Abstract
By introducing oxygen doping, the structure of an AlCrNbSiTiN coating was optimized, and its high-temperature oxidation resistance was improved. As the oxygen content incorporated increases, the coating changes from an FCC structure to an amorphous or spinel structure. Meanwhile, stress relaxation occurred, and the hardness of the coating dropped to 12 gpa. Oxygen-doped coatings exhibit excellent oxidation resistance; this is especially the case for oxidized coatings, whose structure remains stable up to 900 °C in an oxidizing environment.
1. Introduction
The demand for in situ structural health monitoring in extreme environments (>500 °C)—including power generation, aerospace, and oil/gas industries—has intensified the need for reliable high-temperature piezoelectric devices [1,2,3]. While ultrasonic techniques offer non-destructive testing advantages, conventional piezoelectric transducers face irreversible performance degradation due to oxidation, stress-induced delamination, and coupling agent failure beyond 400 °C [4]. Although materials such as ZnO and LiNbO3 exhibit piezoelectricity, their limited thermal stability (ZnO < 430 °C) and oxygen-deficiency issues (LiNbO3) restrict high-temperature operation [5,6]. Aluminum nitride (AlN) emerges as a promising candidate with its high Curie temperature (>1150 °C) and chemical inertness [7], yet uncontrolled interfacial oxidation and mechanical stress accumulation during thermal cycling remain unresolved [8].
To address this challenge, protective coatings for piezoelectric films must simultaneously achieve thermal stability, oxidation resistance, and mechanical durability. Conventional nitride coatings (e.g., AlCrN) offer limited protection above 900 °C due to accelerated oxidative spallation and phase transformation [9,10,11]. High-entropy oxides (HEOs), leveraging multi-principal component designs, have emerged as a frontier material class with superior high-temperature tolerance through entropy-stabilized structures [12,13]. Kirnbauer et al. [14] synthesized (AlCrNbTaTi)O2 oxide coatings by reactive magnetron sputtering. When the O2 flow rate increases from 30% to 80%, the hardness increases from 22 GPa to 24 GPa. Chen et al. [15] prepared AlxCoCrCuFeNi (x = 0.5, 1, 2) oxide coatings by reactive magnetron sputtering. The coatings have a cubic spinel crystal structure with a maximum hardness of 22.6 ± 1.6 GPa. When annealed at 500 °C, the hardness of the coatings increased, with the maximum hardness reaching 25.7 ± 2.3 GPa [16]. However, existing HEO coatings face challenges in balancing oxygen diffusion resistance with piezoelectric compatibility—excessive oxygen content degrades mechanical properties, while insufficient doping compromises oxidation barrier efficacy.
In this study, we propose a novel AlCrNbSiTiN oxygen-doped HEO protective coating for piezoelectric thin films. By modulating oxygen flow during arc ion plating, we prepared HEO coatings doped with different oxygen contents. The coating’s role in blocking oxygen diffusion is quantitatively investigated. The HEO protective coatings extend the service life of piezoelectric transducers to over 900 °C in an oxidizing environment, enabling reliable ultrasonic monitoring in applications such as high-temperature bolt preload detection.
2. Experimental Details
2.1. Coating Preparation
All the substrates were cleaned in acetone and alcohol for 15 min using an ultrasonic cleaning instrument. The vacuum chamber was evacuated to 6 × 10−4 Pa and heated to 300 °C to perform ion etching cleaning on the substrates. After the etching was completed, Cr and CrN transition layers were deposited. Subsequently, the AlCrNbSiTiN oxygen-doped coatings were deposited. The specific deposition parameters are shown in Table 1.

Table 1.
Deposition parameters of AlCrNbSiTiN oxygen-doped coatings.
2.2. Coating Characterization
The surface and cross-sectional morphology were tested by using scanning electron microscopy (SEM, TESCANMIRA3,TESCANBrno, Ltd., Oxford, UK) and atomic force microscopy (AFM, Shimadzu, SPM-9700HT, Kyoto, Japan). The composition was determined using an energy dispersive spectrometer (EDS, Oxford Instruments X-MAXN, Oxford, UK). The structure of the coatings was characterized by X-ray diffraction (XRD, PANalytical, XPert Pro, Xi’an, China). The hardness and elastic modulus were measured using a nano-indentation instrument (NANO, KLA, G200, Milpitas, CA, USA). The oxidation experiment was conducted in a muffle furnace with an oxidation temperature of 600–900 °C. The heating rate was set at 3 °C/min, and the holding time was 2 h. After the oxidation was completed, the sample was taken out after cooling to room temperature in the furnace.
