Abstract
This study reports the preparation of benzalkonium chloride-modified montmorillonite (MMT-1227) via a wet chemical method and systematically investigates its structural characteristics and antimicrobial/antifungal properties. The modified montmorillonite was comprehensively characterized using X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), thermogravimetric analysis (TGA), and Brunauer–Emmett–Teller (BET) surface area analysis. The results confirmed the successful intercalation of benzalkonium chloride into montmorillonite layers, leading to altered surface morphology, increased interlayer spacing, and enhanced hydrophobicity. Antimicrobial assays demonstrated that MMT-1227 exhibits potent activity against both Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus, with inhibition zone diameters of 15.6 ± 0.2 mm and 17.7 ± 0.2 mm, respectively, and minimum inhibitory concentrations (MIC) of 1 mg/mL and 0.5 mg/mL. When incorporated into latex paint at a mass fraction of 0.3%, MMT-1227 achieved a 99.9% antibacterial rate against both strains after 24 h. Additionally, fungal resistance testing in accordance with GB/T 1741-2020 revealed that the modified paint films completely inhibited the growth of eight common mold strains (e.g., Aspergillus niger, Trichoderma viride), achieving a resistance grade of 0. These findings validate that benzalkonium chloride modification endows montmorillonite with excellent antimicrobial and antifungal properties, highlighting its potential as a high-performance additive for functional coatings and related antimicrobial materials.
1. Introduction
With the heightened awareness and concerns regarding environmental and health issues, the quest for safe and effective antibacterial materials has gained significant attention in recent years. One such material that holds promise is montmorillonite, a naturally occurring mineral with wide-ranging applications in various domains. It exhibits exceptional adsorption capabilities and chemical stability, rendering it highly suitable for potential applications in environmental protection, pharmaceuticals, and the food industry. However, the inherent limited antibacterial activity of montmorillonite hinders its advancement in the field of antibacterial applications. Consequently, researchers have turned their attention towards modifying montmorillonite through the incorporation of antibacterial agents, with the goal of developing composite materials that exhibit enhanced antibacterial activity.
Sodium bentonite, primarily composed of the mineral smectite, is a non-metallic mineral resource. The smectite structure is characterized by a 2:1 layered crystalline structure, which consists of two tetrahedral silica sheets interleaved with an octahedral alumina sheet. Owing to the labile nature of certain cations within the smectite lattice that are prone to exchange with other cations, numerous researchers have employed cation exchange methods to modify smectite. By intercalating Cu2+ and Ag+ ions into the smectite matrix, these modified forms of smectite have been evaluated for their antimicrobial activity. Specifically, smectites loaded with Cu2+ and Ag+ have demonstrated potent antibacterial effects against Escherichia coli and Staphylococcus aureus [1]. Metal ions can be utilized not only for individual modification but also in conjunction with organic compounds to modify montmorillonite. Shameli, Kamyar et al. [2] synthesized a silver/montmorillonite/chitosan bionanocomposite material (Ag/MMT/Cts BNC) via a wet chemical reduction method. All synthesized Ag/MMT/Cts BNCs exhibited significant antimicrobial activity against both Gram-positive bacteria, such as Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA), and Gram-negative bacteria, including Escherichia coli, E. coli O157:H7, and Pseudomonas aeruginosa. Capitalizing on the cation exchange capacity of montmorillonite, certain cationic antimicrobial agents effectively modify the montmorillonite, endowing the modified montmorillonite with superior antibacterial activity against Gram-positive bacteria (Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli). The antimicrobial efficacy is found to intensify with an increase in the mass fraction of the organic substance [3]. The modification of montmorillonite with cetylpyridinium (CP) confers antimicrobial activity to the clay mineral, reducing its irritancy and enhancing the durability of its antimicrobial effect [4]. A novel material has been prepared by the adsorption of sodium lauroyl sarcosinate (SR) onto cetylpyridinium-modified montmorillonite. This material exhibits favorable antibacterial effects against Staphylococcus aureus and Escherichia coli, making it suitable for applications in antimicrobial liquid soaps, toothpaste formulations, personal care products, and topical treatments for acne and wounds without adversely affecting the physicochemical and washing properties of the materials. Verma, Anurakshee et al. [5] have successfully achieved the intercalation and in situ polymerization of poly(o-phenylenediamine) (POPD) within montmorillonite (MMT) using sonochemical techniques, resulting in the formation of montmorillonite/poly(o-phenylenediamine) (MMT/POPD) nanocomposites (NCs). The effective concentration (EC_(50)) and minimum inhibitory concentration (MIC) of the NCs have been determined, suggesting their potential as antimicrobial agents. Modified Bentonite-Intercalated MMT (MBI-MMT) exhibits high efficacy against Gram-positive bacteria (e.g., Staphylococcus aureus) and fungi, while demonstrating limited effectiveness against Gram-negative bacteria (e.g., Escherichia coli). The increased interlayer spacing of modified MBI-MMT confirms successful molecular intercalation [6]. The ultrasonication-assisted preparation of ε-polylysine-modified montmorillonite (PL-Mt) enhances the antimicrobial efficacy of this food-grade agent, particularly against common pathogens [7].
The antimicrobial efficacy of cotton fibers treated with quaternary ammonium-modified montmorillonite (A-MMT) demonstrates concentration-dependent enhancement, with optimal performance against both Staphylococcus aureus and Escherichia coli achieved at 10% A-MMT loading [8].
Benzalkonium chloride, recognized for its broad-spectrum antimicrobial activity and enduring efficacy, is extensively utilized in the medical and agricultural sectors. Consequently, the modification of montmorillonite with benzalkonium chloride through a wet chemical method has been employed to fabricate composite materials with antimicrobial properties. Subsequently, the modified montmorillonite samples will undergo characterization analysis and antimicrobial performance assessment. These endeavors aim to explore novel preparation methods for antimicrobial composite materials, holding promise for a significant enhancement of the antimicrobial capabilities of montmorillonite.
2. Materials and Methods
2.1. Experimental Materials
Montmorillonite was purchased from Zhejiang Fenghong New Materials Co., Ltd. (Huzhou, China). Benzalkonium chloride (1227, 99%) was purchased from Wuhan Kemike Biomedical Technology Co., Ltd. (Wuhan, China). Polycarboxylate sodium salt dispersant (SN5040) was purchased from San Nopco (Shanghai) Trading Co., Ltd. (Shanghai, China). Wetting agent (BD109), cellulose ether (HBR250), and multi-functional additive (AMP-95) were purchased from Dow Chemical (China) Investment Co., Ltd. (Shanghai, China). Mineral defoamer (BYK-1630) was purchased from BYK Additives & Instruments (Shanghai) Co., Ltd. (Shanghai, China). It should be noted that in order to test antimicrobial activity, bacterial strains were obtained from BeNa Culture Collection (Suzhou, China). All other chemicals were commercially obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and used without any further purification.
