Abstract
To enhance the molten salt corrosion resistance of Ni200 alloy plasma arc welds, the welds were subjected to tensile deformation followed by heat treatment. The grain boundary character distribution (GBCD) was analyzed using electron backscatter diffraction (EBSD) in conjunction with orientation imaging microscopy (OIM). A constant-temperature corrosion test at 900 °C was conducted to evaluate the impact of GBCD on the corrosion resistance of the welds. Results demonstrated that after processing with 6% tensile deformation, and annealing at 950 °C for 30 min, the fraction of low-ΣCSL grain boundaries increased from 1.2% in the as-welded condition to 57.3%, and large grain clusters exhibiting Σ3n orientation relationships were formed. During the heat treatment, an increased number of recrystallization nucleation sites led to a reduction in average grain size from 323.35 μm to 171.38 μm. When exposed to a high-temperature environment of 75% Na2SO4-25% NaCl mixed molten salt, the corrosion behavior was characterized by intergranular attack, with oxidation and sulfidation reactions resulting in the formation of NiO and Ni3S2. The corrosion resistance of Grain boundary engineering (GBE)-treated samples was significantly superior to that of Non-GBE samples, with respective corrosion rates of 0.3397 mg/cm2·h and 0.8484 mg/cm2·h. These findings indicate that grain boundary engineering can effectively modulate the grain boundary character distribution in Ni200 alloy welds, thereby enhancing their resistance to molten salt corrosion.
1. Introduction
Nickel-based alloy Ni200 contains more than 99.5% nickel and demonstrates excellent corrosion resistance in atmospheric, neutral, alkaline, and mildly acidic environments. It is widely utilized as a structural material in various harsh corrosive environments [1]. As a result, Ni200 alloy is frequently subjected to welding during practical applications. However, the weld seam of Ni200 alloy is prone to complex microstructural challenges, such as compositional gradients, residual strains, and heterogeneous microstructures, primarily induced by the thermal cycling inherent in the welding process. Due to its unique microstructural characteristics and exposure to high-temperature molten salt environments, the weld seam becomes increasingly susceptible to environmental degradation, thus representing the most critical failure-prone region in the welded structure. Variations in the microstructure and mechanical properties of the Ni200 weld seam directly influence the reliability and service life of the entire structure [2,3], highlighting the importance of its structural integrity in ensuring the safe and reliable operation of engineering systems.
As the demands for extended service life and improved thermal resistance of hot-end components continue to grow, the requirements for both comprehensive mechanical performance and corrosion resistance of welded structures have become increasingly rigorous. Through rational material selection and optimized processing techniques, the overall performance of weld seams can be significantly enhanced, thereby extending service life and reducing maintenance costs. Therefore, the implementation of effective control strategies to improve the mechanical properties and corrosion resistance of Ni200 alloy weld seams is crucial for enhancing the service performance and operational reliability of the entire structure.
Grain boundary engineering is fundamentally aimed at optimizing the distribution of grain boundary characteristics through the enhancement of annealing twins and twin-related Σ3n grain boundary fractions, representing a critical research direction that has attracted extensive scholarly attention.
Appropriate deformation heat treatment processes can effectively increase the proportion of coincidence site lattice (CSL) grain boundaries in polycrystalline metallic materials. Low-ΣCSL grain boundaries disrupt the connectivity of random grain boundary networks, thereby enhancing grain boundary-related properties [4]. Compared with random grain boundaries, CSL grain boundaries exhibit higher coherent site lattice point density, reduced atomic free volume, and lower grain boundary energy, which collectively reduce the likelihood of intergranular cracking [5]. Grain boundary engineering (GBE) has primarily been applied to face-centered cubic (FCC) metals with medium to low stacking fault energies, yielding significant progress. For instance, Dai et al. [6] demonstrated that grain boundary engineering increased the Σ3 boundary fraction in Incoloy 800H alloy, resulting in improved intergranular corrosion resistance. Jiang et al. [7] investigated the effects of deformation heat treatment on the microstructure and corrosion resistance of nanostructured Incoloy 800 alloy. Their findings revealed that annealing at 600 °C led to clearer grain boundaries and the precipitation of TiC and Cr23C6 phases, while recrystallization occurred at 700 °C, with Cr23C6 phases dispersively distributed in the matrix. The alloy annealed at 600 °C exhibited superior corrosion resistance, whereas excessive Cr23C6 precipitation at 700 °C resulted in deteriorated performance. Xia et al. [8] reported that deformation heat treatment significantly increased the proportion of low-ΣCSL grain boundaries in Inconel 690 alloy, with higher proportions correlating with enhanced resistance to intergranular corrosion. Watanabe et al. [9] observed that low-ΣCSL grain boundaries in polycrystalline copper effectively impeded crack propagation along grain boundaries. Wang Xiaoyan [10] found that grain boundary engineering tripled the capacitive arc radius of Hastelloy X alloy compared to the base material, indicating improved corrosion resistance. These studies collectively demonstrate that grain boundary engineering is an effective strategy for improving the corrosion resistance of FCC metals with medium to low stacking fault energies.