3. Results and Discussion
3.1. Morphology and Chemical Composition Analysis
The surface and cross-sectional morphologies, along with the surface roughness, of the coatings are shown in Figure 1. A small number of large particles were present on the surface of the nitride coating, with a surface roughness of 11.5 nm. After the introduction of oxygen at 30 sccm and 60 sccm, the surface morphology showed no significant change, but the surface roughness decreased markedly, reaching 1.36 nm for the O-60 coating. When the oxygen flow rate reached 100 sccm, the number of large particles on the coating surface increased, their size became larger, and obvious pore defects appeared, resulting in an increased roughness of 3.45 nm. The primary reason for this phenomenon is that at higher oxygen flow rates, the target surface reacts with oxygen to form oxides, leading to target poisoning. Consequently, the active area on the target surface decreases, the arc current density increases, exacerbating the ejection of liquid droplets from the target [17,18]. All coating cross-sections exhibited a dense and uniform morphology, with no detectable significant defects such as pores or cracks.
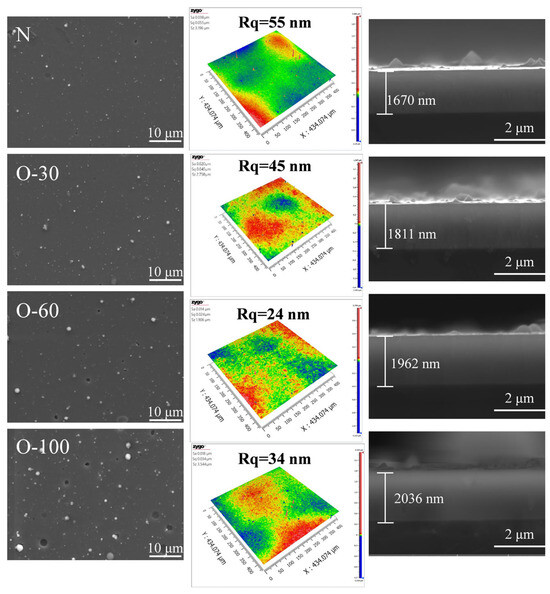
Figure 1.
SEM and AFM morphologies of AlCrNbSiTiN coatings with different oxygen flow rates.
The variation in thickness and deposition rate of the coatings with oxygen flow rate is shown in Figure 2a. It can be observed that the thickness increases with increasing oxygen flow rate. As the oxygen flow rate increased from 0 to 100 sccm, the thickness and deposition rate increased from 1670 nm and 28 nm/min to 2036 nm and 34 nm/min, respectively. Figure 2b shows the changes in elemental content of the AlCrNbSiTiN coatings under different oxygen flow rates. It can be seen that as the oxygen flow rate increases, the nitrogen content in the coatings continuously decreases, while the oxygen content continuously increases. In the O-30 coatings, the N and O contents were 32.3 at.% and 22.2 at.%, respectively. In the O-60 coating, the N content decreased to 7.4 at.%, and the O content increased to 52.9 at.%. No N element was detected in the O-100 coating, and the O content increased to 61.7 at.%, indicating that under these conditions, the coatings had completely transformed into oxide coatings.
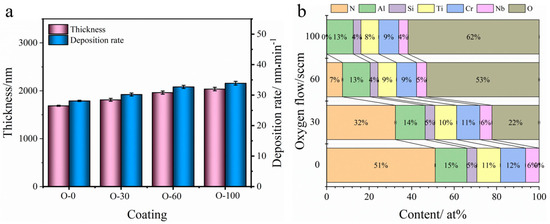
Figure 2.
(a) Thickness and deposition rate and (b) elemental content changes of AlCrNbSiTiN coatings with different oxygen flow rates.
