Abstract
This study investigates the development of silver (Ag)-doped zirconia (ZrO2) coatings deposited on 316LVM stainless steel via the unbalanced magnetron sputtering technique. The oxygen content in the Ar/O2 gas mixture was systematically varied (12.5%, 25%, 37.5%, and 50%) to assess its influence on the resulting coating properties. In response to the growing demand for biomedical implants with improved durability and biocompatibility, the objective was to develop coatings that enhance both wear and corrosion resistance in physiological environments. The effects of silver incorporation and oxygen concentration on the structural, tribological, and electrochemical behavior of the coatings were systematically analyzed. X-ray diffraction (XRD) was employed to identify crystalline phases, while atomic force microscopy (AFM) was used to characterize surface topography prior to wear testing. Wear resistance was evaluated using a ball-on-plane tribometer under simulated prosthetic motion, applying a 5 N load with a bone pin as the counter body. Corrosion resistance was assessed through electrochemical impedance spectroscopy (EIS) in a physiological solution. Additionally, tribocorrosive performance was investigated by coupling tribological and electrochemical tests in Ringer’s lactate solution, simulating dynamic in vivo contact conditions. The results demonstrate that Ag doping, combined with increased oxygen content in the sputtering atmosphere, significantly improves both wear and corrosion resistance. Notably, the ZrO2-Ag coating deposited with 50% O2 exhibited the lowest wear volume (0.086 mm3) and a minimum coefficient of friction (0.0043) under a 5 N load. This same coating also displayed superior electrochemical performance, with the highest charge transfer resistance (38.83 kΩ·cm2) and the lowest corrosion current density (3.32 × 10−8 A/cm2). These findings confirm the high structural integrity and outstanding tribocorrosive behavior of the coating, highlighting its potential for application in biomedical implant technology.
1. Introduction
316LVM stainless steel is one of the most widely used materials for medical implants due to its excellent corrosion resistance, good biocompatibility, and favorable mechanical properties [1,2,3,4]. However, despite these advantages, implants made from 316LVM face several long-term challenges within the human body [5,6,7]. These include the formation of unstable passive layers, the release of potentially toxic metal ions, and inadequate osseointegration. Such issues can trigger adverse biological responses, including inflammation, infection, and implant rejection, thereby compromising implant longevity and patient outcomes [8,9,10].
Zirconium oxide (ZrO2) has garnered significant attention for biomedical applications because of its high mechanical strength, chemical inertness, and superior biocompatibility [11]. These attributes make ZrO2 an attractive candidate for protective coatings on metallic implants, where resistance to wear, corrosion, and degradation in physiological environments is essential [12]. Nonetheless, long-term exposure to bodily fluids and mechanical stress may impair its adhesion and mechanical integrity, limiting long-term functionality.
To address these limitations, the incorporation of silver (Ag) nanoparticles into ZrO2 matrices has been explored as a strategy to enhance both the structural integrity and functional performance of these coatings [13]. Silver exhibits broad-spectrum antimicrobial activity, making it effective in reducing the risk of implant-associated infections, while also contributing to improved corrosion and wear resistance. Despite promising outcomes, the influence of Ag doping on the mechanical, structural, and tribocorrosive behavior of ZrO2 coatings remains an active area of investigation [14,15,16].
Silver-doped zirconium oxide (ZrO2-Ag) coatings represent a class of advanced ceramic nanocomposites with significant potential for biomedical applications. The addition of Ag nanoparticles has been shown to enhance the structural integrity, surface characteristics, and functional durability of ZrO2-based coatings. These improvements are critical for maintaining coating performance under dynamic physiological conditions, where conventional materials often suffer from reduced wear resistance and compromised adhesion [14].
To overcome the limitations of the materials used in medical implants, the use of zirconium oxide (ZrO2) has been explored. ZrO2 is a ceramic material that excels in biomedical applications due to its high mechanical strength, biocompatibility, and stability [16,17,18]. However, its adherence and wear resistance may be compromised under physiological conditions. Doping ZrO2 with silver nanoparticles (Ag NPs) has proven to be an effective strategy for enhancing these properties, increasing corrosion resistance and enhancing the antibacterial characteristics of the material [19]. This optimizes the durability of the implants and prevents infections in the surgical environment. These advancements have led to ceramic nanocomposites such as ZrO2-Ag being considered promising options in the development of advanced coatings for medical implants, as they improve biocompatibility and corrosion resistance and enhance adherence, an essential aspect for ensuring implant longevity without generating toxicity in surrounding tissues [20,21].
Despite these advances, several challenges remain in translating ZrO2-Ag coatings to clinical use. Key concerns include ensuring long-term adhesion to metallic substrates, maintaining mechanical performance under tribological stress, and fully understanding the coatings’ interactions with biological tissues [22]. In this context, the present study aims to evaluate the tribological and electrochemical properties of ZrO2-Ag coatings deposited on 316LVM stainless steel substrates [23].
The use of advanced deposition techniques, such as magnetron sputtering, has enabled the production of homogeneous and adherent ceramic coatings with enhanced performance [24,25]. However, optimizing adhesion, hardness, and wear resistance under physiological and mechanical loading conditions remains a challenge [6]. This study addresses these issues by characterizing the performance of ZrO2-Ag coatings under simulated tribocorrosion conditions.
The coatings were analyzed using X-ray diffraction (XRD) to identify phase composition and [26] atomic force microscopy (AFM) and assess surface morphology and triboelectrochemical testing to simulate fretting corrosion. Electrochemical impedance spectroscopy (EIS) was employed to evaluate corrosion resistance in physiological media [27,28]. By correlating the coating composition and deposition parameters with tribocorrosive performance, this study aims to advance the development of durable, multifunctional coatings for next-generation biomedical implants.
2. Materials and Methods
Silver-doped zirconium oxide (ZrO2-Ag) thin films were deposited on 316LVM stainless steel substrates using the magnetron sputtering technique. Moreover, 316LVM, an austenitic stainless steel widely used in biomedical applications due to its excellent corrosion resistance and biocompatibility, served as the base material. Its chemical composition, determined by energy-dispersive spectroscopy (EDS), includes 16.4 at.% chromium (Cr), 69.9 at.% iron (Fe), 10.1 at.% nickel (Ni), 1.5 at.% manganese (Mn), and 2.1 at.% molybdenum (Mo). This alloy exhibits a yield strength of approximately 200 MPa, tensile strength ranging from 550 to 750 MPa, and an elongation of about 40%, combining mechanical strength with ductility. The sputtering chamber was evacuated to a base pressure of ~10−6 mbar, after which argon (Ar) gas was introduced at a flow rate of 25 SCCM (standard cubic centimeters per minute) and oxygen at 5 SCCM to initiate plasma formation. Deposition was performed at a working pressure of 3.0 × 10−2 mbar and room temperature, using a target power of 40 W. During coating, substrates were rotated at 6 rpm to ensure uniform film coverage.
For deposition, 316LVM stainless steel disks (diameter = 1.27 cm and thickness = 3 mm) and silicon wafers (Si (100)) were used. Silicon substrates were employed for coating thickness measurements. Prior to deposition, steel samples were polished using sandpaper followed by 1 µm and 0.3 μm alumina suspensions, achieving a surface roughness of 37 ± 2 nm.
ZrO2-Ag coatings were deposited using RF magnetron sputtering at 13.56 MHz. A 4-inch Zr target with 99.9% purity was used, positioned 4.3 cm from the substrate. The pressure in the chamber was set at 2 × 10−2 mbar, with a cathode power of 80 W. The deposition conditions were maintained at 50 W power, 2.6 × 10−3 mbar working pressure, and a substrate temperature of 300 °C. The oxygen content in the Ar/O2 gas mixture was varied (12.5%, 25%, 37.5%, and 50%) to evaluate its effect on coating properties.
The microstructure and nanocomposite formation of the ZrO2-Ag coatings were analyzed using JEOL JEM 2100 transmission electron microscopy (TEM) at 200 kV, manufactured by JEOL Ltd., Tokyo, Japan. The coatings deposited on 316LVM stainless steel substrates were cross-sectioned for further characterization, transferred to a copper grid, and thinned with a precision microtome to below 100 nm. The imaging parameters were optimized to capture high-resolution structural details.