Currently, extensive research has been devoted to improving the microstructure and mechanical properties of nickel-based alloys through deformation and heat treatment techniques. Nevertheless, studies focusing on the application of these treatments to alloy welds are relatively limited, and investigations into their effectiveness in enhancing the corrosion resistance of nickel-based alloy welds remain sparse. Moreover, the impact of grain boundary engineering on the corrosion behavior and underlying mechanisms in nickel-based alloy welds has yet to be thoroughly understood. In response to these knowledge gaps, this study examines Ni200 alloy welds that have undergone tensile deformation and heat treatment. The hot corrosion behavior of the welds was evaluated in a binary mixed molten salt environment, with the objective of clarifying how grain boundary characteristic distributions influence corrosion susceptibility and identifying the mechanism by which specific grain boundary structures mitigate molten salt-induced hot corrosion. This research offers a robust theoretical basis and practical guidance for enhancing the corrosion resistance of welds in other medium- and low-stacking-fault-energy face-centered cubic metals [9,11,12,13,14] through grain boundary engineering, and it possesses considerable scientific and engineering significance.
2. Materials and Methods
2.1. Experimental Materials
The 6 mm thick Ni200 nickel-based alloy plates were obtained from Jinchuan Group Co., Ltd. (Jinchang, China). Welding was performed using the LHM-315 plasma arc welding system manufactured by Sanli Yisheng Welding Technology Co., Ltd. (Beijing, China). Ni200 alloy weld joints exhibiting sound formation and free of surface defects such as porosity and cracks were selected as the experimental specimens. The measured chemical composition of the Ni200 alloy plates is summarized in Table 1.

Table 1.
Chemical composition of nickel-based alloy Ni200 welds (wt.%).
2.2. Experimental Methods
The weld-seam-containing test plates were sectioned into long strips measuring 100 mm × 20 mm × 6 mm using a DK77 wire-cutting machine (Taizhou Tongfang CNC Machine Tool Co., Ltd., Taizhou, China). Two groups of weld-integrated strips were subjected to 6% tensile deformation on a UTM5205 microcomputer-controlled electronic universal testing machine (Shandong Wancheng Testing Machine Co., Ltd., Jinan, China). Subsequently, one group was annealed in an SX2-4-10 box-type resistance furnace (Xinghua City Chenghua Industrial Electric Furnace Factory, Taizhou, China) and designated as GBE samples. The annealing process parameters were as follows: 6% tensile deformation, 950 °C annealing temperature, and water quenching after 30 min of heat treatment. The other group, without undergoing the annealing treatment, was labeled as Non-GBE samples.
The samples were electrolytically polished using a 20% H2SO4 + 80% CH3OH electrolyte to prepare electron backscatter diffraction (EBSD) specimens. Cross-sectional micro-regions of the samples were systematically scanned using a Zeiss GeminiSEM 300 scanning electron microscope (manufactured by Carl Zeiss AG, Oberkochen, Germany), equipped with an HKL-EBSD detector. Scanning was conducted with a step size of 2.5 μm over a defined area of 1500 μm × 1000 μm. Data analysis was performed using Channel 5 software (version 5.2), and grain boundary types were classified in accordance with the Palumbo-Aust criteria [15].
