Photocatalytic Performance of 3D-Printed Triply Periodic Minimal Surface Photocatalytic Reactors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
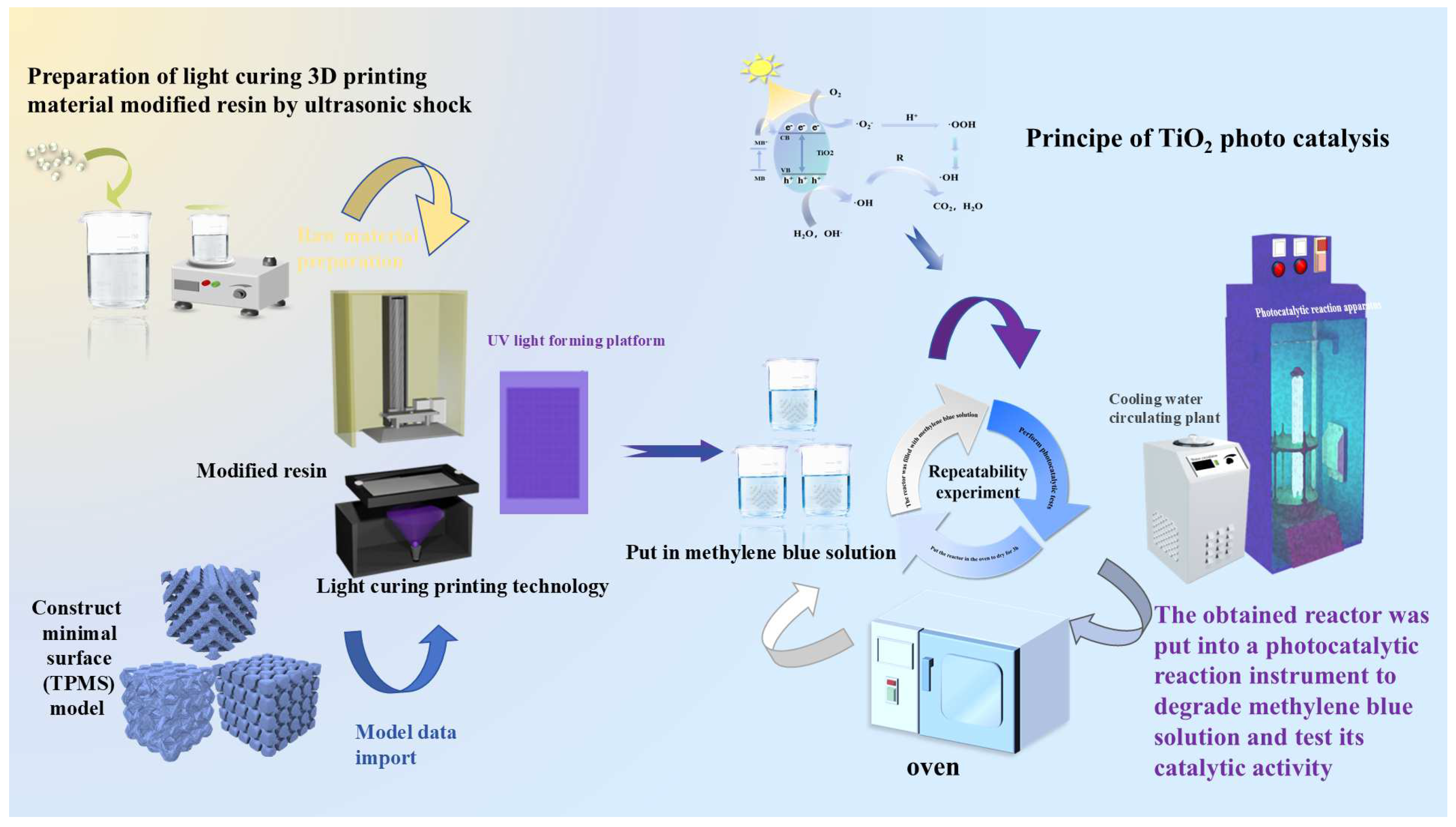
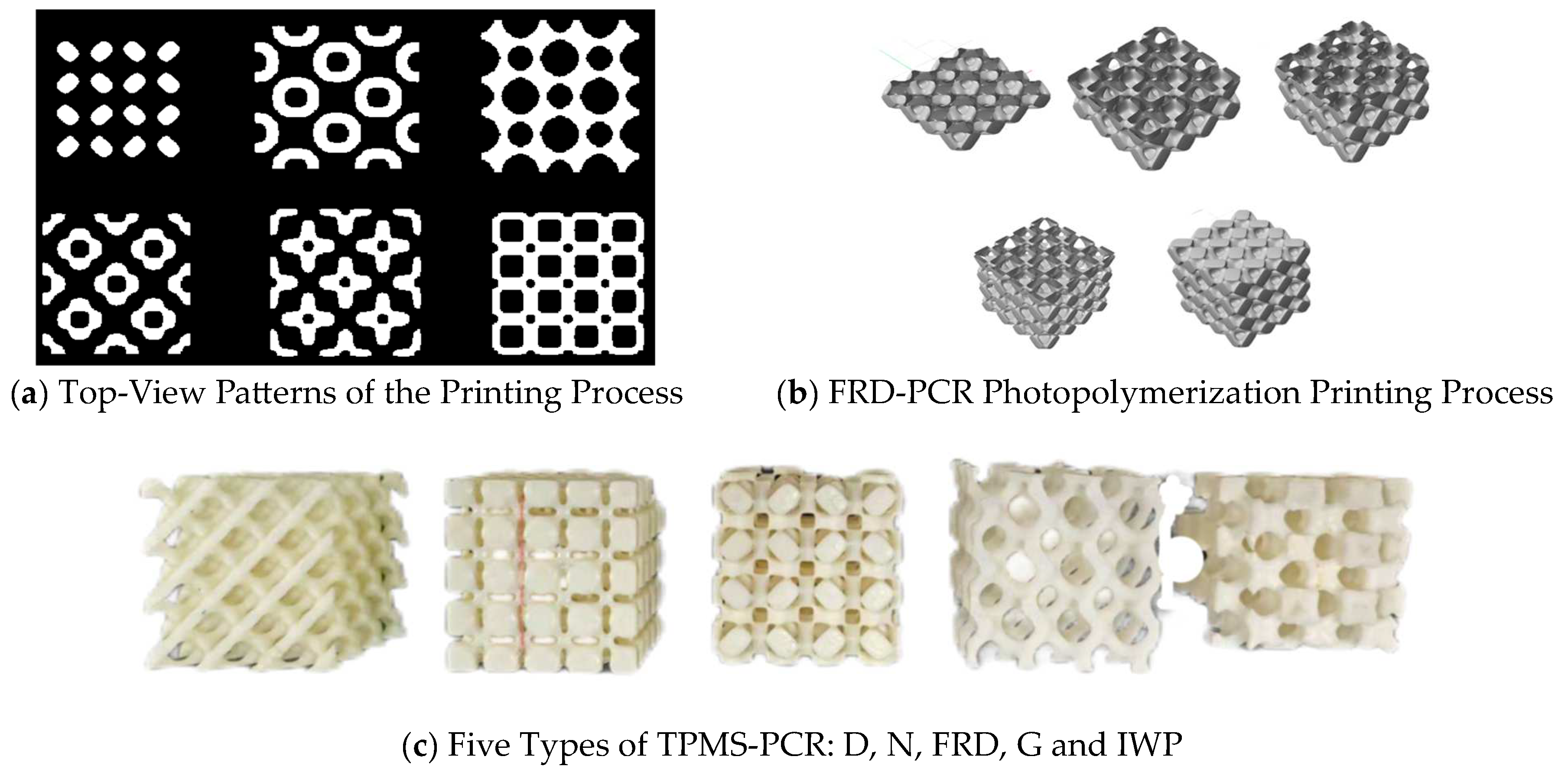
2.2.1. Fabrication of Triply Periodic Minimal Surface Models
2.2.2. Photocatalytic Performance Evaluation Under Controlled Flow Conditions