Crystallographic characterization was conducted via X-ray diffraction (XRD) using an EMPYREAN-PANalytical manufactured by PANalytical B.V., Almelo, The Netherlands. System with monochromatic Cu-Kα radiation (λ = 1.5406 Å) in Bragg–Brentano geometry. Spectra were acquired in a 2θ range of 10° to 90°, with a step size of 0.02° and a step time of 2 s. The crystalline phases were identified by comparing peak positions with entries from the Crystallography Open Database (COD-2022).
The surface morphology prior to wear testing was examined via atomic force microscopy (AFM, Asylum Research MFP-3D, manufactured by Asylum Research, an Oxford Instruments Company, Santa Barbara, CA, USA) in contact mode over a 45 µm × 45 µm area.
The wear resistance was evaluated using a linearly reciprocating ball-on-flat sliding tribometer (Nanovea T2000, manufactured by Nanovea, Irvine, CA, USA). The counter-body consisted of a 3 mm-diameter cylindrical pin fabricated via a micromachining tool, with a total distance of 6 cm from the bone. Although designed to simulate microscopic-scale contact conditions, the 5 N load applied corresponds to a significantly higher pressure than typical microscale applications (mN range). This experimental setup was chosen to replicate localized contact stresses encountered in biomedical implants. A sliding speed of 2 mm/s was applied over a total sliding distance of 888 m.
Electrochemical impedance spectroscopy (EIS) was conducted to assess corrosion resistance in a physiological medium using a Gamry’s Interface 1010E potensiostat, Interface 1010E, manufactured by Gamry Instruments, Warminster, PA, USA. Ringer’s physiological solution (9 g/L of NaCl, 0.4 g/L of CaCl2, and 2.1 g/L of NaHCO3) at 37 °C was used as the electrolyte. Measurements were performed with a 5 mV AC perturbation across a frequency range of 10 kHz to 0.001 Hz. The data were analyzed using Bode plots analyzing the impedance (Z) and phase angle (φ) responses, and modeled using an equivalent electrical circuit consisting of solution resistance (RΩ), double layer resistance (Rdl), polarization resistance (Rp), and associated capacitive elements, with impedance represented as complex values (real and imaginary components).
To assess tribocorrosion behavior, combined tribological and electrochemical testing was conducted in Ringer’s lactate solution under reciprocating sliding motion. This setup simulated the mechanical and electrochemical conditions experienced by implants in vivo.
A custom-modified Nanovea T2000, manufactured by Nanovea, Irvine, CA, USA tribometer, integrated with an electrochemical cell, was used for the tribocorrsion tests, as shown in Figure 1. A three-electrode configuration was employed, consisting of an Ag/AgCl reference electrode, a platinum counter-electrode, and a working electrode comprising the coated sample (1 cm2 exposed area), as depicted in Figure 1. The electrochemical data, including polarization curves and corrosion current densities, were recorded in real time using the Gamry Interface 1010E potentiostat.
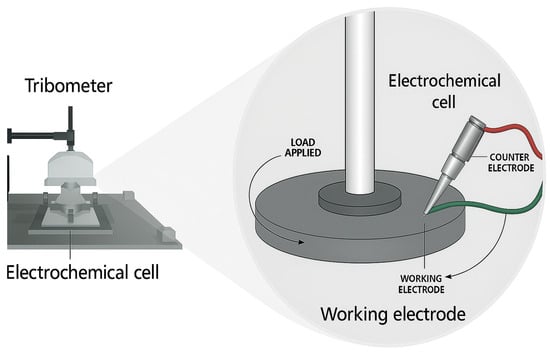
Figure 1.
Experimental setup of the tribocorrosion test: reciprocating ball-on-flat tribometer coupled with an electrochemical cell.
Prior to testing, samples were immersed in Ringer’s lactate solution for 120 min to stabilize the electrochemical interface. Subsequently, reciprocating sliding tests were conducted while simultaneously recording tribological and electrochemical responses. To ensure reproducibility, each test was repeated three times under identical experimental conditions.
3. Results and Discussion
3.1. Structural Characterization
Figure 2 presents four cross-sectional transmission electron microscopy (TEM) views of zirconium oxide coatings doped with silver (ZrO2-Ag), deposited on silicon substrates. The coatings exhibit a dense and compact structure with no evident defects. A columnar microstructure is clearly visible, which can be attributed to bombardment by energetic particles (ions and neutrals) during the sputtering process. This bombardment enhances adatom mobility on the growing surface, thereby minimizing vacancy formation and promoting ordered growth.
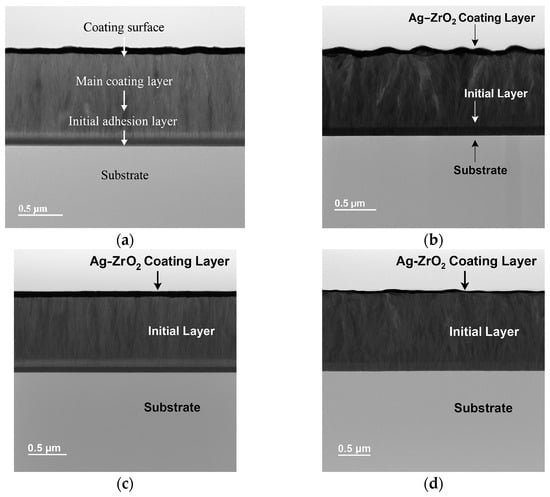
Figure 2.
Cross-sectional TEM micrographs of ZrO2-Ag coatings deposited with varying Ar/O2 gas mixture ratios: (a) 12.5%, (b) 25%, (c) 37.5%, and (d) 50%.
The TEM micrographs display regions with varying contrast: brighter areas correspond to silver-enriched zones, while darker regions represent the ZrO2 ceramic matrix. This contrast indicates a relatively homogeneous elemental distribution throughout the coating, in line with the established deposition parameters.
A distinct ZrO2 adhesion-promoting interlayer, approximately 100 nm thick, is visible in all samples. This layer enhances adhesion between the coating and the substrate, as previously reported [20,28]. The images correspond to coatings deposited with different oxygen concentrations in the Ar/O2 gas mixture: 12.5% (a), 25% (b), 37.5% (c), and 50% (d). The total coating thickness is approximately 1.2 µm, as confirmed via transmission electron microscopy measurements.
Figure 3 presents a morphological and compositional characterization of the ZrO2-Ag coating synthesized under an Ar/O2 atmosphere with 50% oxygen content. In part (a), the cross-sectional SEM image reveals a compact and homogeneous structure with a well-defined interface between the coating and the substrate. The observed microstructure suggests good densification and strong interfacial adhesion, attributes that are critical for enhanced mechanical performance and long-term protective behavior.
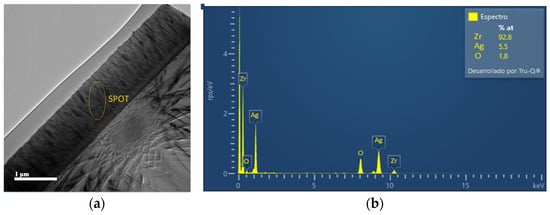
Figure 3.
(a) Cross-sectional SEM micrograph of the ZrO2-Ag coating deposited under an Ar/O2 atmosphere with 50% oxygen content. The area marked as “SPOT” indicates the region where the EDS analysis was performed. (b) Energy dispersive spectroscopy (EDS) acquired via scanning electron microscopy (SEM) for the sample deposited under an Ar/O2 atmosphere with 50% oxygen content. The spectrum confirms the presence of Zr, O, and Ag in the coating.
A specific region within the coating, labeled as “SPOT”, was selected for detailed elemental analysis. Part (b) shows the corresponding energy-dispersive spectroscopy (EDS) spectrum acquired from this targeted area. The spectrum displays prominent peaks associated with zirconium (Zr), oxygen (O), and silver (Ag), confirming the successful incorporation of these elements into the coating matrix. While EDS is a semi-quantitative technique, its primary utility in this context is to verify the presence of key elements rather than to provide precise compositional ratios. Nevertheless, the clear identification of Zr, O, and Ag supports the intended composition of the nanostructured coating and validates the deposition strategy.
Figure 4 presents the X-ray diffraction (XRD) pattern of the ZrO2-Ag composite coatings, highlighting well-defined peaks associated with distinct crystalline phases. The Ar/O2 gas ratio during deposition plays a critical role in influencing the crystallization behavior of zirconium oxide and the dispersion of metallic silver within the coating matrix [29,30]. Increasing the oxygen content up to 50% induces a phase transformation in the ZrO2 coating, favoring the stabilization of the tetragonal phase. The 316LVM stainless steel substrate retained its characteristic austenitic crystalline structure, exhibiting face-centered cubic (FCC) crystallinity with diffraction peaks corresponding to the (111), (200), and (220) planes.
