Simple Spray Preparation of Multifunctional Organic–Inorganic Hybrid Coatings for Surface Strengthening of Flat Thin-Sheet Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
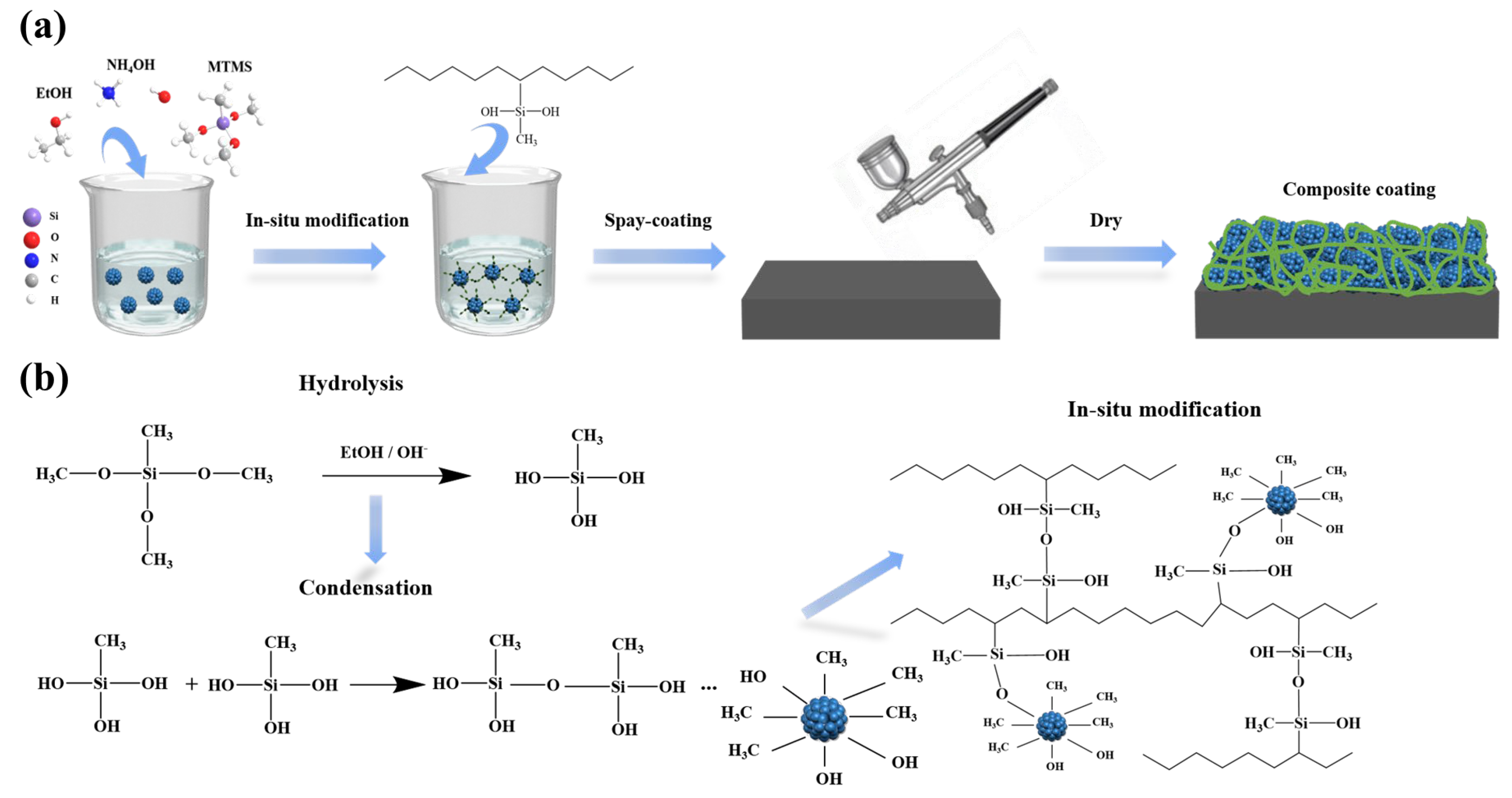
2.2. Preparation of the Multifunctional Organic-Inorganic Hybrid Coatings
2.3. Example Characterization Technology
2.3.1. Hydrophobic Properties Test
2.3.2. Characterization of Surface Morphology and Chemical Composition
2.3.3. Mechanical Strengthening Performance Test
2.3.4. Ultraviolet Resistance Test and Thermal Stability Test
2.3.5. Abrasive Resistance and Chemical Durability
2.3.6. Antifouling and Self-Cleaning Properties
3. Results and Discussion
3.1. Hydrophobic Properties of the Composite Coating
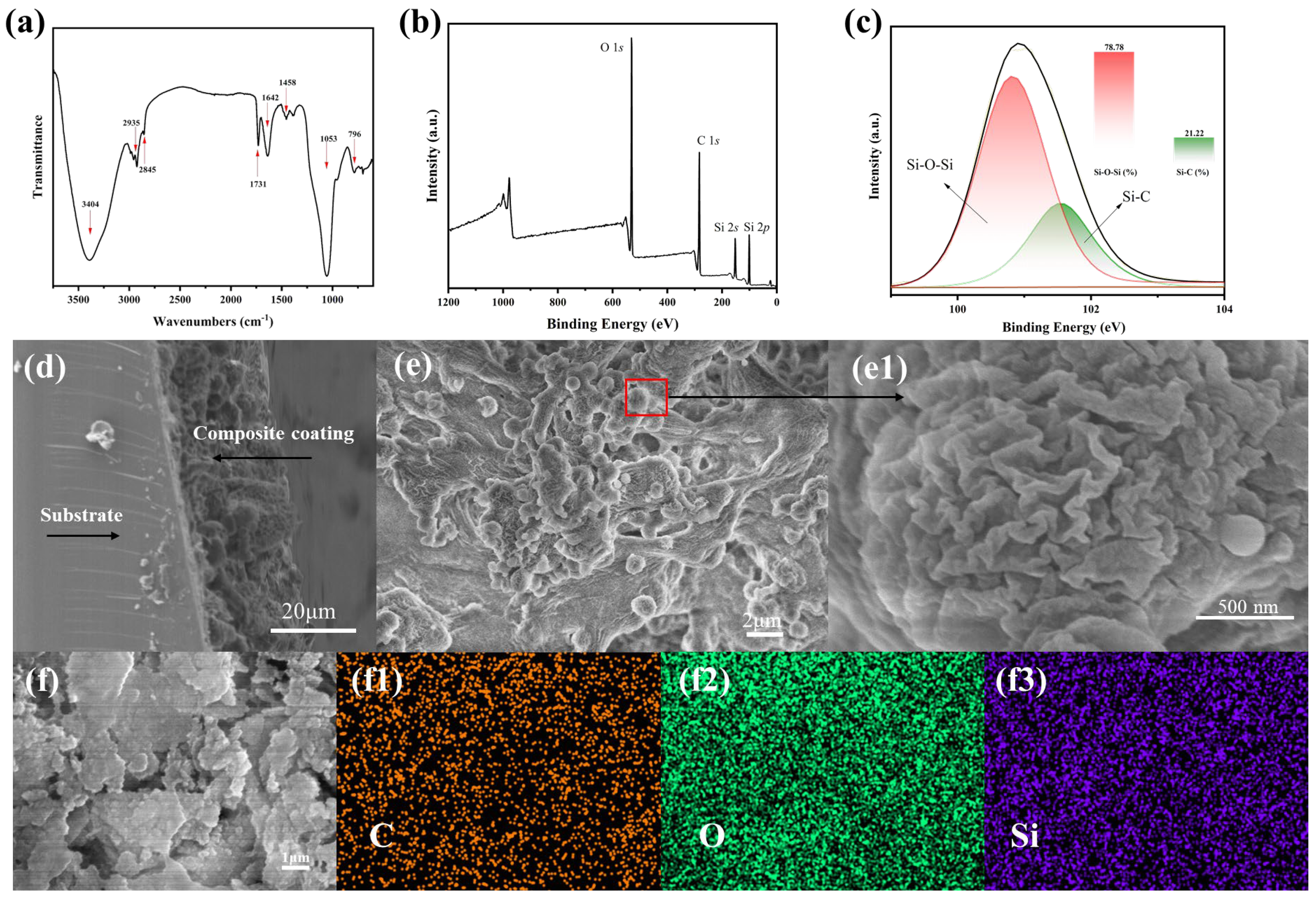
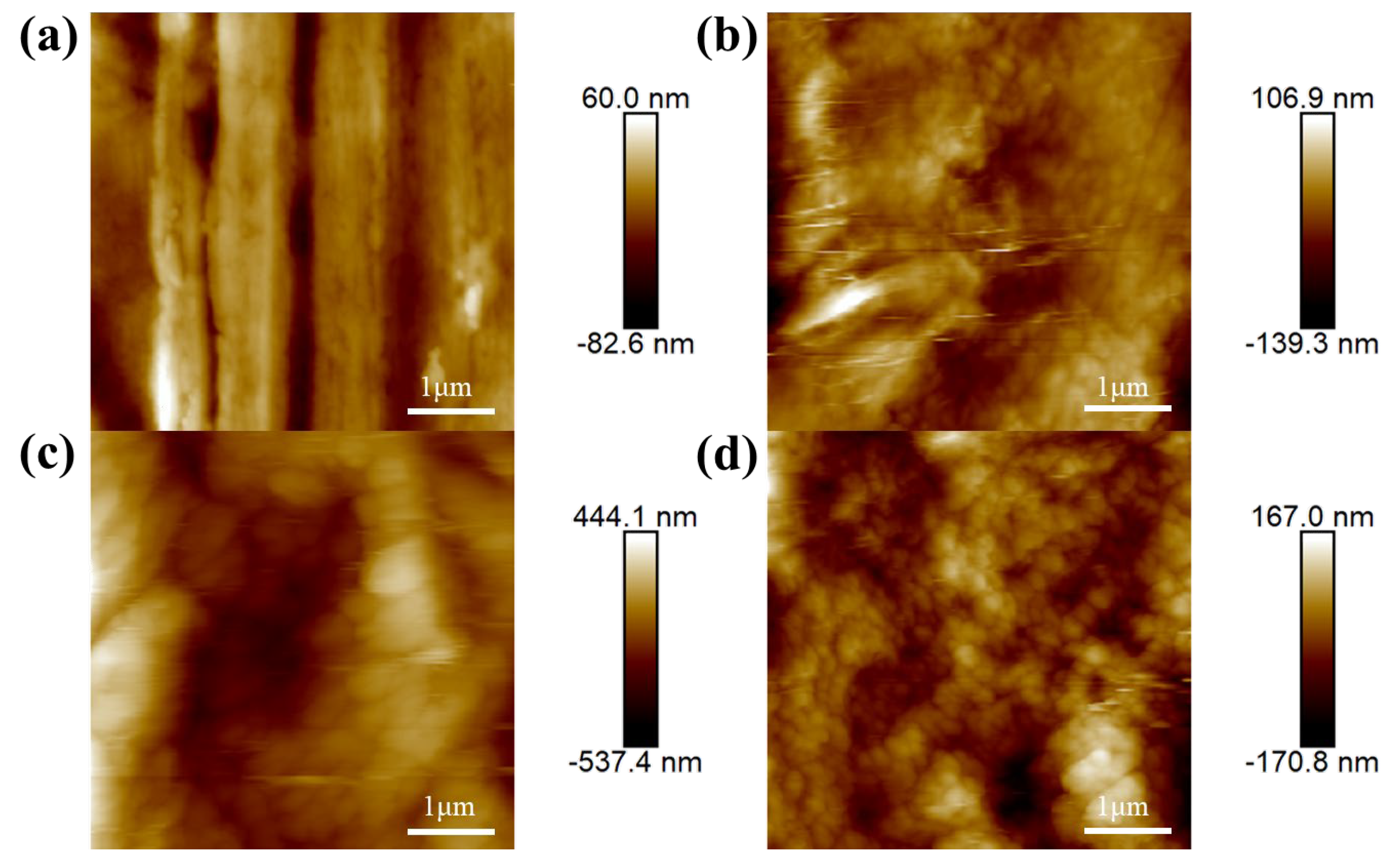
3.2. Surface Morphology and Chemical Composition of the Composite Coating
3.3. Mechanical Strengthening Performance
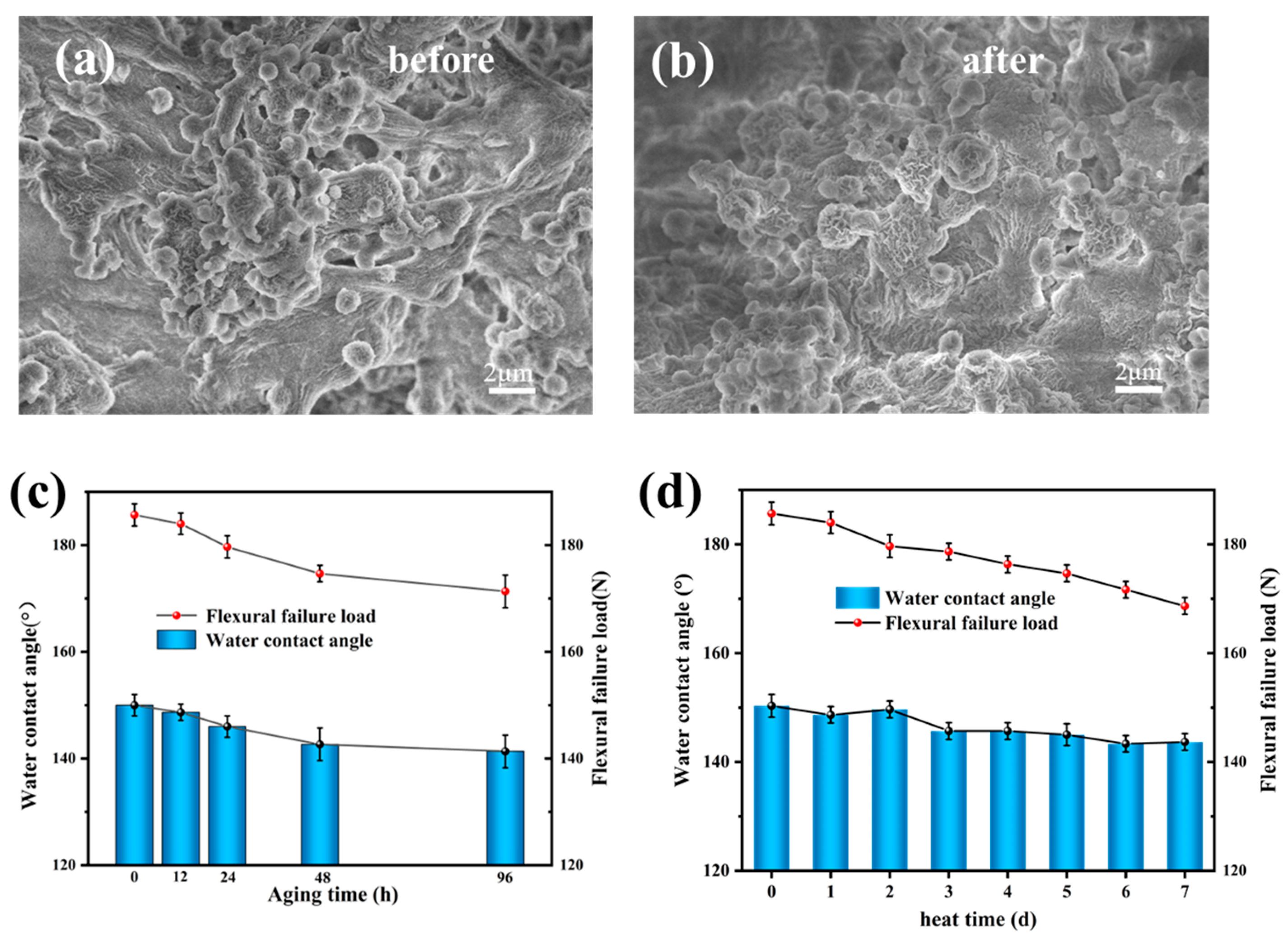
3.4. UV Resistance and Thermal Stability
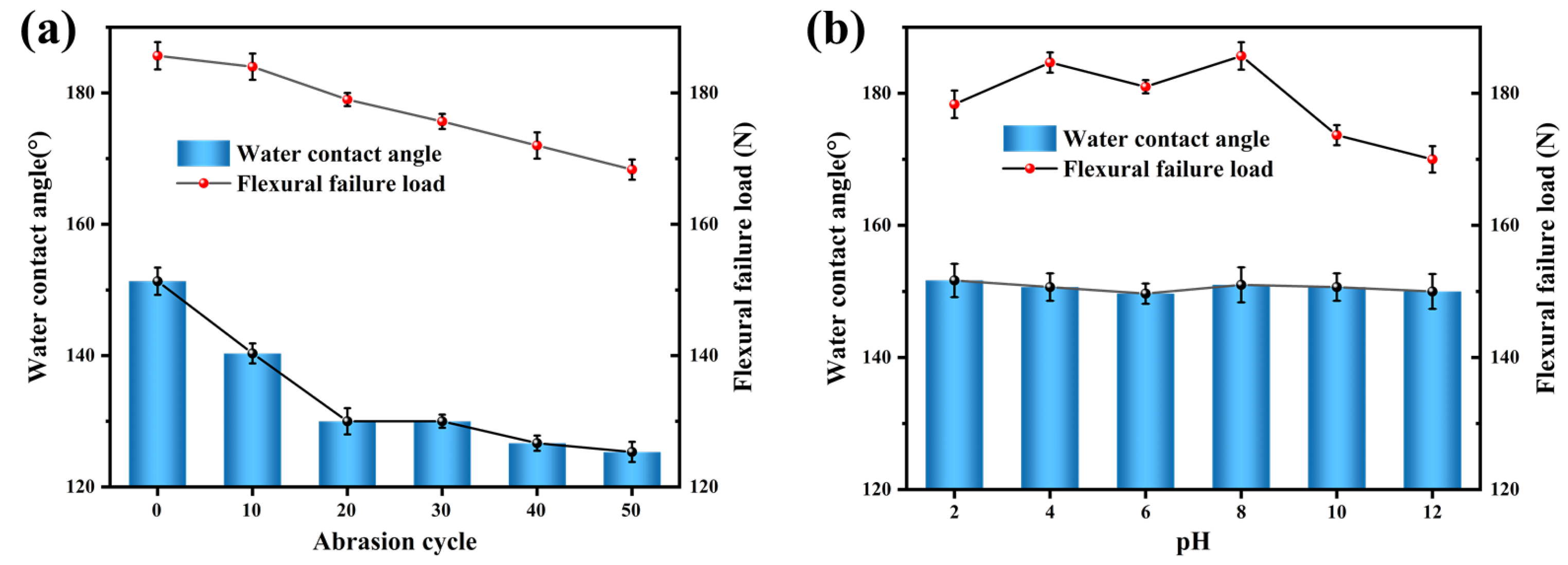
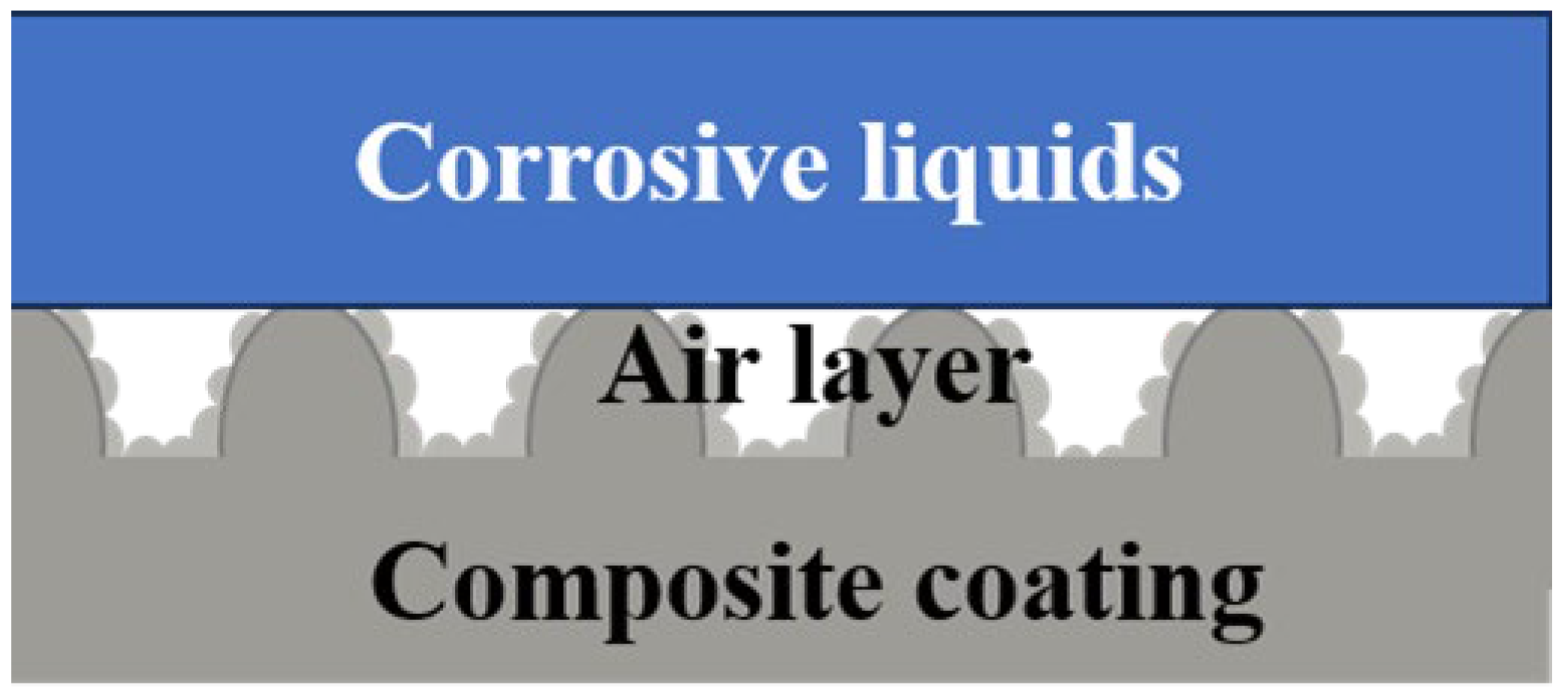
3.5. Abrasion and Chemical Resistance
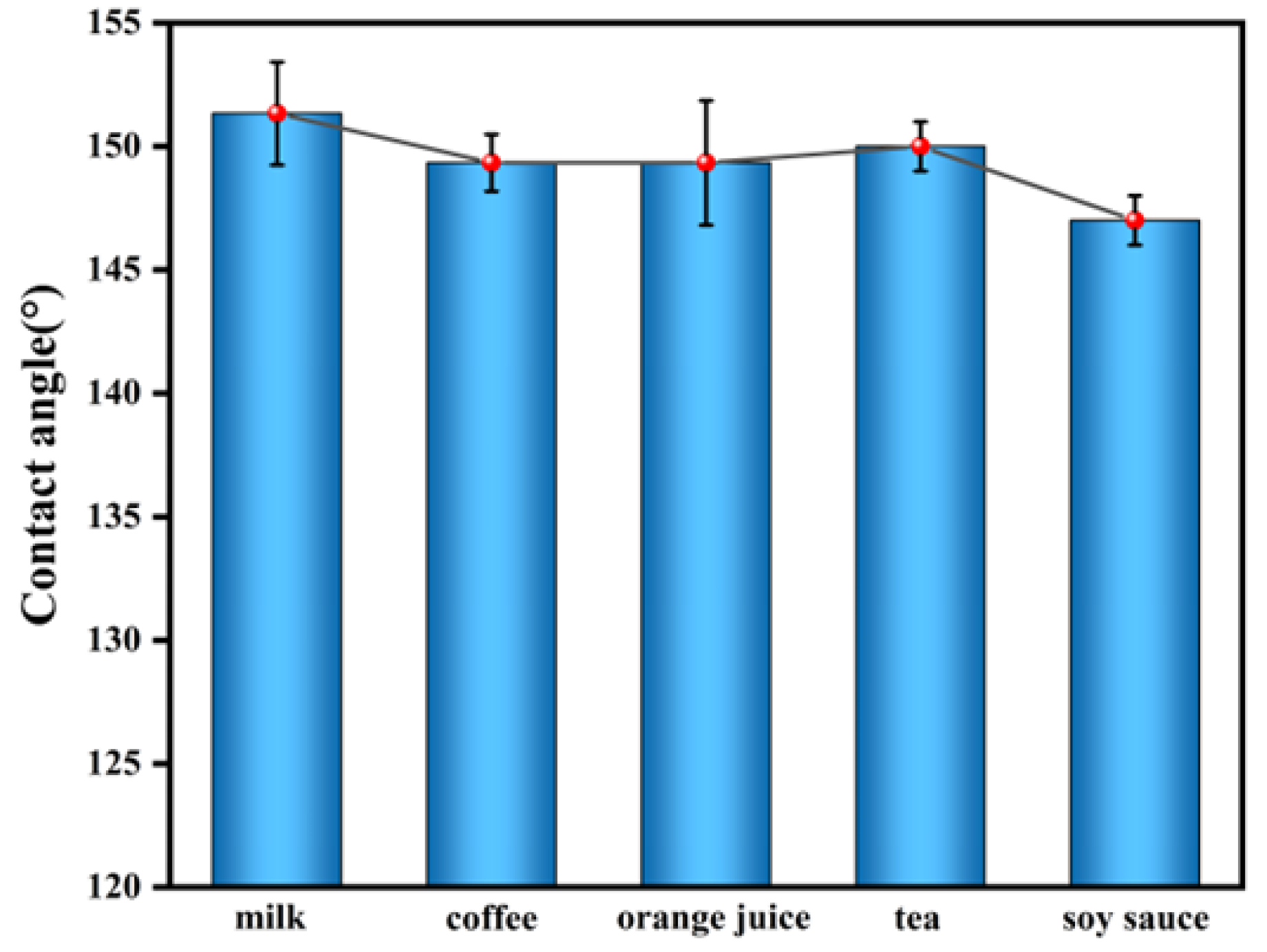
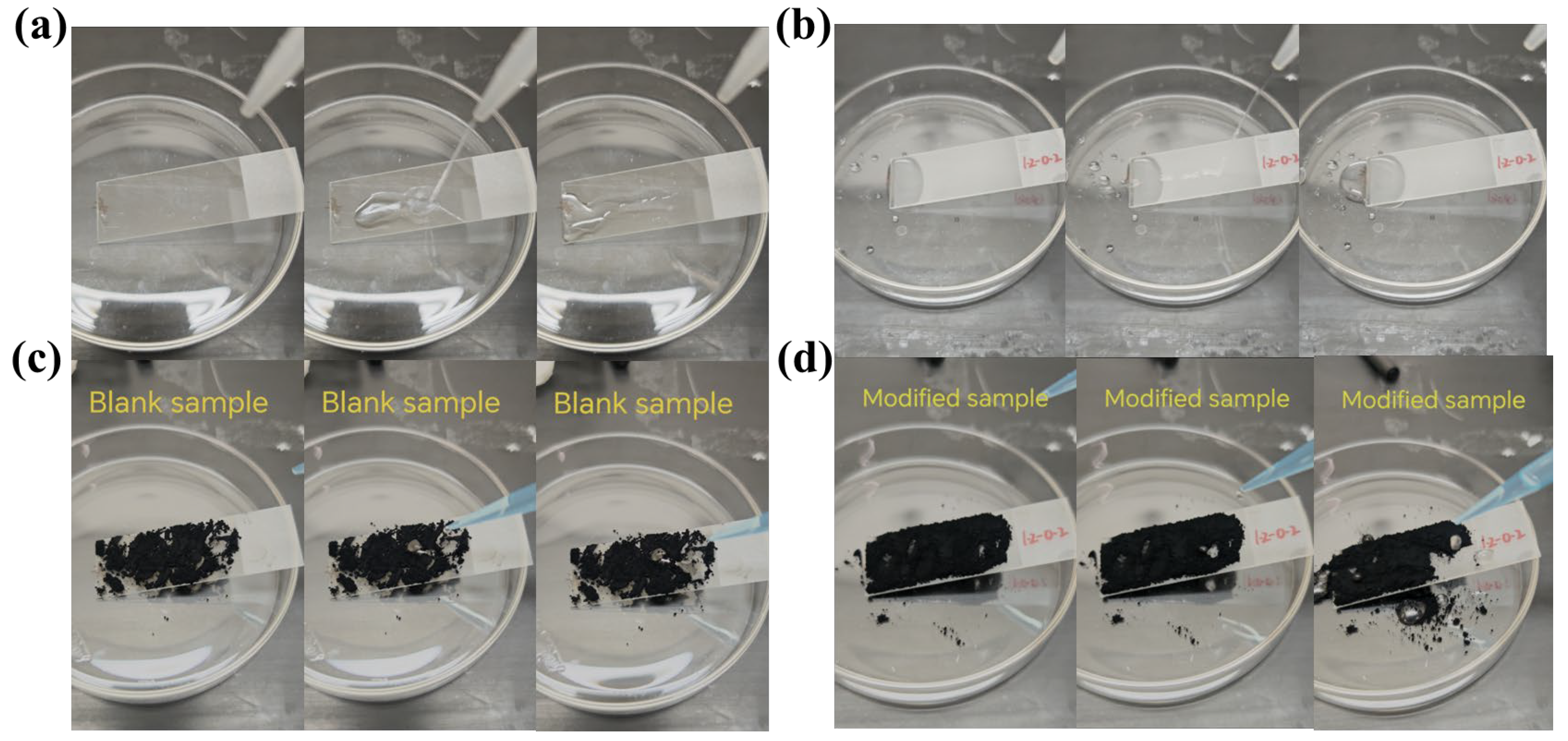
3.6. Anti-Fouling and Self-Cleaning Performance
3.7. Comparison of Preparation Methods and Economic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cong, X.Y.; Khalili, P.; Zhu, C.K.; Li, S.H.; Li, J.J.; Rudd, C.; Liu, X.L. Investigation of Fire Protection Performance and Mechanical Properties of Thin-Ply Bio-Epoxy Composites. Polymers 2021, 13, 731. [Google Scholar] [CrossRef]
- Akkus, M. Hybrid Composite Board Produced from Wood and Mineral Stone Wool Fibers. Bioresources 2022, 17, 6245–6261. [Google Scholar] [CrossRef]