The molten salt-induced hot corrosion kinetics were evaluated using the weight loss method. Both Non-GBE and GBE samples were machined into cubic specimens measuring 10 mm × 10 mm × 6 mm, retaining the weld zone. These specimens were ground sequentially with 800–2000 grit SiC papers (Wuyi Hengyu Instrument Co., Ltd., Jinhua, China) until a mirror-like finish without visible scratches was achieved. Subsequently, the samples were ultrasonically cleaned in deionized water and anhydrous ethanol, dried, and weighed prior to testing. A mixed salt of 75% Na2SO4 and 25% NaCl was placed into two 50 mL alumina crucibles, which were then dried at 200 °C for 5 h in a drying oven. Each type of sample (three per crucible) was placed into one of the crucibles. The crucibles were then transferred to a box-type resistance furnace preheated to 900 °C for isothermal corrosion testing. After each 6 h corrosion cycle, the samples were removed, air-cooled to room temperature, and boiled in deionized water for 30 min to remove residual salts. The cleaned samples were dried, reweighed, and their dimensions measured to calculate the mass loss per unit area. This procedure was repeated six times, resulting in a total exposure time of 36 h.
The corrosion morphology of the sample surface was examined using Gemini SEM 300 scanning electron microscope (SEM) (manufactured by Carl Zeiss AG, Oberkochen, Germany). while the chemical composition of the corroded surface was analyzed via the energy-dispersive X-ray spectroscopy (EDS) system integrated within the SEM, operating at an acceleration voltage of 15 kV. Additionally, the surface of the corroded sample was characterized using a K-Alpha X-ray photoelectron spectrometer (XPS) (Manufactured by Thermo Scientific, Waltham, MA, USA) to investigate the elemental distribution and chemical states of the alloying elements.
3. Results
3.1. Grain Boundary Character Distribution in the Weld Zone
Subsubsection
Figure 1a,c illustrate the grain orientation distribution in the weld zones of the Non-GBE and GBE samples, respectively. As shown in Figure 1a, the average grain size in the weld zone of the Non-GBE sample reaches 323.34 μm, which is primarily due to grain coarsening induced by the welding thermal cycle. In contrast, Figure 1c reveals a significantly refined average grain size of 133.82 μm. Nickel-based alloy Ni200 belongs to the group of medium-to-low stacking fault energy face-centered cubic (FCC) metals. During the process of tensile deformation followed by heat treatment, recrystallization occurs, leading to grain refinement within the weld zone and the formation of numerous transgranular, strip-shaped annealing twins. This phenomenon can be primarily attributed to the relatively low annealing temperature, which provides insufficient thermal activation energy, thereby prolonging the incubation period of recrystallization and inhibiting rapid grain growth.
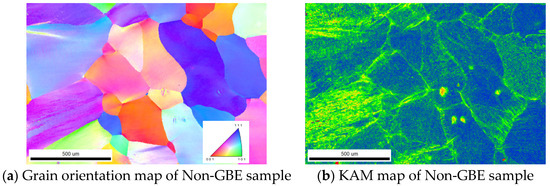
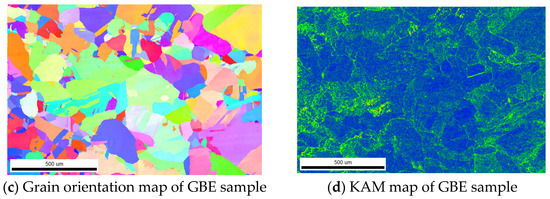
Figure 1.
Grain orientation distribution and KAM distribution in the weld zone of Non-GBE and GBE samples.
Figure 1b,d display the kernel average misorientation (KAM) maps of the Non-GBE and GBE samples, respectively. KAM maps are widely used to evaluate the extent of recrystallization. As shown in Figure 1b,d, the Non-GBE sample surface is predominantly light green, indicating a static recrystallization volume fraction of only 0.41% (see Table 2). In contrast, the GBE sample surface is largely blue, with a significantly higher static recrystallization volume fraction of 45.2%. This suggests that after tensile deformation and heat treatment, the recrystallized region within the weld seam expands substantially, and the proportion of low-ΣCSL grain boundaries is primarily determined by the recrystallization process.