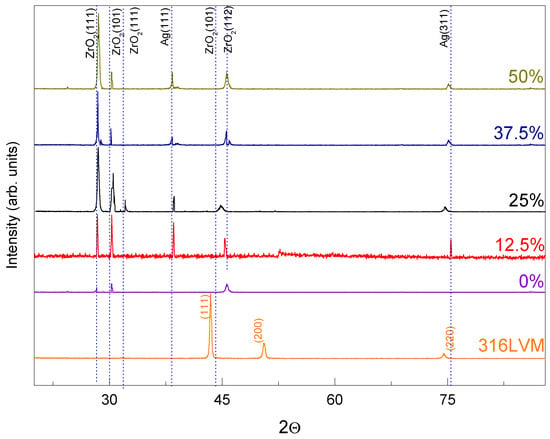
Figure 4.
X-ray diffractogram of Ag-ZrO2 coatings for different Ar/O2 ratios.
The XRD patterns in Figure 4 reveal well-defined peaks corresponding to both the monoclinic and tetragonal phases of ZrO2, along with additional peaks corresponding to metallic silver (Ag), confirming the coexistence of oxide and metal phases within the composite. The crystal planes identified include (111), (101), (111), (112), (101), and (302), located at 2θ values of 27.95°, 30.32°, 32.09°, 37.93°, 44.98°, and 45.68°, respectively. These planes are characteristic of the Ag-ZrO2 system, indicating successful integration between zirconia and silver. The presence of the (112) plane, in accordance with JCPDS card No. 89–7710 [31], is indicative of the tetragonal phase, while the monoclinic contributions align with JCPDS card No. 86–1451, suggesting that the deposition process promotes the formation of the tetragonal phase [32]. The (111) and (311) planes, with 2θ positions at 37.98° and 75.21°, correspond to the face-centered cubic (fcc) structure of metallic silver, aligning with JCPDS card No. 04–0783, which confirms that Ag particles are well crystallized within the composite matrix.
An increase in the oxygen content during deposition enhances the crystallization of the tetragonal phase of ZrO2, as evidenced by the increased intensity of the corresponding diffraction peaks. Variations in the relative intensities of Ag and ZrO2 peaks are likely related to the distribution and dispersion of silver throughout the ceramic matrix, as well as the partial pressure of oxygen during film growth [33,34,35,36]. The simultaneous presence of Ag and ZrO2 peaks without significant peak shifts indicates minimal chemical interaction between the phases, allowing each to retain its distinct crystalline structure.
3.2. Surface Properties and Topography
Figure 5 presents the surface topography of the ZrO2-Ag coatings, as revealed by three-dimensional (3D) atomic force microscopy (AFM) imaging. The analysis indicates that the surface roughness (Ra) of the coatings varies as a function of the Ar/O2 ratio employed during deposition. Notably, the sample deposited with Ar/O2 −12.5% shows the highest average roughness (Figure 5a), with a Ra value of approximately 3.79 ± 0.12 nm, suggesting more pronounced granular growth and surface inhomogeneity [35]. As the oxygen concentration increases, a progressive decrease in surface roughness is observed: Ra ≈ 1.45 ± 0.09 nm for Ar/O2 −25% (Figure 5b), Ra ≈ 1.10 ± 0.06 nm for Ar/O2 37.5% (Figure 5c), and the lowest value of Ra ≈ 0.68 ± 0.04 nm at Ar/O2 −50% (Figure 5d). This trend indicates that higher oxygen concentrations favor the formation of smaller grains and promote a more uniform and compact surface morphology, with reduced topographical irregularities [37,38]. Additionally, the observed reduction in roughness may also be attributed to the influence of silver on the nucleation and growth kinetics of the ZrO2 matrix. The incorporation of Ag appears to facilitate the formation of a denser surface with fewer defects, likely by modifying the surface energy conditions and inhibiting abnormal grain growth during film formation.
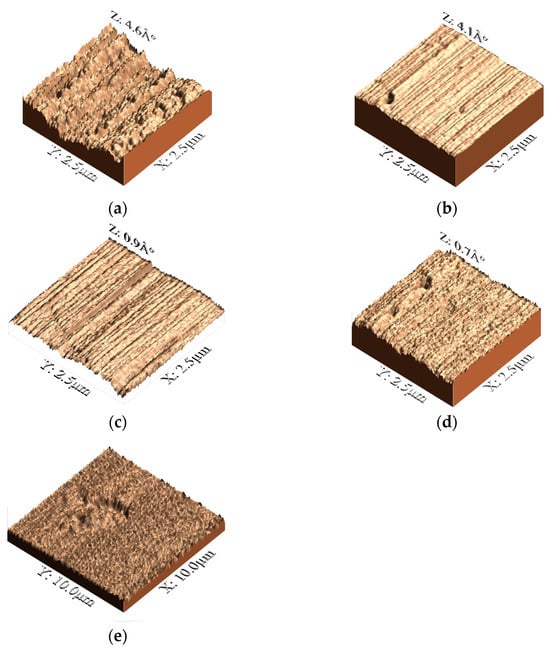
Figure 5.
Three-dimensional topographic images of ZrO2-Ag coatings deposited under different Ar/O2 ratios, illustrating the influence of oxygen content on surface roughness and morphology. (a) 12.5%, (b) 25%, (c) 37.5%, and (d) 50%, (e) substrate 316LVM.
Figure 5 also includes three-dimensional AFM images of ZrO2-Ag nanocomposite coatings deposited under varying Ar/O2 ratios, providing additional qualitative insights into the effect of the oxygen content on surface morphology. Beyond the numerical roughness values, the images reveal a clear evolution in surface features with increasing oxygen content, from pronounced granular structures at 12.5% (Figure 5a) to smoother, more uniform surfaces at 50% (Figure 5d). These morphological changes are indicative of a shift in the grain growth mechanism and improved structural densification at higher oxygen concentrations. To further explore the influence of the coatings on substrate topography, AFM analysis was conducted over a larger scan area of 10 × 10 µm2 (Figure 5e). The uncoated 316LVM substrate exhibits parallel surface features associated with the mechanical polishing process. In contrast, coated surfaces show distinct height distributions that correlate with the oxygen concentration: lower O2 levels result in increased surface irregularities, whereas higher oxygen levels promote the development of a more compact and homogeneous morphology. These observations reinforce the conclusion that the oxygen concentration during deposition not only governs grain refinement, but also plays a critical role in controlling the surface texture and packing density of the coatings, factors that directly impact their tribological and electrochemical behavior.
3.3. Wear
Figure 6 illustrates the results of the wear tests, highlighting a notable improvement in the tribological performance of the Ag-doped ZrO2 coatings as the oxygen content in the Ar/O2 deposition atmosphere increases. A higher oxygen ratio (12.5%, 25%, 37.5%, and 50%) correlates with a progressive reduction in both the average coefficient of friction (COF) and in the wear volume, indicating enhanced wear resistance, particularly for coatings deposited at a 50% Ar/O2 ratio. In this condition, the Ar/O2 50% ratio coating exhibits minimal wear and reduced plastic deformation, consistent with a transition from adhesive to mild abrasive wear mechanisms, as evidenced by shallower grooves and smoother wear track.
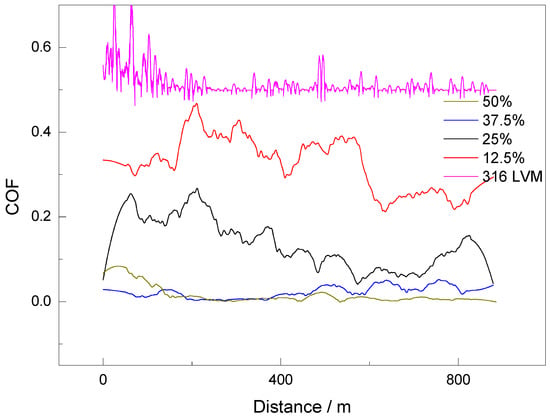
Figure 6.