- Antepara, I.; Pavlik, Z.; Zumar, J.; Pavlikova, M.; Cerny, R. Properties of Hydrophilic Mineral Wool for Desalination of Historical Masonry. Mater. Sci. Medzg. 2016, 22, 88–93. [Google Scholar] [CrossRef][Green Version]
- Simon, T.K.; Mlinárik, L.; Vargha, V. Effect of water vapor on the compressive strength of a mineral wool insulation board. J. Bulid. Phys. 2015, 39, 285–294. [Google Scholar] [CrossRef]
- Liu, W.B.; Yang, X.F.; Wan, Z.; Xia, G.F.; Li, D.; Wang, S.R. Surface strengthening technology for mechanical parts. Surf. Rev. Lett. 2021, 28, 2030006. [Google Scholar] [CrossRef]
- Hua, J.; Liu, J.P.; Liu, P.T.; Zhao, X.J.; Chen, C.H.; Ren, R.M. Investigation on WEA fatigue spalling of U71MnG rail material subject to laser quenching surface treatment. Wear 2023, 512–513, 204560. [Google Scholar] [CrossRef]
- Lu, G.X.; Liu, H.; Lin, C.H.; Zhang, Z.; Shukla, P.; Zhang, Y.K.; Yao, J.H. Improving the fretting performance of aero-engine tenon joint materials using surface strengthening. Mater. Sci. Technol. 2019, 35, 1781–1788. [Google Scholar] [CrossRef]
- Chen, K.Y.; Yang, X.F.; Zhang, Y.F.; Yang, H.; Lv, G.J.; Gao, Y.L. Research progress of improving surface friction properties by surface texture technology. Int. J. Adv. Manuf. Technol. 2021, 116, 2797–2821. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, K.; Li, C.X.; Yasir, M.; Luo, X.T.; Li, C.J. Microstructures of aluminum surfaces reinforced with 316L stainless steel particles via high-speed particle injection and the resulting double-strengthening mechanism. Surf. Coat. Technol. 2020, 385, 125380. [Google Scholar]
- Liu, M.; Wang, S.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Venkataraman, M.; Kremenakova, D.; Militky, J.; Zhou, Y. Progress in Sol-Gel Technology for the Coatings of Fabrics. Materials 2020, 13, 1838. [Google Scholar] [CrossRef]