Table 2.
Average grain size, recrystallization volume fraction, and special grain boundary proportion of Non-GBE and GBE samples.
In the KAM maps, light green regions represent areas with relatively high dislocation density, whereas blue regions correspond to those with the lowest dislocation density. Deformed grains typically exhibit a higher dislocation density, especially at grain boundaries, where dislocations tend to nucleate and multiply during plastic deformation, leading to larger local orientation differences. In contrast, recrystallized grains possess a lower dislocation density, as a significant number of dislocations are eliminated during recrystallization, resulting in smaller local orientation differences and more uniform crystallographic orientations.
Figure 2 presents the grain boundary distribution in Non-GBE and GBE samples, while Table 2 summarizes the average grain size, recrystallization volume fraction, and proportion of special grain boundaries for both sample types. As shown in Figure 2 and Table 2, the proportion of low-ΣCSL grain boundaries in the Non-GBE sample is only 1.2%, with Σ3, Σ9, and Σ27 grain boundaries accounting for 0.8%, 0.3%, and 0.1%, respectively. In contrast, the GBE sample exhibits a significantly higher proportion of low-ΣCSL grain boundaries at 57.3%, where Σ3, Σ9, and Σ27 grain boundaries constitute 50.1%, 4.2%, and 0.3%, respectively.
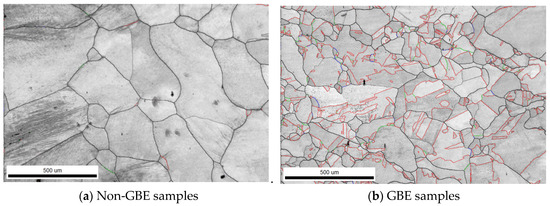
Figure 2.
Distribution of grain boundary characteristics of Non-GBE and GBE samples.
After tensile deformation and heat treatment, the Ni200 nickel-based alloy weld shows a substantial increase in the proportion of low-ΣCSL grain boundaries. These grain boundaries are characterized by lower grain boundary energy, reduced free volume, and higher lattice density, which collectively enhance resistance to intergranular corrosion. The Σ3, Σ9, and Σ27 grain boundaries can form extensive grain clusters that effectively disrupt the connectivity of random grain boundaries, thereby reducing their relative proportion and improving overall corrosion resistance.
Grain boundary engineering introduces a large number of annealing twins into the Ni200 nickel-based alloy weld. This optimization of grain boundary characteristics is primarily achieved through the formation of Σ3n twin boundaries during the recrystallization process. Materials with lower stacking fault energies are more prone to forming annealing twins during thermal treatment, resulting in a higher proportion of Σ3 grain boundaries [16,17]. When twins on different {111} planes intersect, multiple twin boundaries—such as Σ9 and Σ27—are formed [18]. The proportions of Σ9 and Σ27 grain boundaries are closely related to the proportion of Σ3 twins; specifically, a higher Σ3 grain boundary fraction promotes extensive multiple twinning during grain boundary migration. This leads to a relatively higher combined proportion of Σ9 and Σ27 grain boundaries and an increased number of “grain clusters with mutual Σ3n orientation relationships” [19].
3.2. Thermal Corrosion Kinetic Curves
The hot corrosion kinetics curve is a critical parameter for evaluating the resistance of alloys to high-temperature molten salt corrosion. Figure 3 illustrates the corrosion behavior of Non-GBE and GBE samples in a mixed molten salt environment consisting of 75% Na2SO4 and 25% NaCl at 900 °C. As shown in the figure, the corrosion rates of the two samples vary across different time intervals. During the entire 36 h exposure period, the Non-GBE sample exhibited a continuously increasing mass loss trend, with a final corrosion rate of 0.8484 mg/cm2·h. In contrast, the GBE sample showed significantly lower mass loss throughout the test, maintaining a much slower corrosion rate of only 0.3397 mg/cm2·h, indicating its superior resistance to molten salt-induced hot corrosion.
