Tailoring Tribological Properties and Corrosion Resistance of Self-Lubricating Ti-Mo-N Coatings Prepared by Arc Depositions
Abstract
1. Introduction
2. Experimental Section
2.1. Equipment and Experimental Materials
2.2. Coating Preparation
2.3. Coating Characterization
3. Results and Discussion
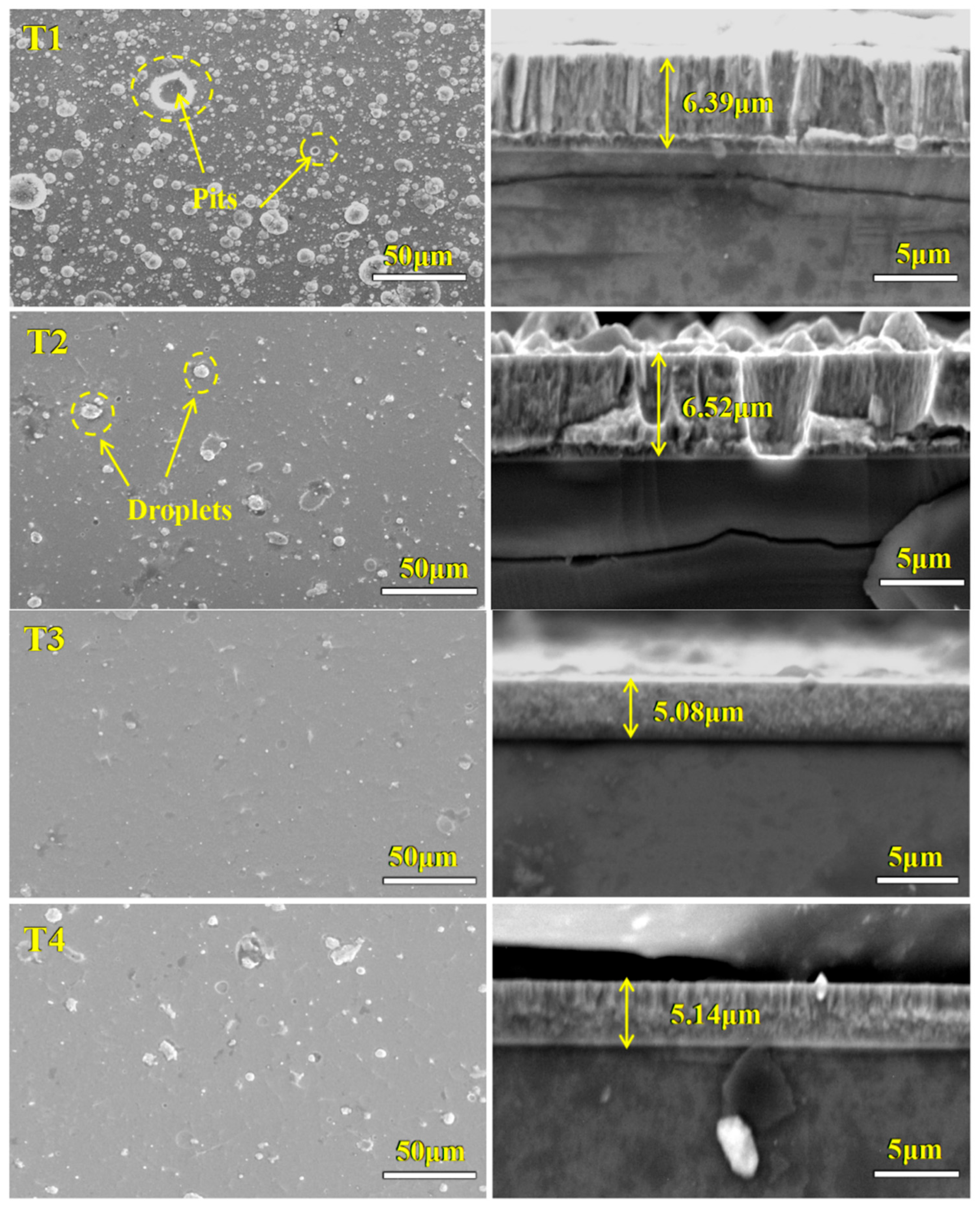
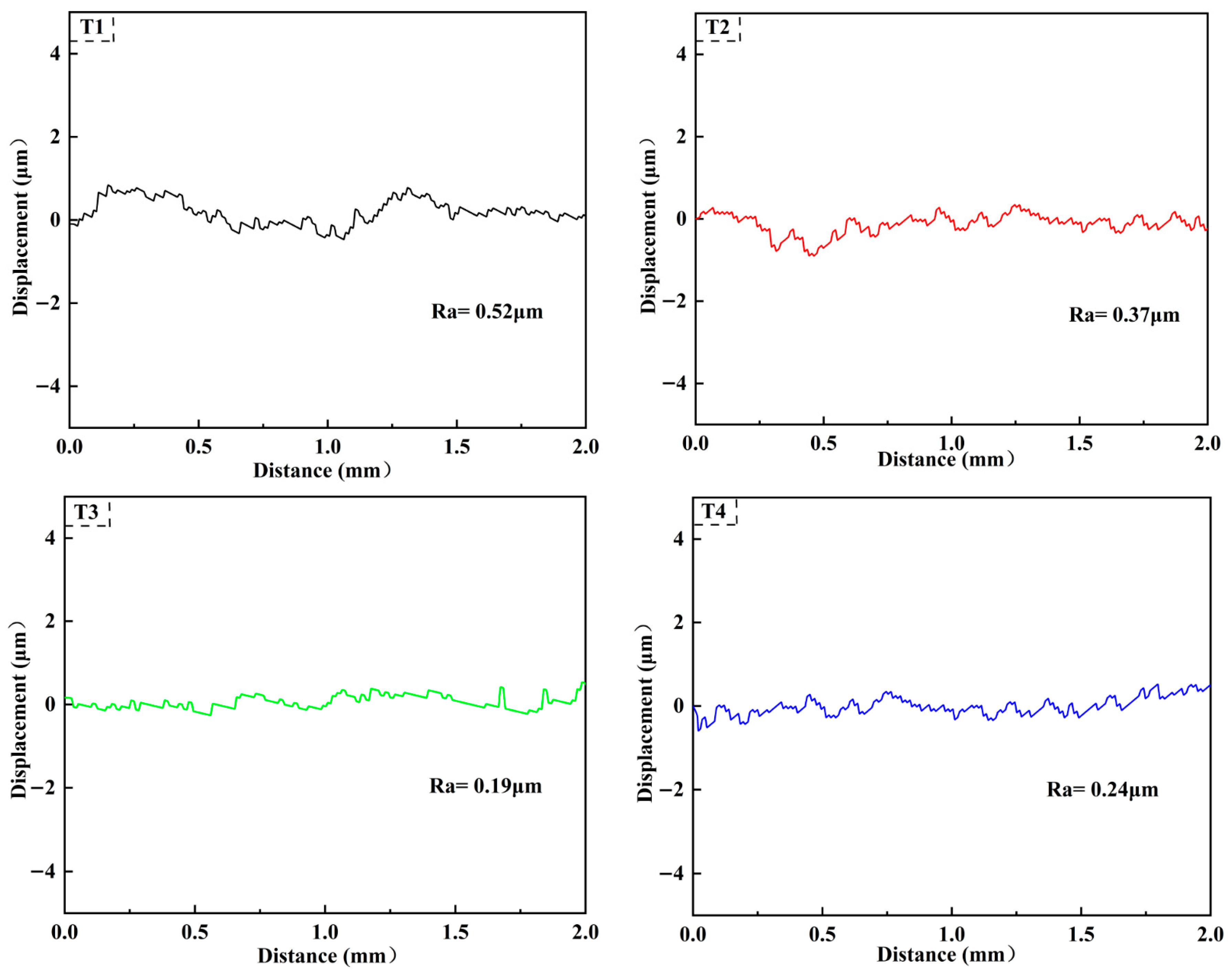
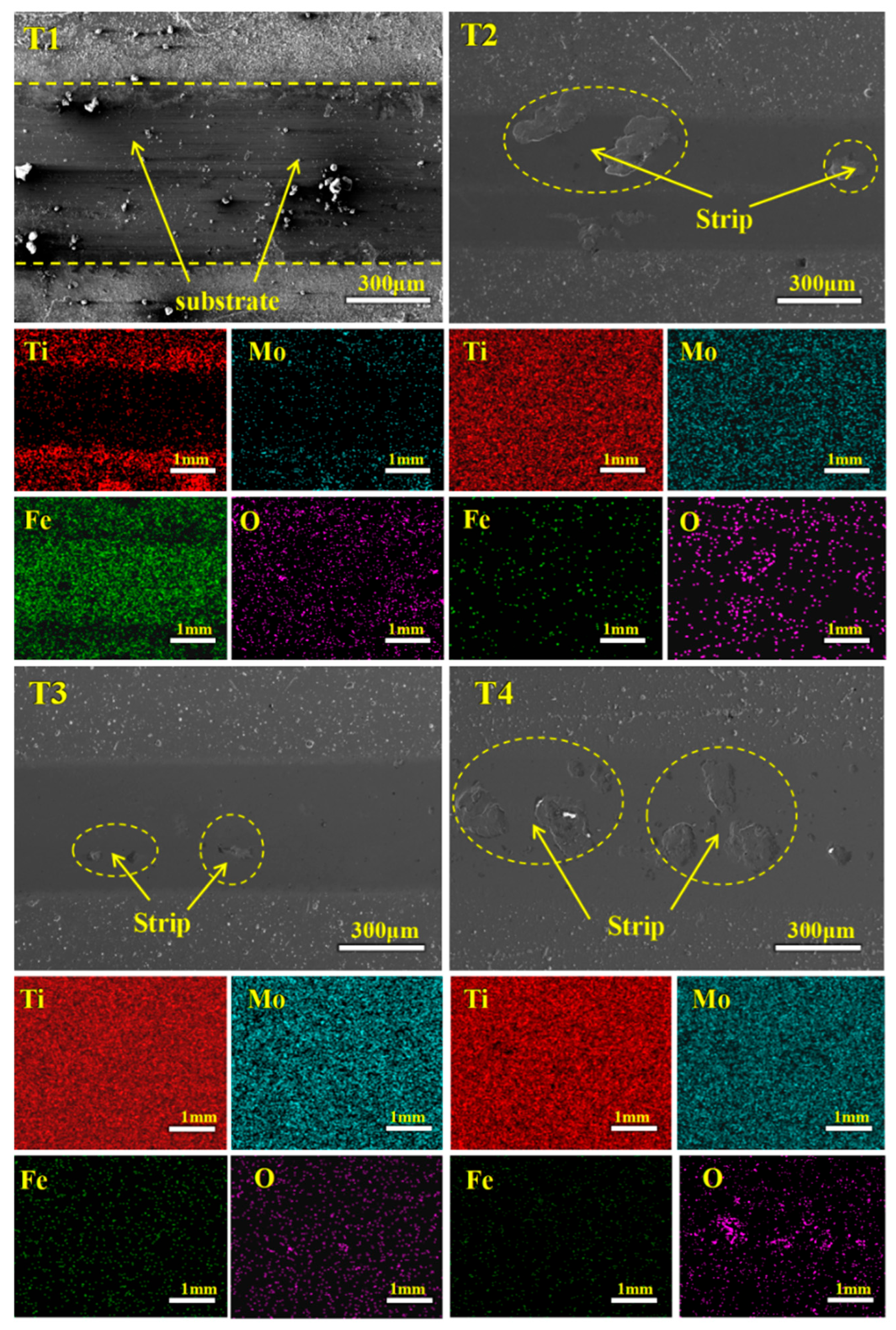
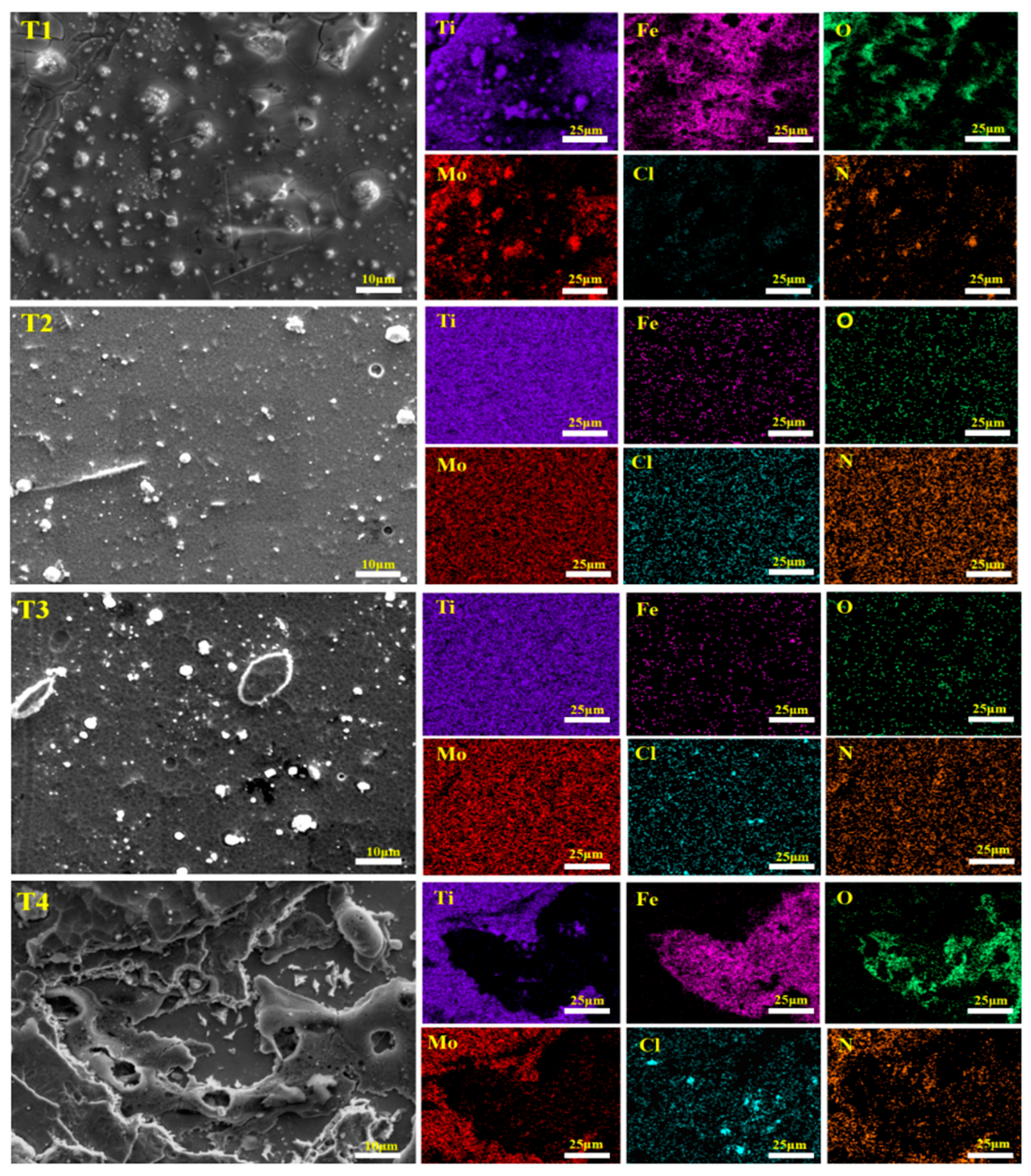
3.1. Surface and Cross-Sectional Morphology
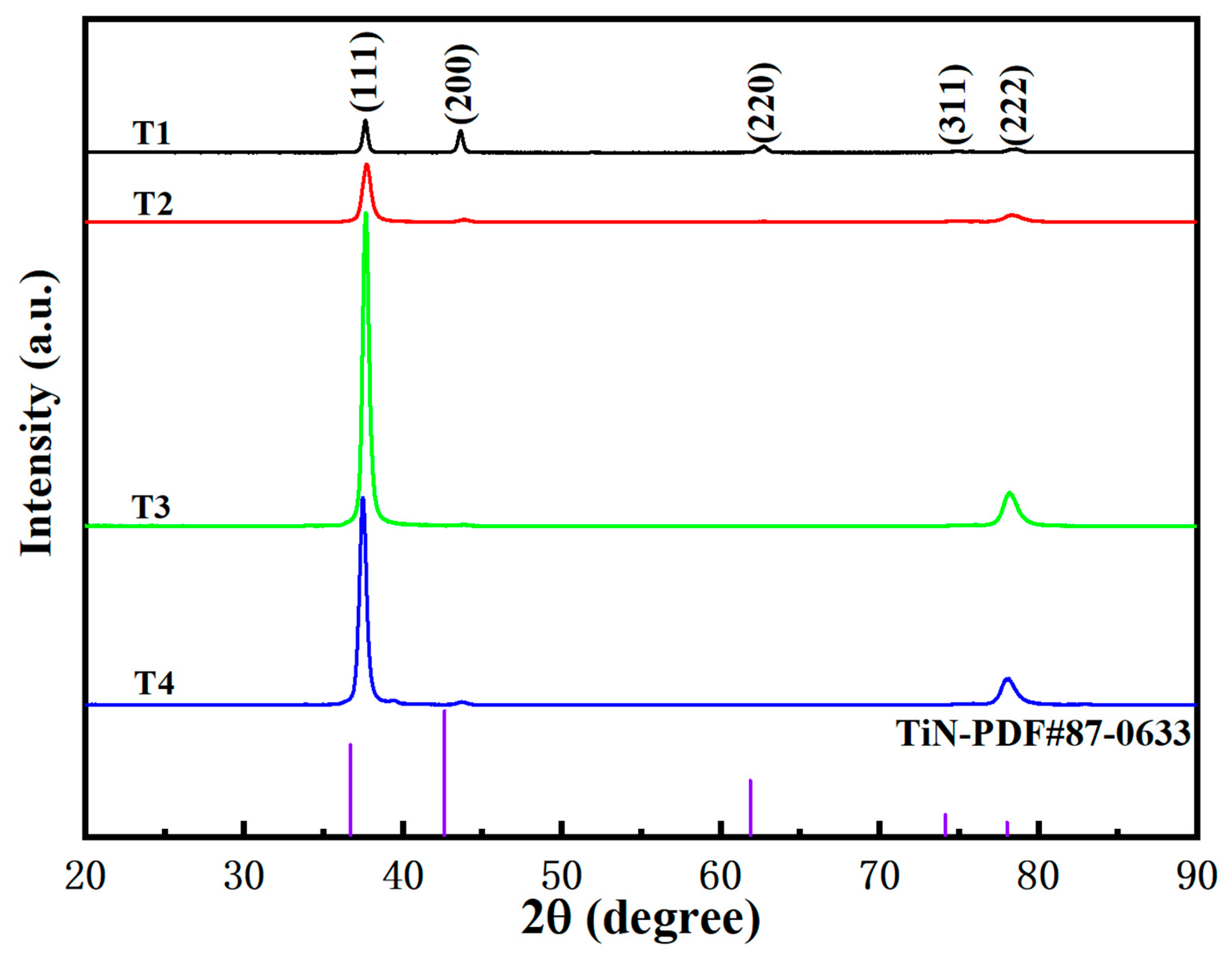
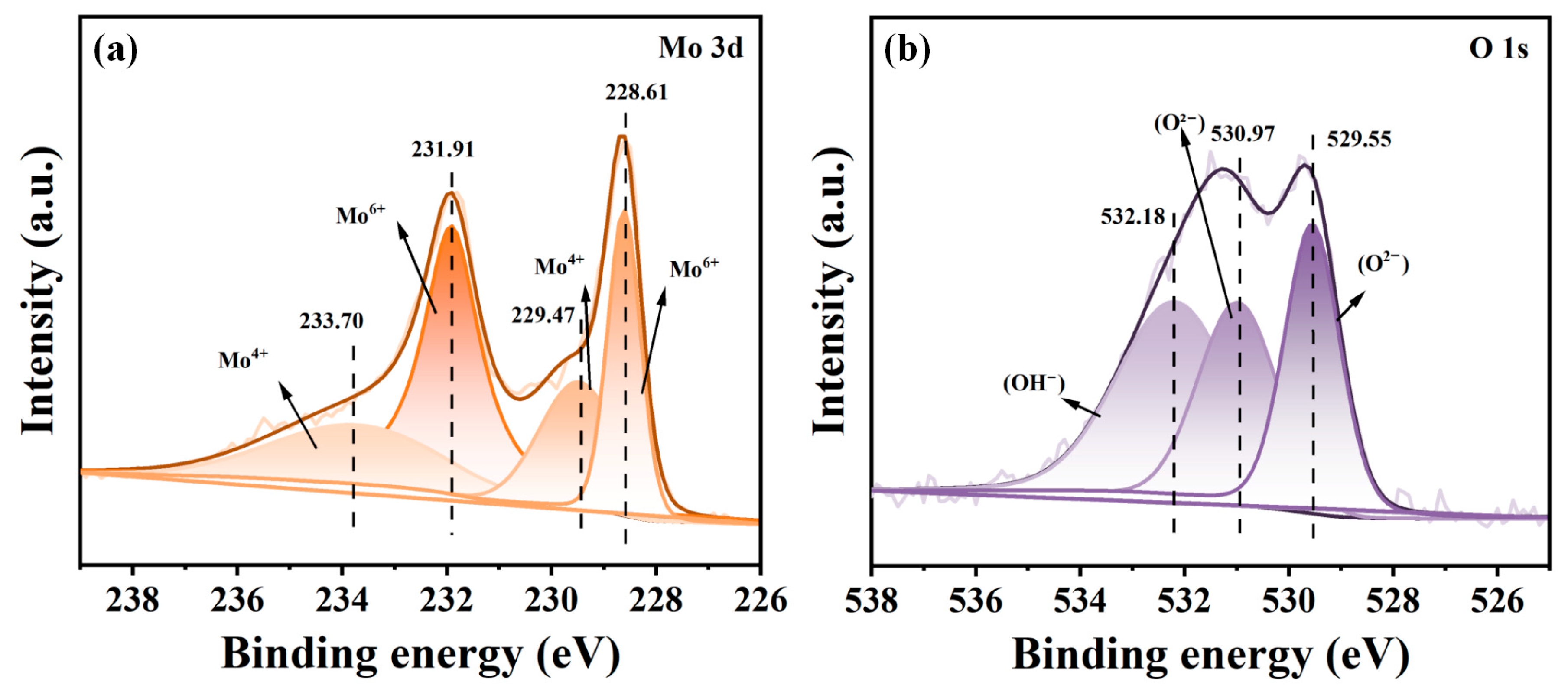
