Street Art in the Rain: Evaluating the Durability of Protective Coatings for Contemporary Muralism Through Accelerated Rain Ageing
Abstract
1. Introduction
2. Materials and Methods
2.1. Painting Mock-Ups Preparation
2.2. Protective Treatments
2.3. Rain Ageing
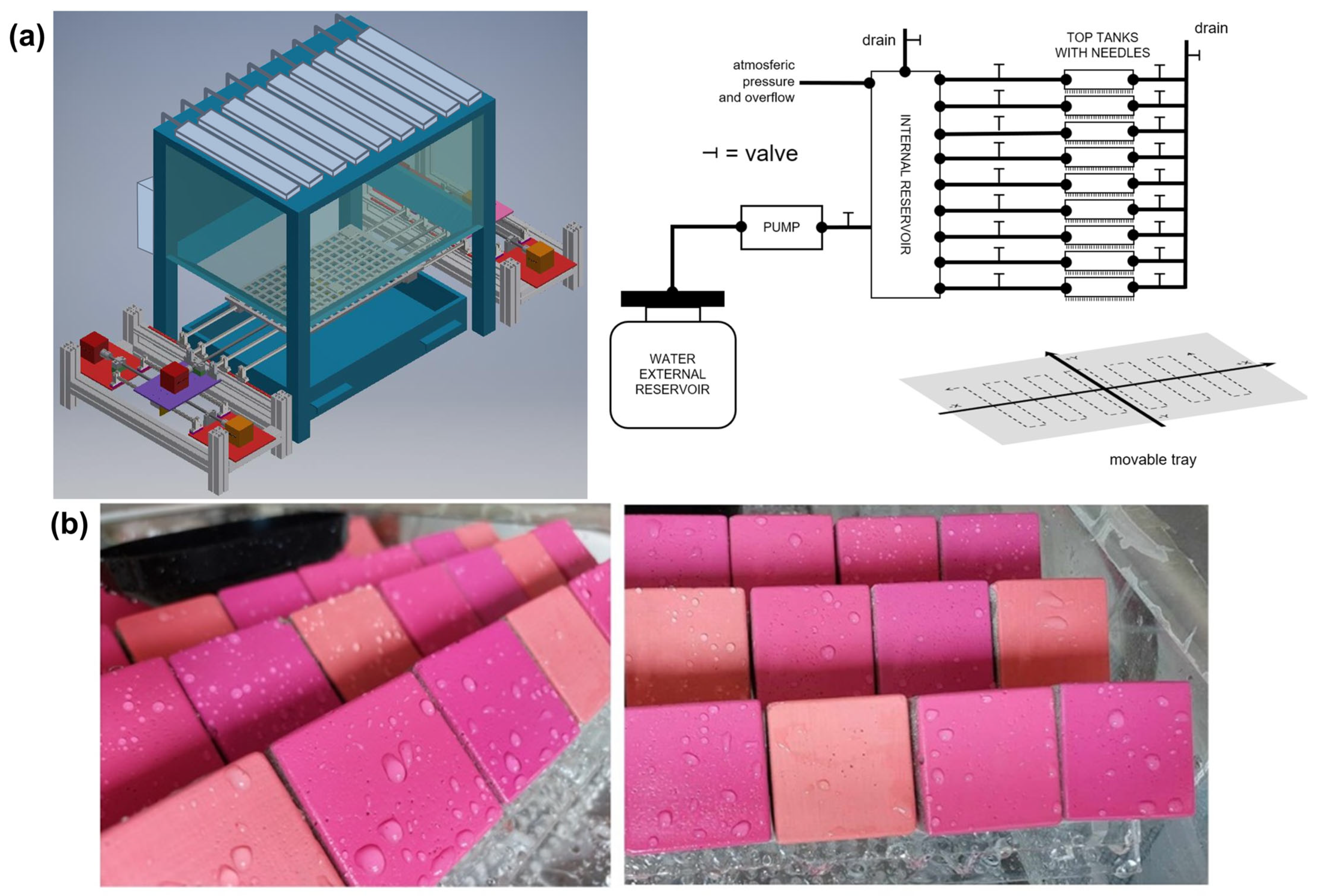
2.3.1. Chamber
2.3.2. Accelerated Rain Ageing Conditions