- Xie, W.-Y.; Song, F.; Wang, X.-L.; Wang, Y.-Z. Development of Copper Phosphate Nanoflowers on Soy Protein toward a Superhydrophobic and Self-Cleaning Film. ACS. Sustain. Chem. Eng. 2017, 5, 869–875. [Google Scholar] [CrossRef]
- Xie, W.-Y.; Wang, F.; Xu, C.; Song, F.; Wang, X.-L.; Wang, Y.-Z. A superhydrophobic and self-cleaning photoluminescent protein film with high weatherability. Chem. Eng. J. 2017, 326, 436–442. [Google Scholar] [CrossRef]
- Hu, C.; Chen, W.; Li, T.; Ding, Y.; Yang, H.; Zhao, S.; Tsiwah, E.A.; Zhao, X.; Xie, Y. Constructing non-fluorinated porous superhydrophobic SiO2-based films with robust mechanical properties. Colloids Surf. A 2018, 551, 65–73. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, H.; Wang, H.; Zhao, Y.; Shao, H.; Xu, Z.; Feng, Z.; Liu, D.; Lin, T. Argon Plasma Treatment of Fluorine-Free Silane Coatings: A Facile, Environment-Friendly Method to Prepare Durable, Superhydrophobic Fabrics. Adv. Mater. Interfaces 2017, 4, 1700027. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Lu, Z.; Chen, L.; Huang, L.; Fan, M. A robust superhydrophobic TiO2 NPs coated cellulose sponge for highly efficient oil-water separation. Sci. Rep. 2017, 7, 9428. [Google Scholar] [CrossRef]
- He, T.; Zhao, H.; Liu, Y.; Zhao, C.; Wang, L.; Wang, H.; Zhao, Y.; Wang, H. Facile fabrication of superhydrophobic Titanium dioxide-composited cotton fabrics to realize oil-water separation with efficiently photocatalytic degradation for water-soluble pollutants. Colloids Surf. A 2020, 585, 124080. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Zerafat, M.M. Fabrication of stable fluorine-free superhydrophobic fabrics for anti-adhesion and self-cleaning properties. Appl. Surf. Sci. 2019, 464, 458–471. [Google Scholar] [CrossRef]
- Walter, T.; Hein, T.; Weichselgartner, M.; Wommer, K.; Aust, M.; Vogel, N. Dispersion-based, scalable fabrication of repellent superhydrophobic and liquid-infused coatings under ambient conditions. Green Chem. 2022, 24, 3009–3016. [Google Scholar] [CrossRef]