Coefficient of friction as a function of sliding distance for Ag-doped ZrO2 coatings deposited with different Ar/O2 ratios. The curves correspond to 50% (olive), 37.5% (blue), 25% (black), 12.5% (red), and uncoated 316LVM substrate (violet).
The coating deposited under an Ar/O2 atmosphere containing 50% oxygen exhibited the lowest coefficient of friction (0.0043) and the smallest wear volume (0.086 mm3). This behavior is attributed to improved phase consolidation, which enhances the coating’s abrasion resistance and facilitates the formation of a dense, adherent layer with potential lubricating properties [39]. Conversely, coatings grown under lower oxygen concentrations, particularly 12.5%, show poor structural refinement, leading to inhomogeneous particle distribution, pronounced plastic deformation, and deeper grooves, confirming more severe wear, as corroborated by AFM analyses.
Coatings obtained at intermediate O2 ratios (25% and 37.5%) display transitional behavior, with moderate wear resistance improvements. The sample prepared at 37.5% O2 achieved a COF of 0.032, significantly higher than that of the 50% O2 coating, despite exhibiting similar roughness values. This suggests that the friction behavior is influenced more by mechanical properties than by surface topography alone. These intermediate coatings indicate that increasing the oxygen content contributes to a more efficient deposition process and improved microstructure, enhancing wear resistance relative to the 12.5% O2 sample.
During sliding, the adaptation of the softer counter body (pin) to the surface morphology of the ZrO2-Ag coatings prepared at 50% Ar/O2 results in a gradual decrease in COF, reaching a steady state after approximately 150 m of sliding [40]. This stabilization is attributed to the interplay between adhesive and interfacial mechanisms, with the final COF primarily governed by the elastoplastic response and surface roughness of the coating. Coatings deposited at lower O2 ratios fail to reach this steady state, exhibiting fluctuating COF profiles due to unstable contact conditions.
To quantify wear resistance, the Archard equation was employed [41], relating the volume of the material removed to the applied normal load and sliding distance. The wear volumes, calculated from profilometric measurements of the wear tracks, confirmed the inverse relationship between the oxygen content and wear rate. For instance, coatings deposited with 12.5% and 25% O2 showed wear volumes of 1.072 mm3 and 0.086 mm3, respectively, while those deposited at 37.5% and 50% O2 exhibited significantly reduced volumes of 0.248 mm3 and 0.086 mm3, respectively. These results underscore that increasing the oxygen concentration during deposition leads to dense and more uniform coatings, thereby reducing material loss under sliding conditions [42]. This suggests that a lower oxygen concentration in the deposition atmosphere hinders the formation of a stable and homogeneous ZrO2 layer, negatively impacting the wear resistance of the coating. Under these conditions, ZrO2 particles tend to be less uniformly distributed, leading to higher wear rates during tribological tests, with friction coefficients reaching 0.16 for 25% and 0.38 for 12.5% ratios. These coatings exhibit a more aggressive wear mechanism, favoring plastic deformation and deep groove formation.
Coatings with 25% and Ar/O2 37.5% gas mixtures show a transition in their tribological properties, with an evident but less pronounced improvement in wear resistance compared to the 50% coating. In these cases, a higher oxygen content in the atmosphere facilitates a more efficient deposition process and better microstructure, contributing to a more homogeneous ZrO2 particle distribution. This allows the coating to maintain more efficient and durable wear behavior compared to Ar/O2 12.5% coatings.
The tribological profiles also reveal that COF fluctuations are strongly linked to the dominant wear mechanisms. For example, the 316LVM substrate exhibits significant COF variability due to the accumulation of wear debris and unstable contact. Repeatability was confirmed by conducting three wear tests per sample, all yielding consistent COF trends in both amplitude and curve morphology, indicating reliable tribological performance under the experimental conditions.
Finally, considering the relationship between COF, applied load, and sliding distance, it can be inferred that as long as the applied load remains below the critical threshold for surface failure, the frictional behavior stabilizes and the COF decreases due to improved contact conditions and coating integrity.
3.4. Electrochemical Evaluation
Figure 7 shows the Bode plots of the ZrO2-Ag coatings, revealing the electrochemical response of each sample across a frequency range from 100 kHz to 0.01 Hz. An increase in the magnitude of the impedance modulus (|Z|) is observed for coatings deposited with a higher oxygen content, particularly in the low-frequency region. This behavior indicates enhanced barrier properties and improved resistance to electrolyte penetration.
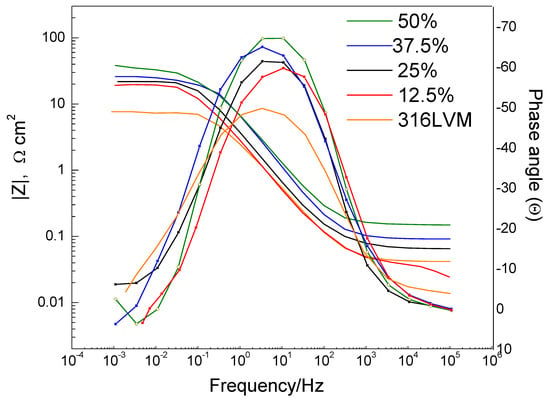
Figure 7.
Bode plots showing impedance magnitude and phase angle as a function of frequency for ZrO2-Ag coatings deposited with different Ar/O2 ratios. Curves correspond to 50% (green), 37.5% (blue), 25% (black), 12.5% (red), and uncoated 316LVM substrate (orange).
The plots display characteristics consistent with the presence of two distinct time constants, suggesting the contribution of two interfacial processes: one associated with the outer coating–electrolyte interface and the other with the inner coating–substrate interface. The increased phase angle and broader plateau in the mid-to-low frequency region for the coatings deposited at 37.5% and 50% O2 are indicative of superior electrochemical stability and capacitive behavior.
These results confirm that oxygen concentrations during deposition promote the formation of more compact and homogeneous coatings, which effectively hinder charge transfer and ionic diffusion. Consequently, coatings synthesized under higher Ar/O2 ratios demonstrate optimized protective performance against electrochemical degradation in simulated physiological environments [43].
Analysis of the Bode plots confirms a progressive decrease in total impedance as the Ar/O2 ratio in the deposition atmosphere is reduced (from 50% to 12.5%). This trend is attributed to the less effective formation of a continuous and compact oxide layer, which alters the coating’s electrochemical behavior and compromises its protective capabilities [39]. The impedance response across the frequency spectrum enables the detailed characterization of the system’s charge transfer resistance and interfacial capacitance, two key parameters for evaluating coating stability under simulated physiological conditions.
From an electrochemical impedance spectroscopy (EIS) standpoint, high- and medium-frequency regions reflect processes occurring at the coating–electrolyte interface, whereas medium to low frequencies are dominated by charge transfer kinetics and ionic diffusion mechanisms. At the lowest frequencies, the impedance response is primarily influenced by interactions between the coating and the underlying substrate. The presence of a partial semicircle in the Nyquist representation indicates surface-controlled conduction, whose diameter diminishes as the oxygen content decreases. This reduction suggests that denser and more uniform films, typically obtained at higher oxygen ratios, enhance the overall system impedance and thereby improve corrosion resistance.
Coatings synthesized under higher Ar/O2 ratios display markedly higher impedance magnitudes, in agreement with previous tribomechanical findings, where these systems also demonstrated superior wear resistance. This correlation implies that controlled oxygen incorporation during deposition promotes the formation of dense, adherent, and electrochemically stable coatings, contributing to improved durability under aggressive environmental conditions.
Additionally, variations in time constants derived from the EIS spectra suggest that the electrochemical behavior of the coatings evolves with prolonged exposure to the corrosive medium. In the ZrO2-Ag system, shifts in interfacial capacitance are observed, potentially related to the passivation of exposed substrate regions in areas where the coating may contain minor discontinuities [44]. This self-passivation process favors the formation of a protective oxide layer, thereby enhancing corrosion resistance and improving coating–substrate adhesion.
The slight displacement of the time constants over time also indicates modifications in the dielectric response due to both the coating and the substrate, likely associated with progressive passivation effects. As shown in the equivalent circuit model (Figure 8), two distinct time constants are observed during the first hour of immersion, with the second remaining stable thereafter, suggesting that the system reaches electrochemical equilibrium. Furthermore, the low root mean square (RMS) roughness values observed through AFM support this interpretation, indicating that a homogeneous surface morphology contributes to improving resistance to electrochemical degradation.