3. Results and Discussion
3.1. FTIR Characterization of TPMS PCRs
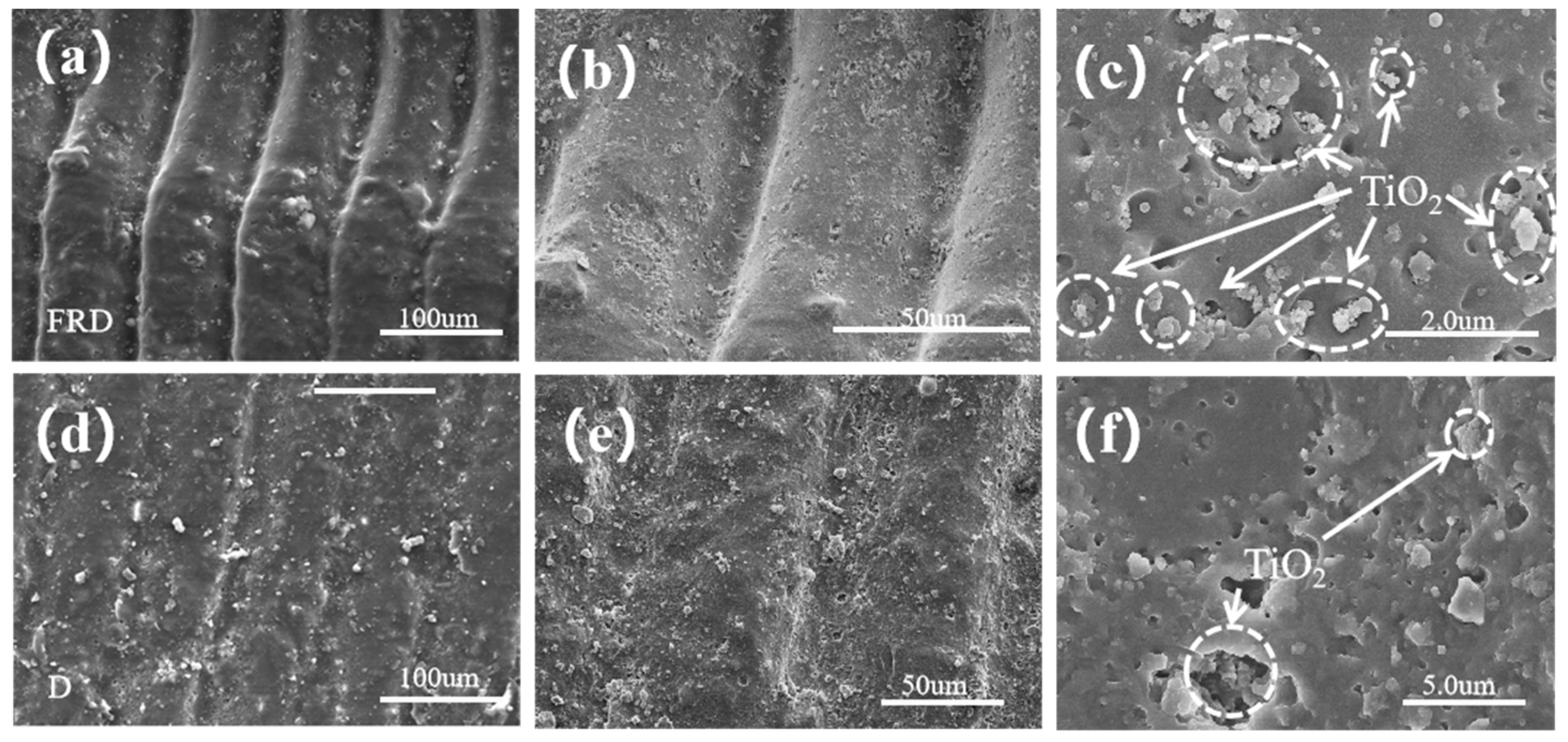
3.2. SEM and EDS Characterization of TPMS PCRs
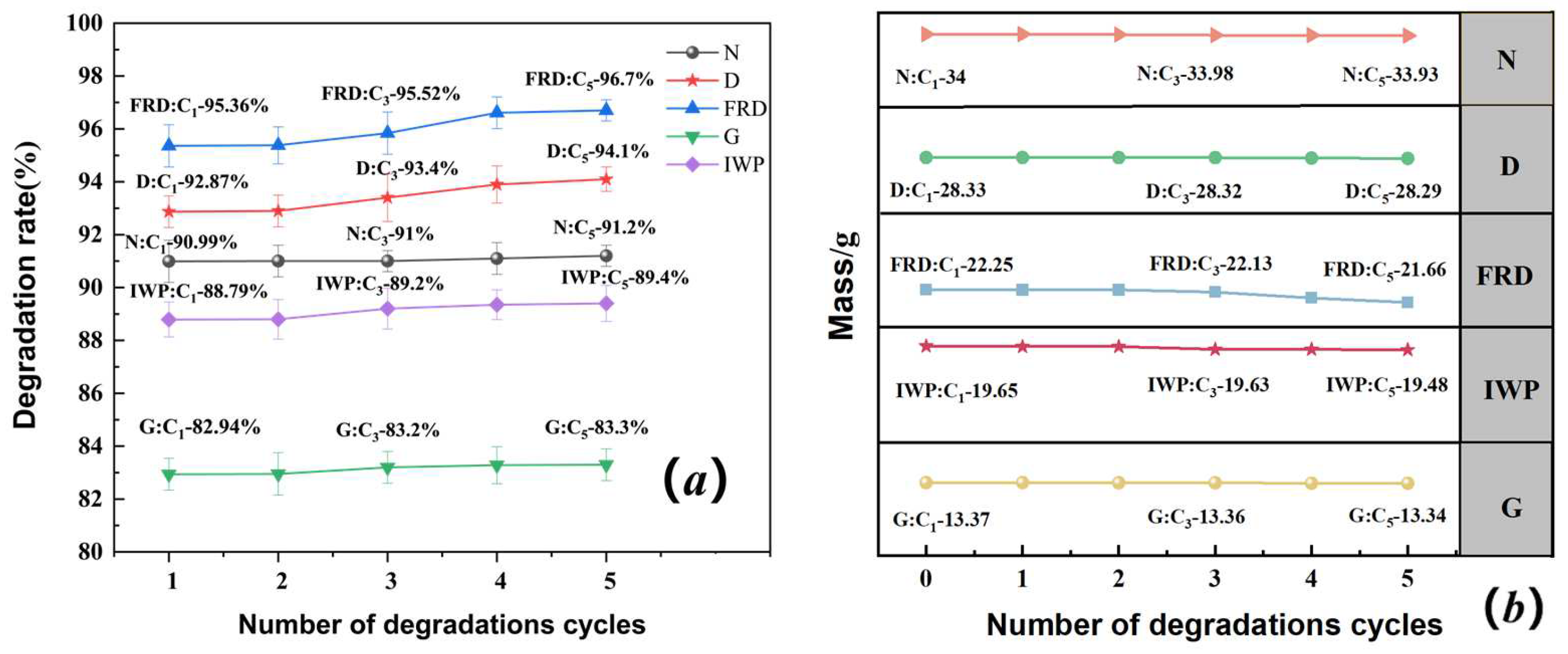
3.3. Photocatalytic Performance Evaluation of SLA-Printed TPMS PCR
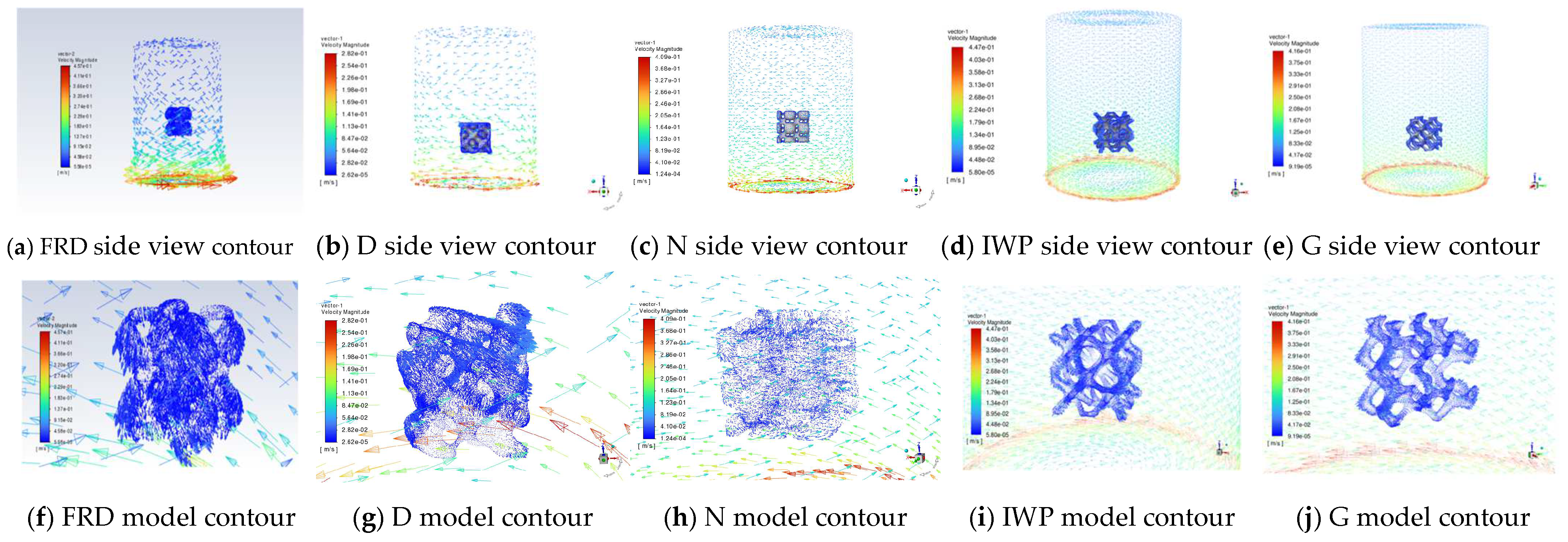
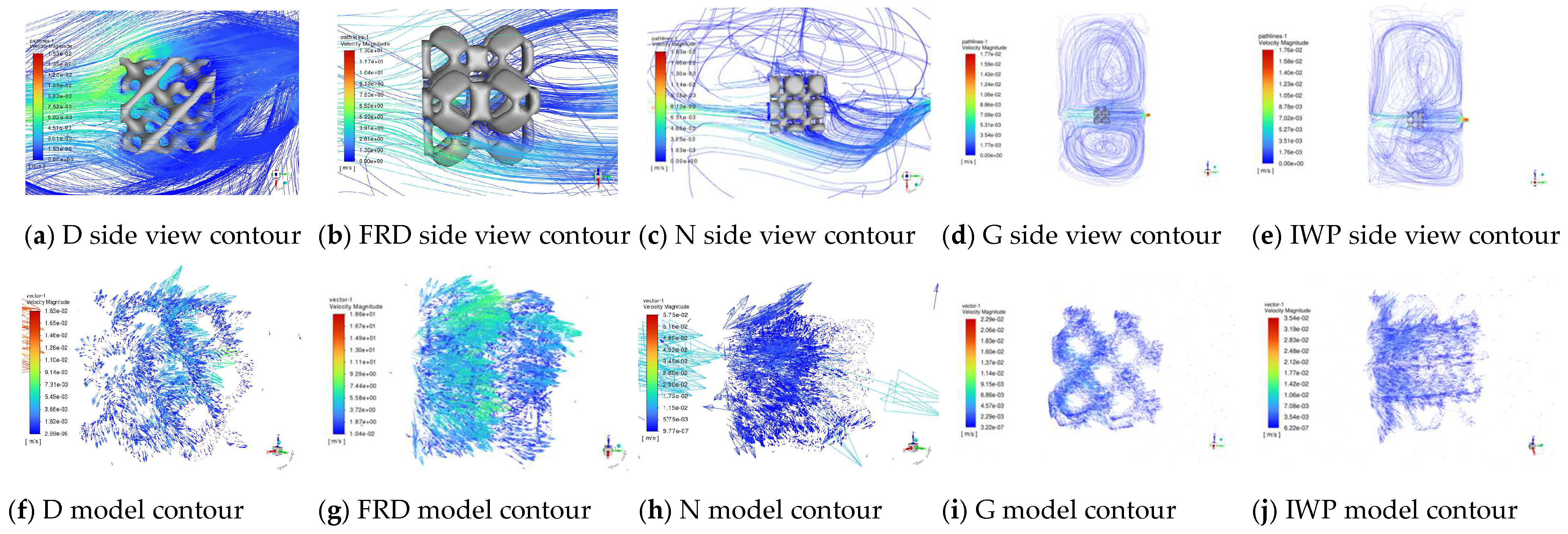
3.4. CFD Hydrodynamic Simulation Analysis of Degradation Process in TPMS PCRs
4. Conclusions
- Structural Optimization: The FRD-type TPMS-PCR exhibited the highest photocatalytic efficiency (95.36% in 2.5 h under rotational flow), attributed to its balanced surface area (252.83 cm2), hierarchical porosity, and optimized flow-channel design.