- Na, J.Y.; Kang, B.; Sin, D.H.; Cho, K.; Park, Y.D. Understanding Solidification of Polythiophene Thin Films during Spin-Coating: Effects of Spin-Coating Time and Processing Additives. Sci. Rep. 2015, 5, 13288. [Google Scholar] [CrossRef]
- Dong, T.; Zhou, Y.; Hu, D.; Xiao, P.; Wang, Q.; Wang, J.; Pei, J.; Cao, Y. Free-standing, flexible, multifunctional, and environmentally stable superhydrophobic composite film made of self-assembled organic micro/super-nanostructures through solution process. J. Colloid Interface Sci. 2015, 445, 213–218. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, H.; Liu, Z.; Zhang, X.; Zhang, W.; Chen, X.; Zhu, Y. Durable fluorine-free superhydrophobic polyethersulfone (PES) composite coating with uniquely weathering stability, anti-corrosion and wear-resistance. Prog. Org. Coat. 2019, 127, 16–26. [Google Scholar]
- Zhou, K.J.; Bao, M.X.; Fang, Y.K.; He, P.; Ren, J.G.; Zong, W.; Zhang, L.S.; Liu, T.X. Single-Atom Zirconium Coordination Polyimide Aerogel as Separator Coating Toward High-Rate Lithium Metal Battery. Adv. Funct. Mater. 2025, 35, 2411963. [Google Scholar]
- Guo, X.; Zhang, Q.; Ding, X.; Shen, Q.; Wu, C.; Zhang, L.; Yang, H. Synthesis and application of several sol–gel-derived materials via sol–gel process combining with other technologies: A review. J. Sol-Gel Sci. Technol. 2016, 79, 328–358. [Google Scholar] [CrossRef]
- Yang, M.; Liu, W.; Jiang, C.; He, S.; Xie, Y.; Wang, Z. Fabrication of superhydrophobic cotton fabric with fluorinated TiO2 sol by a green and one-step sol-gel process. Carbohydr. Polym. 2018, 197, 75–82. [Google Scholar]