2.2. Experimental Equipment
Vertical pressure steam sterilizer (Jiangsu Dengguan Medical Equipment Co., Ltd., Changzhou, China), Biochemical incubator and Ultrapure water machine and Constant temperature and humidity chamber (Shanghai Lichengbangxi Instrument Technology Co., Ltd., Shanghai, China), Laminar flow cabinet (Shanghai Shangdao Instrument Manufacturing Co., Ltd., Shanghai, China), Centrifuge (Hunan Kaida Scientific Instrument Co., Ltd., Changsha, China), Electronic balance (Ohaus Instrument Changzhou Co., Ltd., Changzhou, China), High-speed disperser (Biaoguda Precision Instrument Co., Ltd., Guangzhou, China).
2.3. Latex Paint Formulation (Mass Fraction)
Tap water30%, SN5040 0.4%, Dow BD109 0.1%, Mineral defoamer 0.2%, Propylene glycol 1%, AMP-95 0.1%, Titanium dioxide 7%, Heavy calcium carbonate 41%, Acrylic emulsion 4%, Film-forming aid (C-12 alcohol ester) 1%, Propylene glycol 1%, MMT-1227 0.3%.
2.4. Preparation of Ag-Exchanged Montmorillonites
Montmorillonite was dispersed in tap water and mixed with stirring for 10 min, followed by pH adjustment to 6. The resulting slurry was heated to 75 °C with continuous stirring. Afterwards, 10 wt% Benzalkonium chloride (1227) was added and reacted for 2 h; a dispersant agent was then introduced, the reaction continued for 0.5 h. Upon reaction completion, the product underwent thorough washing, suction filtration, and drying. Final processing included pulverization to obtain modified montmorillonite designated as MMT-1227.
2.5. Multimodal Characterization: SEM, XRD, FTIR, TGA, and BET
Scanning electron microscope (SEM) (Thermofisher FEI Apreo 2S, Thermo Fisher Scientific, Waltham, MA, USA) was evaluated to catch SEM images. X-ray diffraction (XRD) analysis was performed on the samples at ambient temperature using a D8-Advance diffractometer (Bruker AXS GmbH, Ettlingen, Germany) with Cu Kα radiation (λ = 1.5406 Å). The measurements were conducted at 40 kV tube voltage and 40 mA tube current, scanning 10–80° 2θ at 0.0057° steps. The ATR-FTIR was performed using a PerkinElmer Spectrum IR spectrometer (PerkinElmer, Waltham, MA, USA), which is equipped with an ATR accessory covering a spectral range from 4000 to 450 cm−1. For each sample, 16 scans were recorded in transmission mode with a resolution of 4 cm−1. Approximately 10 mg of dried modified montmorillonite was weighed. Thermogravimetric analysis (TGA) was performed using a simultaneous DSC-TGA instrument (SDT Q600, TA Instruments, New Castle, DE, USA). Measurements were conducted under ultra-high-purity N2 atmosphere (50 mL/min flow rate). The temperature program executed a constant heating ramp of 10 °C/min from 25 °C to 800 °C. The surface area and total pore volume of dried montmorillonite powder were analyzed using a full-automatic surface area and pore size analyzer (Micromeritics ASAP 2460 Version 3.01) based on isothermal N2 adsorption at 77 K.
2.6. Antibacterial Activity Tests
2.6.1. Determination of the Diameter of the Inhibition Zone
The antimicrobial activity was assessed using the disk diffusion (Kirby–Bauer) method. Sample powders were formed into circular disks measuring 6mm in diameter, with triplicate trials conducted for each test.
The dried MMT-1227 and unmodified MMT samples were individually placed in an agate mortar and manually ground for 20 min until a fine, particle-free powder was obtained. The resulting powder was sieved through a 200-mesh (pore size 75 μm) nylon sieve. The sieved powder was transferred to a glass Petri dish and dried in a vacuum oven at 60 °C for 2 h to remove adsorbed trace moisture, followed by cooling to room temperature for subsequent use.
A custom-made stainless-steel tableting mold was employed, consisting of a bottom gasket (diameter: 6.0 mm) and a cylindrical punch (diameter: 5.9 mm, slightly smaller than the inner diameter of the gasket to reserve space for powder filling). Prior to use, the mold was disinfected by wiping with 75% ethanol, followed by autoclaving at 121 °C for 20 min, and then dried for standby.
An electronic balance (precision: 0.1 mg) was used to weigh the pretreated sample powder, with each tablet controlled to a mass of 20 ± 1 mg. Preliminary pre-experiments verified that this mass ensures sufficient release of antibacterial components from the compressed tablet while avoiding the blurred edges of the inhibition zone caused by excessive sample loading. The weighed powder was uniformly poured into the bottom gasket of the mold, which was then placed on a manual tablet press (pressure range: 0–50 kN). A pressure of 5 kN was applied slowly and maintained for 30 s, followed by gradual pressure release. The formed circular tablets were removed and stored in sterile Petri dishes for subsequent experiments.
The bacterial strains E. coli ATCC8739 and S. aureus 6538P were inoculated onto Mueller–Hinton (MH) agar plates and incubated at 37 °C for 24 h. Three to four pure colonies were selected and suspended in sterile 0.9% sodium chloride solution to create a uniform suspension, which was then matched in turbidity to a 0.5 McFarland standard unit.
A sterile cotton swab was used to apply a small amount of bacterial solution evenly onto the agar surface. Once dry, sterile forceps were used to place the test samples uniformly onto the surface of the medium, pressing gently in the center to ensure secure attachment. The plates were then incubated at 35 °C ± 1 °C for 18 h, after which the plates were retrieved, and the size of the inhibition zones was measured.
2.6.2. Determination of the Minimum Inhibitory Concentration (MIC)
The MIC of the modified montmorillonite against different bacterial strains was determined using the microdilution method. Initially, the modified montmorillonite was mixed with broth at varying concentrations, and then each mixture was inoculated with the corresponding bacterial strain. After a set incubation period, the lowest concentration of the mixture showing no bacterial growth was identified to determine the MIC.
2.6.3. Antimicrobial Testing of Paint Films
The antimicrobial testing of paint films was performed in accordance with ISO 22196-2011 [9]. Bacteria were quantitatively inoculated onto the test panels, and a film overlay method was used to ensure uniform contact between the bacteria and the panels. After a specified period of incubation, the viable bacterial count on the panels was determined, and the antibacterial rate was calculated.