- Flow Field Dominance: Rotational flow fields enhanced degradation efficiency by approximately sixfold compared to horizontal flow, with the D-type reactor achieving the highest surface velocity (5.3 × 10−2 m/s).
- Performance Correlation: Photocatalytic efficiency followed FRD > D > N > IWP > G, directly linked to pore connectivity, specific surface area, and hydrodynamic characteristics.
- Cyclic Stability: The FRD reactor maintained 96.7% efficiency after five cycles, with SEM revealing surface roughening and TiO2 exposure as key mechanisms for improved recyclability.
- CFD Insights: Model-specific velocity attenuation (surface > internal) and flow field type (rotational > horizontal) critically determined mass transfer and catalytic activity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TPMS | Triply Periodic Minimal Surface |
| PCR | Photocatalytic Reactors |
Appendix A
Appendix A.1. Governing Equations and Numerical Methods
Appendix A.1.1. Governing Equations and Steady-State Assumption
Appendix A.1.2. Core Equations of the Realizable k-ε Model
Appendix A.2. Convergence Analysis
Appendix B
Appendix B.1. Stereolithographic Fabrication of TPMS-PCRs with Varied TiO2
| Parameter | Pure Resin | 1 wt% TiO2/Resin | 1.5 wt% TiO2/Resin | 2 wt% TiO2/Resin | 2.5 wt% TiO2/Resin |
|---|---|---|---|---|---|
| Layer thickness (mm) | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Normal exposure time (s) | 3 | 3 | 3 | 3 | 3 |
| Light-off delay (s) | 2 | 2 | 2 | 2 | 2 |
| Bottom exposure time (s) | 30 | 30 | 40 | 60 | 60 |
| Bottom layers | 6 | 8 | 8 | 8 | 8 |
| Anti-aliasing level | 1 | 1 | 1 | 1 | 1 |
| Z-lift height (mm) | 10 | 10 | 10 | 10 | 10 |
| Z-lift speed (mm/s) | 4 | 4 | 4 | 4 | 4 |
| Z-retract speed (mm/s) | 4 | 4 | 4 | 4 | 4 |
Appendix B.2. Photocatalytic Degradation Performance of TPMS-PCRs with Varied TiO2 Loadings
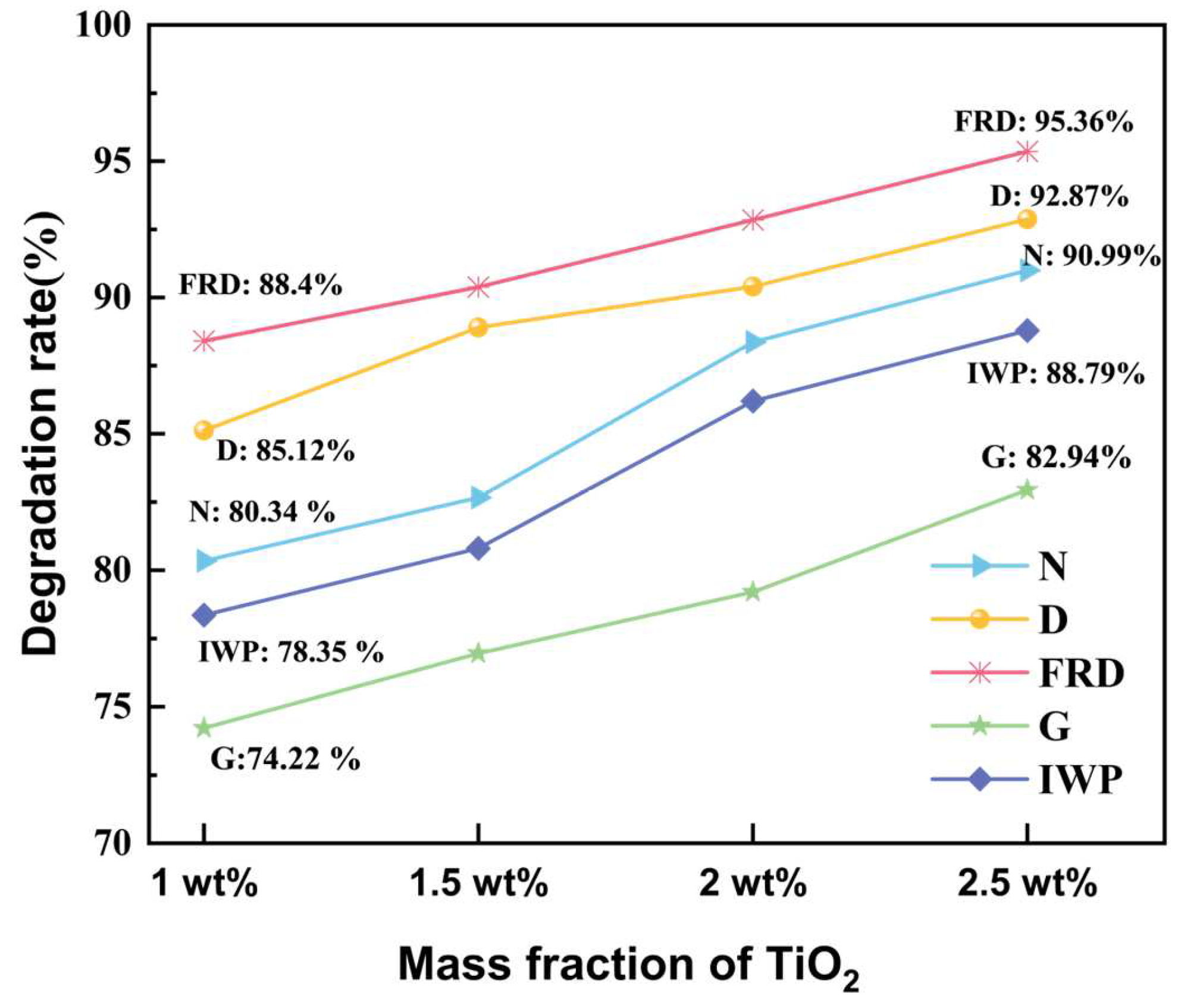
Appendix B.3. The EDS Analyses of N, D, FRD, G, and IWP TPMS-PCRs

References
- Zondag, S.D.; Mazzarella, D.; Noël, T. Scale-Up of Photochemical Reactions: Transitioning from Lab Scale to Industrial Production. Annu. Rev. Chem. Biomol. Eng. 2023, 14, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Akram, S.; Khalid, S.; Lal, B.; Hassan, S.U.; Ashraf, R.; Kezembayeva, G.; Mushtaq, M.; Chinibayeva, N.; Hosseini-Bandegharaei, A. Advanced photocatalysis as a viable and sustainable wastewater treatment process: A comprehensive review. Environ. Res. 2024, 253, 118947. [Google Scholar] [CrossRef] [PubMed]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2019, 27, 2522–2565. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C.; Tong, Y.; Wang, X.; Chen, X.; Yang, Y.; Liu, J.; Chen, Q.; Li, N. Preparation of high-strength ceramsite from coal gangue, fly ash, and steel slag. RSC Adv. 2025, 15, 4332–4341. [Google Scholar] [CrossRef]
- Pham, D.C.; Cao, T.M.D.; Nguyen, M.C.; Nguyen, V.H.; Bui, V.H.; Nguyen, T.T.T. Integrating Photocatalysis and Microfiltration for Methylene Blue Degradation: Kinetics and Cost Estimation. Chem. Eng. Technol. 2022, 45, 1748–1758. [Google Scholar] [CrossRef]