3.2. Components and Phase Structure
3.3. Mechanical Properties
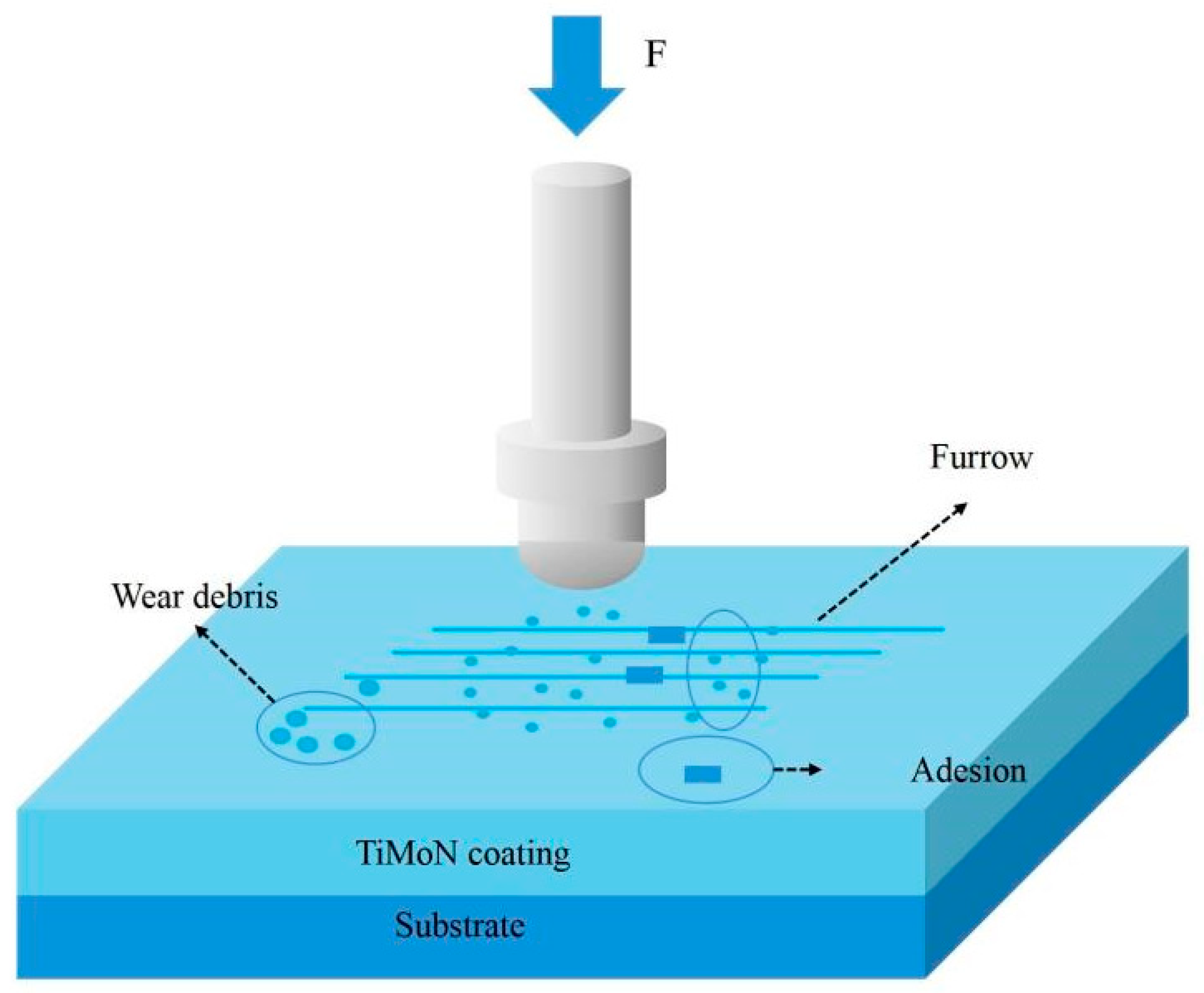
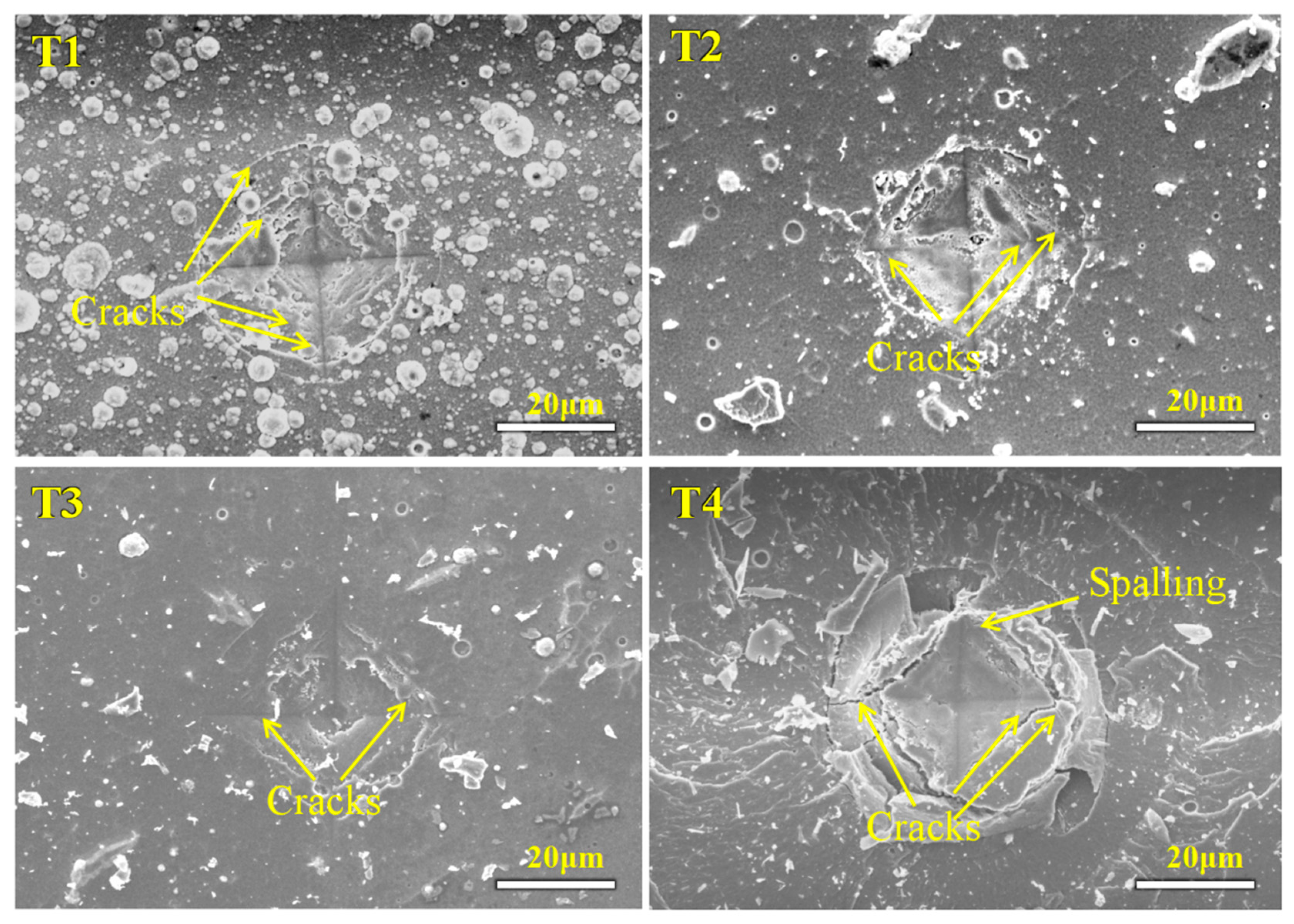
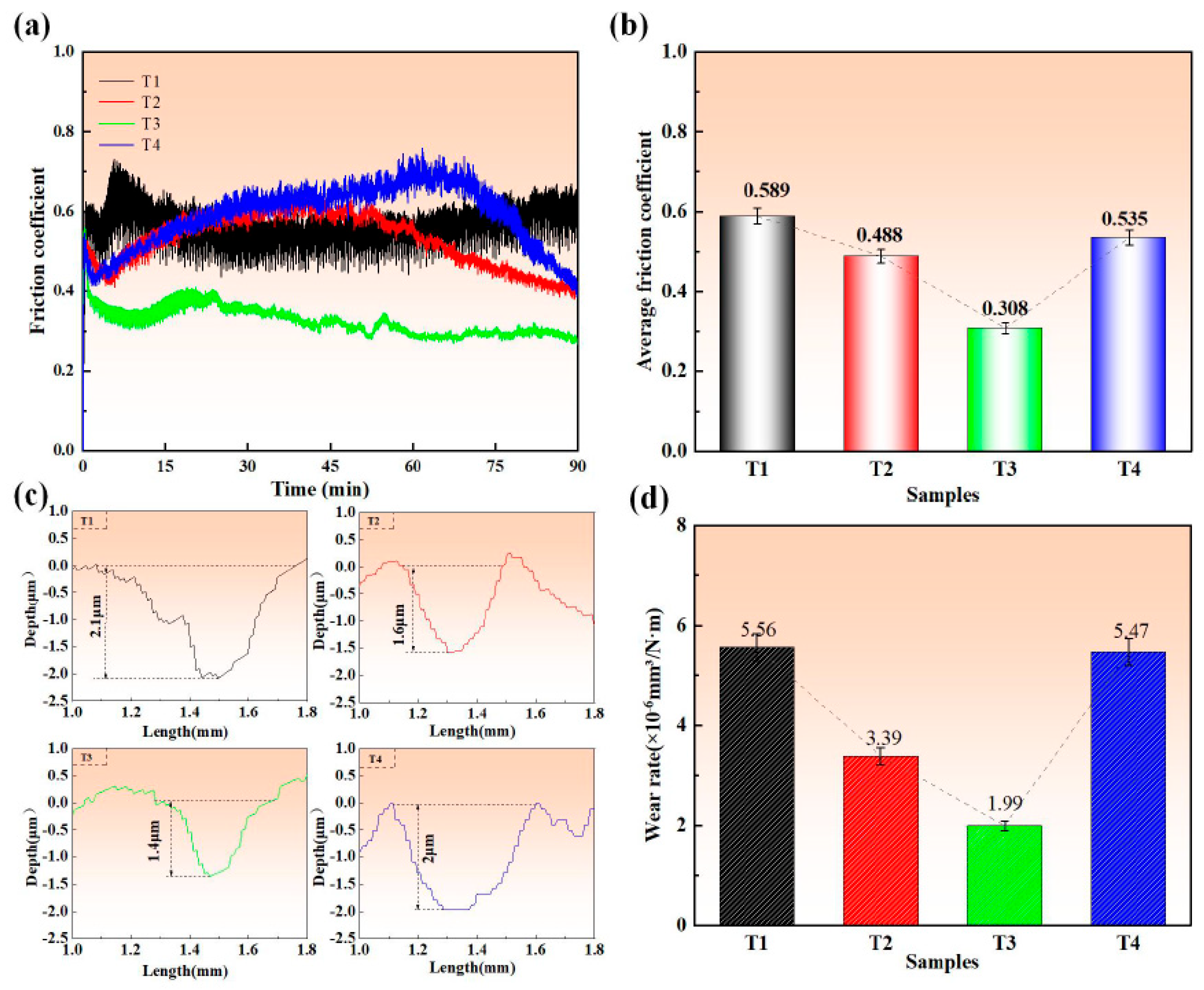
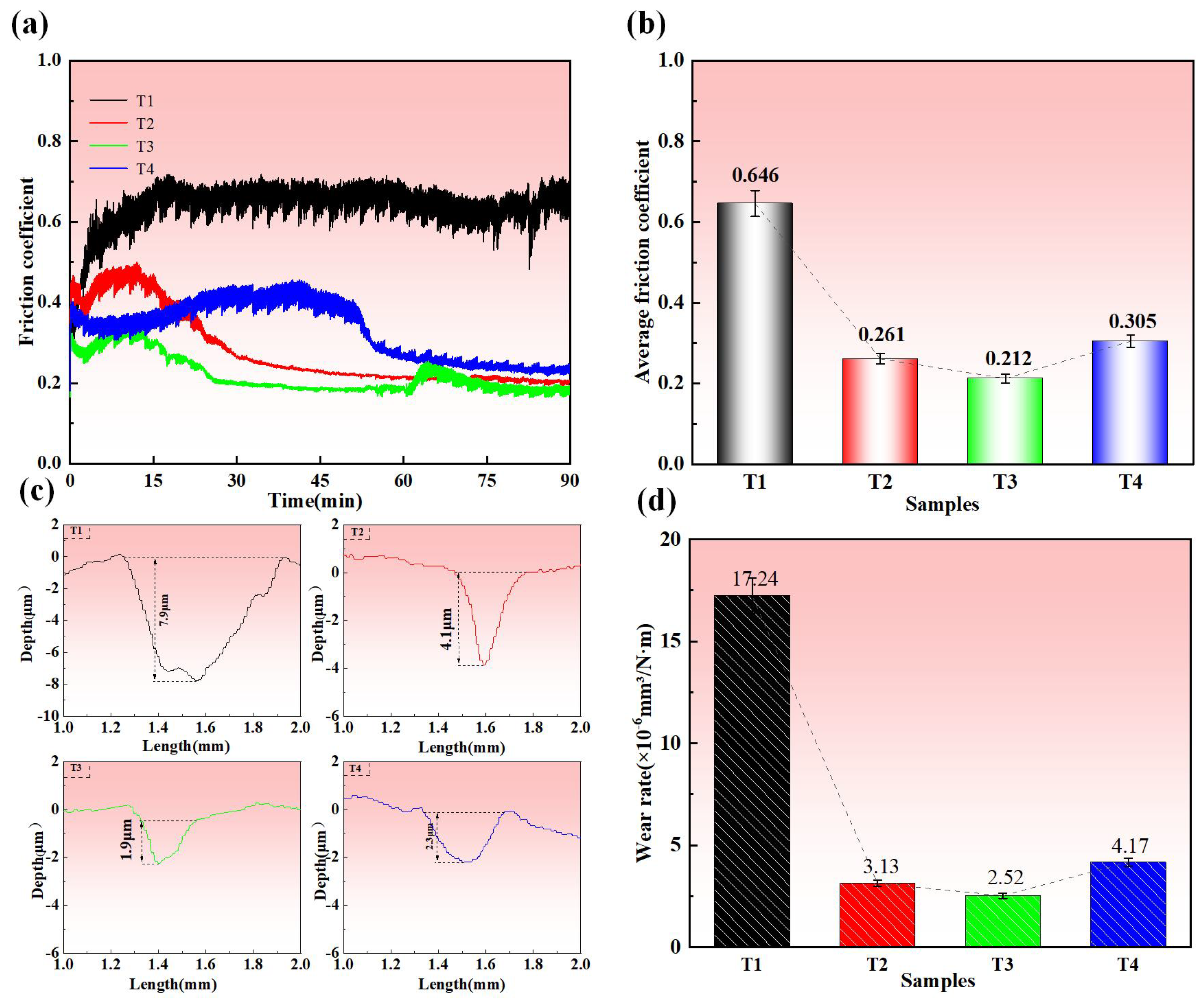
3.4. Tribological Properties