Escherichia coli and Staphylococcus aureus strains were inoculated onto Mueller–Hinton (MH) agar plates and incubated at 37 °C for 24 h. Three to four pure colonies were selected and suspended in sterile 0.9% sodium chloride solution to form a homogeneous suspension. The turbidity of the suspension was adjusted to match a 0.5 McFarland standard. The bacterial suspension was then diluted to a concentration of 1–5 × 105 CFU/mL. Four hundred microliters (400 µL) of the diluted bacterial suspension was dropped onto different latex paint panels, covered with a sterilized overlay film, and incubated in a constant temperature and humidity chamber at (35 ± 1) °C with a relative humidity (RH) of 95% for 24 h. After incubation, the panels were rinsed repeatedly with SCDPL solution to elute the bacteria, and the viable bacterial count was determined.
The antibacterial rate was calculated using the following formula:
antibacterial rate (%) = [(Ct − Tt)/Ct] × 100
Ct: The mean viable bacterial count of the control samples after 24 h (CFU/cm2).
Tt: The mean viable bacterial count of the test samples after 24 h (CFU/cm2).
2.6.4. Fungal Resistance Testing of Paint Films
The fungal resistance of paint films was evaluated in accordance with GB/T 1741-2020 [10]. The test simulated the environmental conditions conducive to fungal growth. Fungal spores were inoculated onto the surface of the test samples, which were then cultured under conditions favorable for fungal growth. The growth of fungi on the sample surfaces was observed, and the fungal resistance of the paint films was assessed and graded based on the degree of fungal growth on the samples.
Spores of Aspergillus niger, Aspergillus flavus, Penicillium verrucosum, Penicillium aurantiogriseum, Penicillium citrinum, Trichoderma viride, Aureobasidium pullulans, and Alternaria alternata were selected from the preserved strains and inoculated onto fresh PDA (Potato Dextrose Agar) culture media. The cultures were allowed to develop until the surfaces were covered with spores. Fungal spore suspensions were prepared under sterile conditions using sterile water. The spore concentration was adjusted to 1.0 × 106 spores per milliliter through centrifugation and dilution.
Latex paint samples were applied to test panels measuring 50 mm × 50 mm. After curing at room temperature for 7 days, the mixed spore suspension was inoculated onto the surface of the test samples using either the Petri dish method or the hanging method. The samples were placed in Petri dishes containing nutrient salt agar medium. Four hundred microliters (400 µL) of the spore suspension was sprayed onto the samples, which were then cultured. The inoculated samples were placed in a constant temperature and humidity incubator maintained at 25 °C with a relative humidity (RH) of 90%. After 7 days, the fungal growth on the control samples was examined to ensure the validity of the test. The samples were then further cultured for a total of 28 days. Subsequently, the test samples were visually inspected and examined under a microscope. The area of fungal growth was calculated using ImageJ 1.8.0 software. The fungal resistance grade of the samples was determined based on the percentage of the surface covered by fungi, in accordance with the fungal resistance grading table (Table 1).

Table 1.
Evaluation of Results.
3. Results and Discussion
3.1. SEM Analysis
Figure 1 presents the SEM morphologies of unmodified MMT and 1227-modified MMT (MMT-1227). Unmodified MMT (Figure 1a–c) displays irregular aggregates with coarse surfaces and scattered pores, where particle stacking occurs due to interlayer forces and surface adsorption. After 1227-modification (Figure 1d–f), the material exhibits reduced agglomeration, enhanced dispersion, and a more compact texture with regularly arranged pores. MMT-1227 has a narrower size distribution of 1–5 μm, compared to pristine MMT with a size distribution of 5–20 μm, which confirms improved dispersion. The cationic quaternary ammonium salt 1227 binds to negatively charged MMT layers, reducing electrostatic attraction while introducing steric hindrance, thereby improving platelet dispersion. These morphological alterations confirm successful MMT modification by 1227.
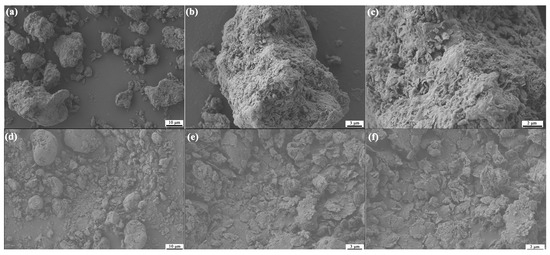
Figure 1.
The SEM micrographs of MMT and MMT-1227 ((a–c): MMT; (d–f): MMT-1227).
3.2. XRD Analysis
XRD analysis of benzalkonium chloride-modified MMT reveals the disappearance of characteristic peaks at 38.82° (SiO2 (101)), 56.07° (CaCO3 (202)), and 59.86° (Fe2O3 (214)). This phenomenon is attributed to a tripartite structural evolution: (i) acidic dissolution of impurity phases (silicates/carbonates/oxides); (ii) cation exchange where C21H38N+ displaces interlayer cations, altering electrostatic environments and reducing long-range order through delamination-restacking reorientation; (iii) local phase transformations and crystal defect generation during modification [11]. Collectively, these changes confirm successful 1227 intercalation into MMT interlayers (Figure 2).
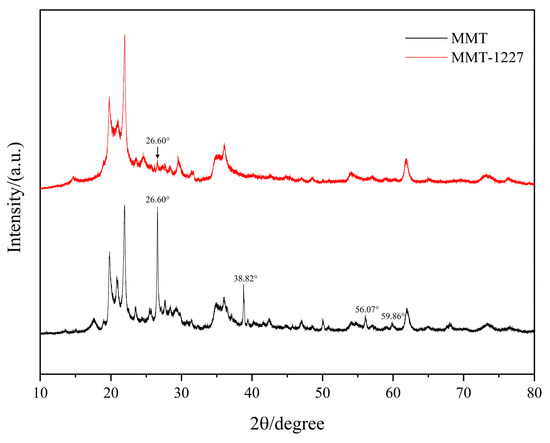
Figure 2.
The Comparative XRD Patterns of MMT and MMT-1227.
3.3. ATR-FTIR Study
Natural montmorillonite (MMT) exhibits intense absorption bands in the low-wavenumber region (1200–500 cm−1), characteristic of Si-O vibrations. The intense peak near 1000 cm−1 is primarily assigned to Si-O-Si stretching vibrations, while peaks in the 500-600 cm−1 region correspond to Si-O bending modes [12]. These characteristic sharp and intense bands reflect the inherent structure of MMT’s tetrahedral sheets, indicating a well-ordered crystalline framework. In the mid-to-high wavenumber region (2500–4000 cm−1), the MMT spectrum exhibits relatively weak features, with characteristic peaks at 3400 cm−1 and 3623 cm−1 corresponding to O-H stretching of adsorbed interlayer/surface water and structural hydroxyl groups (crystallographic O-H), respectively [13].