- Cheng, W.; Liu, W.; Wang, Q.; Wang, P.; Zhou, M.; Yu, Y. Durable hydrophobic and antibacterial textile coating via PDA/AgNPs/ODA in situ assembly. Cellulose 2022, 29, 1175–1187. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, M.; Cai, L. Improvement of mechanical, humidity resistance and thermal properties of heat-treated rubber wood by impregnation of SiO2 precursor. Sci. Rep. 2019, 9, 982. [Google Scholar]
- Li, S.; Page, K.; Sathasivam, S.; Heale, F.; He, G.; Lu, Y.; Lai, Y.; Chen, G.; Carmalt, C.J.; Parkin, I.P. Efficiently texturing hierarchical superhydrophobic fluoride-free translucent films by AACVD with excellent durability and self-cleaning ability. J. Mater. Chem. A 2018, 6, 17633–17641. [Google Scholar]
- Sun, W.; Wang, L.; Yang, Z.; Li, S.; Wu, T.; Liu, G. Fabrication of polydimethylsiloxane-derived superhydrophobic surface on aluminium via chemical vapour deposition technique for corrosion protection. Corros. Sci. 2017, 128, 176–185. [Google Scholar] [CrossRef]
- Celia, E.; Darmanin, T.; Taffin de Givenchy, E.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid Interface Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Fu, Q.; Li, M.; Hu, W.; Wang, Q.; Du, M.; Liu, X.; Chen, Z. Fabrication of self-cleaning superhydrophobic surface on aluminum alloys with excellent corrosion resistance. Surf. Coat. Technol. 2015, 276, 341–348. [Google Scholar]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Bio-inspired sustainable and durable superhydrophobic materials: From nature to market. J. Mater. Chem. A 2019, 7, 16643–16670. [Google Scholar] [CrossRef]
- Sri, A.K.; Deeksha, P.; Deepika, G.; Nishanthini, J.; Hikku, G.S.; Shilpa, S.S.; Jeyasubramanian, K.; Murugesan, R. Super-hydrophobicity: Mechanism, fabrication and its application in medical implants to prevent biomaterial associated infections. J. Ind. Eng. Chem. 2020, 92, 1–17. [Google Scholar] [CrossRef]