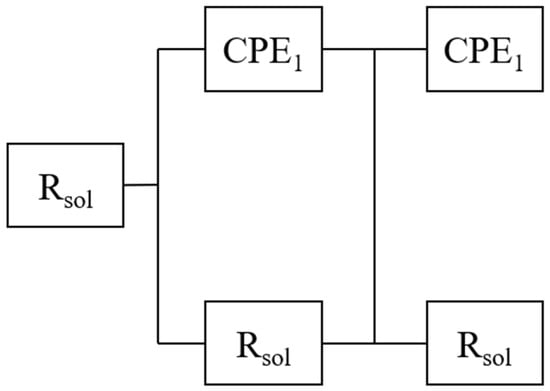
Figure 8.
Equivalent circuit used to model the electrochemical response of the coatings, considering electrolyte resistance (Rsol), coating resistance (Rpo), charge transfer resistance (Rct), and constant phase elements (CPE1 and CPE2).
Two sets of constant phase elements (CPEs) in parallel were incorporated into the equivalent circuit model to simulate the dielectric and electrochemical behavior of the ZrO2-Ag coatings. The first pair (CPE1 and Rpo) represents the barrier properties of the coating. Here, Rpo accounts for the pore resistance, indicating the coating’s ability to resist electrolyte penetration through structural defects such as pores or microcracks. CPE1 describes the non-ideal capacitive behavior of the coating–electrolyte interface.
The second pair (CPE2 and Rct) simulates the electrochemical processes occurring at the metal–coating interface, primarily through localized defects. The Rct (charge transfer resistance) reflects the kinetics of the corrosion reaction, while CPE2 captures the dielectric properties of the double layer formed at these active sites.
In the equivalent circuit model, Rsol represents the solution resistance between the working and reference electrodes. The use of constant phase elements allows for the more accurate modeling of non-ideal capacitive behavior, typically observed in real electrochemical systems with surface roughness, inhomogeneities, or interfacial porosity. This modeling approach enables a refined interpretation of the charge transfer and mass transport phenomena governing coating performance under corrosive conditions.
The fitted parameter values for the ZrO2-Ag coatings are consistent with the trends observed in the Bode plots. Notably, both the Rpo and Rct increase with higher Ar/O2 ratios (12.5%, 25%, 37.5%, and 50%), suggesting the formation of a more compact, stable, and protective passive oxide layer. An increase in the Rct reflects a slower charge transfer process and enhanced corrosion resistance, while a higher Rpo value indicates reduced electrolyte access to the substrate through coating defects [45].
Table 1 summarizes the electrical parameters obtained from fitting the EIS data. The solution resistance (Rsol) shows minor variation across the coated samples, ranging from 45 to 64 Ω·cm2. In contrast, the uncoated 316LVM substrate exhibits a significantly lower Rsol value (12.05 Ω·cm2), likely due to its direct exposure to the electrolyte, which facilitates ionic exchange. The double-layer capacitance values (C1 and C2), along with their associated dispersion factors (α1 and α2), provide insights into the coatings’ dielectric properties and surface uniformity. Notably, C2 decreases steadily as the oxygen content increases, from 610.24 µF·cm−2 (12.5% Ar/O2) to 287.39 µF·cm−2 (50% Ar/O2), suggesting the formation of a denser and less porous passive film.

Table 1.
Parameters used in the simulation of impedance data.
The most critical indicators of corrosion resistance, R1 and R2, exhibit a clear increasing trend with a higher oxygen content. Specifically, R2 rises from 8.14 kΩ·cm2 (12.5%) to 38.83 kΩ·cm2 (50%), reflecting enhanced charge transfer resistance and superior barrier performance. This trend is inversely correlated with the C2 values, reinforcing the conclusion that increased oxygen during deposition promotes the formation of a compact and electrochemically stable oxide layer. The highest R2 and α2 values observed in the 50% Ar/O2 coating further confirm the optimized microstructure and improved homogeneity of this system.
3.5. Triboelectrochemical Wear on Corrosion Resistance
Figure 9 presents the electrochemical behavior of the ZrO2-Ag coatings under tribocorrosive conditions, represented through Tafel plots. These curves allow for the evaluation of the synergistic effects between wear and corrosion and illustrate how the electrochemical response varies with different Ar/O2 gas ratios during deposition. The sample deposited under the highest Ar/O2 50% ratio exhibits the most favorable electrochemical performance, characterized by the lowest corrosion current density (Icorr) and the highest polarization resistance (Rp). This indicates that the coating provides superior protection against corrosion, even under mechanical stress [46,47]. This enhanced behavior is attributed to the formation of a dense and chemically stable ZrO2 passive layer, which serves as an effective barrier to aggressive ionic species and simultaneously improves wear resistance. The high oxygen content during deposition likely promotes better crystallinity and homogeneity of the ceramic matrix, thereby enhancing its electrochemical stability.
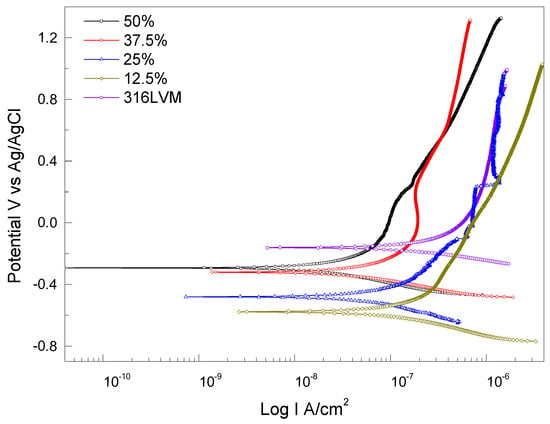
Figure 9.
Tafel curves obtained from electrochemical tests, illustrating the synergy between wear and corrosion in ZrO2-Ag coatings with different Ar/O2 ratios. Curves are distinguished as follows: 50% (□, solid black), 37.5% (◯, solid red), 25% (△, solid blue), 12.5% (◇, solid olive), and 316LVM (◯, solid violet).
In contrast, the coating deposited with an Ar/O2 12.5% ratio demonstrates the poorest electrochemical performance, with significantly higher corrosion current density values and reduced polarization resistance, as summarized in Table 2. This inferior behavior is indicative of a less protective and possibly discontinuous passive layer, which is more susceptible to breakdown under tribological loading [48]. The absence of a compact and robust passive layer increases its vulnerability to localized corrosion, ultimately accelerating material degradation.

Table 2.
Electrochemical parameters obtained from Tafel analysis for ZrO2-Ag coatings deposited under different Ar/O2 ratios, highlighting the influence of deposition conditions on corrosion resistance.
The Tafel analysis clearly demonstrates that the quality of the coating significantly affects the wear–corrosion synergy [48]. Coatings with more robust and stable passive layers not only minimize corrosion, but also reduce the coefficient of friction and wear volume. Therefore, coatings produced under a higher oxygen content exhibit superior triboelectrochemical performance, both in terms of mechanical durability and corrosion resistance, compared to those deposited at lower oxygen levels.
Additionally, the incorporation of silver into the ZrO2 matrix appears to contribute to enhanced corrosion resistance through a sacrificial anodic mechanism. In chloride-rich environments, such as Ringer’s lactate solution, silver undergoes preferential oxidation, offering cathodic protection to the underlying stainless steel substrate and supporting the stability of the passive layer on the coating surface. This anodic contribution explains the lower corrosion current densities and higher charge transfer resistance observed in samples with an increased silver content and oxygen ratio. Although silver may also act as a solid lubricant, its electrochemical function as a sacrificial component is considered the primary mechanism contributing to the improved tribocorrosive performance.
The inhibition efficiency (IE%) was also calculated based on the corrosion current density, using the uncoated sample (316LVM) as the reference. The equation used was as follows:
IE% = [1 − (Icorr_coated/Icorr_uncoated)] × 100
The results, presented in Table 2, demonstrate a significant improvement in corrosion protection for all coated samples. The trend indicates that increasing the oxygen content in the deposition atmosphere enhances the inhibition efficiency, demonstrating both the effectiveness of silver doping and the beneficial influence of oxygen on the coating performance.
These results are consistent with the observed corrosion rate and corrosion potential values, confirming that a higher oxygen content favors the formation of more stable, dense, and protective coatings.
Therefore, the results suggest that optimizing the Ar/O2 gas ratio in the deposition atmosphere is critical for enhancing the corrosion and wear resistance of ZrO2-Ag coatings, with the added benefit of protecting the substrate from triboelectrochemical degradation.
