CrossRef]
- Tucureanu, V.; Obreja, C.A.; Craciun, G.; Romanitan, C.; Mihailescu, C.M.; Stan, D.; Matei, A. Preparation and evaluation of nanocomposites based on transitional oxides and carbon materials for electrochemical applications. Ceram. Int. 2022, 48, 27201–27212. [Google Scholar] [CrossRef]
- Tucureanu, V.; Obreja, C.A.; Pachiu, C.; Brîncoveanu, O.; Matei, A. Synthesis and Characterization of Nanocomposites Based on Carbon Materials and Transitional Oxides. Mater. Proc. 2023, 14, 8. [Google Scholar]
- Mushahary, M.; Sarkar, A.; Basumatary, F.; Brahma, S.; Das, B.; Basumatary, S. Recent developments on graphene oxide and its composite materials: From fundamentals to applications in biodiesel synthesis, adsorption, photocatalysis, supercapacitors, sensors and antimicrobial activity. Results Surf. Interfaces 2024, 15, 100225. [Google Scholar] [CrossRef]
- Al-Moayid, S.M.; Algarni, H.; Elhosiny Ali, H.; Khairy, Y. Synthesis of reduced graphene oxide decorated with cuprite nanoparticles for energy-storage devices: Dielectric properties and electrical conductivity. Phys. Rev. B Condens. Matter. 2025, 714, 417490. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Bartolomeo, A.D.; Ramezan Zadeh, M.H.; Beitollahi, H.; Tajik, S. Hierarchical nanostructures of MgCo2O4 on reduced graphene oxide as a high-performance catalyst for methanol electro-oxidation. Ceram. Int. 2021, 47, 16079–16085. [Google Scholar] [CrossRef]
- Jagadeesh, P.; Rangappa, S.M.; Siengchin, S. Advanced characterization techniques for nanostructured materials in biomedical applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 122–143. [Google Scholar] [CrossRef]
- Pronin, I.A.; Averin, I.A.; Karmanov, A.A.; Yakushova, N.D.; Komolov, A.S.; Lazneva, E.F.; Sychev, M.M.; Moshnikov, V.A.; Korotcenkov, G. Control over the Surface Properties of Zinc Oxide Powders via Combining Mechanical, Electron Beam, and Thermal Processing. Nanomaterials 2022, 12, 1924. [Google Scholar] [CrossRef]
- Obreja, A.C.; Cristea, D.; Gavrila, R.; Schiopu, V.; Dinescu, A.; Danila, M.; Comanescu, F. Functionalized graphene/poly 3-hexyl thiophene based nanocomposites. In Proceeding of the International Semiconductor Conference CAS, Sinaia, Romania, 17–19 October 2011. [Google Scholar]
- Ayeshamariam, A.; Bououdina, M.; Sanjeeviraja, C. Optical, electrical and sensing properties of In2O3 nanoparticles. Mat. Sci. Semicon. Proc. 2013, 16, 686–695. [Google Scholar] [CrossRef]
- Pawar, K.K.; Desai, D.V.; Bodake, M.; Patil, H.S.; More, S.M.; Nimbalkar, A.S.; Mali, S.S.; Dongale, T.D. Highly reliable multilevel resistive switching in a nanoparticulated In2O3 thin-film memristive device. J. Phys. D Appl. Phys. 2019, 52, 175306. [Google Scholar] [CrossRef]
- Joseph Panneerdoss, I.; Johnson Jeyakumar, S.; Ramalingam, S.; Jothibas, M. Characterization of prepared In2O3 thin films: The FT-IR, FT-Raman, UV–Visible investigation and optical analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 147, 1–13. [Google Scholar] [CrossRef]
- Stokey, M.; Korlacki, R.; Knight, S.; Ruder, A.; Hilfiker, M.; Galazka, Z.; Irmscher, K.; Zhang, Y.; Zhao, H.; Darakchieva, V.; et al. Optical phonon modes, static and high-frequency dielectric constants, and effective electron mass parameter in cubic In2O3. J. Appl. Phys. 2021, 129, 225102. [Google Scholar] [CrossRef]
- Tucureanu, V.; Matei, A.; Avram, A.A. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef] [PubMed]
- All, A.; Abdel-Salam, A.I.; Salim, S.A.; Morsy, M. Tailoring porous structure composed of nano indium oxide and nano zinc ferrite to be act as an efficient humidity sensor. Appl. Phys. A. 2025, 131, 423. [Google Scholar] [CrossRef]
- Nasriddinov, A.; Tokarev, S.; Fedorova, O.; Bozhev, I.; Rumyantseva, M. In2O3 Based Hybrid Materials: Interplay between Microstructure, Photoelectrical and Light Activated NO2 Sensor Properties. Chemosensors 2022, 10, 135. [Google Scholar] [CrossRef]