3.2. Microstructure
The XRD patterns of the coatings are shown in Figure 3. The JCPDS reference numbers for XRD results are 65-2899 and 88-2324. For the nitride coating, only the FCC (200) diffraction peak was detected, indicating a preferred orientation of (200), suggesting that coating growth is primarily controlled by surface energy. The reason is that during preparation, substrate rotation interrupts the continuous bombardment by high-energy ions, thereby reducing the coating’s strain energy and stopping energy, making surface energy the dominant factor controlling coating growth. In the O-30 coating, the intensity of the FCC (200) diffraction peak significantly increased. Similar results were observed by Ahmad et al. [19] in their study on oxygen incorporation into AlCrN coatings. This is related to the reduction in surface energy. Oxygen atoms have higher electronegativity than nitrogen atoms. Therefore, oxygen incorporation enhances polarization, reduces surface energy, and strengthens the preferred orientation of (200) [17]. Additionally, the change in peak shape for O-30 is also related to lattice defects caused by the substitution of nitrogen ions by oxygen ions in the charge balance mechanism [20,21]. No diffraction peaks other than those from the substrate were detected in the O-60 coating, indicating a completely amorphous structure. With a further increase in the oxygen flow rate, diffraction peaks corresponding to α-AlCrNbSiTiO were detected in the coating. Grain sizes were calculated using the Scherrer formula. The grain sizes for the N, O-30, and O-100 coatings were 19.2 nm, 15.6 nm, and 14 nm, respectively. With the increase in oxygen content, oxygen doping leads to severe lattice distortion, and the angle of the diffraction peaks shifts to the right. Therefore, the interplanar spacing decreases. The crystal plane index of the diffraction peaks remains unchanged. Therefore, the lattice parameters are directly proportional to the crystal plane spacing, and the lattice parameters decrease.
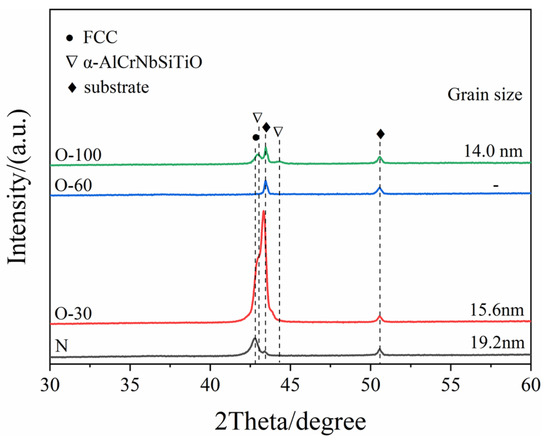
Figure 3.
XRD patterns of the coatings as a function of oxygen flow rate.
3.3. Mechanical Properties
The changes in residual stress, hardness, elastic modulus, and the ratios of H/E and H3/E2 with oxygen flow rate are shown in Figure 4. All coatings exhibited residual compressive stress. The AlCrNbSiTiN coatings had a residual stress of −181 MPa, with hardness and elastic modulus of 34.1 GPa and 436 GPa, respectively. The H/E and H3/E2 ratios were 0.08 and 0.2 GPa, respectively. For coatings after oxygen incorporation, residual stress, hardness, and elastic modulus showed a decreasing trend with increasing oxygen flow rate. When the oxygen flow rate reached 100 sccm, the coating hardness decreased to 12 GPa. Oxygen incorporation can effectively reduce the internal stress of nitride coatings. This stress relaxation can be understood as the formation of a disordered amorphous structure with increasing oxygen incorporation [22,23]. The decrease in hardness occurs because the electronic structure and physical properties of the nitride lattice undergo drastic changes upon oxygen incorporation. To maintain electrical neutrality, three oxygen atoms replace two nitrogen atoms (), generating interstitial anions or forming cation vacancies to compensate for the reduced charge [24] (). The resulting lattice defects lead to a decrease in hardness [25]. Additionally, oxygen replacing nitrogen occupies anion sites, causing the bonding state in the coatings to gradually shift from covalent towards ionic bonding, which also contributes to the decrease in hardness and elastic modulus [21,26]. The changes in the H/E and H3/E2 ratios of the coatings are shown in Figure 4b. Both ratios decreased with increasing oxygen flow rate, indicating that oxygen incorporation reduced toughness.
