The Effect of Crevice Structure on Corrosion Behavior of P110 Carbon Steel in a Carbonated Simulated Concrete Environment
Abstract
1. Introduction
2. Experiment
2.1. Materials and Methods
2.2. Analytical Test Methodology
2.3. Geometrical Model and Control Equations
3. Results
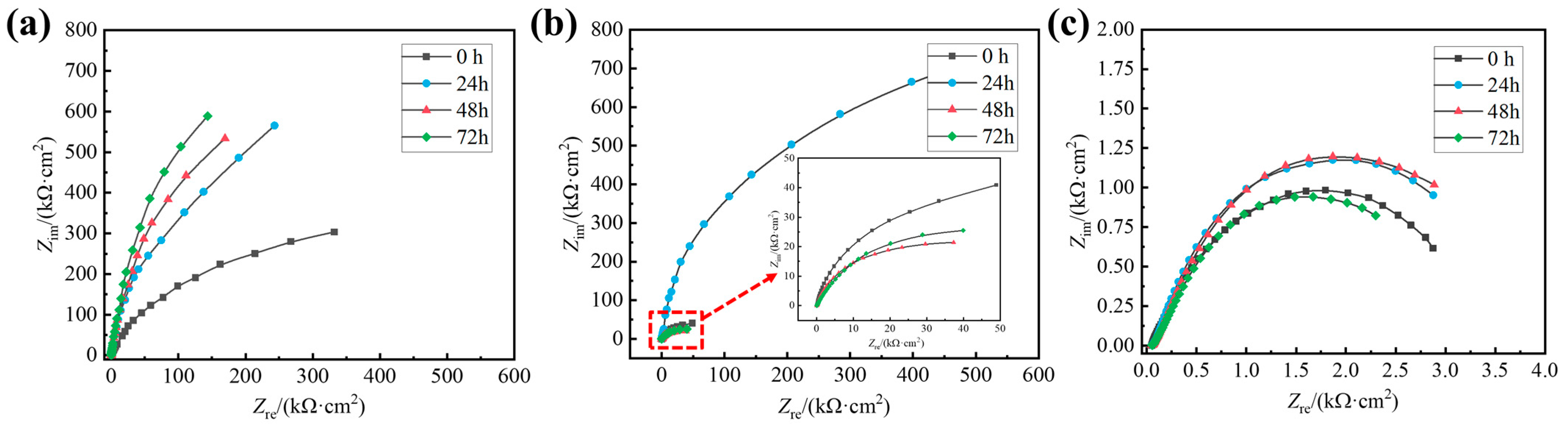
3.1. Electrochemical Experiments
3.2. Surface Morphology and Corrosion Product Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huo, H.B.; Liu, D.D.; Lin, T.; Wang, D.Y.; He, S.M. Integrity challenges and countermeasures of the offshore CCUS based on CO2-EOR. Pet. Drill. Tech. 2023, 51, 74–80. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Young, D.; Nešić, S.; Gray, L.G. Wellbore integrity and corrosion of carbon steel in CO2 geologic storage environments: A literature review. Int. J. Greenh. Gas Control. 2013, 16, S70–S77. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, L.; Mei, K.; Li, X.; Newell, P.; Wang, Y.; Cheng, X.; Zheng, W. CO2-induced evolution of chemical, structural and mechanical properties of reinforced concrete: A review. Constr. Build. Mater. 2022, 353, 129069. [Google Scholar] [CrossRef]
- Wang, S.; Yao, M.; He, X.; Wu, B.; Liu, L.; Wang, S.; Wu, M.; Zhang, X.; Xiang, D. Corrosion Evolution of a Concrete/Casing Steel in Simulated Formation Water under Different CO2 Partial Pressures. Int. J. Electrochem. Sci. 2020, 15, 9948–9970. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Bai, M.X.; Chen, Q.Z. Influencing Factors of Corrosion Behavior of Carbon Dioxide Storage Wellbore. Corros. Prot. 2021, 42, 54–57. [Google Scholar] [CrossRef]
- Gao, D.L.; Dou, H.Y.; Dong, X.L. Research progress in wellbore cement sheath integrity under conditions of CO2 injection and storage. J. Yan’an Univ. (Nat. Sci. Ed.) 2022, 41, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, B.; Zhang, J.; Li, J.; Wang, S.; Dong, L.; Liu, L.; Xiang, D. Corrosion Behavior of P110 Casing Steel in Concrete Exposed to Simulated CO2-Containing Formation Water at 80 °C. Int. J. Electrochem. Sci. 2019, 14, 10693–10706. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, L.; Wang, S.; Lin, Y.; Sun, Y.; Xia, R. Effect of simulated pore solution on passivation characteristic of P110 steel. J. Pet. Sci. Eng. 2018, 167, 949–956. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Z.M.; Zheng, D.; Song, G.-L. The localized corrosion of mild steel in carbonated cement pore solution under supercritical carbon-dioxide in a simulated geothermal environment. Constr. Build. Mater. 2021, 274, 122035. [Google Scholar] [CrossRef]
- Le Saoût, G.; Lothenbach, B.; Hori, A.; Higuchi, T.; Winnefeld, F. Hydration of Portland cement with additions of calcium sulfoaluminates. Cem. Concr. Res. 2013, 43, 81–94. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Wang, S.; Zhang, J.; Deng, T.; Zhang, X.; Zhang, Z. A Study on the CO2 Corrosion Behavior of P110 Steel in High-Density Cement. Int. J. Electrochem. Sci. 2022, 17, 22023. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, X.; Cao, Y.; Yang, H. Natural passivation behavior and its influence on chloride-induced corrosion resistance of stainless steel in simulated concrete pore solution. J. Mater. Res. Technol. 2020, 9, 12378–12390. [Google Scholar] [CrossRef]