2.4. Testing Methods and Measurements
3. Results and Discussion
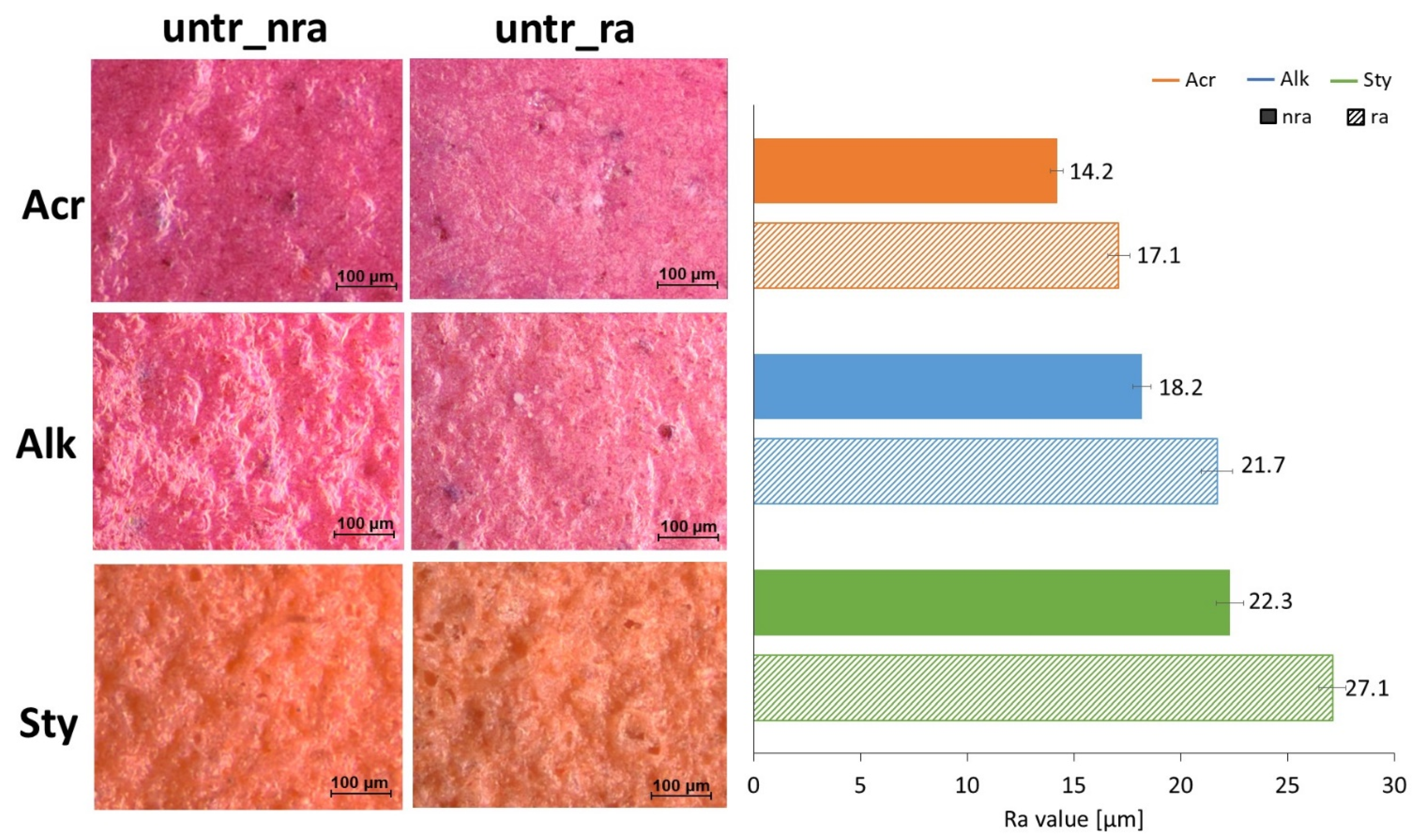
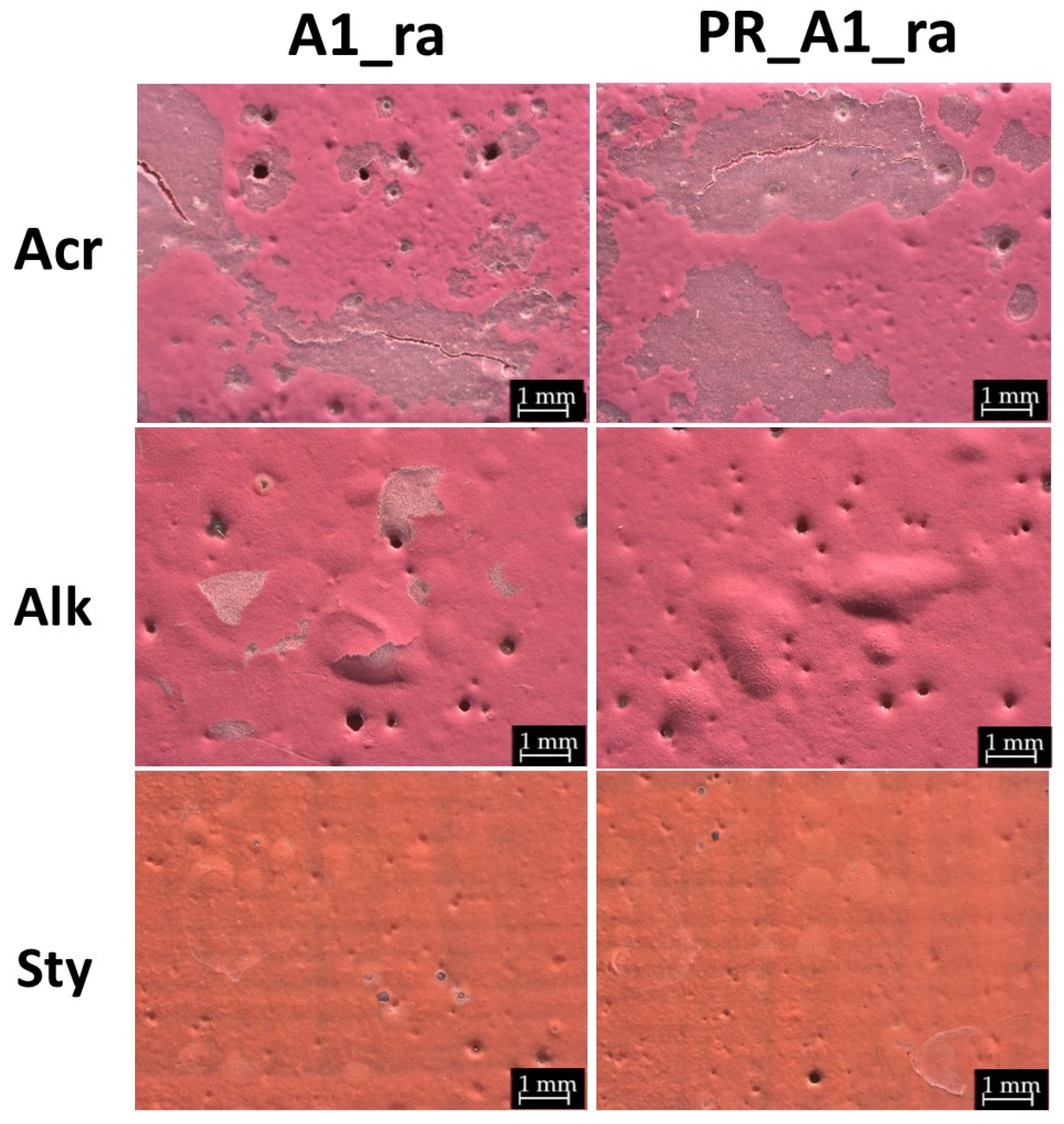
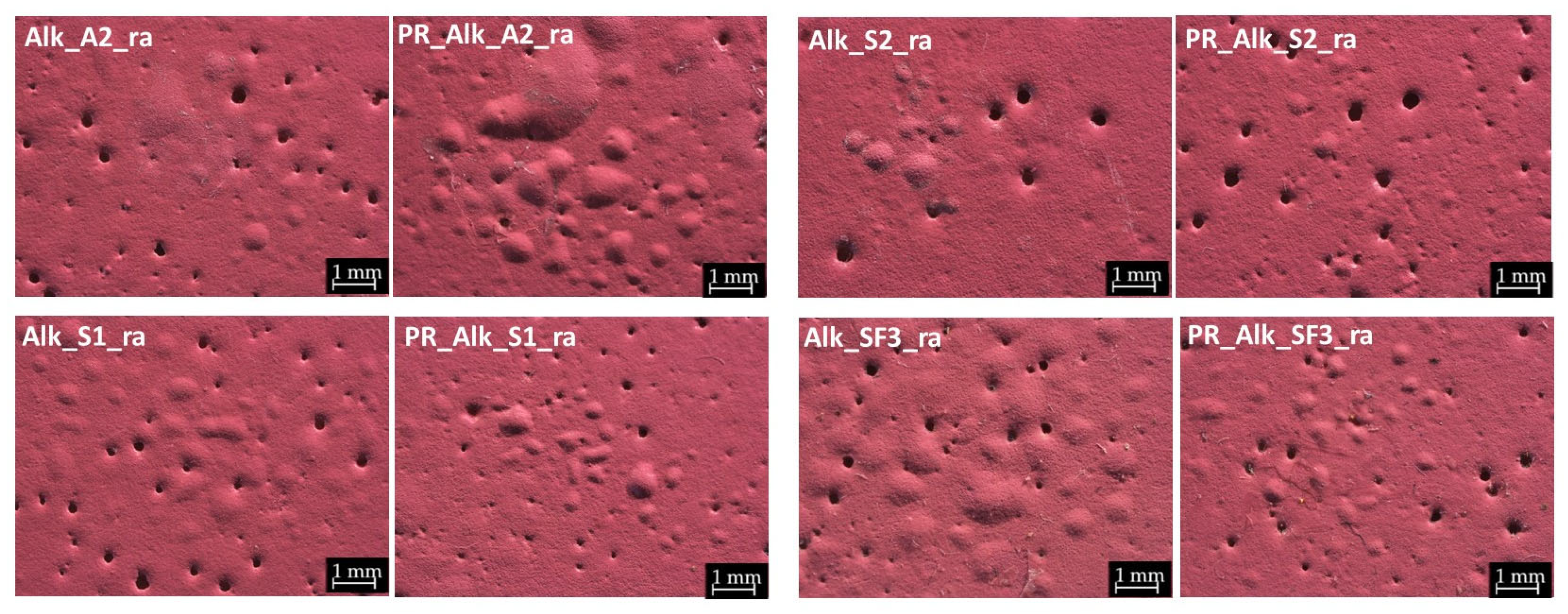
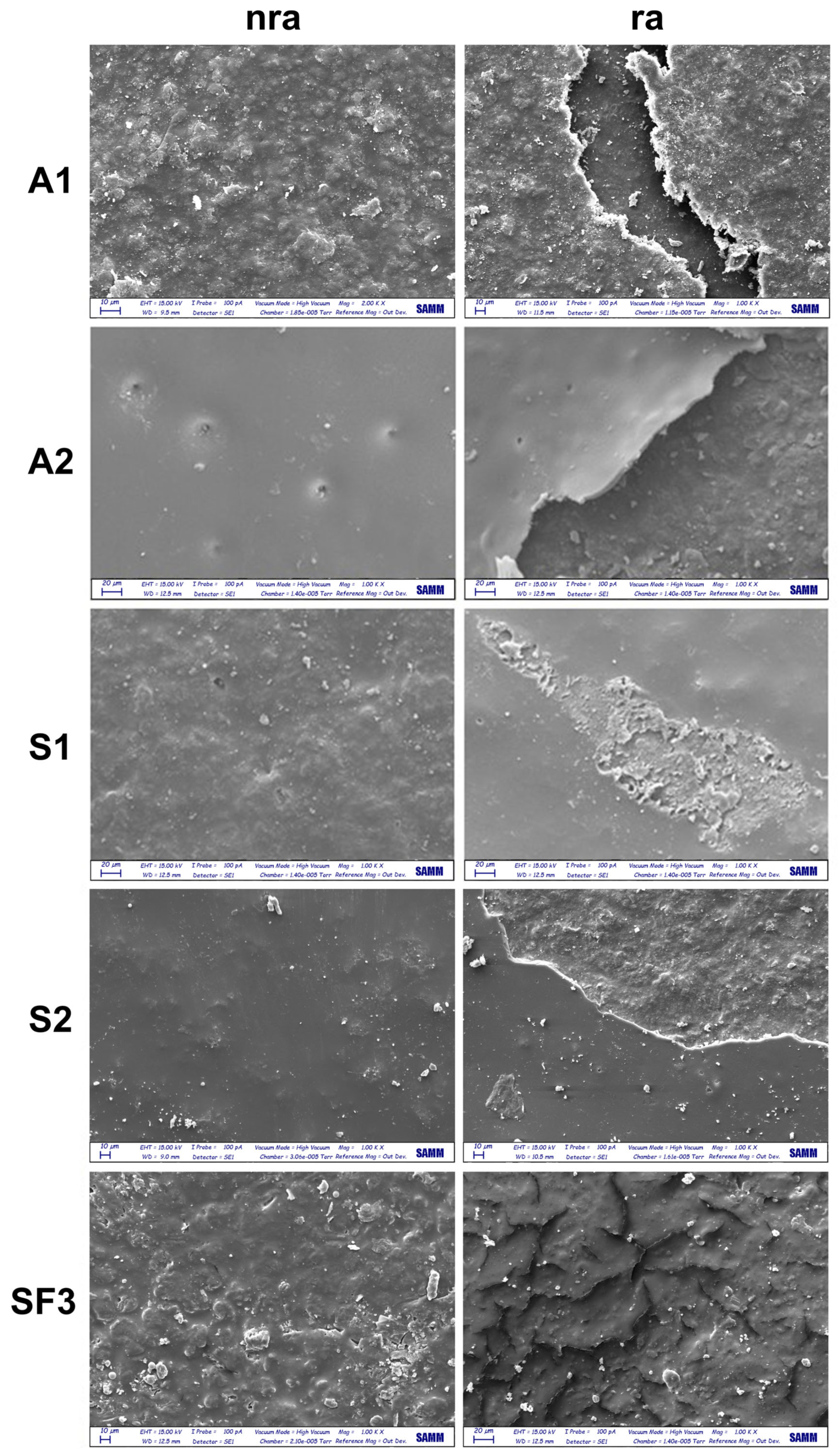
3.1. Morphological Changes After Rain Ageing
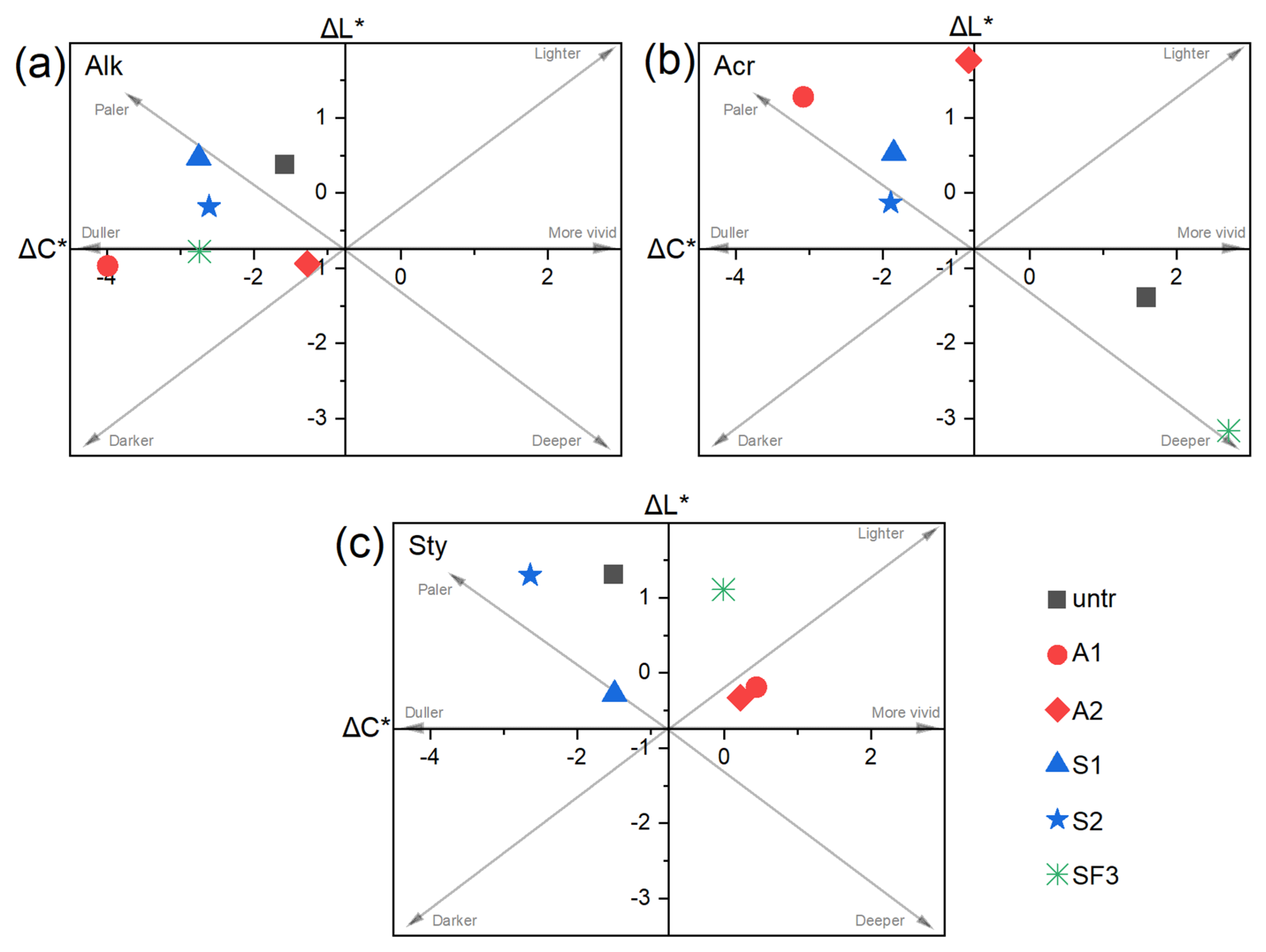
3.2. Colorimetric and Gloss Changes After Rain Ageing