- Rentz, R.; Widerlund, A.; Viklander, M.; Öhlander, B. Impact of Urban Stormwater on Sediment Quality in an Enclosed Bay of the Lule River, Northern Sweden. Water Air Soil Pollut. 2010, 218, 651–666. [Google Scholar] [CrossRef]
- Liu, K.; Xu, X.; Xu, J.; Fang, X.; Liu, L.; Wang, X. The distributions of alkaline earth metal oxides and their promotional effects on Ni/CeO2 for CO2 methanation. J. CO2 Util. 2020, 38, 113–124. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Li, K.; Chen, S.; Yue, D. Photocatalytic Reactor as a Bridge to Link the Commercialization of Photocatalyst in Water and Air Purification. Catalysts 2022, 12, 724. [Google Scholar] [CrossRef]
- Jahn, K.; Bokor, N. Solving the inverse problem of high numerical aperture focusing using vector Slepian harmonics and vector Slepian multipole fields. Opt. Commun. 2013, 288, 13–16. [Google Scholar] [CrossRef]
- Malinowski, S.; Presečki, I.; Jajčinović, I.; Brnardić, I.; Mandić, V.; Grčić, I. Intensification of Dihydroxybenzenes Degradation over Immobilized TiO2 Based Photocatalysts under Simulated Solar Light. Appl. Sci. 2020, 10, 7571. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, K.; Jia, J. Recent Developments of Light-Harvesting Excitation, Macroscope Transfer and Multi-Stage Utilization of Photogenerated Electrons in Rotating Disk Photocatalytic Reactor. Processes 2023, 11, 838. [Google Scholar] [CrossRef]
- Gonçalves, A.G.; Órfão, J.J.; Pereira, M.F.R. Ozonation of bezafibrate over ceria and ceria supported on carbon materials. Environ. Technol. 2014, 36, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhu, Q.; Xi, X.; Xing, M.; Zhang, J. Z-scheme CdS/WO3 on a carbon cloth enabling effective hydrogen evolution. Front. Energy 2021, 15, 678–686. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. The Evolution of Photocatalytic Membrane Reactors over the Last 20 Years: A State of the Art Perspective. Catalysts 2021, 11, 775. [Google Scholar] [CrossRef]
- Barbosa, I.S.O.; Manrique, Y.A.; Paiva, D.; Faria, J.L.; Santos, R.J.; Silva, C.G. Efficient photocatalytic reactors via 3D printing: SLA fabrication and TiO2 hybrid materials. RSC Adv. 2025, 15, 2275–2286. [Google Scholar] [CrossRef]
- Grandcolas, M.; Lind, A. 3D-printed polyamide structures coated with TiO2 nanoparticles, towards a 360-degree rotating photocatalytic reactor. Mater. Lett. 2022, 307. [Google Scholar] [CrossRef]
- Farías, J.J.; Lizondo-Aranda, P.; Thomas, A.H.; Lhiaubet-Vallet, V.; Dántola, M.L. Pterin-lysine photoadduct: A potential candidate for photoallergy. Photochem. Photobiol. Sci. 2022, 21, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Che, S. An Overview of Materials with Triply Periodic Minimal Surfaces and Related Geometry: From Biological Structures to Self-Assembled Systems. Adv. Mater. 2018, 30, e1705708. [Google Scholar] [CrossRef] [PubMed]
- GB/T 1723-1993; Method for Determination of Viscosity of Coatings. The General Administration of Quality Supervision, Inspectionand Quarantine (AQSIQ) of the People’s Republic of China: Beijing, China, 1993.