- Bai, H.; Song, C.; Zheng, L.; Shen, T.; Meng, X.; Ma, J. Overview of rough surface construction technology for cotton fabrics used in oil/water separation. RSC Sustain. 2025, 3, 676–697. [Google Scholar] [CrossRef]
- Guo, F.; Wen, Q.Y.; Peng, Y.B.; Guo, Z.G. Simple one-pot approach toward robust and boiling-water resistant superhydrophobic cotton fabric and the application in oil/water separation. J. Mater. Chem. A 2017, 5, 21866–21874. [Google Scholar] [CrossRef]
- Tao, C.; Yan, H.; Yuan, X.; Yao, C.; Yin, Q.; Zhu, J.; Ni, W.; Yan, L.; Zhang, L. Synthesis of shape-controlled hollow silica nanostructures with a simple soft-templating method and their application as superhydrophobic antireflective coatings with ultralow refractive indices. Colloids Surf. A 2016, 501, 17–23. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Wang, M.; Men, X.; Xue, Q. Environmentally safe, substrate-independent and repairable nanoporous coatings: Large-scale preparation, high transparency and antifouling properties. J. Mater. Chem. A 2017, 5, 20277–20288. [Google Scholar]
- Xie, H.; Zhao, X.; Li, B.C.; Zhang, J.J.; Wei, J.F.; Zhang, J.P. Waterborne, non-fluorinated and durable anti-icing superhydrophobic coatings based on diatomaceous earth. New J. Chem. 2021, 45, 10409–10417. [Google Scholar]
- Tao, C.; Yan, H.; Yuan, X.; Yin, Q.; Zhu, J.; Ni, W.; Yan, L.; Zhang, L. Sol-gel based antireflective coatings with superhydrophobicity and exceptionally low refractive indices built from trimethylsilanized hollow silica nanoparticles. Colloids Surf. A 2016, 509, 307–313. [Google Scholar]
- Xu, T.L.; Zhang, H.Y.; Hess, D.W.; Chai, X.J.; Xu, K.M.; Yang, X.H.; Xie, L.K. A fluorine-free approach for fabricating superhydrophobic coatings on bamboo using methyltrimethoxysilane (MTMS) under alkaline conditions. Prog. Org. Coat. 2025, 203, 109170. [Google Scholar] [CrossRef]