The Ag-doped ZrO2 coatings deposited under varying Ar/O2 gas ratios demonstrated clear passivation behavior, characterized by low corrosion current densities. Notably, coatings deposited at a higher Ar/O2 ratio of 50% (Table 2) exhibited the lowest current densities, indicating the formation of a smoother and more compact surface that restricts ionic penetration and minimizes corrosion through oxidation reactions. In contrast, coatings with increased surface roughness, often resulting from a lower oxygen content, can facilitate crack initiation and propagation, thereby increasing susceptibility to mechanical failure and localized corrosion. The coatings produced at higher Ar/O2 ratios also displayed more positive corrosion potentials and lower corrosion current densities, further supporting their enhanced protective capacity.
Moreover, the incorporation of silver into the coating matrix appears to influence the electrochemical behavior. A general trend of decreasing current density with increasing silver content was observed, potentially due to redox reactions involving metallic silver at the coating surface. The Tafel curves for Ag-containing coatings showed fluctuations in the current density values, indicating the presence of multiple concurrent electrochemical processes. These likely involve chloride or lactate anions interacting with both the exposed metallic substrate and silver particles at the surface.
A comprehensive assessment of triboelectrochemical behavior, evaluating the simultaneous action of mechanical wear and electrochemical corrosion, provides deeper insight into the coating performance under realistic service conditions. During tribocorrosion tests conducted in Ringer’s lactate solution, ZrO2-Ag coatings outperformed uncoated 316LVM stainless steel substrates. This superior behavior is attributed to the synergistic properties of the ZrO2, known for its excellent corrosion resistance and hardness, and silver, which acts as both a solid lubricant and a sacrificial anode. Electrochemical measurements using a three-electrode system confirmed this enhancement, with coated samples exhibiting significantly lower corrosion current densities and higher polarization resistances under sliding wear conditions.
The results of this study highlight a significant synergistic effect between the Ar/O2 deposition ratio and the resulting triboelectrochemical performance of ZrO2-Ag coatings. Coatings fabricated at a high Ar/O2 50% ratio demonstrated the most favorable performance, with a remarkably low coefficient of friction (0.0043), minimal wear volume (0.086 mm3), and a corrosion current density of 3.32 × 10−8 A/cm2. These improvements are attributed to a highly consolidated ZrO2 phase structure, which enhances mechanical integrity, along with a homogeneous distribution of silver that contributes both lubricating and antioxidative functionalities. Conversely, coatings produced with lower Ar/O2 12.5% ratios showed inferior performance, dominated by adhesive wear mechanisms, higher friction coefficients, and greater corrosion susceptibility due to their inhomogeneous microstructure.
The observed interplay between mechanical wear and electrochemical stability highlights the critical importance of controlling deposition parameters to engineer coatings with tailored multifunctional properties. This is particularly relevant for demanding applications such as biomedical implants and marine components, where materials are routinely exposed to simultaneous mechanical and corrosive stressors. Future studies should explore the long-term performance of ZrO2-Ag coatings under cyclic tribocorrosion conditions to further validate their durability and clinical viability.
When compared to other protective coatings such as TiCN, TiSiCN, TiCrSiCN, TiAlSiCN, and ReN, the ZrO2-Ag system reveals superior tribocorrosive performance. Conventional TiCN and TiSiCN coatings, although widely employed, are prone to accelerated wear and brittle fracture under dynamic loading due to structural defects and limited plasticity [49]. Their Cr- and Al-doped counterparts (TiCrSiCN and TiAlSiCN) offer moderate improvements in mechanical performance, but remain susceptible to microcracks and delamination caused by microstructural inhomogeneities. In contrast, the ReN coating have demonstrated outstanding corrosion resistance, particularly as the N2 partial pressure during deposition increases. This behavior is associated with denser film formation and more efficient nitrogen incorporation, resulting in corrosion current density (Icorr) reductions of up to 98.94% [50]. However, while ReN coatings perform well electrochemically, their tribological properties under combined mechanical and corrosive conditions require further validation.
The proposed ZrO2-Ag coating, on the other hand, offers an ideal combination of high corrosion resistance and mechanical durability. Its wear mechanism is governed primarily by controlled plastic deformation, which significantly reduces volume loss. Additionally, the in situ formation of a passive ZrO2 layer, reinforced by the uniformly distributed silver phase, provides sustained protection even in chloride-rich environments. This dual functionality makes the ZrO2-Ag coating a promising and robust alternative to conventional protective systems for use in biomedical and other aggressive application settings.
The influence of the Ar/O2 gas mixture ratio on the performance of Ag-ZrO2 coatings is a key parameter that directly impacts the microstructural, tribological, and electrochemical characteristics of the deposited films. Although our results show a consistent improvement in wear resistance and inhibition efficiency with increasing oxygen content, the interpretation of these effects requires further context. In this regard, the observed enhancement in corrosion protection for coatings deposited with higher oxygen concentrations (e.g., 50% O2) can be attributed to the formation of a more homogeneous and fully oxidized zirconia matrix, as also reported by Zhang et al. (2007) [51], who found that high oxygen partial pressures promoted the stabilization of the tetragonal phase, leading to denser and more protective oxide films.
Additionally, previous studies on Ag-doped ZrO2 coatings deposited via reactive sputtering (e.g., Ou et al., 2015) [52] support the notion that increased oxygen flow during deposition favors the better dispersion of silver nanoparticles and suppresses porosity, thereby enhancing the electrochemical barrier properties of the coating. Our results are in agreement with these findings, as evidenced by the increasing charge transfer resistance and decreasing corrosion current densities with the higher O2 content. Furthermore, the improved crystallinity of Ag, confirmed via XRD patterns showing sharper (111) and (311) peaks at higher oxygen ratios, aligns with the conclusions of Biju et al. (2025) [53], who demonstrated that oxygen-rich environments stabilize metallic Ag domains within oxide matrices.
However, it is also important to note that excessively high oxygen flow rates can, in some cases, lead to over-oxidation or poor adhesion due to reduced energy transfer during sputtering. In our study, no such detrimental effects were observed up to 50% O2, suggesting that the selected range of gas mixtures remained within an optimal processing window. This finding underscores the importance of the precise control of the oxygen content during deposition to balance phase formation, coating integrity, and functional performance.
4. Conclusions
The Ar/O2 ratio during deposition significantly influences the microstructure, topography, and tribological performance of Ag-ZrO2 coatings. A higher oxygen content promotes the better crystallization of zirconium oxide, leading to reduced surface roughness and enhanced wear resistance. These characteristics suggest potential applications in systems requiring high durability and low friction coefficients.
X-ray diffraction analysis confirmed the presence of monoclinic and tetragonal phases of ZrO2, as well as crystallized metallic silver. The tetragonal phase was more prevalent at higher Ar/O2 ratios, indicating that the deposition process modulates the crystalline structure of the coating.
Surface roughness measurements indicated that coatings deposited with a higher Ar/O2 ratio exhibited lower Ra values, resulting in a more compact and homogeneous morphology. This suggests that the nucleation and grain growth processes are strongly affected by the deposition atmosphere.
Tribological tests demonstrated that coatings deposited with a higher Ar/O2 ratio exhibited lower friction coefficients and reduced volume loss, indicating improved wear resistance. The best tribological performance was observed in coatings with 50% Ar/O2, which showed the lowest volume loss and friction coefficient.
Electrochemical tests revealed that coatings deposited with higher Ar/O2 ratios exhibited lower corrosion current densities and higher polarization resistance. This improvement is attributed to the formation of a stable and dense passive ZrO2 layer, which enhances the coating’s resistance to corrosion.
The optimized Ag-ZrO2 coatings developed in this study demonstrate potential for biomedical applications, particularly in implants where enhanced durability and corrosion resistance are critical.
Future research should focus on long-term in vitro and in vivo studies to evaluate the biocompatibility and stability of these coatings in physiological environments.
Author Contributions
Conceptualization and methodology, G.O.-H. and W.A.; validation, W.A.; formal analysis, G.O.-H.; investigation, W.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The initial characterization data are reported in the following repository: IOP Publishing dataset. https://iopscience.iop.org/article/10.1088/1742-6596/687/1/012031.