Following modification with benzalkonium chloride (1227), the MMT-1227 spectrum exhibits distinctive new bands in the 2800–3000 cm−1 region. The absorption peaks at 2926.86 cm−1 and 2852 cm−1 are assigned to C-H stretching vibrations of saturated alkyl groups (-CH2-, -CH3) [14]. These diagnostic infrared bands constitute direct evidence of 1227 incorporation, with the characteristic C-H stretching vibrations confirming successful intercalation and surface adsorption via cation exchange within the MMT structure. Concurrently, the disappearance of the 3400 cm−1 band suggests reduced water absorption, likely resulting from 1227-induced polarity modification of MMT surfaces and interlayers [15]. The incorporated long-chain alkyl groups not only impart hydrophobicity but may also enhance antimicrobial efficacy through charge interactions and membrane disruption mechanisms [16]. Benzalkonium chloride modification fundamentally alters MMT’s surface chemistry and inorganic framework vibrations by introducing alkyl functionalities, thereby establishing the molecular basis for enhanced antibacterial performance in MMT-1227 (Figure 3).
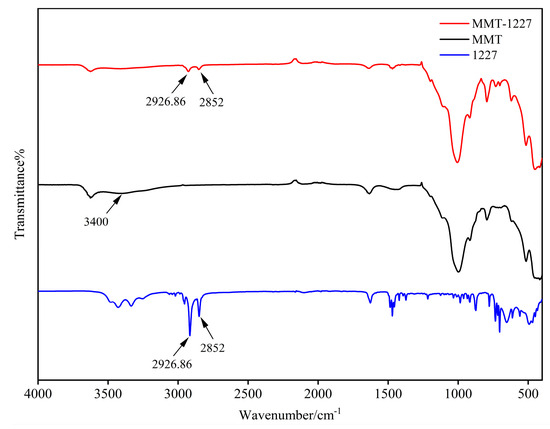
Figure 3.
Infrared spectroscopy of MMT, MMT-1227, and 1227.
3.4. Thermogravimetric Analysis (TGA)
MMT exhibits a three-stage thermal decomposition profile: (1) 7% mass loss at 32–100 °C due to the desorption of physisorbed water from hydrophilic surfaces/pores; [17] (2) 2% mass loss at 100–160 °C from release of weakly bound interlayer water; (3) gradual 3% mass loss at 160–800 °C via structural dehydroxylation (OH− elimination as H2O from octahedral sheets) accompanied by silicate framework reorganization [18].
MMT-1227 exhibits a distinctive four-stage thermal decomposition profile, contrasting with MMT and reflecting benzalkonium chloride (1227) modification effects: (1) at 32–75 °C, 2% mass loss (100%→98%) from reduced surface water adsorption due to hydrophobic alkyl chains; (2) a thermal plateau (98%→97%) between 75 and 180 °C signifying stabilized organic-water complexes through weak alkyl-water interactions; (3) rapid 14% mass loss (97%→83%) during 180–410 °C resulting from 1227 decomposition via alkyl chain pyrolysis/oxidation and quaternary ammonium degradation; (4) gradual 6% mass loss (83%→77%) from 410 to 800 °C arising from combined structural dehydroxylation and carbonaceous residue decomposition, where char-mineral interactions retard degradation while lowering final mass retention versus MMT [19,20].
TGA curves distinctly reveal thermal behavior differences post-modification: the rapid mass loss in MMT-1227’s intermediate-temperature regime (180–410 °C) confirms successful benzalkonium chloride grafting, while the shifted decomposition stage and increased mass loss rate demonstrate that 1227 modification significantly alters montmorillonite’s decomposition pathways and thermal stability profile through introduced organic moieties (Figure 4).
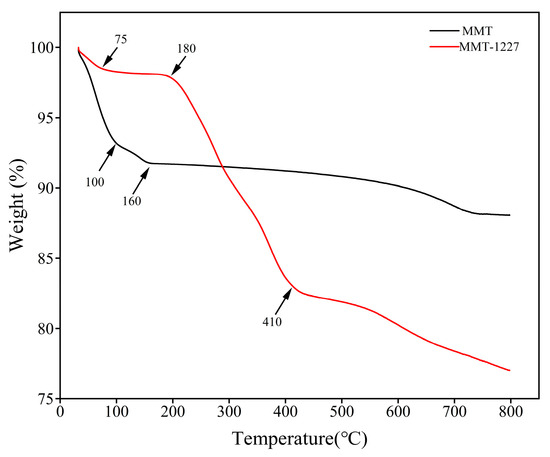
Figure 4.
The TGA/TG curve of MMT and MMT-1227.
3.5. Gas Sorption Analysis: SSA and PSD by BET/BJH Methods
MMT exhibits a Type IV isotherm with an H3 hysteresis loop per the IUPAC classification [21]. The gradual uptake at low relative pressures (p/p0 < 0.4) indicates monolayer-multilayer adsorption on homogeneous active sites (e.g., hydroxyls, unsaturations), reflecting low surface porosity [22]. At intermediate-high pressures (0.4 < p/p0 < 1.0), rapid adsorption increase corresponds to capillary condensation within slit-shaped mesopores (2–50 nm) and macropores (>50 nm) in interlayer domains and particle-stacked channels. The H3 hysteresis—characterized by delayed desorption from ink-bottle pores (narrow-necked/wide-bodied structures) due to necking effects—confirms slit-shaped and disordered pores as dominant geometries in platelet-stacked MMT [23].
The 1227-MMT similarly exhibits a Type IV isotherm but with distinct modifications reflecting pore structure regulation by benzalkonium chloride: at low relative pressures (p/p0 < 0.4), reduced adsorption including negative initial uptake (~−10 cm3/g STP) suggests nitrogen displacement due to enhanced hydrophobicity. The long alkyl chains create hydrophobic surfaces with weaker N2 interactions versus hydrophilic MMT, impeding adsorption, and potentially causing negative adsorption from residual hydrophobic microenvironments post-degassing [24,25]. At intermediate-high pressures (0.4 < p/p0 < 1.0), diminished adsorption and significantly reduced hysteresis loop area arise from the following: (1) alkyl chain occupation of interlayer spaces reducing effective pore volume [26,27]; (2) tighter platelet stacking via hydrophobic interactions decreasing particle-stacked channels. Consequently, attenuated capillary condensation and contracted hysteresis indicate more uniform pore geometries from organic modification [28].