- Caldona, E.B.; De Leon, A.C.C.; Thomas, P.G.; Naylor, D.F., III; Pajarito, B.B.; Advincula, R.C. Superhydrophobic Rubber-Modified Polybenzoxazine/SiO2 Nanocomposite Coating with Anticorrosion, Anti-Ice, and Superoleophilicity Properties. Ind. Eng. Chem. Res. 2017, 56, 1485–1497. [Google Scholar] [CrossRef]
- Xue, C.H.; Zhang, Z.D.; Zhang, J.; Jia, S.T. Lasting and self-healing superhydrophobic surfaces by coating of polystyrene/SiO2 nanoparticles and polydimethylsiloxane. J. Mater. Chem. A 2014, 2, 15001–15007. [Google Scholar] [CrossRef]
- Ye, H.; Zhu, L.Q.; Li, W.P.; Liu, H.C.; Chen, H.N. Simple spray deposition of a water-based superhydrophobic coating with high stability for flexible applications. J. Mater. Chem. A 2017, 5, 9882–9890. [Google Scholar] [CrossRef]
- Gorrochategui, E.; Pérez-Albaladejo, E.; Casas, J.; Lacorte, S.; Porte, C. Perfluorinated chemicals: Differential toxicity, inhibition of aromatase activity and alteration of cellular lipids in human placental cells. Toxicol. Appl. Pharmacol. 2014, 277, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Morrissette, J.M.; Carroll, P.J.; Bayer, I.S.; Qin, J.; Waldroup, D.; Megaridis, C.M. A methodology to produce eco-friendly superhydrophobic coatings produced from all-water-processed plant-based filler materials. Green Chem. 2018, 20, 5169–5178. [Google Scholar] [CrossRef]
- Allcock, H.R. Fluorinated Polyphosphazenes. In Handbook of Fluoropolymer Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 1–20. [Google Scholar]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef]
- Zhang, L.S.; Zhou, A.G.; Sun, B.R.; Chen, K.S.; Yu, H.Z. Functional and versatile superhydrophobic coatings via stoichiometric silanization. Nat. Commun. 2021, 12, 982. [Google Scholar] [CrossRef]
- GB/T 25998-2020; Mineral Wool Decorating and Acoustic Ceilings. Standardization Administration of China: Beijing, China, 2020.