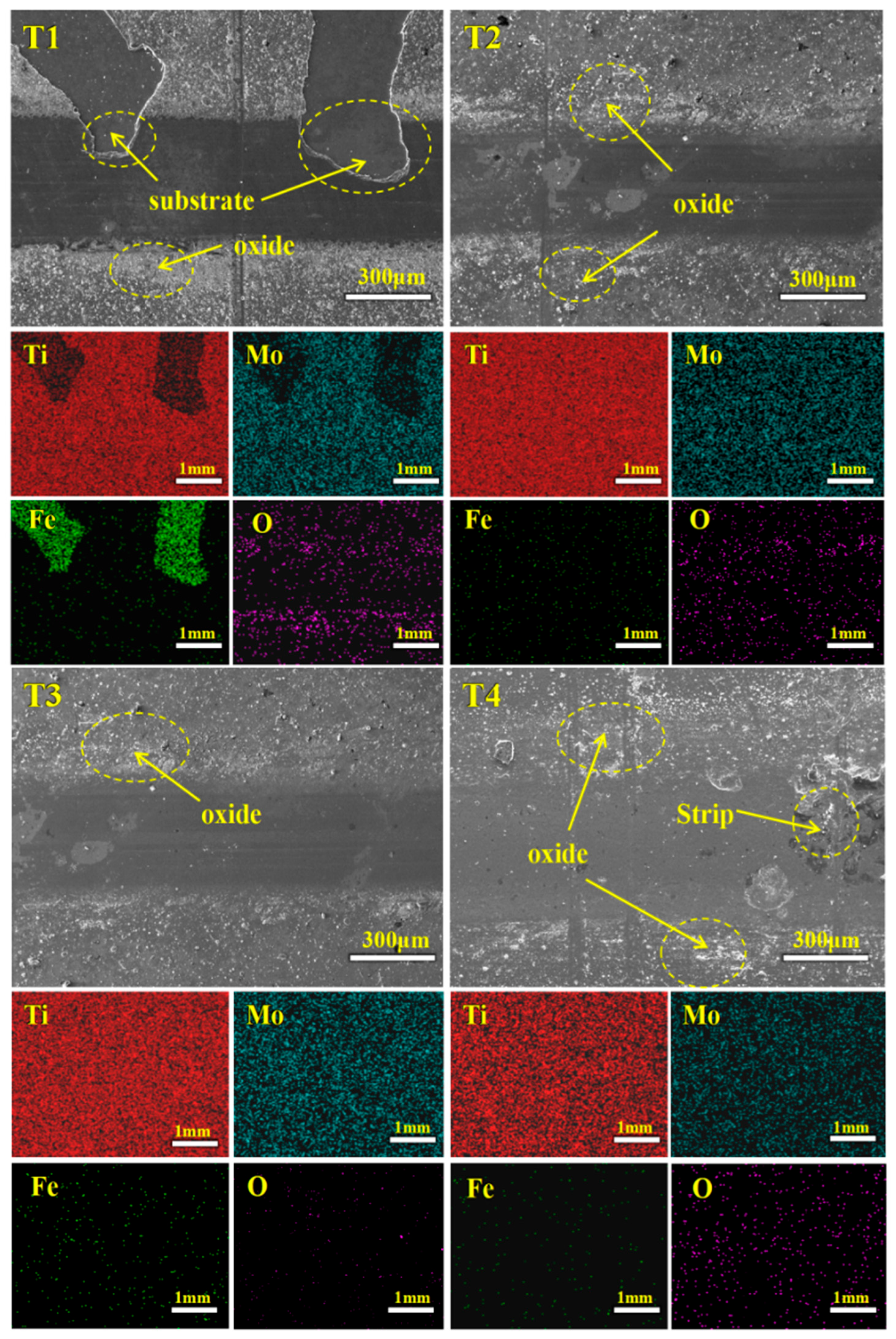
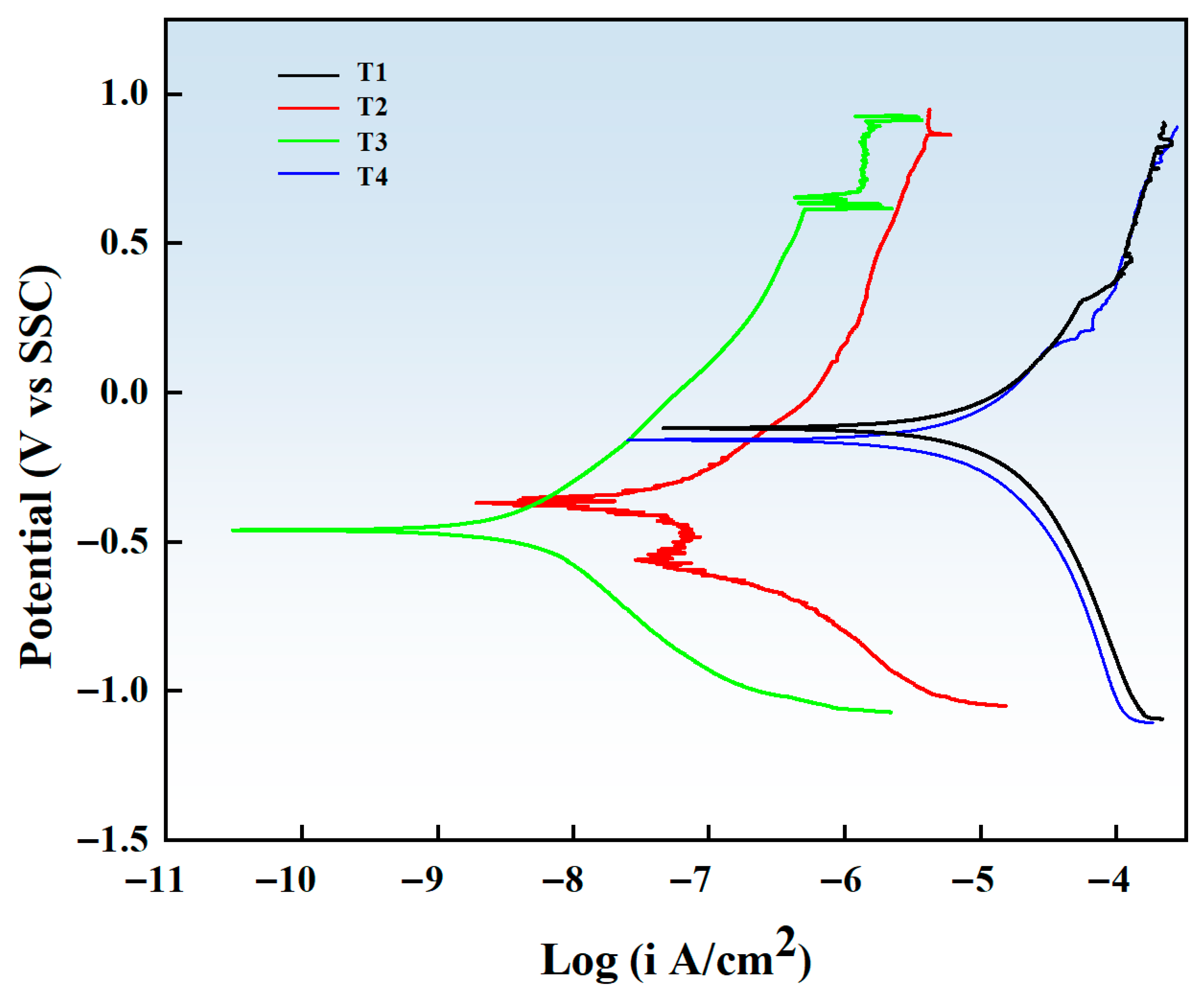
3.5. Electrochemical Test
4. Conclusions
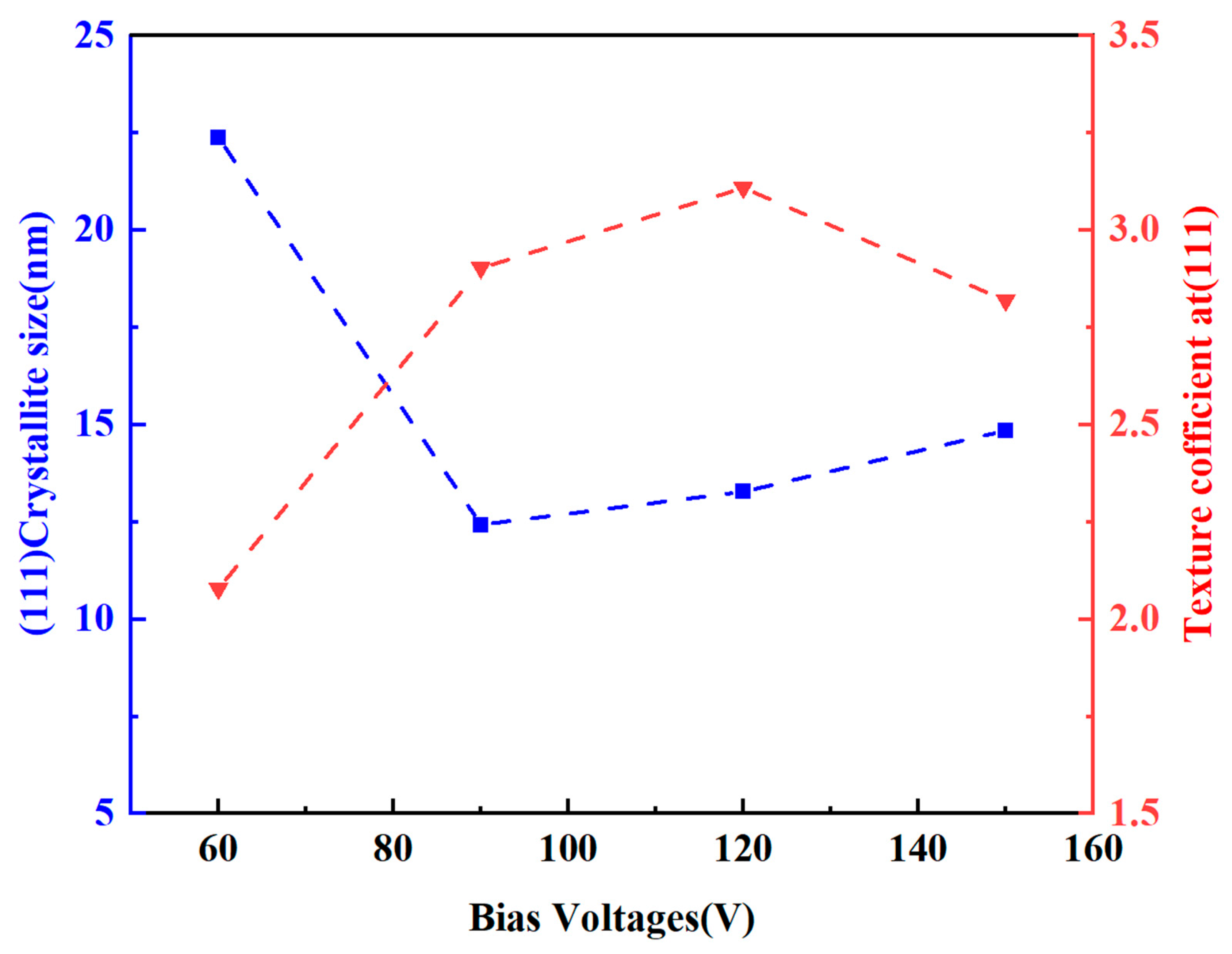
- The deposition bias voltage is the core process parameter for regulating the microstructure and properties of the Ti-Mo-N