Nitrogen adsorption–desorption isotherm analysis demonstrates that pristine montmorillonite (MMT) displays a characteristic Type IV isotherm with an H3 hysteresis loop, attributable to its hydrophilic surface and slit-shaped mesopores formed by lamellar stacking, resulting in high adsorption capacity ideal for applications demanding a high specific surface area and mesoporosity (e.g., wastewater treatment adsorbents). Conversely, benzalkonium chloride-modified MMT (1227-MMT) exhibits reduced adsorption capacity and a constricted hysteresis loop due to altered surface properties and pore structure following the incorporation of hydrophobic alkyl chains. This modification reflects pore structure alteration through organic-inorganic interfacial interactions, including pore filling and structural reorganization. These findings provide fundamental surface science insights into the structure–property relationships of organo-modified clays and offer valuable guidance for optimizing their applications in adsorption processes and composite materials.
Pore size distribution analysis reveals that montmorillonite (MMT) exhibits a unimodal profile with a highly constrained dominant pore size range (10–20 Å), corresponding to a differential pore volume peak of approximately 0.0020 cm3/g·Å. This characteristic stems from MMT’s lamellar silicate structure, where slit-shaped pores formed within interlayer spaces through cation exchange and water molecule adsorption exhibit size limitations dictated by basal spacing (typically 10–20 Å, depending on hydration state). Macropores (>500 Å) originating from particle stacking contribute negligibly, as evidenced by the rapid attenuation of differential pore volume. Collectively, these features confirm that interlayer slit pores constitute the principal pore type in MMT, resulting in exceptionally narrow and homogeneous pore size distribution [29,30].
Pore size distribution analysis indicates that benzalkonium chloride-modified montmorillonite (1227-MMT) exhibits a broadened peak profile, with the dominant pore size shifting to the mesoporous range (50–200 Å). This is evidenced by a significantly reduced differential pore volume peak (≈0.0004 cm3/g·Å) and wider distribution compared to pristine MMT. The pore structure modification mechanism involves two concurrent processes: (1) Interlayer pore filling and restructuring—benzalkonium chloride molecules (long-chain alkyl quaternary ammonium salts) occupy interlayer spaces through cation exchange, reducing small-sized interlayer pores (<50 Å) and diminishing the differential pore volume peak; (2) Altered particle stacking—hydrophobic interactions between alkyl chains induce the restructuring of lamellar stacking configurations, transforming interparticle pores from slit-shaped geometries to irregular mesopores. This dual mechanism consequently increases pore volume contribution within the 50-200 Å range and broadens the overall pore size distribution [31].
Pore size distribution analysis demonstrates that benzalkonium chloride modification induces the intercalation-driven restructuring of the montmorillonite pore architecture, resulting in broadened dominant pore size and widened distribution. This process facilitates a distinct transformation from narrowly distributed interlayer pores to broadly dispersed mesopores.
3.6. Antibacterial Tests
The antimicrobial effect of MMT-1227 against Escherichia coli shows that a clear inhibition zone is formed around the MMT-1227 sample with neat edges (without serrations or blurred areas), indicating the uniform diffusion of antimicrobial components and a stable bacteriostatic effect. The diameter of the inhibition zone is 15.6 ± 0.2 mm (Figure 5a). MMT-1227 exhibits a significant inhibitory effect on Escherichia coli. The antimicrobial effect of MMT-1227 against Staphylococcus aureus shows that the diameter of the inhibition zone of MMT-1227 against Staphylococcus aureus is 17.7 ± 0.2 mm (Figure 5b). The edge of the inhibition zone is also neat, and there is no residual bacterial growth in the transparent area, indicating a stronger inhibitory effect against Gram-positive bacteria. In contrast, no inhibition zone is formed around the unmodified MMT sample (the diameter is recorded as NA), and bacteria on the agar surface grow uniformly to the edge of the sample (Figure 5a,b), confirming that unmodified montmorillonite has no antimicrobial activity per se, and the antimicrobial property of MMT-1227 is entirely attributed to the introduction of benzalkonium chloride.

Figure 5.
The analysis curves of the specific surface area and pore size distribution of MMT and MMT-1227. (a) Nitrogen adsorption-desorption isotherms and (b) pore size distribution curves of MMT and MMT-1227.
The difference in the inhibition zones of MMT-1227 against the two bacteria (Staphylococcus aureus > Escherichia coli) is closely related to the structure of bacterial cell walls. The cell wall of Gram-positive bacteria (such as Staphylococcus aureus) is mainly composed of a thick layer of peptidoglycan (accounting for 50%–80% of the dry weight of the cell wall). Peptidoglycan chains form a network structure through cross-linking, and the surface is rich in negatively charged teichoic acids. In contrast, the cell wall of Gram-negative bacteria (such as Escherichia coli) has a thin peptidoglycan layer (accounting for only 10%–20%) with an outer lipopolysaccharide membrane, which exhibits lower permeability to cationic substances [32].
Benzalkonium chloride (1227), as a cationic surfactant, can preferentially adsorb to the teichoic acids on the surface of Gram-positive bacteria through electrostatic interactions, more easily disrupting the integrity of their cell walls, resulting in a larger diffusion range of antimicrobial components and thus forming a wider inhibition zone. In addition, SEM analysis shows that the dispersibility of MMT-1227 particles is better than that of unmodified MMT (Figure 1), reducing agglomeration, which makes it easier for benzalkonium chloride to be released from the interlayers of montmorillonite and diffuse into the agar—this is also an important reason for the formation of a clear inhibition zone.
BET analysis (Figure 4) shows that unmodified MMT is dominated by interlayer pores with a narrow distribution of 10–20 Å, while the pore size of MMT-1227 is widened to the range of mesopores (50–200 Å) with a more uniform pore distribution. This change in pore structure is due to the filling and stacking of long-chain alkyl groups of 1227 between the layers. On one hand, mesopores can serve as “storage channels” for 1227, slowing down its release rate; on the other hand, the more open pore structure reduces the diffusion resistance of the antimicrobial agent, enabling 1227 to diffuse more efficiently from the interior of the material to the bacterial surface. For Staphylococcus aureus with a thicker cell wall (containing a large amount of negatively charged teichoic acids), this optimized diffusion efficiency can accelerate the electrostatic adsorption and membrane disruption processes of 1227.
MMT-1227 exhibited distinct inhibitory effects against E. coli and S. aureus, with inhibition zone diameters measuring 15.6 mm and 17.7 mm, respectively (Table 2). In contrast, the control group with untreated MMT demonstrated no inhibition zones, indicating the pronounced antimicrobial efficacy of MMT-122 (Figure 6).