- Long, H.; Chen, L.; Dong, B.; Sun, Y.; Yan, Y.; Chen, C. The electronic properties and surface chemistry of passive film on reinforcement: Effect of composition of simulated concrete pore solution. Constr. Build. Mater. 2022, 360, 129567. [Google Scholar] [CrossRef]
- Goyal, A.; Pouya, H.S.; Ganjian, E.; Claisse, P. A Review of Corrosion and Protection of Steel in Concrete. Arab. J. Sci. Eng. 2018, 43, 5035–5055. [Google Scholar] [CrossRef]
- Bonfil, D.; Veleva, L.; Feliu, S.; Escalante-García, J.I. Corrosion Activity of Carbon Steel B450C and Stainless Steel SS430 Exposed to Extract Solution of a Supersulfated Cement. Materials 2022, 15, 8782. [Google Scholar] [CrossRef]
- Ding, S.D.; Lu, P.Q.; Guo, Y.T.; Li, Z.Y.; Lu, Y.H.; Zhou, S.M. Progress and prospect on the study of full life cycle sealing integrity of cement sheath in complex environments. Pet. Drill. Tech. 2023, 51, 104–113. [Google Scholar] [CrossRef]
- Hren, M.; Kosec, T.; Legat, A. An investigation into corrosion around voids at the steel-concrete interface. Cem. Concr. Res. 2024, 181, 107545. [Google Scholar] [CrossRef]
- Shi, J.; Ming, J. Influence of defects at the steel-mortar interface on the corrosion behavior of steel. Constr. Build. Mater. 2017, 136, 118–125. [Google Scholar] [CrossRef]
- Carroll, S.; Carey, J.W.; Dzombak, D.; Huerta, N.J.; Li, L.; Richard, T.; Um, W.; Walsh, S.D.; Zhang, L. Review: Role of chemistry, mechanics, and transport on well integrity in CO2 storage environments. Int. J. Greenh. Gas Control. 2016, 49, 149–160. [Google Scholar] [CrossRef]
- Xiang, Y.; Yuan, Y.; Zhou, P.; Liu, G.; Lv, W.; Li, M.; Zhang, C.; Zhou, Q.; Zhao, X.; Yan, W. Metal Corrosion in Carbon Capture, Utilization, and Storage: Progress and Challenges. Strateg. Study CAE. 2023, 25, 197–208. [Google Scholar] [CrossRef]
- Navas, S.V.; Moraes, C.V.; Della Mea, L.G.; Souto, L.B.; Atz-Dick, P.; Kelly, R.G.; Dick, L.F. Hydrogen permeation due to uniform, pitting, and crevice corrosion of carbon steel in concrete simulated media. Electrochim. Acta 2023, 465, 142999. [Google Scholar] [CrossRef]
- Gong, K.; Liu, C.; Yang, M.; Mao, F.; Wang, J.; Shen, X.; Xiao, L.; Li, M. Effect of multivariable interaction on the corrosion behavior of Q355B steel in simulated concrete pore solutions. Constr. Build. Mater. 2023, 409, 134060. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, C.; Zhang, P.; Sun, M.; Geng, Y.; Zhao, M.; Fan, L.; Liu, N.; Duan, J. Chloride binding of monosulfate hydrate (AFm) and its effect on steel corrosion in simulated concrete pore solution. J. Build. Eng. 2023, 67, 105945. [Google Scholar] [CrossRef]