coating. At a bias voltage of −120 V, the number of surface droplets is the least, the structure is the densest, and the (111) crystal plane has the strongest preferred orientation; deviations from this optimal value result in an increase in surface droplets and a decrease in density.
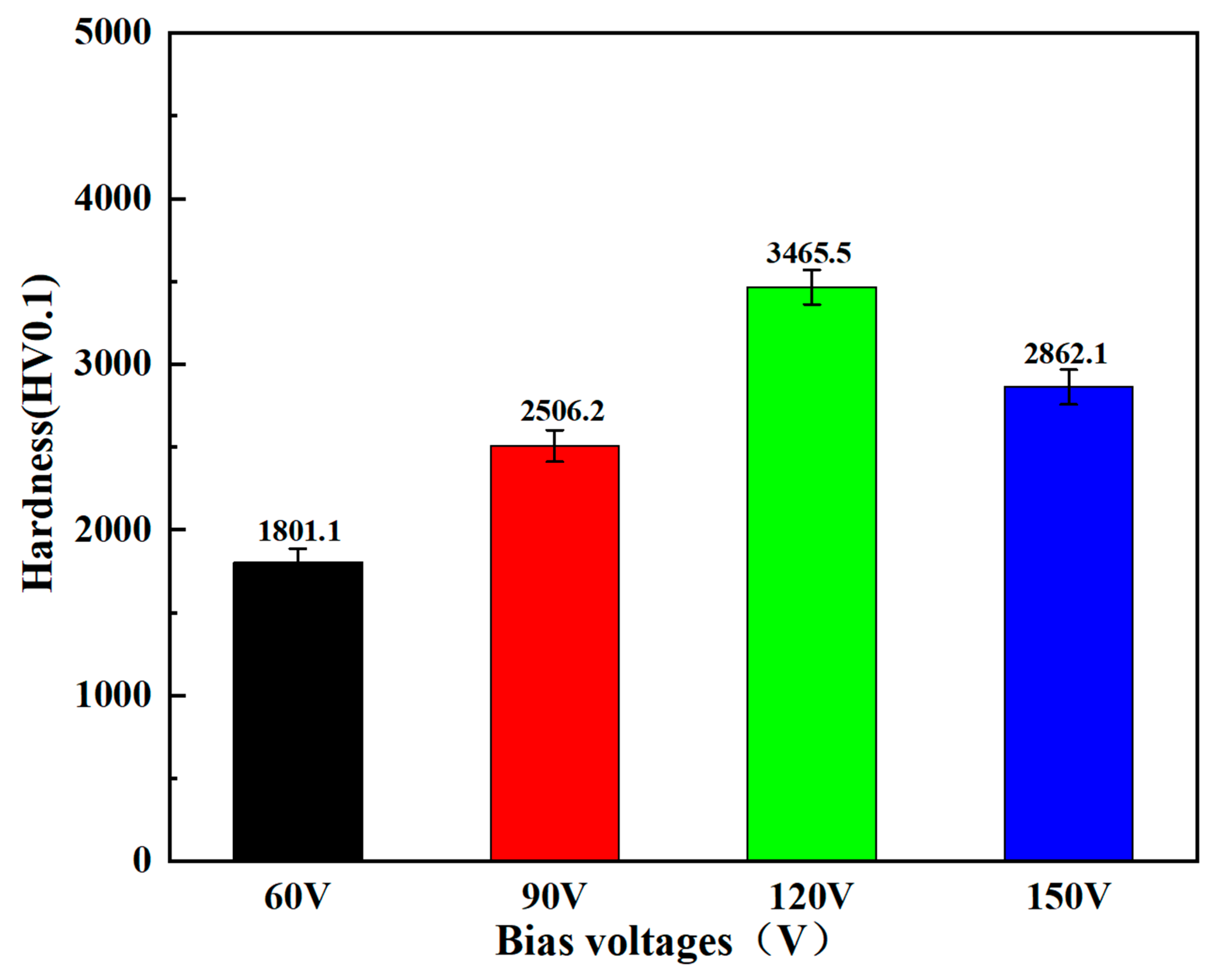
- The hardness and fracture toughness of the coating increase significantly with the increase in bias voltage (from −60 V to −120 V), reaching a peak at −120 V, which is closely related to its densified microstructure and strong (111) texture.