- GB/T 5210-2006; Paints and Varnishes—Pull-Off Test for Adhesion. Standardization Administration of China: Beijing, China, 2006.
- Lin, J.; Cai, X.; Liu, Z.; Liu, N.; Xie, M.; Zhou, B.; Wang, H.; Guo, Z. Anti-liquid-Interfering and Bacterially Antiadhesive Strategy for Highly Stretchable and Ultrasensitive Strain Sensors Based on Cassie-Baxter Wetting State. Adv. Funct. Mater 2020, 30, 2000398. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, J.; Huang, Y.X.; Lin, Q.Q.; Yu, W.J. Facile preparation of multifunctional bamboo with superhydrophobic, conductive, and flame-retardant properties. Ind. Crop. Prod. 2022, 188, 115676. [Google Scholar] [CrossRef]
- Li, D.W.; Ma, L.J.; Zhang, B.; Chen, S.H. Large-scale fabrication of a durable and self-healing super-hydrophobic coating with high thermal stability and long-term corrosion resistance. Nanoscale 2021, 13, 7810–7821. [Google Scholar] [CrossRef]
- Tian, X.L.; Verho, T.; Ras, R.H.A. Moving superhydrophobic surfaces toward real-world applications. Science 2016, 352, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Sun, Q.Q.; Hokkanen, M.J.; Zhang, C.L.; Lin, F.Y.; Liu, Q.; Zhu, S.P.; Zhou, T.F.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, T.; Li, B.; Wei, J.; Zhang, J. Totally Waterborne and Highly Durable Superamphiphobic Coatings for Anti-Icing and Anticorrosion. Adv. Mater. Interfaces 2019, 6, 1901255. [Google Scholar] [CrossRef]
- Hu, C.; Liu, S.; Li, B.; Yang, H.; Fan, C.; Cui, W. Micro-/Nanometer Rough Structure of a Superhydrophobic Biodegradable Coating by Electrospraying for Initial Anti-Bioadhesion. Adv. Healthc. Mater. 2013, 2, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.; Emelyanenko, A. A wetting experiment as a tool to study the physicochemical processes accompanying the contact of hydrophobic and superhydrophobic materials with aqueous media. Adv. Colloid Interface Sci. 2012, 179–182, 133–141. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhang, C.Y.; Zhang, X.G.; Wang, C.J.; Liu, F.T.; Yuan, R.X.; Wang, H.Y. Durable superhydrophobic PVDF/FEVE/GO@TiO2 composite coating with excellent anti-scaling and UV resistance properties. Chem. Eng. J. 2021, 411, 128632. [Google Scholar] [CrossRef]
- Jin, C.D.; Li, J.P.; Han, S.J.; Wang, J.; Sun, Q.F. A durable, superhydrophobic, superoleophobic and corrosion-resistant coating with rose-like ZnO nanoflowers on a bamboo surface. Appl. Surf. Sci. 2014, 320, 322–327. [Google Scholar] [CrossRef]
- Jin, C.D.; Li, J.P.; Han, S.J.; Wang, J.; Yao, Q.F.; Sun, Q.F. Silver mirror reaction as an approach to construct a durable, robust superhydrophobic surface of bamboo timber with high conductivity. J. Alloys Compd. 2015, 635, 300–306. [Google Scholar] [CrossRef]


| Samples | SFE (mJ·m−2) | WCA (°) |
|---|---|---|
| 0.4 MTMS-0.2 SAC | 35 ± 1.6 | 99.5 |
| 0.6 MTMS-0.2 SAC | 16 ± 1.5 | 118.5 |
| 1.2 MTMS-0.2 SAC | 0.9 ± 0.3 | 150.2 |
| 1.5 MTMS-0.2 SAC | 40 ± 1.0 | 100.1 |
| 3.0 MTMS-0.2 SAC | 45 ± 1.2 | 94.4 |
| 5.0 MTMS-0.2 SAC | 65 ± 1.5 | 61.6 |
| Sample | Ra (nm) | Rq (nm) | WCA (°) |
|---|---|---|---|
| Blank sample | 13.6 | 17.4 | 99.5 |
| 0.6 MTMS-0.2 SAC | 34.5 | 44.0 | 118.5 |
| 1.2 MTMS-0.2 SAC | 88.4 | 109.0 | 150.2 |
| 1.5 MTMS-0.2 SAC | 20.7 | 27.2 | 100.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Li, H.; Chen, H.; Xie, S.; Han, W.; Xi, X.; Hu, Z.; Yue, X.; Xiang, J. Simple Spray Preparation of Multifunctional Organic–Inorganic Hybrid Coatings for Surface Strengthening of Flat Thin-Sheet Materials. Coatings 2025, 15, 1267. https://doi.org/10.3390/coatings15111267
Yu X, Li H, Chen H, Xie S, Han W, Xi X, Hu Z, Yue X, Xiang J. Simple Spray Preparation of Multifunctional Organic–Inorganic Hybrid Coatings for Surface Strengthening of Flat Thin-Sheet Materials. Coatings. 2025; 15(11):1267. https://doi.org/10.3390/coatings15111267
Chicago/Turabian StyleYu, Xianbo, Huaxin Li, Hu Chen, Shuao Xie, Wei Han, Xiaoxue Xi, Zhongbo Hu, Xian Yue, and Junhui Xiang. 2025. "Simple Spray Preparation of Multifunctional Organic–Inorganic Hybrid Coatings for Surface Strengthening of Flat Thin-Sheet Materials" Coatings 15, no. 11: 1267. https://doi.org/10.3390/coatings15111267
APA StyleYu, X., Li, H., Chen, H., Xie, S., Han, W., Xi, X., Hu, Z., Yue, X., & Xiang, J. (2025). Simple Spray Preparation of Multifunctional Organic–Inorganic Hybrid Coatings for Surface Strengthening of Flat Thin-Sheet Materials. Coatings, 15(11), 1267. https://doi.org/10.3390/coatings15111267