Acknowledgments
W. Aperador acknowledges the support of Universidad Militar Nueva Granada. G. Orozco acknowledges the support of Universidad ECCI.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maver, U.; Xhanari, K.; Žižek, M.; Gradišnik, L.; Repnik, K.; Potočnik, U.; Finšgar, M. Carboxymethyl cellulose/diclofenac bioactive coatings on AISI 316LVM for controlled drug delivery, and improved osteogenic potential. Carbohydr. Polym. 2020, 230, 115612. [Google Scholar] [CrossRef] [PubMed]
- Pachla, W.; Skiba, J.; Kulczyk, M.; Przybysz, S.; Przybysz, M.; Wróblewska, M.; Diduszko, R.; Stępniak, R.; Bajorek, J.; Radomski, M.; et al. Nanostructurization of 316L type austenitic stainless steels by hydrostatic extrusion. Mater. Sci. Eng. A 2014, 615, 116–127. [Google Scholar] [CrossRef]
- Carreon, H.; Barriuso, S.; Barrera, G.; González-Carrasco, J.L.; Caballero, F.G. Assessment of blasting induced effects on medical 316 LVM stainless steel by contacting and non-contacting thermoelectric power techniques. Surf. Coat. Technol. 2012, 206, 2941–2946. [Google Scholar] [CrossRef]
- López, R.; Menéndez, M.; Fernández, C.; Chmiela, A.; Bernardo-Sánchez, A. The Influence of Carbon Coatings on the Functional Properties of X39Cr13 and 316LVM Steels Intended for Biomedical Applications. Metals 2019, 9, 815. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Hamedi, H.; Isfahani, T. Wear and corrosion properties of mechanically coated 316 stainless Steel-TiC nanocomposites. Results Eng. 2024, 24, 102966. [Google Scholar] [CrossRef]
- Freitas Filho, A.; Silva, G.C.; Rodrigues, S.C.S.; Santos, A.J. Evaluation of the effect of surface modification of Ti64 and 316 L by addition of calcium phosphate through electrical discharge machining process. Tribol. Int. 2023, 180, 108245. [Google Scholar] [CrossRef]
- Thambapillary, S.; Dimitriou, R.; Makridis, K.G.; Fragkakis, E.M.; Bobak, P.; Giannoudis, P.V. Implant longevity, complications and functional outcome following proximal femoral arthroplasty for musculoskeletal tumors: A systematic review. J. Arthroplast. 2013, 28, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Nasiri-Tabrizi, B.; Basirun, W.J.; Walvekar, R.; Yeong, C.H.; Phang, S.W. Exploring the potential of intermetallic alloys as implantable biomaterials: A comprehensive review. Biomater. Adv. 2024, 161, 213854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef] [PubMed]
- Elsa, G.; Hanan, A.; Walvekar, R.; Numan, A.; Khalid, M. Zirconium–Based MXenes: Synthesis, Properties, Applications, and Prospects. Coord. Chem. Rev. 2025, 526, 216355. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Foyez, T.; Krishna, S.B.N.; Poda, S.; Imran, A.B. Recent Advances of Silver Nanoparticle-Based Polymer Nanocomposites for Biomedical Applications. RSC Adv. 2025, 15, 8480–8505. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, C.; Shen, Z.; Zhou, L.; Sheng, L.; Xu, D.; Zheng, Y.; Chu, P.K.; Xiao, S.; Ying, T.; et al. Simultaneous Improvement of Wear and Corrosion Resistance of Microarc Oxidation Coatings on ZK61 Mg Alloy by Doping with ZrO2 Nanoparticles. J. Mater. Sci. Technol. 2025, 224, 312–327. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Yu, J.-G.; Ren, Q.-Y.; Zheng, M.-Y.; Cai, Z.-B.; Jiao, Y.-J. Study on the Fretting Corrosion Behavior of Zirconium Alloy in Simulated Primary Coolant Condition. Wear 2025, 570, 206057. [Google Scholar] [CrossRef]
- Niu, D.; Zhang, C.; Sui, X.; Lu, X.; Zhang, X.; Wang, C.; Hao, J.; Shi, Z. Microstructure, mechanical properties and tribo-corrosion mechanism of (CrNbTiAlVMo) coated 316L stainless steel in 3.5 wt% NaCl solution. Tribol. Int. 2022, 173, 107638. [Google Scholar] [CrossRef]
- Davoodi, F.; Atapour, M.; Ashrafizadeh, F.; Rikhtehgaran, R. Dry sliding wear characteristics of NiP/TiN duplex coated aluminium alloy and wear analysis using response surface method. J. Mater. Eng. Perform. 2022, 31, 6360–6372. [Google Scholar] [CrossRef]
- Fook, P.; Berger, D.; Riemer, O.; Karpuschewski, B. Structuring of Bioceramics by Micro-Grinding for Dental Implant Applications. Micromachines 2019, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, S.; Li, J.; Tang, W.; Yu, M.; Ahmed, M.H.; Liang, S.; Zhang, F.; Inokoshi, M.; Yao, C.; et al. Influence of surface treatments on highly translucent zirconia: Mechanical, optical properties and bonding performance. J. Dent. 2025, 154, 105580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Van Meerbeek, B.; Vleugels, J. Importance of tetragonal phase in high-translucent partially stabilized zirconia for dental restorations. Dent. Mater. 2020, 36, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, J.; Zhang, W.; You, L.; Li, J. Ag nanoparticles interlayered Fe3O4/Ag/m(TiO2-ZrO2) magnetic photocatalysts with enhanced stability and photocatalytic performance for Cr(VI) reduction. Appl. Surf. Sci. 2023, 607, 155076. [Google Scholar] [CrossRef]
- Shao, Y.; Jiang, Y.; Wang, Y.; Dong, Q.; Wang, C.; Wang, Y.; Feng, X.; Chu, C.; Bai, J. Electrodepositing Ag on anodized stainless steel for enhanced antibacterial properties and corrosion resistance. J. Funct. Biomater. 2025, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Aissi, M.; Tayyaba, Q.; Er-Ramly, A.; Hermawan, H.; Merzouk, N. Improving the clinical performance of dental implants through advanced surface treatments: The case of Ti and ZrO2 coatings. Metals 2025, 15, 320. [Google Scholar] [CrossRef]
- Bai, L.; Yi, G.; Wan, S.; Wang, W.; Sun, H. Comparison of tribological performances of plasma sprayed YSZ, YSZ/Ag, YSZ/MoO3 and YSZ/Ag/MoO3 coatings from 25 to 800 °C. Wear 2023, 526–527, 204944. [Google Scholar] [CrossRef]
- González-Hernández, A.; Aperador, W.; Flores, M.; Onofre-Bustamante, E.; Bermea, J.E.; Bautista-García, R.; Gamboa-Soto, F. Influence of Deposition Parameters on Structural and Electrochemical Properties of Ti/Ti2N Films Deposited by RF-Magnetron Sputtering. Metals 2022, 12, 1237. [Google Scholar] [CrossRef]
- Guzmán, P.; Yate, L.; Sandoval, M.; Caballero, J.; Aperador, W. Characterization of the Micro-Abrasive Wear in Coatings of TaC-HfC/Au for Biomedical Implants. Materials 2017, 10, 842. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Liu, B.; Wang, Y.; Yang, Z.; Wang, Y. Interfacial microstructure and mechanical properties of joints between ZrO2 ceramic and 316 stainless steel brazed using Ag–Cu–In–Ti filler. Vacuum 2023, 218, 112668. [Google Scholar] [CrossRef]
- Irshad, M.; Ibrahim, M.M.; Siddique, S.; Younas, U.; Mersal, G.A.M.; Al-Juaid, S.S.; Irshad, A.; Warsi, M.F. Boosting the properties of Ag-decorated Ni3V2O8 via 1D-CNTs integration for advanced photocatalytic and anti-bacterial performance. Ceram. Int. 2025, 51, 19704–19714. [Google Scholar] [CrossRef]