- The Ti-Mo-N coating prepared at −120 V bias voltage exhibits the best tribological performance, with the lowest friction coefficient (0.308) and the lowest wear rate (1.99 × 10−6 mm3/N·m). This excellent performance is attributed to the layered MoO3 lubricating phase formed during the friction process, and the coating substrate can continuously transport Mo elements to the friction interface to compensate for consumption, thereby dynamically maintaining an effective lubricating film, demonstrating a significant self-lubrication effect.
- In terms of electrochemical corrosion behavior, the coating with −120 V bias voltage shows the lowest corrosion current density (3.62 × 10−9 A/cm2) and the best corrosion resistance. No obvious damage was observed in the corrosion morphology, indicating that it has good surface protection capability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, F.; Chen, X.; Xu, H.; Dong, H.; Weng, Y.; Cao, W. Current Situation of Metallurgical Quality and Fatigue Properties of Rolling Bearing Steel and the Development Direction of High-end Bearing Steel. Acta Metall. Sin. 2020, 56, 513–522. [Google Scholar]
- Hu, X.; Wang, L.; Li, F.; Zhang, H.; Zheng, Z. Analysis of Failure Causes and Optimization Measures of Main Drive Bearings of Loaders. Constr. Mach. 2025, 56, 187–190+114. [Google Scholar]
- Wei, A.; Ma, G.; Li, G.; Yong, Q.; Guo, W.; Zhao, H.; Huang, Y.; Han, C.; Wang, H. Current Situation and Prospect of Research Methods on Wear Life of Self-lubricating Spherical Plain Bearings. Surf. Technol. 2023, 52, 31–46. [Google Scholar]
- Zhang, Y.; Feng, S.; Qi, Y.; Zhu, J. The Main Failure Modes of the Main Bearings of Aero Engines. Mech. Manag. Dev. 2024, 39, 268–270. [Google Scholar]
- Ma, B.; Fu, L.; Bao, S.; Du, S.; Yue, Y.; Zhang, Y. Research on Microstructure and Friction and Wear Propertiesof GCr15 and G20CrNi2Mo Bearing Steels. Mater. Rev. 2021, 35, 16120–16125. [Google Scholar]
- Ebrahimzadeh, P.; Peral, L.B.; González-Martínez, R.; Mardaras, E.; Fernández-Pariente, I. Influence of Severe Surface Plastic Deformation Induced by Shot Peening on Microstructure and Corrosion Resistance of fine grained 316 L stainless steel. Corros. Sci. 2024, 231, 111988. [Google Scholar] [CrossRef]
- Hoche, H.; Pusch, C.; Oechsner, M. Corrosion and wear protection of mild steel substrates by innovative PVD coatings. Surf. Coat. Technol. 2020, 391, 125659. [Google Scholar] [CrossRef]
- Wen, X.; Cui, X.; Jin, G.; Liu, Y.; Zhang, Y.; Zhang, X.; Liu, E.; Tian, H.; Fang, Y. Corrosion and tribo-corrosion behaviors of nano-lamellar Ni1.5CrCoFe0.5Mo0.1Nbx eutectic high-entropy alloy coatings: The role of dual-phase microstructure. Corros. Sci. 2022, 201, 110305. [Google Scholar] [CrossRef]
- Bisht, A.K.; Vaishya, R.O.; Walia, R.; Singh, G. Nitrides ceramic coatings for tribological applications: A journey from binary to high-entropy compositions. Ceram. Int. 2024, 50, 8553–8585. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, W.; Li, B.; Wang, C.; Kuang, T.; Li, Y. Physical vapor deposition technology for coated cutting tools: A review. Ceram. Int. 2020, 46, 18373–18390. [Google Scholar] [CrossRef]
- Movassagh-Alanagh, F.; Abdollah-Zadeh, A.; Aghababaei, R. Effects of B and C elements on microstructure, mechanical and tribological properties of TiN coating deposited by PACVD. Mater. Charact. 2025, 225, 115178. [Google Scholar] [CrossRef]
- Wang, X. Research on the Microstructure and Properties of Ti-Mo-N Nanostructured Composite Films. Bachelor’s Thesis, Jiangsu University Of Science And Technology, Zhenjiang, China, July 2011. [Google Scholar]
- Zhou, S.; Zhao, W.; Qiu, Z.; Lin, S.; Zheng, Z.; Zeng, D. Improved load-bearing capacity of Mo-doped Ti-N coatings: Effects of Mo alloying and GB plasticity. Surf. Coat. Technol. 2021, 424, 127630. [Google Scholar] [CrossRef]
- Moussaoui, A.; Abboudi, A.; Aissani, L.; Belgroune, A.; Cheriet, A.; Alhussein, A.; Rtimi, S. Effect of Mo addition on the mechanical and tribological properties of magnetron sputtered TiN films. Surf. Coat. Technol. 2023, 470, 129862. [Google Scholar] [CrossRef]
- Othman, M.F.; Bushroa, A.R.; Abdullah, W.N.R. Evaluation techniques and improvements of adhesion strength for TiN coating in tool applications: A review. J. Adhes. Sci. Technol. 2015, 29, 569–591. [Google Scholar] [CrossRef]
- Santecchia, E.; Hamouda, A.; Musharavati, F.; Zalnezhad, E.; Cabibbo, M.; Spigarelli, S. Wear resistance investigation of titanium nitride-based coatings. Ceram. Int. 2015, 41, 10349–10379. [Google Scholar] [CrossRef]
- Zuo, J.; Xie, Y.; Zhang, J.; Wei, Q.; Zhou, B.; Luo, J.; Wang, Y.; Yu, Z.; Tang, Z. TiN coated stainless steel bracket: Tribological, corrosion resistance, biocompatibility and mechanical performance. Surf. Coat. Technol. 2015, 277, 227–233. [Google Scholar] [CrossRef]
- Olbrich, W.; Fessmann, J.; Kampschulte, G.; Ebberink, J. Improved control of TiN coating properties using cathodic arc evaporation with a pulsed bias. Surf. Coat. Technol. 1991, 49, 258–262. [Google Scholar] [CrossRef]
- Bozyaz, E.; Rgen, M.; Akr, A.F. Comparison of reciprocating wear behaviour of electrolytic hard chrome and arc-PVD CrN coatings. Wear 2004, 256, 832–839. [Google Scholar] [CrossRef]
- He, C.; Zhang, J.; Wang, J.; Ma, G.; Zhao, D.; Cai, Q. Effect of structural defects on corrosion initiation of TiN nanocrystalline films. Appl. Surf. Sci. 2013, 276, 667–671. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, X.-G.; Yang, X.-X.; Xu, F.-F.; Kim, K.-H.; Shao, Z.-G. Influence of substrate bias on microstructure and morphology of ZrN thin films deposited by arc ion plating. Trans. Nonferrous Met. Soc. China 2012, 22 (Suppl. S1), s115–s119. [Google Scholar] [CrossRef]
- Qi, Z.B.; Zhu, F.P.; Wang, Z.C.; Peng, C.; Wu, D.L. The inverse Hall–Petch effect in nanocrystalline ZrN coatings. Surf. Coat. Technol. 2011, 205, 3692–3697. [Google Scholar] [CrossRef]
- Xue, L.; Hu, X.; Xi, Y.; Qiu, L.; Pan, X.; Zhang, Y. Effect of N2 partial pressure on ZrN coating orientation and tribocorrosion behavior and mechanism. Ceram. Int. 2024, 50, 24847–24863. [Google Scholar] [CrossRef]
- Liu, A.; Deng, J.; Cui, H.; Chen, Y.; Zhao, J. Friction and wear properties of TiN, TiAlN, AlTiN and CrAlN PVD nitride coatings. Int. J. Refract. Met. Hard Mater. 2012, 31, 82–88. [Google Scholar]