- Wu, P.-P.; Gong, Y.-P.; Zhang, S.-H.; Zhang, Y.-Z.; Liu, B.-S.; Song, G.-L. Crevice corrosion of reinforcing steel in carbonated simulated concrete pore solutions contaminated by chloride. J. Iron Steel Res. Int. 2024, 32, 293–311. [Google Scholar] [CrossRef]
- Tran, D.T.; Lee, H.-S.; Singh, J.K. Chloride threshold determination of hybrid inhibitor immersed in simulated concrete pore solution. Constr. Build. Mater. 2023, 384, 131446. [Google Scholar] [CrossRef]
- Shi, J.; Wu, M.; Ming, J. Degradation effect of carbonation on electrochemical behavior of 2304 duplex stainless steel in simulated concrete pore solutions. Corros. Sci. 2020, 177, 109006. [Google Scholar] [CrossRef]
- Niu, Z.; Lu, X.; Luo, Y. The Effects of a Multifunctional Rust Inhibitor on the Rust Resistance Mechanism of Carbon Steel and the Properties of Concrete. Coatings 2023, 13, 1375. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, Y.; Xia, R.; Dai, Z.; Zhang, W.; Liu, W. Electrochemical Investigation of Chloride Ion-Induced Breakdown of Passive Film on P110 Casing Steel Surface in Simulated Pore Solution: Behavior and Critical Value Determination. Metals 2024, 14, 93. [Google Scholar] [CrossRef]
- Gong, K.; Yang, M.; Liu, C.; Shen, X.; Xiao, L.; Li, M.; Mao, F. Synergistic effect of chloride ions and surface film on depassivation mechanism of Q355B steel in simulated concrete pore solution. J. Build. Eng. 2023, 78, 107742. [Google Scholar] [CrossRef]
- Li, K.; Li, X.; Zhao, Y.; Wang, K.; Song, S.; Jin, W.; Xia, D.; Xu, Y.; Huang, Y. Influence of Partial Rust Layer on the Passivation and Chloride-Induced Corrosion of Q235b Steel in the Carbonated Simulated Concrete Pore Solution. Metals 2022, 12, 1064. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, M.; Chen, M.; Xie, H.; Ding, F.; Yin, H.; Liu, F.; Li, W. Corrosion behavior analysis of steel in simulated carbonated concrete pore solution based on 3D optical microscopy method. Mater. Today Commun. 2024, 39, 109110. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Han, E.-H. A multiphysics model for studying transient crevice corrosion of stainless steel. J. Mater. Sci. Technol. 2021, 60, 186–196. [Google Scholar] [CrossRef]
- Chen, D.; Han, E.-H.; Wu, X. Effects of crevice geometry on corrosion behavior of 304 stainless steel during crevice corrosion in high temperature pure water. Corros. Sci. 2016, 111, 518–530. [Google Scholar] [CrossRef]
- Sun, W.; Wang, L.; Wu, T.; Liu, G. An arbitrary Lagrangian–Eulerian model for modelling the time-dependent evolution of crevice corrosion. Corros. Sci. 2014, 78, 233–243. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Hua, Y.; Barker, R.; Neville, A. Effect of calcium on X65 carbon steel pitting in saturated CO2 environment. Electrochim. Acta 2022, 407, 139899. [Google Scholar] [CrossRef]
- Ren, X.; Lu, Y.; Wei, Q.; Yu, L.; Zhai, K.; Tang, J.; Wang, H.; Xie, J. The influence of Ca2+ on the growth mechanism of corrosion product film on N80 steel in CO2 corrosion environments. Corros. Sci. 2023, 218, 111168. [Google Scholar] [CrossRef]