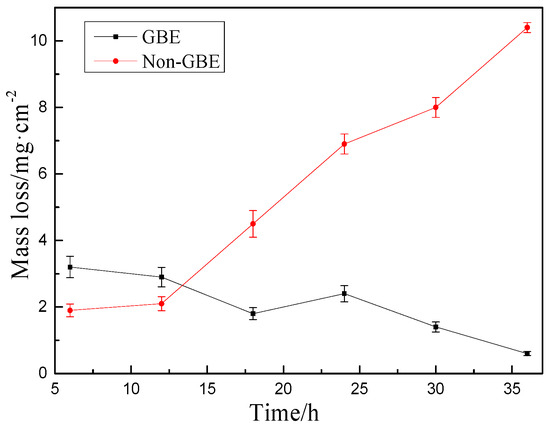
Figure 3.
Hot corrosion kinetics curves.
Intergranular corrosion is the primary degradation mode in nickel-based alloy welds under such aggressive conditions. Following grain boundary engineering (GBE) treatment of the N6 nickel-based alloy weld, the proportion of low-energy, low-ΣCSL grain boundaries—particularly Σ3, Σ9, and Σ27 types—was substantially increased. These grain boundaries form interconnected networks that effectively disrupt the continuity of random grain boundary pathways, thereby impeding the progression of corrosive attack [20,21]. These findings demonstrate that grain boundary engineering can significantly improve the molten salt corrosion resistance of N6 nickel-based alloy welds.
3.3. Corrosion Morphology and Composition Analysis
Figure 4 presents the SEM micrographs of Non-GBE and GBE samples after 36 h of molten salt corrosion at 900 °C. As shown in the figure, both sample groups underwent intergranular corrosion. The weld surface of the Non-GBE sample exhibited severe degradation, characterized by extensive spallation, uneven gullies, and deep corrosion fissures (Figure 4a). In contrast, the GBE sample (Figure 4b) displayed a relatively smooth surface with only localized deep crevices; the majority of the surface showed mild corrosion, and no significant inward penetration into the substrate was observed.
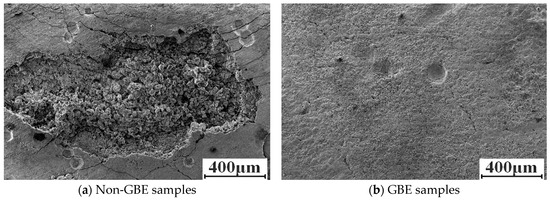
Figure 4.
Corrosion morphology of Non-GBE and GBE sample surfaces.
The corrosion rate data and surface morphology collectively indicate that grain boundary engineering (GBE) significantly enhances the resistance of nickel-based alloy welds to molten salt corrosion. Following tensile deformation and heat treatment, the number of high-angle grain boundaries in the weld zone was markedly reduced, with many being replaced by low-energy, low-ΣCSL grain boundaries. This structural transformation effectively disrupts the connectivity of the random grain boundary network, thereby limiting the available diffusion pathways for corrosive ions into the matrix. Consequently, the propagation of corrosion along high-angle grain boundaries is impeded, which suppresses intergranular attack and crack extension, ultimately improving the overall grain boundary corrosion resistance of the weld [21].
The surface of the GBE sample, following 36 h of corrosion in molten salt at 900 ℃, was analyzed using EDS. As illustrated in Figure 5, the corroded surface of the Ni200 alloy weld primarily contains Ni, O, and S, with no detectable Cl. These results indicate that the surface is predominantly covered with sulfides and oxides.
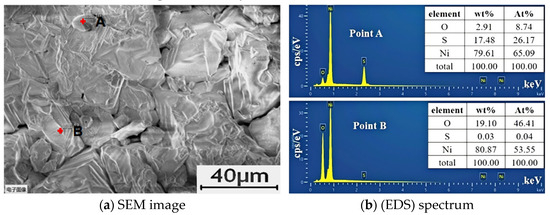
Figure 5.
Composition distribution on the surface of the corroded sample.