- Wang, L.; Jin, Y.; Lin, X.; Chen, C. Effects of Substrate Bias Duty Cycle on Morphology and Mechanical Properties of TiAlSiN Coatings Prepared by Multi-Arc Ion Plating. Surf. Technol. 2017, 46, 237–240. [Google Scholar]
- Du, J.; Tao, G.; Chen, H.; Yi, J.; Wan, H.; Liu, S.; Zhang, Y. The Influence of Bias Voltage on the Structure and Properties of TiAlSiN Coating Prepared by HiPIMS. Mater. Prot. 2024, 57, 30–42. [Google Scholar]
- Gu, W.; Li, S.; Wang, H.; Chen, C.; Li, P.; Huang, F. The influence of substrate bias voltage on the structure and properties of TiB2 coating prepared by magnetron sputtering. J. Aeronaut. Mater. 2014, 34, 37–42. [Google Scholar]
- Li, Z.; Munroe, P.; Jiang, Z.; Zhao, X.; Xu, J.; Zhou, Z.; Jiang, J.; Fang, F.; Xie, Z. Designing superhard, self-toughening CrAlN coatings through grain boundary engineering. Acta Mater. 2012, 60, 5735–5744. [Google Scholar] [CrossRef]
- Chou, W.J.; Yu, G.P.; Huang, J.H. Mechanical properties of TiN thin film coatings on 304 stainless steel substrates. Surf. Coat. Technol. 2002, 149, 7–13. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, S.; Fang, L.; Zhang, Y.; Li, X.; Zhou, Z. Influence of Microhardness and Wear Resistance of CrAlN Coating Deposited by Multi-Arc Ion Plating on the Surface of H13 Steel. Equip. Environ. Eng. 2023, 20, 115–120. [Google Scholar]
- Fillot, N.; Iordanoff, I.; Berthier, Y. Modelling third body flows with a Discrete Element Method a tool for understanding wear with adhesive particles. Tribol. Int. 2007, 40, 973–981. [Google Scholar] [CrossRef]
- Raaif, M. Investigating the structure and tribo-mechanical performance of PVD TiN on bearing TiN substrate constructed by rf plasma. Mater. Chem. Phys. 2019, 224, 117–123. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, G.; Jiang, B. Comparison in mechanical and tribological properties of CrTiAlMoN and CrTiAlN nano-multilayer coatings deposited by magnetron sputtering. Appl. Surf. Sci. 2016, 363, 217–224. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, L.; Patnaik, P.; Zeng, X. Wear Resistant TiMoN Coatings Deposited by Magnetron Sputtering. Wear 2006, 261, 119–125. [Google Scholar] [CrossRef]
- Chen, X.; Lin, Z.; Xiao, K.; Han, Y.; Ding, W.; Li, T.; Yuan, F.; Fang, S.; Liang, Z.; Zhang, L. Superior wear performance of titanium alloys from TiN coatings and ultrafine-grained gradient structures. Surface and Coatings Technology. Surf. Coat. Technol. 2025, 513, 132514. [Google Scholar] [CrossRef]
- Jiang, S.; Che, X.; Huang, J.; Huang, D. High temperature oxidation behavior of Mo-Zr alloys in pure oxygen condition. Int. J. Refract. Met. Hard Mater. 2024, 124, 106820. [Google Scholar] [CrossRef]
- Liu, C.; Park, S.; Nagao, S.; Nogi, M.; Koga, H.; Ma, J.-S.; Zhang, G.; Suganuma, K. The role of Zn precipitates and Cl− anions in pitting corrosion of Sn–Zn solder alloys. Corros. Sci. 2015, 92, 263–271. [Google Scholar] [CrossRef]
- Guan, X.; Wang, Y.; Zhang, G.; Jiang, X.; Wang, L.; Xue, Q. Microstructures and properties of Zr/CrN multilayer coatings fabricated by multi-arc ion plating. Tribol. Int. 2016, 106, 78–87. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, L.; Zhang, X.; Han, Y. A Hierarchically Structured Coating on 2a12-T4 Aluminum Alloy for Anti-Wear and Corrosion. SSRN Electron. J. 2022, 207, 110598. [Google Scholar]
- He, C.-L.; Zhang, J.-L.; Ma, G.-F.; Du, Z.-F.; Wang, J.-M.; Zhao, D.-L. Influence of bias voltage on structure, mechanical and corrosion properties of reactively sputtered nanocrystalline TiN films. J. Iron Steel Res. 2017, 24, 1223–1230. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Wang, J.; Zheng, M.; Guo, P.; Zhang, Y.; Ren, Y.; Liu, J.; Huang, W. Effect of stress-relief annealing on anodic dissolution behaviour of additive manufactured Ti-6Al-4V via laser solid forming. Corros. Sci. 2019, 153, 314–326. [Google Scholar] [CrossRef]
- Qin, Q.; Fu, Z.; Wang, Z. The influence of matrix bias pressure on the surface TiAlCrN film structure and properties of H13 steel. Electroplat. Precis. Finish. 2023, 45, 19–25. [Google Scholar]


| Parameter | Adhesion Layer | Intermediate Layer | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|---|
| N2/Ar Flow [ccm] | 0/1500 | 400/800 | 400/800 | 400/800 | 400/800 | 400/800 |
| Bias Voltage [V] | 100 | 100 | 60 | 90 | 120 | 150 |
| Deposition Time [min] | 15 | 15 | 120 | 120 | 120 | 120 |
| Target Current [A] | 130 | 130 | 100 | 100 | 100 | 100 |
| Targets | Ti | Ti | Ti/TiMo | Ti/TiMo | Ti/TiMo | Ti/TiMo |
| Temperature [℃] | 300 | 300 | 300 | 300 | 300 | 300 |
| Rotation Speed [rpm] | 2 | 2 | 2 | 2 | 2 | 2 |
| Sample | Ti/Mo | Element Composition (at. %) | ||
|---|---|---|---|---|
| Ti | Mo | N | ||
| T1 | 7.05 | 53.91 | 7.65 | 38.44 |
| T2 | 8.38 | 53.44 | 6.38 | 38.19 |
| T3 | 6.99 | 54.11 | 7.74 | 38.15 |
| T4 | 7.57 | 54.63 | 7.22 | 38.15 |
| Sample | Rs (Ω) | CPE (F·cm−2) | n | Rct (Ω) | W | Rf (Ω) | CPEdl (F·cm−2) | nd |
|---|---|---|---|---|---|---|---|---|
| 60 V | 15.23 | 26.63 | 0.497 | 8.7 × 103 | — | — | 135.2 | 0.742 |
| 90 V | 271.4 | 1.58 | 0.520 | 3.4 × 105 | 3.6 × 10−6 | — | — | — |
| 120 V | 622.2 | 1.45 | 0.602 | 4.6 × 105 | 1.9 × 10−5 | — | — | — |
| 150 V | 70.9 | 90.52 | 0.816 | 9.3 × 103 | — | — | 149.1 | 0.837 |
| Specimens | Ecorr/V | Icorr/(A/cm2) |
|---|---|---|
| T1 | −0.17 | 8.43 × 10−6 |
| T2 | −0.39 | 3.97 × 10−8 |
| T3 | −0.44 | 3.62 × 10−9 |
| T4 | −0.18 | 5.67 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Liu, J.; Liu, G.; Xue, L.; Zhang, K. Tailoring Tribological Properties and Corrosion Resistance of Self-Lubricating Ti-Mo-N Coatings Prepared by Arc Depositions. Coatings 2025, 15, 956. https://doi.org/10.3390/coatings15080956
Wang C, Liu J, Liu G, Xue L, Zhang K. Tailoring Tribological Properties and Corrosion Resistance of Self-Lubricating Ti-Mo-N Coatings Prepared by Arc Depositions. Coatings. 2025; 15(8):956. https://doi.org/10.3390/coatings15080956
Chicago/Turabian StyleWang, Chenwei, Jing Liu, Gang Liu, Liyuan Xue, and Keren Zhang. 2025. "Tailoring Tribological Properties and Corrosion Resistance of Self-Lubricating Ti-Mo-N Coatings Prepared by Arc Depositions" Coatings 15, no. 8: 956. https://doi.org/10.3390/coatings15080956
APA StyleWang, C., Liu, J., Liu, G., Xue, L., & Zhang, K. (2025). Tailoring Tribological Properties and Corrosion Resistance of Self-Lubricating Ti-Mo-N Coatings Prepared by Arc Depositions. Coatings, 15(8), 956. https://doi.org/10.3390/coatings15080956