- Halbos, R.J.; Al-Algawi, S.; Rasheed, R.T.; Hassan, R.A.; Mahdi, R.R.; Azeez, H.; Fayad, F.A. A Study of In2O3 Nano Particles for Gas Sensor Application. J. Fuzzy Syst. Control. 2024, 2, 135–139. [Google Scholar] [CrossRef]
- Shinde, D.V.; Ahn, D.Y.; Jadhav, V.V.; Lee, D.Y.; Shrestha, N.K.; Lee, J.K.; Lee, H.Y.; Mane, R.S.; Han, S.H. A coordination chemistry approach for shape-controlled synthesis of indium oxide nanostructures and their photoelectrochemical properties. J. Mater. Chem. A 2014, 2, 5490–5498. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, S.; Xie, P.; Gao, Z.; Zou, R. Preparation, characterization and photocatalytic activity of juglans-like indium oxide (In2O3) nanospheres. Mater. Lett. 2017, 192, 76–79. [Google Scholar] [CrossRef]
- Carvalho, M.H.; Piton, M.R.; Lemine, O.M.; Bououdina, M.; Galet, H.V.A.; Pereira, E.C.; Galvão Gobato, Y.; Ade Oliveira, A.J. Effects of strain, defects and crystal phase transition in mechanically milled nanocrystalline In2O3 powder. Mater. Res. Express 2019, 6, 025017. [Google Scholar] [CrossRef]
- Uma, K.; Chong, S.; Mohan, S.C.; Jothivenkatachalam, K.; Yang, T.C.K.; Lin, J.H. Multi-functional RGO-supported α-Fe2O3 nanocomposites for high-performance pseudocapacitors and visible light–driven photocatalytic applications. Ionics 2020, 26, 3491–3500. [Google Scholar] [CrossRef]
- Romanitan, C.; Tudose, I.V.; Mouratis, K.; Popescu, M.C.; Pachiu, C.; Couris, S.; Koudoumas, E.; Suchea, M. Structural Investigations in Electrochromic Vanadium Pentoxide Thin Films. Phys. Status Solidi A 2022, 219, 2100431. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Samoila, P.; Airinei, A.; Olaru, N.; Rotaru, A.; Romanitan, C.; Tudoran, L.B.; Suchea, M. Cu/TiO2 composite nanofibers with improved photocatalytic performance under UV and UV–visible light irradiation. Surf. Interfaces 2022, 28, 101644. [Google Scholar] [CrossRef]
- Marinas, I.C.; Gradisteanu Pircalabioru, G.; Oprea, E.; Geana, E.I.; Zgura, I.; Romanitan, C.; Matei, E.; Angheloiu, M.; Brincoveanu, O.; Georgescu, M.; et al. Physico-chemical and pro-wound healing properties of microporous cellulosic sponge from Gleditsia triacanthos pods functionalized with Phytolacca americana fruit extract. Cellulose 2023, 30, 10313–10339. [Google Scholar] [CrossRef]
- De Lima, B.S.; Komorizono, A.A.; Ndiaye, A.L.; Bernardi, M.I.B.; Brunet, J.; Mastelaro, V.R. Tunning the Gas Sensing Properties of rGO with In2O3 Nanoparticles. Surfaces 2022, 5, 127–142. [Google Scholar] [CrossRef]
- Song, J.W.; Fan, L.W. Temperature dependence of the contact angle of water: A review of research progress, theoretical understanding, and implications for boiling heat transfer. Adv. Colloid Interface Sci. 2021, 288, 102339. [Google Scholar] [CrossRef]
- Ubuo, E.E.; Udoetok, I.A.; Tyowua, A.T.; Ekwere, I.O.; Al-Shehri, H.S. The Direct cause of Amplified Wettability: Roughness or Surface Chemistry? J. Compos. Sci. 2021, 5, 213. [Google Scholar] [CrossRef]
- Zulkharnay, R.; Ualibek, O.; Toktarbaiuly, O.; May, P.W. Hydrophobic behaviour of reduced graphene oxide thin film fabricated via electrostatic spray deposition. Bull. Mater. Sci. 2021, 44, 112. [Google Scholar] [CrossRef]
- Ryl, J.; Cieslik, M.; Zielinski, A.; Ficek, M.; Dec, B.; Darowicki, K.; Bogdanowicz, R. High-Temperature Oxidation of Heavy Boron-Doped Diamond Electrodes: Microstructural and Electrochemical Performance Modification. Materials 2020, 13, 964. [Google Scholar] [CrossRef]
- Metikos-Hukovic, M.; Omanovic, S. Thin indium oxide film formation and growth: Impedance spectroscopy and cyclic voltammetry investigations. J. Electroanal. Chem. 1998, 455, 181–189. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).