3.3. Protective Effectiveness After Rain Ageing
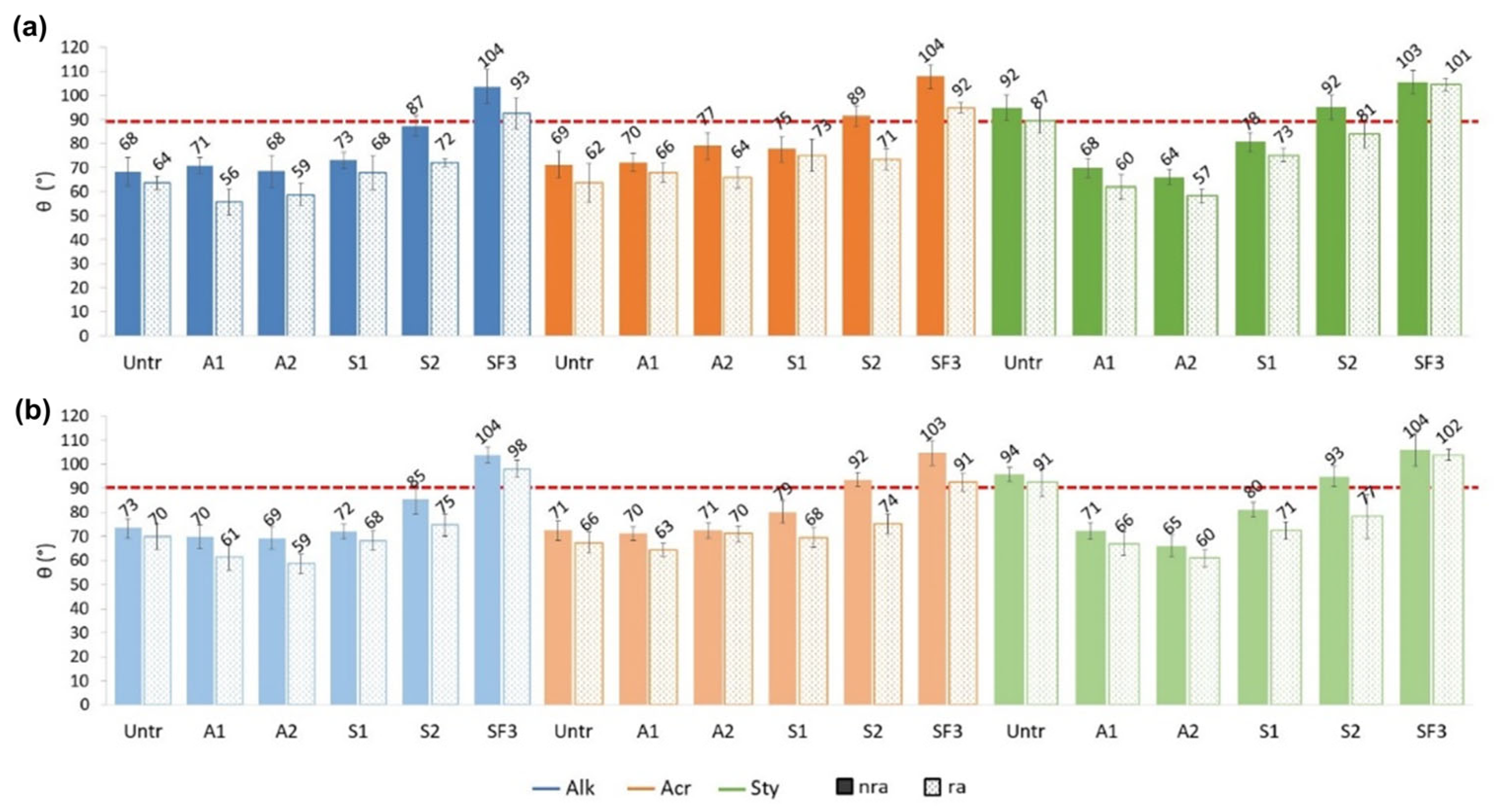
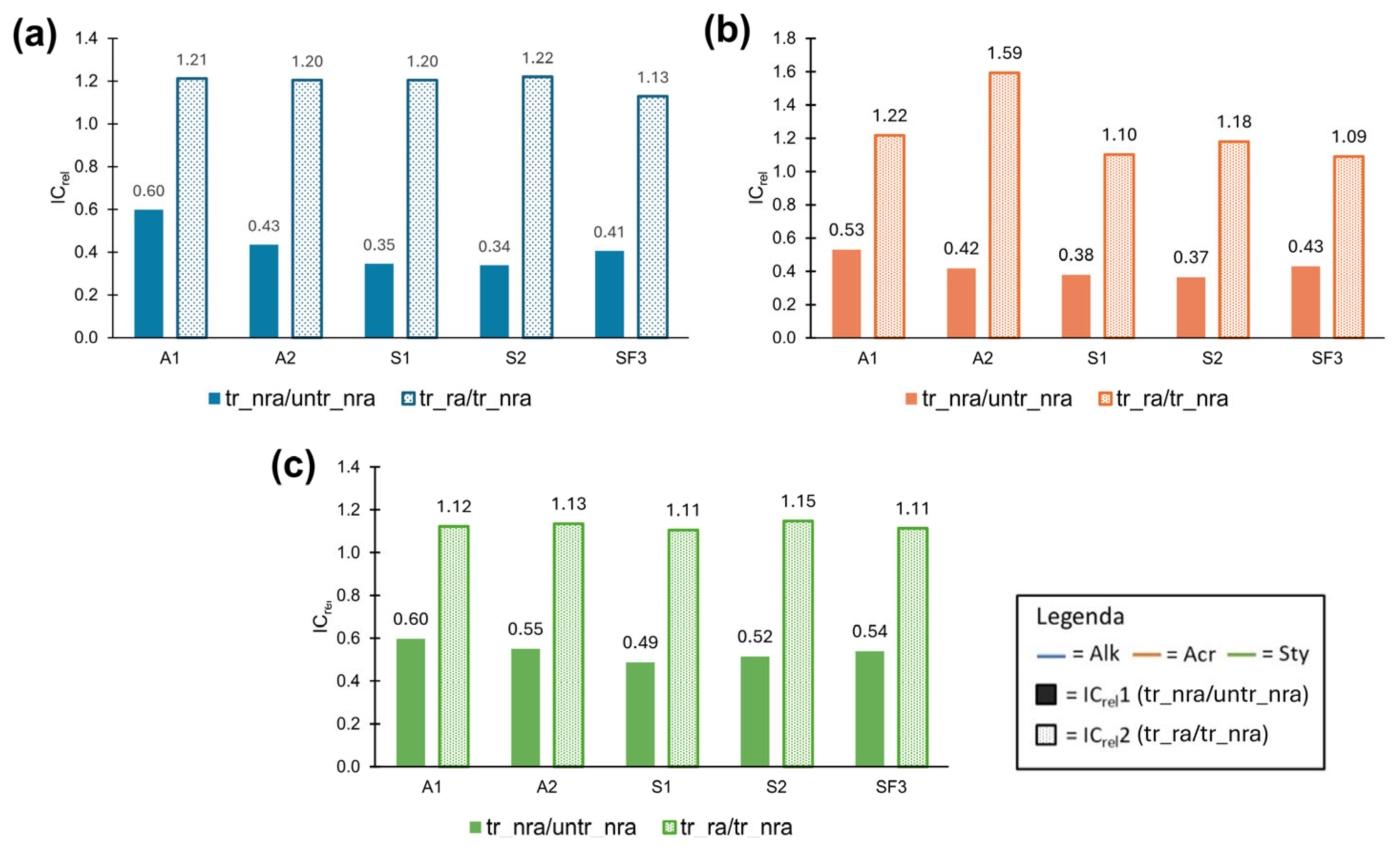
3.3.1. Static Contact Angles (WCA) Change After Rain Ageing
- (1)
- On Alk painted mock-ups, the most pronounced decrease in WCA was observed for A1 and S2, followed by A2 and SF3. S1 and the untreated samples showed the smallest WCA variation.
- (2)
- On Acr painted mock-ups, S2 showed the highest WCA reduction, followed by SF3 and the untreated sample. The smallest variation was observed for A2, A1 and S1.
- (3)
- On Sty paint, S2, A2 and A1 showed the greatest decrease in WCA values, followed by S1 and the untreated. The smallest variation was observed for SF3.