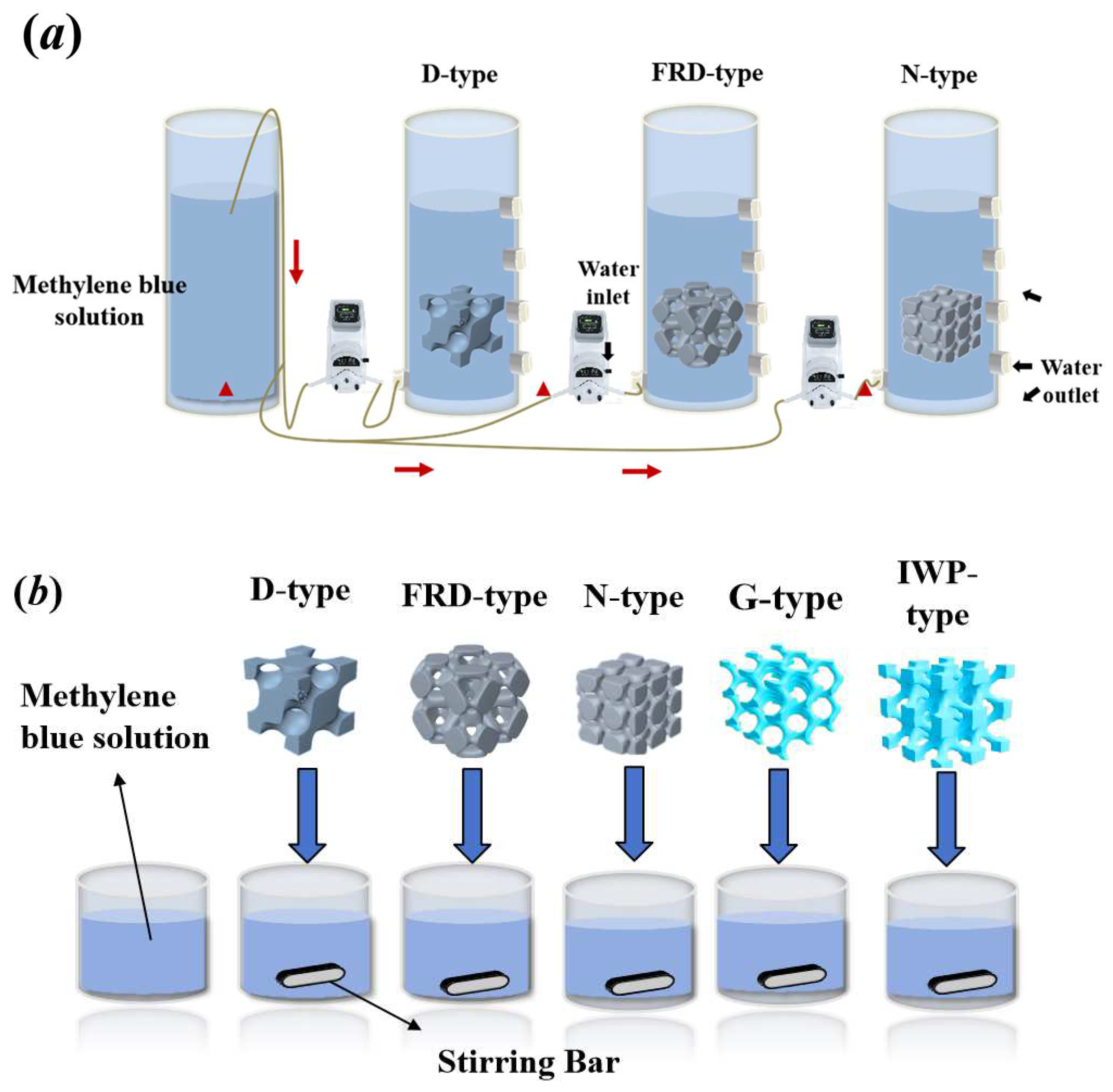
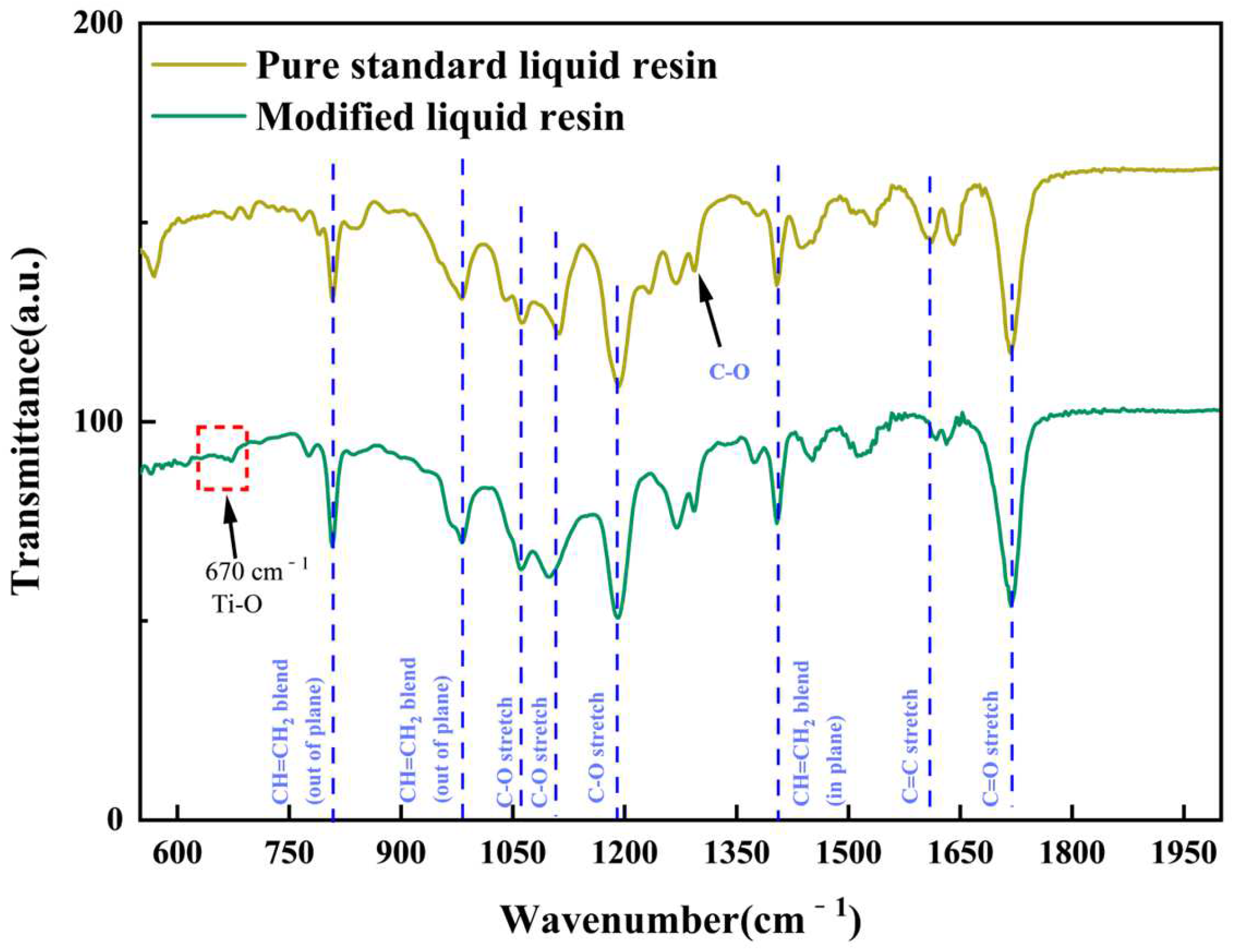
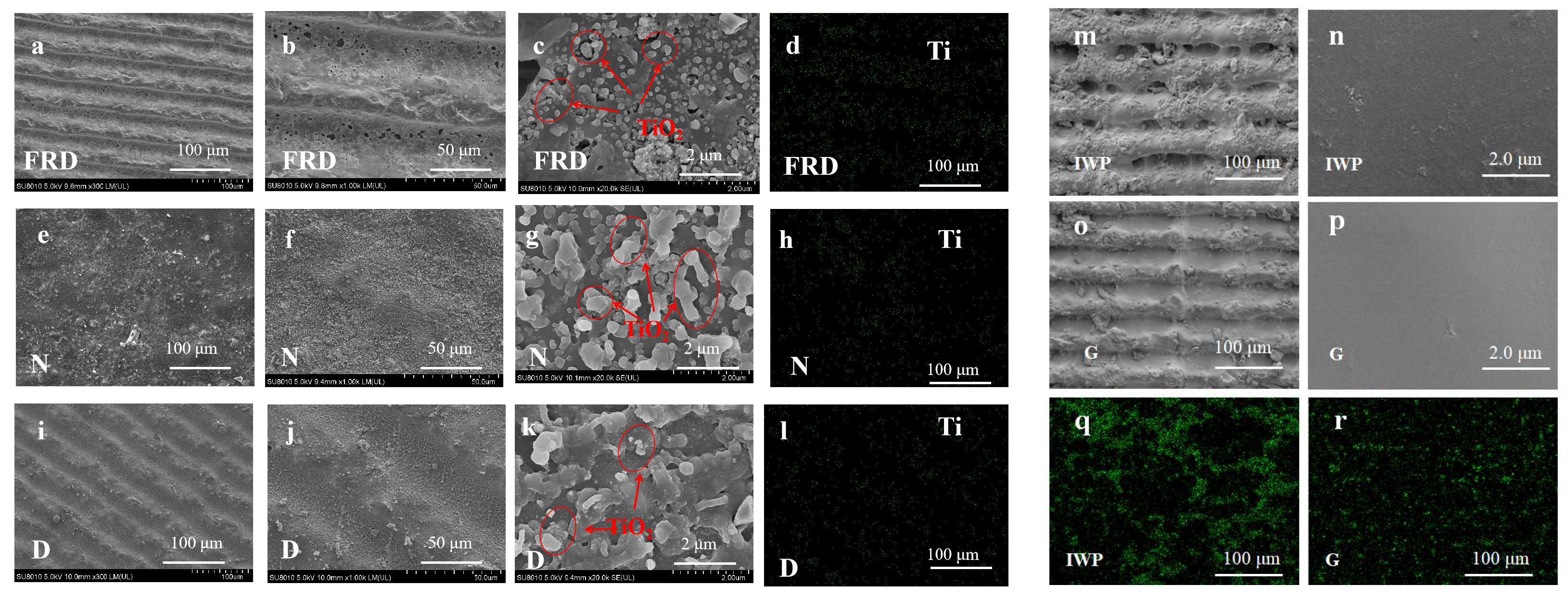
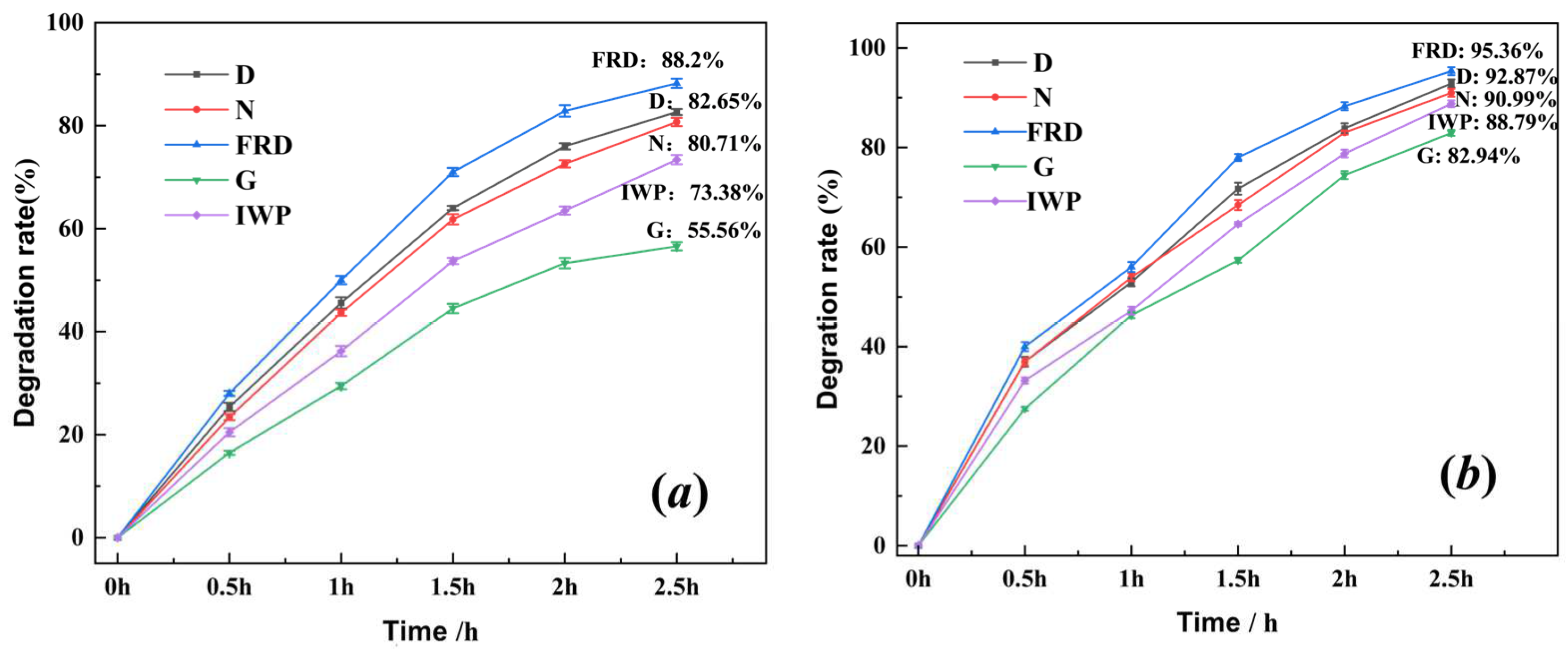
| Reactor Type | Advantages | Disadvantages | References |
|---|---|---|---|
| Suspended reactor | Nanoscale catalyst layer enhances light absorption | Membrane fouling during long-term operation requiring frequent cleaning | [5] |
| Fixed-bed reactor | Immobilized catalysts minimize loss with operational stability | Low mass transfer efficiency and catalyst surface passivation | [6] |
| Thin-film reactor | Ultra-thin (nanoscale) catalyst layer improves light utilization | Prone to membrane fouling during prolonged operation | [7] |
| Annular reactor | Large photocatalytic surface area; CFD-optimizable flow patterns suitable for continuous operation | Potential non-uniform light distribution requiring complex hydrodynamic control | [8] |
| Microstructured fiber reactor | Uniform light distribution with low energy loss | Complex fabrication process | [9] |
| Flat-plate reactor | Simple structure for easy maintenance; ideal for lab-scale studies | Limited reaction volume and shallow light penetration depth | [10] |
| Rotating disk reactor | Enhanced mass transfer via forced convection | High energy consumption | [11] |
| Fluidized-bed reactor | Excellent particle suspension and mass transfer; suitable for high-turbidity water | Catalyst loss requiring replenishment; high energy input | [12] |
| Tubular reactor | Suitable for continuous processing | Significant scale-up effects and clogging risks | [13] |
| Hollow fiber membrane reactor | Reduces secondary pollution | Requires regular cleaning and maintenance | [14] |
| TPMS | Mathematical Function |
|---|---|
| FRD | F(x,y,z) = cos(ωxx)cos(ωyy)cos(ωzz) + cos(2ωxx)cos(2ωyy)cos(2ωzz) − cos(2ωxx) cos(2ωyy) − cos(2ωyx)cos(2ωzy) − cos(2ωxx)cos(2ωzz) = C |
| D | F(x,y,z) = cos(ωxx)cos(ωyy)cos(ωzz) − sin(ωxx)sin(ωyy)sin(ωzz) = C |