- Hang, P.; Zhao, B.; Zhou, J.; Ding, Y. Effect of Heat Treatment on Crevice Corrosion Behavior of 304 Stainless Steel Clad Plate in Seawater Environment. Materials 2023, 16, 3952. [Google Scholar] [CrossRef]
- Yeh, C.-P.; Tsai, K.-C.; Huang, J.-Y. Influence of Chloride Concentration on Stress Corrosion Cracking and Crevice Corrosion of Austenitic Stainless Steel in Saline Environments. Materials 2020, 13, 5640. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Tian, Y.W.; Wang, G.; Hu, J.Z. Electrochemical Behavior of Steel Corrosion in Microporous Environment of Marine Concrete. J. Guandong Ocean. Univ. 2022, 42, 126–134. [Google Scholar] [CrossRef]










| Element | C | Si | Mn | Ni | Cr | Mo | Ti | Fe |
|---|---|---|---|---|---|---|---|---|
| Content(wt.%) | 0.27 | 0.23 | 1.7 | 0.02 | 0.05 | 0.01 | 0.01 | Balance |
| pH | Icorr/(A·cm−2) | Ecorr/V | Rp/(Ω·cm2) | Corrosion Rate/(mm·y−1) | |
|---|---|---|---|---|---|
| Without crevice electrode | 12.70 | 1.84 × 10−7 | −0.351 | 2.78 × 105 | 0.001 |
| 11.50 | 1.31 × 10−6 | −0.546 | 9.36 × 103 | 0.032 | |
| 10.50 | 2.46 × 10−6 | −0.531 | 2.02 × 103 | 0.150 | |
| Half crevice electrode | 12.70 | 2.14 × 10−7 | −0.344 | 3.38 × 105 | 0.001 |
| 11.50 | 2.93 × 10−6 | −0.735 | 4.81 × 103 | 0.063 | |
| 10.50 | 4.84 × 10−6 | −0.781 | 1.23 × 103 | 0.245 |
| pH | Rs/ (Ω·cm2) | Qf/ (Ω−1·cm−2·s−nf) | nf | Rf/ (Ω·cm2) | Qdl/ (Ω−1·cm−2·s−ndl) | ndl | Rct/ (Ω·cm2) | |
|---|---|---|---|---|---|---|---|---|
| Without crevice electrode | 12.70 | 16.0 | 2.10 × 10−5 | 0.954 | 2.99 × 105 | - | - | - |
| 11.50 | 24.6 | 4.01 × 10−5 | 0.950 | 236.4 | 7.50 × 10−5 | 0.750 | 1.38 × 104 | |
| 10.50 | 22.0 | 1.57 × 10−4 | 0.908 | 68.1 | 1.61 × 10−4 | 0.952 | 1.31 × 103 | |
| Half crevice electrode | 12.70 | 42.3 | 1.32 × 10−5 | 0.975 | 1.13 × 106 | - | - | - |
| 11.50 | 41.7 | 1.40 × 10−4 | 0.806 | 429.3 | 1.32 × 10−4 | 0.954 | 8.17 × 103 | |
| 10.50 | 46.4 | 1.63 × 10−4 | 0.820 | 34.1 | 1.06 × 10−4 | 0.909 | 3.18 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, F.; Li, C.; Guo, H.; Xiang, Y. The Effect of Crevice Structure on Corrosion Behavior of P110 Carbon Steel in a Carbonated Simulated Concrete Environment. Coatings 2025, 15, 919. https://doi.org/10.3390/coatings15080919
Ling F, Li C, Guo H, Xiang Y. The Effect of Crevice Structure on Corrosion Behavior of P110 Carbon Steel in a Carbonated Simulated Concrete Environment. Coatings. 2025; 15(8):919. https://doi.org/10.3390/coatings15080919
Chicago/Turabian StyleLing, Fanghai, Chen Li, Hailin Guo, and Yong Xiang. 2025. "The Effect of Crevice Structure on Corrosion Behavior of P110 Carbon Steel in a Carbonated Simulated Concrete Environment" Coatings 15, no. 8: 919. https://doi.org/10.3390/coatings15080919
APA StyleLing, F., Li, C., Guo, H., & Xiang, Y. (2025). The Effect of Crevice Structure on Corrosion Behavior of P110 Carbon Steel in a Carbonated Simulated Concrete Environment. Coatings, 15(8), 919. https://doi.org/10.3390/coatings15080919