3.4. XPS Analysis
The surface corrosion products of both Non-GBE and GBE samples in the molten salt environment are identical, consisting primarily of NiO and Ni3S2. Figure 6 presents the XPS full spectrum and high-resolution spectra of the samples after 36 h of exposure to molten salt at 900 °C. As shown in Figure 6a, distinct peaks corresponding to S 2p, C 1s, O 1s, and Ni 2p3/2 can be clearly identified, indicating that the corrosion products on the sample surface are mainly nickel sulfides and oxides. Figure 6b displays the deconvoluted Ni 2p3/2 spectrum, where the peak at 529.4 eV corresponds to NiO. In Figure 6c, a characteristic peak at approximately 163 eV confirms the presence of Ni3S2.
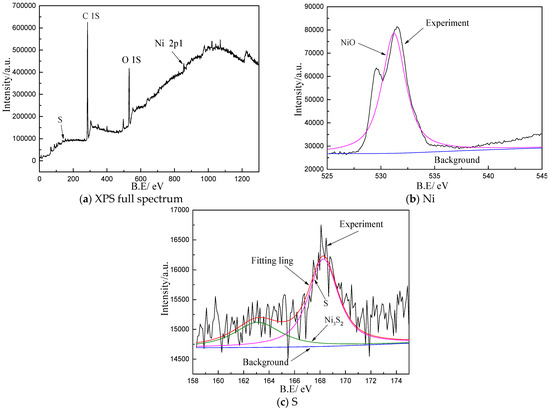
Figure 6.
XPS survey spectrum and high-resolution spectra of the sample after 36 h of corrosion in molten salt at 900 °C.
Corrosion mechanism of nickel-based alloy welds in a 75% Na2SO4-25% NaCl mixed salt system: The surface corrosion products formed during molten salt thermal corrosion consist of NiO and Ni3S2, indicating that both oxidation and sulfidation reactions occur. Initially, a thin NiO oxide film forms on the sample surface. However, this protective layer is gradually dissolved by the aggressive molten salt environment, leading to localized rupture. Subsequently, sulfur and oxygen species diffuse inward along grain boundaries, promoting further oxidation and sulfidation reactions to form NiO and Ni3S2, respectively.
Na2SO4, one of the main components of the molten salt, has a melting point of approximately 884 °C. Upon addition of NaCl, the eutectic composition of the mixture is approached, lowering the melting point to around 675 °C. At 900 °C, the salt fully melts and reacts with the exposed nickel-based alloy surface. During this process, Ni undergoes oxidation: Ni → Ni2+ + 2e−. Oxygen from the surrounding atmosphere is reduced at the salt surface: O2 + 4e− → 2O2−. The Ni2+ ions then combine with O2− to form NiO via an oxidation reaction. Meanwhile, sulfur released from the decomposition of Na2SO4 reacts with Ni to form Ni3S2. This sulfide may further react with Ni to form a low-melting-point Ni–Ni3S2 eutectic phase. The eutectic compound subsequently reacts with oxygen: Ni3S2 + 2O2 → 3Ni + 2SO2. Released SO2 can react with outward-diffusing Ni atoms to regenerate Ni3S2, sustaining the corrosion cycle.
Despite the presence of NaCl in the molten salt, no chlorine-containing compounds were detected in the final corrosion products. This is attributed to the high penetration ability of Cl− ions at elevated temperatures. NiO generated during oxidation reacts with molten NaCl to produce volatile NiCl2 and Cl2 gas. Due to its high vapor pressure, NiCl2 readily evaporates through defects in the oxide film during thermal exposure. Newly formed Cl2 or Cl− ions may re-enter the alloy matrix and react with Ni to regenerate NiCl2, which again volatilizes. Over time, and particularly in the absence of salt replenishment during the experiment, the NaCl content in the melt is progressively consumed.
4. Conclusions
To enhance the molten salt corrosion resistance of Ni200 alloy plasma arc welds, the welds were subjected to tensile deformation and heat treatment. The effect of low-ΣCSL grain boundaries on the corrosion behavior of the welds was investigated. The main findings of this study are summarized as follows:
- Following tensile deformation and heat treatment of the Ni200 nickel-based alloy weld, the proportion of low-ΣCSL grain boundaries increased significantly from 1.2% in the as-welded state to 57.3%. This enhancement was achieved through grain boundary engineering (GBE), which effectively promoted the formation of low-ΣCSL grain boundaries and improved the microstructural stability of the weld.