| N | F(x,y,z) = 3[cos(ωxx) + cos(ωyy) + cos(ωzz)] + 4cos(ωxx)cos(ωyy)cos(ωzz) = C |
| G | F(x,y,z) = sin(ωxx)cos(ωyy) + sin(ωzz)cos(ωxx) + sin(ωyy)cos(ωzz) = C |
| IWP | 2 × [cos(ωxx)cos(ωyy) + cos(ωyy)cos(ωzz) + cos(ωzz)cos(ωxx) − [cos(2ωxx) + cos(2ωyy) + cos(2ωzz)] = C |
| Parameter | Pure Photosensitive Resin | 2.5 wt% TiO2/Resin |
|---|---|---|
| Layer thickness (mm) | 0.05 | 0.05 |
| Normal exposure time (s) | 3.0 | 3.0 |
| Light-off time (s) | 2.0 | 2.0 |
| Bottom exposure time (s) | 30.0 | 60.0 |
| Bottom layer count | 6 | 8 |
| Anti-aliasing level | 1 | 1 |
| Z-axis lift height (mm) | 10.0 | 10.0 |
| Z-axis lift speed (mm/s) | 4.0 | 4.0 |
| Z-axis retract speed (mm/s) | 4.0 | 4.0 |
| TPMS Type | Dimensions (mm) | Volume (cm3) | Surface Area (cm2) | Porosity (%) | Specific Surface Area (cm2/cm3) |
|---|---|---|---|---|---|
| N | 4 × 4 × 4 | 28.01 | 276.15 | 78.02 | 14.17 |
| D | 4 × 4 × 4 | 13.19 | 186.90 | 53.32 | 9.86 |
| FRD | 4 × 4 × 4 | 20.28 | 252.83 | 66.20 | 12.47 |
| G | 4 × 4 × 4 | 6.27 | 112.39 | 89.56 | 17.94 |
| IWP | 4 × 4 × 4 | 14.93 | 172.66 | 75.12 | 11.57 |
| TPMS Type | Ti Content (wt%) | Avgerage Size of TiO2 (nm) |
|---|---|---|
| N | 2.52 | 28.2 ± 1.5 |
| D | 2.60 | 26.7 ± 2.1 |
| FRD | 2.23 | 25.8 ± 1.8 |
| G | 2.38 | 24.5 ± 1.1 |
| IWP | 2.27 | 29.8 ± 2.6 |
| TPMS Type | Horizontal Flow Field | Rotational Flow Field | ||
|---|---|---|---|---|
| Surface Velocity (10−3 m/s) | Internal Velocity (10−3 m/s) | Surface Velocity (10−3 m/s) | Internal Velocity (10−3 m/s) | |
| N | 3.7 | 1.5 | 1.96 | 0.76 |
| D | 4.5 | 1.78 | 5.3 | 2.5 |
| FRD | 6.5 | 4.65 | 5.11 | 2.21 |
| IWP | 4.9 | 3.18 | 3.7 | 2.1 |
| G | 4.5 | 2.5 | 3.66 | 1.98 |
| TPMS Type | Velocities in Horizontal Flow Field | Velocities in Rotational Flow Field | Optimal Flow Conditions | Recommended Applications |
|---|---|---|---|---|