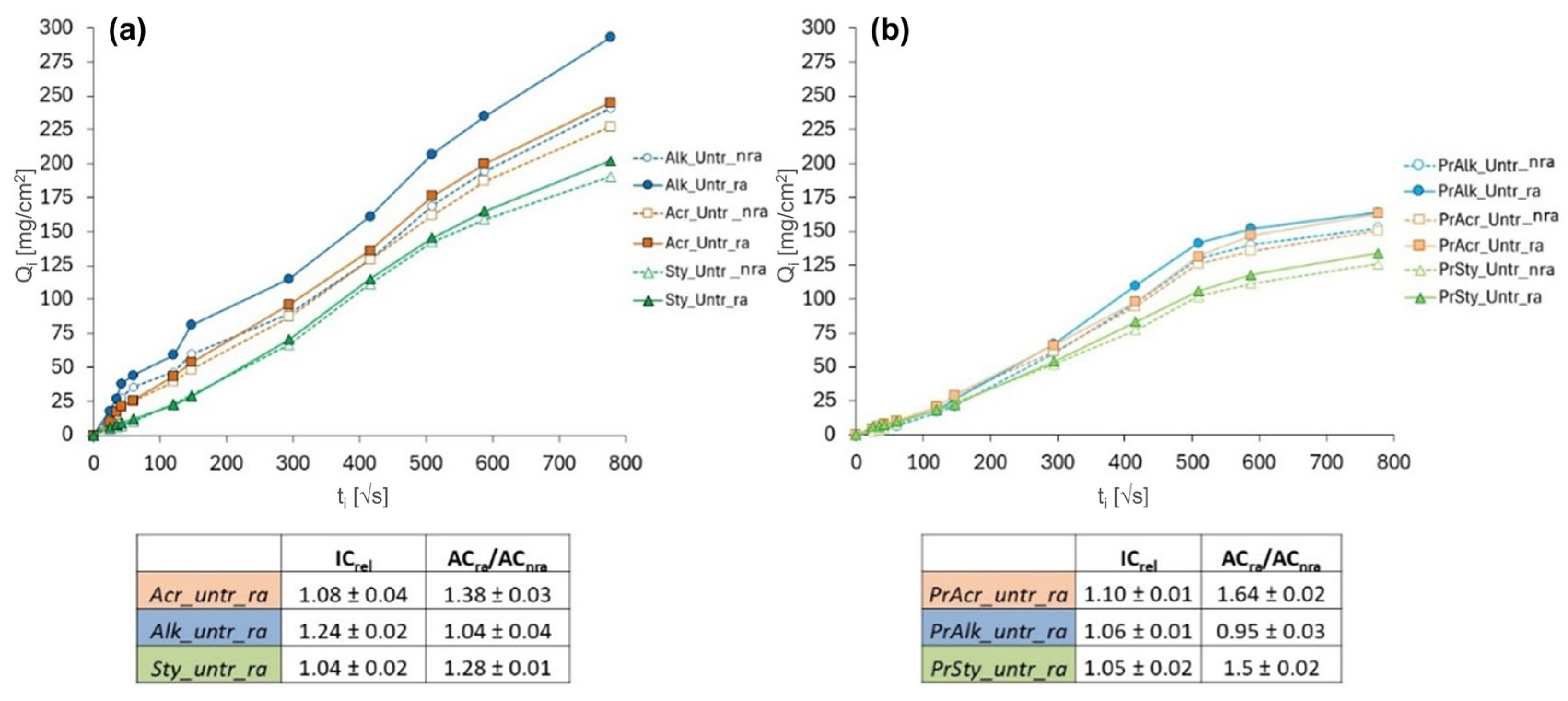
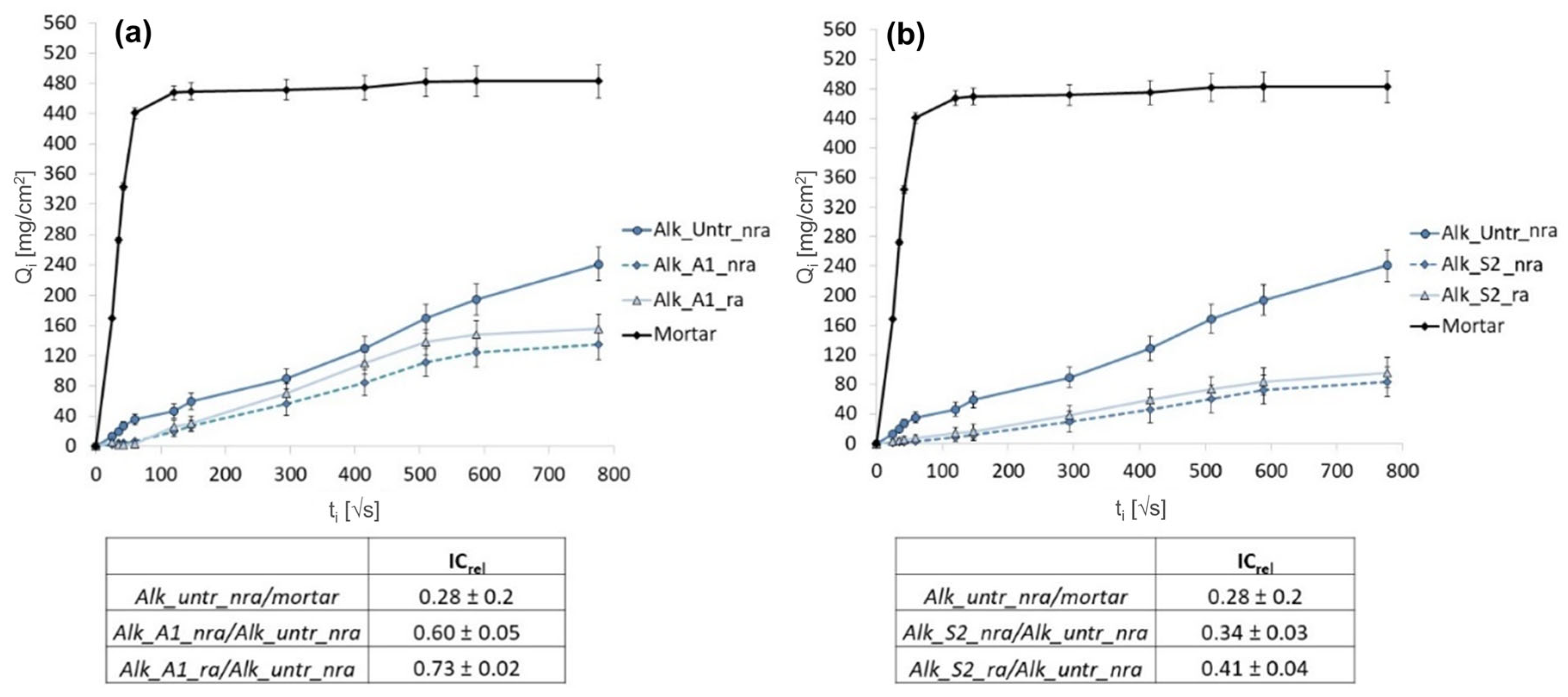
3.3.2. Water Absorption by Capillarity
3.4. Chemical Changes After Rain Ageing
4. Final Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cadetti, A. Conservare La Street Art: Le Problematiche Del Muralismo Contemporaneo in Italia; Edifir Edizioni Firenze: Firenze, Italy, 2020; ISBN 978-88-9280-020-5. [Google Scholar]
- Mezzadri, P. Contemporary Murals in the Street and Urban Art Field: Critical Reflections between Preventive Conservation and Restoration of Public Art. Heritage 2021, 4, 2515–2525. [Google Scholar] [CrossRef]
- Rainer, L.; Paul, T.J.; Trust, G. The Conservation of Outdoor Contemporary Murals. Conserv. Getty Conserv. Inst. Newsl. 2003, 18, 4–9. [Google Scholar]
- Pagnin, L.; Guarnieri, N.; Izzo, F.C.; Goidanich, S.; Toniolo, L. Protecting Street Art from Outdoor Environmental Threats: What Are the Challenges? Coatings 2023, 13, 2044. [Google Scholar] [CrossRef]
- Cimino, D.; Lamuraglia, R.; Saccani, I.; Berzioli, M.; Izzo, F.C. Assessing the (In) Stability of Urban Art Paints: From Real Case Studies to Laboratory Investigations of Degradation Processes and Preservation Possibilities. Heritage 2022, 5, 581–609. [Google Scholar] [CrossRef]
- Cianci, C.; Andriulo, F.; Giorgi, R. Formulation of a New Sustainable Hybrid Coating for the Conservation of Street-Art: Characterization and Application. Prog. Org. Coat. 2025, 200, 109026. [Google Scholar] [CrossRef]
- Bosi, A.; Ciccola, A.; Serafini, I.; Guiso, M.; Ripanti, F.; Postorino, P.; Curini, R.; Bianco, A. Street Art Graffiti: Discovering Their Composition and Alteration by FTIR and Micro-Raman Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117474. [Google Scholar] [CrossRef] [PubMed]
- Pagnin, L.; Calvini, R.; Wiesinger, R.; Schreiner, M. SO2- and NOx- Initiated Atmospheric Degradation of Polymeric Films: Morphological and Chemical Changes, Influence of Relative Humidity and Inorganic Pigments. Microchem. J. 2021, 164, 106087. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Rivas, T.; González, N.; Alonso-Villar, E.M. Deterioration of Graffiti Spray Paints Applied on Granite after a Decade of Natural Environment. Sci. Total Environ. 2022, 826, 154169. [Google Scholar] [CrossRef]
- Pagnin, L.; Zendri, E.; Izzo, F.C. How Can Ozone and Relative Humidity Affect Artists’ Alkyd Paints? A FT-IR and Py-GC/MS Systematic Study. Polymers 2022, 14, 1831. [Google Scholar] [CrossRef]
- Guarnieri, N.; Di Benedetto, A.; Comelli, D.; Mirani, F.; Dellasega, D.; Pagnin, L.; Goidanich, S.; Toniolo, L. Rapid Chromatic Alteration of Street Art: Mechanisms of Deterioration of the Painting Materials of the 20 Years of Freedom and Democracy Mural. Dye. Pigment. 2025, 239, 112733. [Google Scholar] [CrossRef]
- Pagnin, L.; Goidanich, S.; Izzo, F.C.; Zhang, Y.; Scalarone, D.; Toniolo, L. Compatibility and Efficacy Evaluations of Organic Protective Coatings for Contemporary Muralism. Coatings 2025, 15, 166. [Google Scholar] [CrossRef]
- UNI EN 16581:2015; Conservation of Cultural Heritage—Surface Protection for Porous Inorganic Materials—Laboratory Test Methods for Evaluating the Performance of Water-Repellent Products. Italian Organization for Standardization: Milan, Italy, 2015.