- In a mixed molten salt environment composed of 75% Na2SO4 and 25% NaCl at 900 °C, the dominant corrosion mode of the N6 weld is intergranular corrosion. The thermal corrosion rate of the Non-GBE sample (0.8484 mg/cm2·h) is notably higher than that of the GBE sample (0.3397 mg/cm2·h), indicating that the GBE-treated weld exhibits superior resistance to molten salt-induced hot corrosion. The low-ΣCSL grain boundaries possess lower energy and higher lattice coherence, which effectively disrupt the continuity of random grain boundary networks. This reduces the diffusion pathways for corrosive ions into the matrix, thereby inhibiting the propagation of intergranular corrosion along high-angle grain boundaries and suppressing overall corrosion kinetics.
- After 36 h of exposure to the binary mixed molten salt, the Ni200 nickel-based alloy weld undergoes both oxidation and sulfidation reactions, resulting in the formation of NiO and Ni3S2 as the primary corrosion products. Although NaCl is present in the molten salt, no chlorine-containing species were detected in the final corrosion products. This is attributed to the high volatility of NaCl and its reaction products (such as NiCl2 and Cl2) under elevated temperature conditions, which facilitates their evaporation and prevents the accumulation of Cl within the corrosion layer.
Author Contributions
Conceptualization, T.C. and Y.Y.; methodology, Y.Y.; software, J.H.; validation, T.C., J.H. and L.Y.; formal analysis, H.X. and T.C.; investigation, J.H.; resources, Y.Y.; data curation, H.X., J.H. and L.Y.; writing—original draft preparation, H.X.; writing—review and editing, T.C.; visualization, T.C.; supervision, Y.Y.; project administration, T.C.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [Scientific Research Projects of Higher Education Institutions in Gansu Province] grant number [2023A-119] And The APC was funded by [Discipline Construction Project of Lanzhou City University].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated in this study, and therefore data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jia, Z.; Wei, B.L.; Sun, X.; Ji, J.J.; Wang, Y.J.; Yu, L.D. Hot workability and dynamic recrystallization mechanisms of pure nickel N6. Trans. Nonferrous Met. Soc. China 2022, 32, 3259–3275. [Google Scholar] [CrossRef]
- Chai, T.X.; Wang, X.J.; Wei, W.K.; Zhang, J.Y.; Wang, B.S. Analysis of the Corrosion Resistance of Pure Nickel and Welding. Mater. Prot. 2016, 49, 91–94. [Google Scholar]
- Zhou, C.S.; Huang, Q.Y.; Guo, Q.; Zheng, J.; Chen, X.; Zhu, J.; Zhang, L. Sulphide stress cracking behaviour of the dissimilar metal welded joint of X60 pipeline steel and Inconel 625 alloy. Corros. Sci. 2016, 110, 242–252. [Google Scholar] [CrossRef]
- Jia, T.; Fu, S.L.; Dong, J.L. Effect of grain boundary characteristic distribution on corrosion resistence performance of B10 cupronickel. Nonferrous Met. Mater. Eng. 2023, 44, 15–23. [Google Scholar]
- Gao, Y.B.; Ding, Y.T.; Chen, J.J.; Xu, J.; Ma, Y.; Wang, X. Effect of Thermo-Mechanical Processing on Grain Boundary Character Distribution of GH3625 Superalloy. Rare Met. Mater. Eng. 2019, 48, 3585–3592. [Google Scholar]
- Dai, Q.; Ye, X.X.; Ai, H.; Chen, S.; Jiang, L.; Liang, J.; Yu, K.; Leng, B.; Li, Z.; Zhou, X. Corrosion of Incoloy 800H alloys with nickel cladding in FLiNaK salts at 850 °C. Corros. Sci. 2018, 133, 349–357. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, H.; Qiang, Y.L.; Zhang, Z.X. Effect of thermomechanical treatment on microstructure and corrosion resistance of Incoloy 800 alloy. J. Plast. Eng. 2025, 32, 228–234. [Google Scholar]