- Rosalbino, F.; Macciò, D.; Scavino, G. Corrosion behaviour of Zr–Ag alloys for dental implant application. Mater. Sci. Appl. 2023, 14, 501–514. [Google Scholar] [CrossRef]
- Lee, D.W.; Seo, D.-S. Surface Functionalization of Ag-Doped Zirconium Oxide Layers for Molecular Alignment. FlatChem 2025, 50, 100831. [Google Scholar] [CrossRef]
- Nossova, L.; Caravaggio, G. Effect of Dopants on Soot Oxidation over Doped Ag/ZrO2 Catalysts for Catalyzed Gasoline Particulate Filter. Catal. Commun. 2023, 182, 106744. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, N.; Chen, B.; Ma, J.; Song, T.; Wu, X.; Yang, T.; Zhang, X.; Li, Q. Tailoring Microstructure, Mechanical Properties, and Biocompatibility of Zr Alloys via the Addition of Ag. Mater. Sci. Eng. A 2024, 900, 146486. [Google Scholar] [CrossRef]
- Nasir, N.; Rashid, M.H.; Cheema, S.A.; Rasheed, A.; Sabir, N.; Tanveer, Z.; Hassan, T.; Anjam, Q. An Experimental and Simulation Evaluation of the Structural, Morphological and Optical Characters of ZnO-Based Nano-Fibers Doped with Ag and ZrO2. Optik 2022, 265, 169383. [Google Scholar] [CrossRef]
- Kumar, P.; Saravanan, P.; Baskar, G.; Chitrashalini, S.; Omer, S.N.; Subashini, S.; Rajeshkannan, R.; Venkatkumar, S. Synthesis and Characterization of Ag-Decorated ZnO/MgO Nanocomposite Using a Novel Phyto-Assisted Biomimetic Approach for Anti-Microbial and Anti-Biofilm Applications. Inorg. Chem. Commun. 2024, 170, 113443. [Google Scholar] [CrossRef]
- Khan, A.; Zaid, M.; Ameen, F.; Khan, M.A.; Kumar, S.; Al-Masri, A.A.; Islam, M.A. Colossal Antibacterial, Antibiofilm and Solar Light-Driven Photocatalytic Activity of Nanoenhanced Conjugate of Bimetallic Ag-Zr Nanoparticles with Graphene Oxide. J. Mol. Struct. 2024, 1300, 137223. [Google Scholar] [CrossRef]
- Simon, S.M.; Prakashan, V.P.; Sajna, M.S.; Chandran, A.; George, G.; Barmiah, E.K.; Jose, G.; Biju, P.R.; Joseph, C.; Unnikrishnan, N.V. Development and Characterizations of Ag Nanoparticles Decorated TiO2-ZrO2 Coatings as Electrode Material for Supercapacitors. Results Surf. Interfaces 2023, 10, 100098. [Google Scholar] [CrossRef]
- Chouhan, L.; Bouzerar, G.; Srivastava, S.K. d0 Ferromagnetism in Ag-Doped Monoclinic ZrO2 Compounds. Vacuum 2020, 182, 109716. [Google Scholar] [CrossRef]
- Saleh, M.; Isik, Z.; Belibagli, P.; Arslan, H.; Gonca, S.; Özdemir, S.; Kudaibergenov, N.; Khataee, A.; Dizge, N. Fabrication of Ag Nanoparticles Coated Leonardite Basalt Ceramic Membrane with Improved Antimicrobial Properties for DNA Cleavage, E. coli Removal and Antibiofilm Effects. J. Ind. Eng. Chem. 2023, 128, 532–541. [Google Scholar] [CrossRef]
- Gambardella, A.; Berni, M.; Graziani, G.; Kovtun, A.; Liscio, A.; Russo, A.; Visani, A.; Bianchi, M. Nanostructured Ag Thin Films Deposited by Pulsed Electron Ablation. Appl. Surf. Sci. 2019, 475, 917–925. [Google Scholar] [CrossRef]
- Shang, X.; Liang, Y.; Wang, P.; Wu, Y. Synergistic Mechanism of the Fretting Wear Resistance of Fe2O3/Ag Nanostructured Coatings Prepared by Sliding Friction and Magnetron Sputtering. Wear 2024, 546–547, 205313. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Hu, X.; Zhang, L.; Shi, X.; Li, Z. Simulation and Experimental Study on Frictional Wear of Plough Blades in Soil Cultivation Process Based on the Archard Model. Biosyst. Eng. 2024, 248, 190–205. [Google Scholar] [CrossRef]
- Elahi Haghighi, N.; Hadianfard, M.J. Fabrication of Ni–ZrO2 Nanocomposites through a New Electroforming Bath and Assessment of Their Morphology, Wear, and Corrosion Resistance. Heliyon 2024, 10, e35779. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xie, F.; Wu, X.; Li, L.; Luo, R.; Yang, H.; Wang, S. Preparation and Friction Wear Performance of ZrO2/MoS2 PEO Composite Coating. Tribol. Int. 2025, 202, 110312. [Google Scholar] [CrossRef]
- Pogodin, A.; Filep, M.; Malakhovska, T.; Vakulchak, V.; Komanicky, V.; Vorobiov, S.; Izai, V.; Shender, I.; Bilanych, V.; Kokhan, O.; et al. Recrystallization Effect on Mechanical Parameters and Increasing of Ag⁺ Ionic Conductivity in Ag7(Si1−xGex)S5I Ceramic Materials. Solid State Sci. 2023, 140, 107203. [Google Scholar] [CrossRef]
- Mourya, A.K.; Gaikwad, G.S.; Singh, R.P.; Khagar, P.S.; Uke, S.J.; Wankhade, A.V. ZnO/Ag2ZrO3 Nanocomposites: A Tailored Nanostructure for Enhanced Supercapacitor, Photocatalytic & Antimicrobial Applications. J. Alloys Compd. 2024, 1004, 175792. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Q.; Xia, S.; Yang, X.; Lei, J.; Sun, Q.; Chen, X.; Shao, J.; Tang, X.; Zhou, G. A Cu-Ag Double-Layer Coating Strategy for Stable and Reversible Zn Metal Anodes. J. Colloid Interface Sci. 2024, 665, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Moreno Amado, M.; Alfonso, J.E.; Olaya Florez, J.J. Effect of Al and Ag Dopants on the Corrosion Resistance of the AISI 316L-YSZ System. Ceram. Int. 2019, 45, 566–572. [Google Scholar] [CrossRef]
- Moayedee, Y.; Nikzad, L.; Fakhraei, O.; Paykar, Z.; Zekavat, E. Improvement of Mechanical, Biological, and Electrochemical Properties of Ti6Al4V Alloy Modified with Nb and Ag for Biomedical Applications. J. Alloys Compd. 2024, 972, 172736. [Google Scholar] [CrossRef]
- Mina, A.; Caicedo, J.C.; Aperador, W. Sequential Analysis of Electrochemical Properties of Β–Tricalcium Phosphate/Chitosan Coatings Obtained on 316L Stainless Steel. Int. J. Electrochem. Sci. 2013, 8, 11186–11200. [Google Scholar] [CrossRef]
- Kuptsov, K.A.; Kiryukhantsev-Korneev, P.V.; Sheveyko, A.N.; Shtansky, D.V. Comparative Study of Electrochemical and Impact Wear Behavior of TiCN, TiSiCN, TiCrSiCN, and TiAlSiCN Coatings. Surf. Coat. Technol. 2013, 216, 273–281. [Google Scholar] [CrossRef]
- Aperador, W.; Orozco-Hernández, G.; Aperador, J.; Bautista-Ruiz, J. Microstructural, Electrochemical, Mechanical, and Biocompatibility Characterization of ReN Thin Films Synthesized by DC Sputtering on Ti6Al4V Substrates. Metals 2025, 15, 272. [Google Scholar] [CrossRef]
- Zhang, J.P.; He, G.; Zhu, L.Q.; Liu, M.; Pan, S.S.; Zhang, L.D. Effect of oxygen partial pressure on the structural and optical properties of ZnO film deposited by reactive sputtering. Appl. Surf. Sci. 2007, 253, 9414–9421. [Google Scholar] [CrossRef]
- Ou, S.-F.; Chung, R.-J.; Lin, L.-H.; Chiang, Y.-C.; Huang, C.-F.; Ou, K.-L. A mechanistic study on the antibacterial behavior of silver doped bioceramic. J. Alloys Compd. 2015, 629, 362–367. [Google Scholar] [CrossRef]
- Biju, R.F.; Jaffrin, G.; Jobisha, J.; Matharasi, A.; Prabha, S.; Vinisha, V.; Linet, M.; Mani, A.M. Structural, spectroscopic, thermal and morphological evaluation of biogenic ZnO/Ag nanocomposite using Moringa oleifera seed extract for enhanced antimicrobial efficacy. Chem. Phys. Impact 2025, 10, 100850. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).