- Chiantore, O.; Lazzari, M. Photo-Oxidative Stability of Paraloid Acrylic Protective Polymers. Polymer 2001, 42, 17–27. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; De Simone, N.; Santarelli, M.L.; Frigione, M. Protective Properties and Durability Characteristics of Experimental and Commercial Organic Coatings for the Preservation of Porous Stone. Prog. Org. Coat. 2017, 103, 193–203. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Alonso-Villar, E.M.; Rivas, T.; Márquez, I. Evaluation of a Protective Acrylic Finish Applied to Surfaces Painted with Acrylic Paints for Outdoor or Indoor Uses. Dye. Pigment. 2023, 212, 111141. [Google Scholar] [CrossRef]
- Alonso-Villar, E.M.; Rivas, T.; Pozo-Antonio, J.S.; Pellis, G.; Scalarone, D. Efficacy of Colour Protectors in Urban Art Paintings under Different Conditions: From a Real Mural to the Laboratory. Heritage 2023, 6, 3475–3498. [Google Scholar] [CrossRef]
- Tidblad, J.; Kucera, V.; Ferm, M.; Kreislova, K.; Brüggerhoff, S.; Doytchinov, S.; Screpanti, A.; Grøntoft, T.; Yates, T.; De La Fuente, D.; et al. Effects of Air Pollution on Materials and Cultural Heritage: ICP Materials Celebrates 25 Years of Research. Int. J. Corros. 2012, 2012, 496321. [Google Scholar] [CrossRef]
- Hamilton, R.; Crabbe, H. Environment, Pollution and Effects. In The Effects of Air Pollution on Cultural Heritage; Hamilton, R., Kucera, V., Tidblad, J., Watt, J., Eds.; Springer: Boston, MA, USA, 2009; ISBN 978-0-387-84893-8. [Google Scholar]
- Timoncini, A.; Brattich, E.; Bernardi, E.; Chiavari, C.; Tositti, L. Safeguarding Outdoor Cultural Heritage Materials in an Ever-Changing Troposphere: Challenges and New Guidelines for Artificial Ageing Test. J. Cult. Herit. 2023, 59, 190–201. [Google Scholar] [CrossRef]
- Brunelli, A.; Calgaro, L.; Semenzin, E.; Cazzagon, V.; Giubilato, E.; Marcomini, A.; Badetti, E. Leaching of Nanoparticles from Nano-Enabled Products for the Protection of Cultural Heritage Surfaces: A Review. Environ. Sci. Eur. 2021, 33, 48. [Google Scholar] [CrossRef]
- Gonçalves, T.D.; Pel, L.; Rodrigues, J.D. Influence of Paints on Drying and Salt Distribution Processes in Porous Building Materials. Constr. Build. Mater. 2009, 23, 1751–1759. [Google Scholar] [CrossRef]
- Arias, P.A.; Bellouin, N.; Coppola, E.; Jones, R.G.; Krinner, G.; Marotzke, J.; Naik, V.; Palmer, M.D.; Plattner, G.-K.; Rogelj, J.; et al. Technical Summary. In Climate Change 2021: The Physical Science Basis: Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Intergovernmental Panel on Climate Change (IPCC), Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2023; pp. 35–144. ISBN 978-1-009-15788-9. [Google Scholar] [CrossRef]
- Cacciotti, R.; Kaiser, A.; Sardella, A.; De Nuntiis, P.; Drdácký, M.; Hanus, C.; Bonazza, A. Climate Change-Induced Disasters and Cultural Heritage: Optimizing Management Strategies in Central Europe. Clim. Risk Manag. 2021, 32, 100301. [Google Scholar] [CrossRef]
- Moreno, M.; Barea, R.; Castro, L.; Cagigas, D.; Ortiz, R.; Ortiz, P. Climate Change Monitoring with Art-Risk 5: New Approach for Environmental Hazard Assessment in Seville and Almería Historic Centres (Spain). Procedia Struct. Integr. 2024, 55, 9–17. [Google Scholar] [CrossRef]
- Art-Risk 5.0 Atlas. Available online: https://artrisk50.users.earthengine.app/view/art-risk5-english (accessed on 10 January 2025).
- De La Fuente, D.; Vega, J.M.; Viejo, F.; Díaz, I.; Morcillo, M. Mapping Air Pollution Effects on Atmospheric Degradation of Cultural Heritage. J. Cult. Herit. 2013, 14, 138–145. [Google Scholar] [CrossRef]
- Roveri, M.; Goidanich, S.; Toniolo, L. Artificial Ageing of Photocatalytic Nanocomposites for the Protection of Natural Stones. Coatings 2020, 10, 729. [Google Scholar] [CrossRef]
- Technical Datasheet Impregnante Alphatex SF-Sikkens. Available online: https://www.sikkens.it/it/prodotti/impregnante-alphatex-sf?size=1l (accessed on 28 July 2025).