| FRD | Highest surface (6.5 × 10−3 m/s) and internal (4.65 × 10−3 m/s) velocities | Significant velocity reduction at surface (5.11→2.21 × 10−2 m/s) | High-turbulence | Industrial wastewater treatment; High-flow recirculation systems; Processes requiring frequent catalyst-surface contact |
| D | Relatively high surface velocity (4.5 × 10−3 m/s) | Notable internal velocity decrease (5.3→2.5 × 10−2 m/s) | Moderate-high turbulence | Medium-scale water treatment; Scenarios requiring high surface flow velocity |
| IWP | Balanced velocity distribution (surface: 4.9→3.18 × 10−3 m/s) | Maintains relatively stable internal flow (3.7→2.1 × 10−2 m/s) | Stable flow | Continuous flow systems; Applications needing consistent internal flow |
| G | Moderate surface velocity (4.5→2.5 × 10−3 m/s) | Minimal internal velocity attenuation (3.66→1.98 × 10−2 m/s) | Low-flow | Laboratory-scale reactions; Low-energy systems; Light-limited conditions |
| N | Low energy dissipation (3.7→1.5 × 10−3 m/s) | Relatively small internal velocity reduction (1.96→0.76 × 10−2 m/s) | Static/low-flow | Batch processing; Small-volume degradation; High-surface-area applications |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhang, C.; Chen, Q.; Chen, X.; Li, N. Photocatalytic Performance of 3D-Printed Triply Periodic Minimal Surface Photocatalytic Reactors. Coatings 2025, 15, 953. https://doi.org/10.3390/coatings15080953
Chen X, Zhang C, Chen Q, Chen X, Li N. Photocatalytic Performance of 3D-Printed Triply Periodic Minimal Surface Photocatalytic Reactors. Coatings. 2025; 15(8):953. https://doi.org/10.3390/coatings15080953
Chicago/Turabian StyleChen, Xi, Chenxi Zhang, Qi Chen, Xiao Chen, and Ningning Li. 2025. "Photocatalytic Performance of 3D-Printed Triply Periodic Minimal Surface Photocatalytic Reactors" Coatings 15, no. 8: 953. https://doi.org/10.3390/coatings15080953
APA StyleChen, X., Zhang, C., Chen, Q., Chen, X., & Li, N. (2025). Photocatalytic Performance of 3D-Printed Triply Periodic Minimal Surface Photocatalytic Reactors. Coatings, 15(8), 953. https://doi.org/10.3390/coatings15080953