Table 2.
Zone of inhibition test results, all reported data correspond to the mean value.
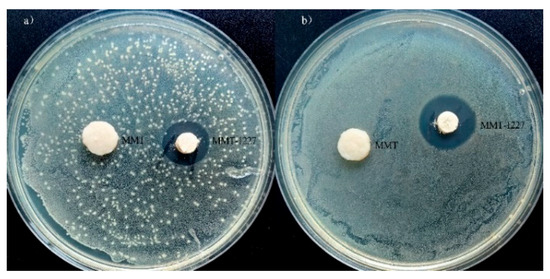
Figure 6.
Inhibition zone test results for (a) E. coli and (b) S. aureus.
3.7. Minimum Inhibitory Concentration
The Minimum Inhibitory Concentration (MIC) of MMT-1227 against the two bacterial strains was determined using the dilution method. The experiment set up a concentration gradient ranging from 0.125 to 128 mg/mL, with each gradient concentration inoculated with a bacterial suspension of 5 × 105 CFU/mL. After incubation at 37 °C for 24 h, the lowest concentration at which no visible bacterial growth was observed was defined as the MIC.
The results (Table 3) showed that the MIC of MMT-1227 against Escherichia coli was 1 mg/mL, and against Staphylococcus aureus was 0.5 mg/mL, which were significantly lower than that of unmodified MMT (>128 mg/mL). This result quantitatively confirms the substantial enhancement of the antimicrobial activity of the modified material, with the MIC value reduced by approximately 128 to 256 times. The lower MIC value for Staphylococcus aureus further confirms its high sensitivity to MMT-1227, which is presumably related to the difference in the interaction strength between the cationic properties of benzalkonium chloride (C21H38N+) and the anionic sites on the bacterial cell membrane. The cell membrane of Gram-positive bacteria contains more teichoic acids, which form tighter electrostatic bonds with cationic antimicrobial agents, resulting in the inhibition of their growth at lower concentrations.

Table 3.
Minimum Inhibitory Concentration (MIC) results.
The Minimum Inhibitory Concentration (MIC) of MMT-1227 against E. coli and S. aureus was determined to be 1 mg/mL and 0.5 mg/mL, respectively, whereas the MIC for the unmodified montmorillonite (MMT) was greater than 128 mg/mL for both organisms. These results indicate that the modification of montmorillonite with MMT-1227 confers a significant antimicrobial effect.
3.8. Antimicrobial Test
In accordance with the ISO 22196-2011 standard, the bactericidal effect of latex paint films containing 0.3% MMT-1227 against bacteria was evaluated. The latex paint was applied to test panels of 50 mm × 50 mm, and after curing for 7 days, 400 μL of bacterial suspension with a concentration of 1–5 × 105 CFU/mL was inoculated. A sterile film was covered to ensure sufficient contact, and the panels were incubated at 35 ± 1 °C with a relative humidity of 95% for 24 h.
The results (Table 4) showed that the number of Escherichia coli and Staphylococcus aureus in the control group proliferated from the initial 1.5 × 105 CFU/cm2 and 2.3 × 105 CFU/cm2 to 5.6 × 106 CFU/cm2 and 7.6 × 106 CFU/cm2 within 24 h, respectively. In contrast, the number of viable bacteria in the test group containing 0.3% MMT-1227 was only 0.79 CFU/cm2 and 0.63 CFU/cm2, with an antibacterial rate of 99.9% for both.

Table 4.
The antimicrobial performance of the dried latex paint film.
This result confirms that MMT-1227 can effectively inhibit bacterial proliferation in the latex paint system, and its antibacterial activity is not significantly reduced due to the encapsulation of the coating matrix, indicating that this modified montmorillonite has good applicability in practical applications such as architectural coatings.
3.9. Fungal Resistance Testing
The following eight typical mold strains were selected: Aspergillus niger, Aspergillus flavus, Cladosporium herbarum, Paecilomyces varioti, Penicillium citrinum, Trichoderma viride, Aureobasidium pullulans, and Alternaria alternata. These strains are widely present in the natural environment and are the main microorganisms causing mildew in materials such as coatings and building materials. Their metabolites (such as mycotoxins) may also pose health risks, thus having important testing representativeness. After 28 days of cultivation, through visual observation combined with optical microscope (10× objective lens) observation, ImageJ image analysis software was used to quantitatively calculate the proportion of mold growth area on the paint film surface, and the rating was performed in accordance with the grading standard of GB/T 1741-2020 (Table 1).
The surface of the paint film in the control group (without MMT-1227 added) was covered with a large number of molds, and the mycelia spread in a cotton-like manner. ImageJ analysis showed that the mold growth area accounted for 57%, corresponding to an antifungal grade of 3 (“moderate growth”, 30%–60% coverage) (Table 5). This indicates that the blank latex paint film has no antifungal ability, and is easily colonized and rapidly reproduced by molds in high-humidity environments. Long-term use may cause the paint film to chalk and peel off, affecting the material performance.

Table 5.
Fungal Resistance Results of Latex Paint Dry Films.
In the experimental group (containing 0.3% MMT-1227 by mass), no visible mold growth was observed on the surface of the paint film, and no spore germination or mycelium formation was observed under the microscope. The mold growth area accounted for 0%, corresponding to an antifungal grade of 0 (“no growth”). This result confirms that the introduction of MMT-1227 can completely inhibit the growth of the above eight types of molds, and its antifungal effect is significant.
The antifungal performance of MMT-1227 is due to the synergistic effect of benzalkonium chloride (1227) and montmorillonite. As a cationic surfactant, benzalkonium chloride can be adsorbed on the negatively charged fungal cell membrane (containing a phospholipid bilayer and surface anionic polysaccharides) through electrostatic interaction, destroying the integrity of the membrane structure, leading to the leakage of intracellular substances (such as electrolytes and proteins). At the same time, its long-chain alkyl groups can insert into the lipid regions of the fungal cell membrane, interfering with membrane permeability and energy metabolism, and ultimately inhibiting spore germination and mycelial growth [33]. The layered structure of montmorillonite provides a stable loading platform for benzalkonium chloride, delaying its release rate, thereby achieving long-term antifungal effects.
Therefore, the latex paint film containing 0.3% MMT-1227 showed a complete inhibitory effect on eight common molds within the 28-day test period, with an antifungal grade of 0, which was significantly better than the blank control group, indicating that this modified montmorillonite has important application value in the fields of mildew-proof coatings and building materials in humid environments (Figure 7).