- Xia, S.; Zhou, B.X.; Chen, W.J. Grain boundary character distribution of Alloy 690 and its effect on intergranular corrosion. J. Chin. Electron Microsc. Soc. 2008, 27, 461–468. [Google Scholar]
- Watanabe, T.; Tsurelawa, S. Toughening of brittle materials by grain boundary engineering. Mater. Sci. Eng. A 2004, 387–389, 447–455. [Google Scholar] [CrossRef]
- Wang, X.Y. Research on the optimization of grain boundary character distribution and its effect on properties in Hastelloy X. Master’s Thesis, Nanjing University of Science and Technology, Nanjing, China, 2017. [Google Scholar][Green Version]
- Liu, T.G.; Bai, Q.; Ru, X.K.; Xia, S.; Zhong, X.; Lu, Y.; Shoji, T. Grain boundary engineering for improving stress corrosion cracking of 304 stainless steel. Mater. Sci. Technol. 2019, 35, 477–487. [Google Scholar] [CrossRef]
- Liu, T.; Xia, S.; Du, D.; Bai, Q.; Zhang, L.; Lu, Y. Grain boundary engineering of large-size 316 stainless steel via warm-rolling for improving resistance to intergranular attack. Mater. Lett. 2019, 234, 201–204. [Google Scholar] [CrossRef]
- Detrois, M.; Rotella, J.; Goetz, R.L.; Helmink, R.C.; Tin, S. Grain boundary engineering of powder processed Ni-base superalloy RR1000: Influence of the deformation parameters. Mater. Sci. Eng. A 2015, 627, 95–105. [Google Scholar] [CrossRef]
- Guan, X.J.; Shi, F.; Ji, H.M.; Li, X.W. Gain boundary character distribution optimization of Cu-16at. % Al alloy by thermome-chanical process: Critical role of deformation microstructure. Mater. Sci. Eng. A 2019, 765, 138299. [Google Scholar] [CrossRef]
- Palumbo, G.; Aust, K.T. Structure-Dependence of Intergranular Corrosion in High Purity Nickel. Acta Metall. Et Mater. 1990, 38, 2343–2352. [Google Scholar] [CrossRef]
- Fang, X.Y.; Wang, W.G.; Guo, H.; Zhang, X. ∑3n Special Boundary Distributions in the Cold-Rolled and Annealed 304 Stainless Steel. Acta Metall. Sin. 2007, 12, 9–14. [Google Scholar]
- Huang, X.Y.; Wang, W.G.; Rohrer, G.S.; Xu, G.; Chen, S.; Feng, X.; Zhang, H.; Chen, W.; Zhou, B. The {0 1 1}/{0 1 1} near singular boundaries in 00Cr12 ferritic stainless steel after rolling and recrystallization. Sci. Sin. Technol. 2024, 54, 459–476. [Google Scholar] [CrossRef]
- Zhao, Q.; Xia, S.; Zhou, B.X.; Bai, Q.; Su, Q.; Wang, B.S.; Cai, Z.G. Effect of Deformation and Thermomechanical Processing on Grain Boundary Character Distribution of Alloy 825 Tubes. Acta Metall. Sin. 2015, 51, 1465–1471. [Google Scholar]
- Bai, Q.; Zhao, Q.; Xia, S.; Wang, B.; Zhou, B.; Su, C. Evolution of grain boundary character distributions in alloy 825 tubes during high temperature annealing: Is grain boundary engineering achieved through recrystallization or grain growth. Mater. Charact. 2017, 123, 178–188. [Google Scholar] [CrossRef]
- Bai, Q.; Bian, L.; Zhao, Q.; Wang, B.; Yang, C.; Xia, S.; Zhou, B. Effect of Grain Boundary Engineering on Intergranular Corrosion Resistance of Incoloy825 Alloy. Corros. Prot. 2019, 40, 705–709. [Google Scholar]
- Ding, Y.T.; Sun, F.H.; Xu, J.Y.; Liu, B.; Gao, Y.B.; Zhang, D. Controlling Corrosion Behavior of Inconel 718 via Additive Manufacture Based on Grain Boundary Engineering. Chin. J. Rare Met. 2024, 48, 640–650. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).