- Blocken, B.; Derome, D.; Carmeliet, J. Rainwater Runoff from Building Facades: A Review. Build. Environ. 2013, 60, 339–361. [Google Scholar] [CrossRef]
- Abuku, M.; Blocken, B.; Roels, S. Moisture Response of Building Facades to Wind-Driven Rain: Field Measurements Compared with Numerical Simulations. J. Wind. Eng. Ind. Aerodyn. 2009, 97, 197–207. [Google Scholar] [CrossRef]
- Erkal, A.; D’Ayala, D.; Sequeira, L. Assessment of Wind-Driven Rain Impact, Related Surface Erosion and Surface Strength Reduction of Historic Building Materials. Build. Environ. 2012, 57, 336–348. [Google Scholar] [CrossRef]
- BS EN ISO 4287:2000; Geometrical Product Specifications (Gps)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland, 2000.
- EN 15886:2010; Conservation of Cultural Property—Test Methods—Colour Measurement of Surfaces. European Committee for Standardization: Brussels, Belgium, 2010.
- UNI EN ISO 2813:2016; Paints and Varnishes-Determination of Gloss Value at 20 Degrees, 60 Degrees and 85 Degrees. Italian Organization for Standardization: Milan, Italy, 2016.
- EN 15802:2009; Conservation of Cultural Property—Test Methods—Determination of Static Contact Angle. European Committee for Standardization: Brussels, Belgium, 2009.
- UNI 10859:2000; Cultural Heritage—Natural and Artificial Stone Materials—Determination of Water Absorption by Capillarity. Italian Organization for Standardization: Milan, Italy, 2000.
- Conservation of Art in Public Spaces (CAPuS)—Illustrated Glossary. Available online: http://www.capusproject.eu/glossary/ (accessed on 10 January 2025).
- Abdullah, M.N.; Mustapha, F.; Mustapha, M.; Khan, T.; Sebaey, T.A. Study on Adhesive and Microstructure Properties of Rice Husk Ash-Based Geopolymer Hybrid Alkyd Coating. J. Nat. Fibers 2024, 21, 2317427c. [Google Scholar] [CrossRef]
- Vevere, L.; Fridrihsone, A.; Kirpluks, M.; Cabulis, U. A Review of Wood Biomass-Based Fatty Acids and Rosin Acids Use in Polymeric Materials. Polymers 2020, 12, 2706. [Google Scholar] [CrossRef] [PubMed]
- Duce, C.; Della Porta, V.; Tiné, M.R.; Spepi, A.; Ghezzi, L.; Colombini, M.P.; Bramanti, E. FTIR Study of Ageing of Fast Drying Oil Colour (FDOC) Alkyd Paint Replicas. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 214–221. [Google Scholar] [CrossRef]
- Osmond, G.; Boon, J.J.; Puskar, L.; Drennan, J. Metal Stearate Distributions in Modern Artists’ Oil Paints: Surface and Cross-Sectional Investigation of Reference Paint Films Using Conventional and Synchrotron Infrared Microspectroscopy. Appl. Spectrosc. 2012, 66, 1136–1144. [Google Scholar] [CrossRef]
- Garrappa, S.; Kočí, E.; Švarcová, S.; Bezdička, P.; Hradil, D. Initial Stages of Metal Soaps` Formation in Model Paints: The Role of Humidity. Microchem. J. 2020, 156, 104842. [Google Scholar] [CrossRef]
- Weeks, C.; Hand, R.J.; Sharp, J.H. Retardation of Cement Hydration Caused by Heavy Metals Present in ISF Slag Used as Aggregate. Cem. Concr. Compos. 2008, 30, 970–978. [Google Scholar] [CrossRef]
- Izzo, F.C.; Kratter, M.; Nevin, A.; Zendri, E. A Critical Review on the Analysis of Metal Soaps in Oil Paintings. ChemistryOpen 2021, 10, 904–921. [Google Scholar] [CrossRef] [PubMed]
- Misovski, T.; Nichols, M.; Hardcastle, H. The Influence of Water on the Weathering of Automotive Paint Systems. In Service Life Prediction of Polymeric Materials: Global Perspectives; Springer: New York, NY, USA, 2009; pp. 295–308. ISBN 978-0-387-84875-4. [Google Scholar]
- Hunt, F.Y.; Galler, M.A.; Martin, J.W. Microstructure of Weathered Paint and Its Relation to Gloss Loss: Computer Simulation and Modeling. J. Coat. Technol. 1998, 70, 45–53. [Google Scholar] [CrossRef]
- Peruzzi, R.; Poli, T.; Toniolo, L. The Experimental Test for the Evaluation of Protective Treatments: A Critical Survey of the “Capillary Absorption Index”. J. Cult. Herit. 2003, 4, 251–254. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; An, M.; Sun, Y.; Yu, Z.; Huang, H. Factors Influencing the Capillary Water Absorption Characteristics of Concrete and Their Relationship to Pore Structure. Appl. Sci. 2022, 12, 2211. [Google Scholar] [CrossRef]
- Van der Weerd, J.; van Loon, A.; Boon, J.J. FTIR Studies of the Effects of Pigments on the Aging of Oil. Stud. Conserv. 2005, 50, 3–22. [Google Scholar] [CrossRef]
- Ziraldo, I.; Watts, K.; Luk, A.; Lagalante, A.F.; Wolbers, R.C. The Influence of Temperature and Humidity on Swelling and Surfactant Migration in Acrylic Emulsion Paint Films. Stud. Conserv. 2016, 61, 209–221. [Google Scholar] [CrossRef]
- Chabane, R.; Sahli, S.; Raynaud, P. CF4 Doping Effects on SiOF Thin Films: Chemical, Structural and Dielectric Properties in PECVD-Deposited Films from HMDSO/O2 Vapor Mixtures. Eur. Phys. J. B 2025, 98, 112. [Google Scholar] [CrossRef]
- Sah, A.; Castricum, H.L.; Bliek, A.; Blank, D.H.A.; Ten Elshof, J.E. Hydrophobic Modification of γ-Alumina Membranes with Organochlorosilanes. J. Membr. Sci. 2004, 243, 125–132. [Google Scholar] [CrossRef][Green Version]
- Sharifzadeh, S.; Hassanajili, S.; Rahimpour, M.R. Wettability Alteration of Gas Condensate Reservoir Rocks to Gas Wetness by Sol–Gel Process Using Fluoroalkylsilane. J. Appl. Polym. Sci. 2013, 128, 4077–4085. [Google Scholar] [CrossRef]


| Paint Abbreviation | Chemical Composition from Technical Datasheet | Chemical Identification * |
|---|---|---|
| Acr | Acrylic emulsion | Acrylic emulsion + PR122 + PR254 + PW6 |
| Alk | Alkyd resin | Alkyd resin + PR122 + PW6 + Talc (filler) |