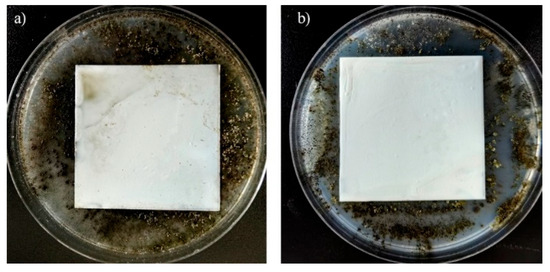
Figure 7.
Fungal resistance test results after 28 days of Control and MMT-1227 ((a): Control; (b): with the addition of 0.3% MMT-1227).
4. Conclusions
- (1)
- Multiple characterization techniques collectively confirm the successful modification of montmorillonite by benzalkonium chloride (1227). Scanning electron microscopy (SEM) reveals reduced particle aggregation and enhanced dispersion with densified surface morphology in 1227-MMT compared to pristine MMT. X-ray diffraction (XRD) analysis demonstrates the elimination of impurity-phase diffraction peaks and confirms 1227 intercalation into interlayer spaces. Fourier-transform infrared (FTIR) spectroscopy exhibits new absorption bands at 2926.86 cm−1 and 2852 cm−1, corresponding to C-H stretching vibrations of alkyl chains in 1227, providing direct evidence of organic modification. Thermogravimetric analysis (TGA) identifies a predominant weight loss stage at 180–410 °C attributable to 1227 thermal decomposition. Consistently, nitrogen adsorption–desorption (BET) analysis reveals altered pore architecture and surface hydrophobicity, manifested as reduced adsorption capacity and constricted hysteresis loop—findings that align with our prior pore structure characterization.
- (2)
- Antibacterial assessment confirms that benzalkonium chloride-modified montmorillonite (1227-MMT) exhibits potent efficacy against both Gram-negative (Escherichia coli) and Gram-positive (Staphylococcus aureus) bacteria. Quantitative analysis reveals inhibition zones of 15.6 mm and 17.7 mm for E. coli and S. aureus, respectively, whereas pristine MMT shows no inhibitory activity. The minimum inhibitory concentration (MIC) values further demonstrate enhanced antimicrobial potency, with 1227-MMT exhibiting MICs of 1 mg/mL and 0.5 mg/mL against E. coli and S. aureus—significantly lower than those of unmodified MMT (>128 mg/mL for both strains).
- (3)
- MMT-1227 demonstrates significant application potential in latex coatings. Incorporating 0.3 wt% of 1227-MMT yields dry films exhibiting 99.9% antibacterial efficacy against both Escherichia coli and Staphylococcus aureus. Furthermore, these films achieve mold resistance Grade 0 (according to the GB/T 1741-2020 standard) with no fungal growth observed, representing substantial enhancement over the control group without additives (Grade 3).
Author Contributions
Conceptualization, S.X., F.Y., and C.L.; methodology, S.X. and F.Y.; software, K.L.; validation, S.X., F.Y., and C.L.; formal analysis, H.S.; investigation, T.Y., Z.Z., and C.L.; resources, M.S.; data curation, X.Z. and S.K.; writing—original draft preparation, S.X.; writing—review and editing, F.Y., C.L., H.B., and G.T.; visualization, H.B. and K.O.; supervision, G.T. and K.O.; project administration, H.S., M.S., and K.O.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the 2024 Jiangsu Province Industry University Research Cooperation Project (BY20240470), the 2024 Jiangsu Province Science and Technology Deputy General Project (FZ20240998), Research Projects for Horizontal Cooperation in Universities (11130200125029), and the 2025 Flexible Energy Storage Material Design and Performance Optimization Team (30130800225001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Authors Zexiong Zhou, Hong Sun, Xiaoli Zhan, Mingkui Shi, and Soyeon Kim were employed by the company Zhejiang Fenghong New Material Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ma, Y.; Yang, H.; Zhang, J.; Zhao, Q.; Gao, H. Study on antibacterial properties of Cu(2+)-carrying and Ag+-carrying montmorillonite. China Feed. 2014, 14, 25–28, 37. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Zargar, M.; Yunus, W.M.Z.W.; Ibrahim, N.A.; Shabanzadeh, P.; Moghaddam, M.G. Synthesis and characterization of silver/montmorillonite/chitosan bionanocomposites by chemical reduction method and their antibacterial activity. Int. J. Nanomed. 2009, 6, 271–284. [Google Scholar] [CrossRef]
- Zhang, K.; Tan, S.; Liu, Y. Antimicrobial activity and antimicrobial mechanism of modified montmorillonite with quaternary ammonium salts. J. Chin. Ceram. Soc. 2006, 34, 87–92. [Google Scholar] [CrossRef]
- Özdemir, G.; Saadet, Y.; Mine, H.L. Preparation of cetylpyridinium montmorillonite for antibacterial applications. Appl. Clay Sci. 2013, 72, 201–205. [Google Scholar] [CrossRef]
- Verma, A.; Parveen, R.; Shamsi, T.N.; Khan, A.A.; Fatima, S.; Aazam, E.S.; Riaz, U. Ed .A Novel Strategy to Arrest Bacterial Pathogen Infestation Using Poly(o-Phenylenediamine)/ Montmorillonite Nanocomposites. Chem. Sel. 2022, 7, e202200797. [Google Scholar] [CrossRef]
- Edraki, M.; Zaarei, D. Modification of montmorillonite clay with 2-mercaptobenzimidazole and investigation of their antimicrobial properties. Asian J. Green Chem. 2018, 2, 189–200. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, J.; Zhang, R.; Liu, J.; Wang, W.; Hou, H. Ultrasound-Assisted Preparation, Characterization, and Antibacterial Activity of Montmorillonite Modified by epsilon-Polylysine Hydrochloride. Materials 2019, 12, 4148. [Google Scholar] [CrossRef]
- Maryan, A.S.; Montazer, M.; Rashidi, A.; Rahimi, M. Antibacterial Properties of Clay Layers Silicate: A Special Study of Montmorillonite on Cotton Fiber. Asian J. Chem. 2013, 25, 2889–2892. [Google Scholar] [CrossRef]
- ISO 22196-2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2011.
- GB/T 1741-2020; Test Method for Determining the Resistance of Paints Film to Mold. The Standardization Administration of the People’s Republic of China: Beijing, China, 2020.
- Olszewski, A.; Ławniczak, A.; Kosmela, P.; Strąkowski, M.; Mielewczyk-Gryń, A.; Hejna, A.; Piszczyk, Ł. Influence of Surface-Modified Montmorillonite Clays on the Properties of Elastomeric Thin Layer Nanocomposites. Materials 2023, 16, 1703. [Google Scholar] [CrossRef]
- Nascimento, D.S.D.; Etcheverry, M.; Orduz, A.E.; Waiman, C.V.; Zanini, G.P. Adsorption of cationic surfactant as a probe of the montmorillonite surface reactivity in the alginate hydrogel composites. RSC Adv. 2022, 12, 35469–35476. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, J.; Guo, N.; Sun, J.; Hu, H.; Chi, X. Nonlinear Conductivity and Thermal Stability of Anti-Corona Epoxy Resin Nanocomposites. Polymers 2024, 16, 1296. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, D.; Wang, Y.; Chen, G.; Jiang, S.; Li, G.; Wang, Y.; Wang, W.; Wu, P.; Liu, X.; et al. Comprehensive Characterization of the Structure and Gel Property of Organo-Montmorillonite: Effect of Layer Charge Density of Montmorillonite and Carbon Chain Length of Alkyl Ammonium. Minerals 2020, 10, 378. [Google Scholar] [CrossRef]
- Kahkha, M.R.R.; Kaykhaii, M.; Kahkha, B.R.; Khosravi, H.; Tohidlou, E. Simultaneous removal of heavy metals from wastewater using modified sodium montmorillonite nanoclay. Anal. Sci. 2020, 36, 1039–1043. [Google Scholar] [CrossRef]
- Tzoumani, I.; Druvari, D.; Evangelidis, M.; Vlamis-Gardikas, A.; Bokias, G.; Kallitsis, J.K. Facile Synthesis of Dual-Functional Cross-Linked Membranes with Contact-Killing Antimicrobial Properties and Humidity-Response. Molecules 2024, 29, 2372. [Google Scholar] [CrossRef]
- Huang, H.; Yin, Y.; Zhang, B.; Feng, G. Effects of the Structure and Composition of Montmorillonite on the Dimerization of Unsaturated Fatty Acids. J. Braz. Chem. Soc. 2018, 29, 1516–1527. [Google Scholar] [CrossRef]
- Alam, N.; Mokaya, R. Strongly acidic mesoporous aluminosilicates prepared via hydrothermal restructuring of a crystalline layered silicate. J. Mater. Chem. A 2015, 3, 7799–7809. [Google Scholar] [CrossRef]
- Xi, Y.; Zhe, D.; He, H.; Frost, R.L. Structure of organoclays--an X-ray diffraction and thermogravimetric analysis study. J. Colloid Interface Sci. 2004, 277, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Martens, M.; He, H.; Frost, R.L. Thermogravimetric analysis of organoclays intercalated with the surfactant octadecyltrimethylammonium bromide. J. Therm. Anal. Calorim. 2005, 81, 91–97. [Google Scholar] [CrossRef]
- Peretich, M. Targeted Synthesis and Characterization of Nanostructured Silicate Building Block Supports and Heterogeneous Catalysts with Tungsten(VI) or Zirconium(IV) Centers. Ph.D. Thesis, The University of Tennessee, Knoxville, TN, USA, 2011. [Google Scholar]
- Krutpijit, C.; Jongsomjit, B. Catalytic Ethanol Dehydration over Different Acid-activated Montmorillonite Clays. J. Oleo Sci. 2016, 65, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Krupskaya, V.V.; Zakusin, S.V.; Tyupina, E.A.; Dorzhieva, O.V.; Zhukhlistov, A.P.; Belousov, P.E.; Timofeeva, M.N. Experimental Study of Montmorillonite Structure and Transformation of Its Properties under Treatment with Inorganic Acid Solutions. Minerals 2017, 7, 49. [Google Scholar] [CrossRef]
- Lv, Z.; Xue, P.; Xie, T.; Zhao, J.; Tian, S.; Liu, H.; Qi, Y.; Sun, S.; Lv, X. High-performing PVDF membranes modified by Na+ MMT/ionic liquids (ILs) with different chain lengths: Dye adsorption and separation from O/W emulsion. Sep. Purif. Technol. 2023, 305, 122516. [Google Scholar] [CrossRef]
- Carson, M.S.; Smith, T.K. Role of bentonite in prevention of T-2 toxicosis in rats. J. Anim. Sci. 1983, 57, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhou, Y.; Lv, G.; Zhou, R. Simultaneous Detoxification of Aflatoxin B1, Zearalenone and Deoxynivalenol by Modified Montmorillonites. Molecules 2022, 27, 315. [Google Scholar] [CrossRef]
- Huang, F.Z.; Wang, Y.Q.; Gao, W.Y.; Cao, X.Q.; Zhang, Y.; Shang, Y.N.; Zhang, Y.Z.; Kan, Y.J. Construction and regulation of high active sites in montmorillonite composite catalyst for the removal of ofloxacin via persulfate activation. Heliyon 2024, 10, e29896. [Google Scholar] [CrossRef]
- Parolo, M.E.; Pettinari, G.R.; Musso, T.B.; Sánchez-Izquierdo, M.P.; Fernández, L.G. Characterization of organo-modified bentonite sorbents: The effect of modification conditions on adsorption performance. Appl. Surf. Sci. 2014, 320, 356–363. [Google Scholar] [CrossRef]
- Qiu, J.; Jiang, S.; Wang, Y.; Chen, G.; Liu, D.; Liu, X.; Wang, G.; Wu, P.; Lyu, X. Crystal chemistry characteristics and dispersion performance of Ca-montmorillonite with different layer charge density. Mater. Res. Express 2020, 7, 075505. [Google Scholar] [CrossRef]
- Kovalchuk, I.; Zakutevskyy, O.; Sydorchuk, V.; Diyuk, O.; Lakhnik, A. The Effect of High-Energy Ball Milling of Montmorillonite for Adsorptive Removal of Cesium, Strontium, and Uranium Ions from Aqueous Solution. Eng 2023, 4, 2812–2825. [Google Scholar] [CrossRef]
- He, H.; Zhou, Q.; Martens, W.N.; Kloprogge, T.J.; Yuan, P.; Xi, Y.; Zhu, J.; Frost, R.L. Microstructure of HDTMA+-Modified Montmorillonite and its Influence on Sorption Characteristics. Clays Clay Miner. 2006, 54, 689–696. [Google Scholar] [CrossRef]
- Carey, A.B.; Ashenden, A.; Kper, I. Model architectures for bacterial membranes. Biophys. Rev. 2022, 14, 111–143. [Google Scholar] [CrossRef] [PubMed]
- Garrido, L.; Lyra, P.; Rodrigues, J.; Viana, J.; Mendes, J.J.; Barroso, H. Revisiting Oral Antiseptics, Microorganism Targets and Effectiveness. J. Pers. Med. 2023, 13, 1332. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).