| Sty | Styrene-acrylic emulsion | Styrene-acrylic emulsion + Eosin B + CaCO3 (filler) |
| Protective Treatment Abbreviation | Product class from Technical Data Sheet | Chemical Identification * |
| A1 | Acrylic | Acrylic polymer, polyurethane, Si-containing filler |
| A2 | Acrylic | Copolymer MA-EMA |
| S1 | Silane | Organic silane, acrylic polymer |
| S2 | Silane/Siloxane | Dimethyl-siloxanes |
| SF3 | Fluoro-silane | Undisclosed formulation (fluoro-silane from technical datasheet) |
| Mock-Ups Abbreviation | Meaning |
|---|---|
| untr | Painted mock-up without protective treatment (untreated) |
| PR | Painted mock-up with primer layer between mortar and paint |
| nra | Mock-up before rain ageing (not rain aged) |
| ra | Mock-up after rain ageing (rain aged) |
| Functional Groups | Peak Wavenumber (cm−1) | Peak Intensity Measurements Range (cm−1) |
|---|---|---|
| OH stretching | 3440 | 3510–3085 |
| C=O stretching | 1720 | 1760–1700 |
| Calcite | 875 | 890–860 |
| Out-of-plane bending (phenyl) | 700 | 730–680 |
| SiCH3 stretching | 880 | 935–870 |
| C-F stretching | 1140 | 1220–1170 |
| C=O stretching of β-diketones | 1635 | 1650–1620 |
| C-H stretching | 2920 | 2930–2910 |
| Selected Peak Intensity Ratios for Untreated Mock-Ups | Selected Peak Intensity Ratios for Treated Mock-Ups | |
|---|---|---|
| C=O/OH | Calcite/C=O | C=O/CH |
| C=O/C=O β-diketones | Phenyl/C=O | Si-CH3/OH |
| CF3/SiO | ||
| ΔE | ΔGU | ΔE | ΔGU | ||
|---|---|---|---|---|---|
| Alk_untr_ra | 1.6 ± 0.5 | −0.05 ± 00.2 | PR_Alk_untr_ra | 0.7 ± 0.2 | −0.02 ± 0.01 |
| Alk_A1_ra | 4.1 ± 1.1 | −0.3 ± 0.05 | PR_Alk_A1_ra | 1.1 ± 0.3 | −0.1 ± 0.02 |
| Alk_A2_ra | 2.2 ± 0.4 | −0.1 ± 0.01 | PR_Alk_A2_ra | 2.5 ± 0.1 | −0.08 ± 0.02 |
| Alk_S1_ra | 3.1 ± 0.6 | −0.03 ± 0.06 | PR_Alk_S1_ra | 0.4 ± 0.2 | −0.02 ± 0.02 |
| Alk_S2_ra | 2.9 ± 1.1 | −0.3 ± 0.04 | PR_Alk_S2_ra | 0.6 ± 0.3 | −0.3 ± 0.01 |
| Alk_SF3_ra | 2.9 ± 1.3 | −0.4 ± 0.02 | PR_Alk_SF3_ra | 0.4 ± 0.1 | −0.2 ± 0.01 |
| Acr_untr_ra | 3.6 ± 0.5 | −0.2 ± 0.02 | PR_Acr_untr_ra | 1.2 ± 0.3 | −0.1 ± 0.03 |
| Acr_A1_ra | 4.4 ± 1.0 | −0.4 ± 0.05 | PR_Acr_A1_ra | 2.4 ± 0.2 | −0.05 ± 0.02 |
| Acr_A2_ra | 2.6 ± 0.9 | −0.06 ± 0.03 | PR_Acr_A2_ra | 2.8 ± 0.5 | −0.06 ± 0.04 |
| Acr_S1_ra | 1.9 ± 0.7 | −0.2 ± 0.08 | PR_Acr_S1_ra | 0.4 ± 0.5 | −0.1 ± 0.03 |
| Acr_S2_ra | 1.9 ± 1.0 | −0.2 ± 0.05 | PR_Acr_S2_ra | 0.2 ± 0.1 | −0.2 ± 0.02 |
| Acr_SF3_ra | 4.2 ± 0.5 | −0.6 ± 0.02 | PR_Acr_SF3_ra | 0.3 ± 0.2 | −0.4 ± 0.03 |
| Sty_untr_ra | 2.0 ± 0.7 | −0.1 ± 0.03 | PR_Sty_untr_ra | 3.1 ± 0.6 | −0.08 ± 0.01 |
| Sty_A1_ra | 0.5 ± 0.5 | −0.4 ± 0.02 | PR_Sty_A1_ra | 1.1 ± 0.7 | −0.1 ± 0.01 |
| Sty_A2_ra | 0.5 ± 1.1 | −0.3 ± 0.04 | PR_Sty_A2_ra | 1.1 ± 0.4 | −0.3 ± 0.02 |
| Sty_S1_ra | 1.5 ± 0.8 | −0.1 ± 0.03 | PR_Sty_S1_ra | 0.5 ± 0.6 | −0.07 ± 0.01 |
| Sty_S2_ra | 3.0 ± 0.6 | −0.5 ± 0.06 | PR_Sty_S2_ra | 0.2 ± 0.1 | −0.4 ± 0.02 |
| Sty_SF3_ra | 1.1 ± 0.9 | −0.4 ± 0.02 | PR_Sty_SF3_ra | 0.2 ± 0.1 | −0.2 ± 0.01 |
| C=O/OH 1720/3440 | C=O/C=O (β-Diketones Formation) 1720/1635 | |||
|---|---|---|---|---|
| Alk_untr | nra | 4.0 | 3.9 | |
| ra | 3.6 | 4.1 | ||
| PR_Alk_untr | nra | 4.5 | 4.3 | |
| ra | 4.4 | 4.6 | ||
| C=O/OH 1720/3440 | C=O/CH 1720/2920 | |||
| Acr_untr | nra | 13.7 | 2.9 | |
| ra | 12.7 | 2.9 | ||
| PR_Acr_untr | nra | 17.3 | 2.9 | |
| ra | 16.4 | 3.0 | ||
| C=O/OH 1720/3440 | Calcite/C=O 785/1720 | Phenyl/C=O 700/1720 | ||
| Sty_untr | nra | 2.3 | 1.4 | 4.1 |
| ra | 3.1 | 0.7 | 2.3 | |
| PR_Sty_untr | nra | 2.5 | 1.2 | 3.8 |
| ra | 2.9 | 0.7 | 2.5 |
| Coating | Type of Paint | C=O/CH 1720/2920 | |
|---|---|---|---|
| A1 | Alk | nra | 1.67 |
| ra | 1.67 | ||
| Acr | nra | 1.70 | |
| ra | 1.68 | ||
| Sty | nra | 1.62 | |
| ra | 1.61 | ||
| A2 | Alk | nra | 6.23 |
| ra | 5.77 | ||
| Acr | nra | 4.66 | |
| ra | 4.36 | ||
| Sty | nra | 4.99 | |
| ra | 4.48 | ||
| Si-CH3/OH 880/3440 | |||
| S1 | Alk | nra | 4.5 |
| ra | 3.3 | ||
| Acr | nra | 4.6 | |
| ra | 3.3 | ||
| Sty | nra | 7.6 | |
| ra | 5.4 | ||
| S2 | Alk | nra | 11.0 |
| ra | 10.0 | ||
| Acr | nra | 10.4 | |
| ra | 8.1 | ||
| Sty | nra | 9.5 | |
| ra | 7.5 | ||
| CF3/SiO 1140/1065 | |||
| SF3 | Alk | nra | 2.1 |
| ra | 2.8 | ||
| Acr | nra | 2.3 | |
| ra | 1.9 | ||
| Sty | nra | 2.1 | |
| ra | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagnin, L.; Goidanich, S.; Guarnieri, N.; Izzo, F.C.; Henriquez, J.J.H.; Toniolo, L. Street Art in the Rain: Evaluating the Durability of Protective Coatings for Contemporary Muralism Through Accelerated Rain Ageing. Coatings 2025, 15, 924. https://doi.org/10.3390/coatings15080924
Pagnin L, Goidanich S, Guarnieri N, Izzo FC, Henriquez JJH, Toniolo L. Street Art in the Rain: Evaluating the Durability of Protective Coatings for Contemporary Muralism Through Accelerated Rain Ageing. Coatings. 2025; 15(8):924. https://doi.org/10.3390/coatings15080924
Chicago/Turabian StylePagnin, Laura, Sara Goidanich, Nicolò Guarnieri, Francesca Caterina Izzo, Jaime Jorge Hormida Henriquez, and Lucia Toniolo. 2025. "Street Art in the Rain: Evaluating the Durability of Protective Coatings for Contemporary Muralism Through Accelerated Rain Ageing" Coatings 15, no. 8: 924. https://doi.org/10.3390/coatings15080924
APA StylePagnin, L., Goidanich, S., Guarnieri, N., Izzo, F. C., Henriquez, J. J. H., & Toniolo, L. (2025). Street Art in the Rain: Evaluating the Durability of Protective Coatings for Contemporary Muralism Through Accelerated Rain Ageing. Coatings, 15(8), 924. https://doi.org/10.3390/coatings15080924


.jpg)