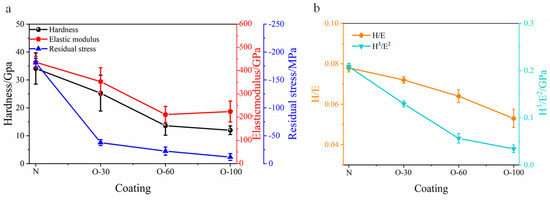
Figure 4.
(a) Residual stress, hardness, and elastic modulus and (b) changes in H/E and H3/E2 ratios for the coatings.
3.4. High-Temperature Oxidation Behavior
Figure 5 shows the surface and cross-sectional morphologies of the coatings after oxidation at 800–1000 °C. For the AlCrNbSiTiN coating, no significant changes were observed on the surface after oxidation at 800–900 °C. After oxidation at 1000 °C, fine oxide particles sized between 100 and 200 nm were observed in high-magnification SEM images of the coating surface. A dense oxide film was detected on the coating cross-section, with a thickness of 71 nm at 800 °C. As the oxidation temperature increased to 1000 °C, the oxide layer thickness increased to 212 nm, demonstrating excellent oxidation resistance. For the oxygen-incorporated coatings, the cross-section of the O-30 coating showed no significant change throughout the oxidation temperature range. Many “blisters” appeared on the surface of the O-60 coating after oxidation at 1000 °C. This is due to poor adhesion between coatings and substrates; the coating partially delaminated from the substrate under thermal stress, forming locally uplifted features. Blisters also appeared on the O-100 coating after oxidation at 900 °C, and signs of coating spallation were found on the surface. No significant changes were observed on the coating cross-sections.
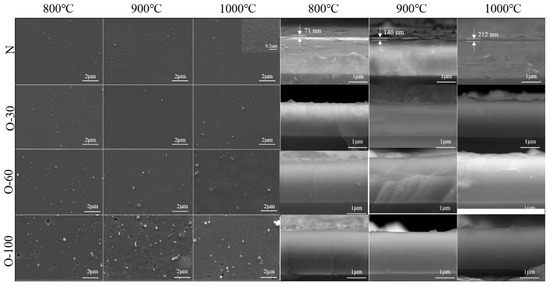
Figure 5.
Surface and cross-sectional morphology changes of the coatings after oxidation.
Changes in coating composition after oxidation are shown in Figure 6. For the nitride coating, as the oxidation temperature increased, the coating continuously reacted with oxygen to form an oxide layer, consuming N within the coating. At 1000 °C, the N content in the coating decreased to 11.5 at.%, while the O content increased to 47.5 at.%. The N content in the O-30 and O-100 coatings dropped to zero at 900 °C and 800 °C, respectively. The oxygen content in the O-30 coating continued to increase with temperature, reaching 52.2 at.%. In the O-60 coating, the oxygen content increased to 63 at.% at 900 °C and remained essentially unchanged upon further temperature increase, indicating oxygen saturation in the coatings. The oxygen content in the O-100 coating remained largely constant with increasing temperature, suggesting an oxygen saturation concentration in the coating of 63 at.%.
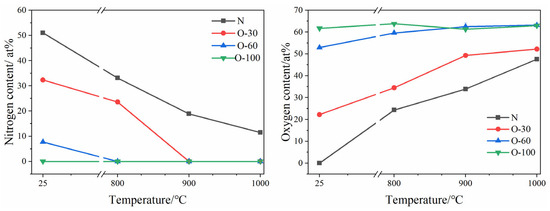
Figure 6.
Changes in N and O content of N, O-30, O-60, and O-100 coatings before and after oxidation.
To further investigate the elemental diffusion behavior during oxidation of the coatings, the AlCrNbSiTiN coating, O-30 coating, and O-100 coating were selected for EDS line scan analysis on their cross-sections after oxidation at 1000 °C. The AlCrNbSiTiN coating sample used a silicon substrate for convenient cross-section preparation. For the O-30 and O-100 coatings, due to poor adhesion to the silicon substrate, coating delamination occurred easily during sample preparation. Therefore, Focused Ion Beam (FIB) technology was used to prepare cross-sections on stainless steel substrates. As shown in Figure 7, for the AlCrNbSiTiN coating, significant enrichment of Al and O elements was observed in the oxide layer, indicating that the coating’s oxide layer is primarily composed of Al2O3.
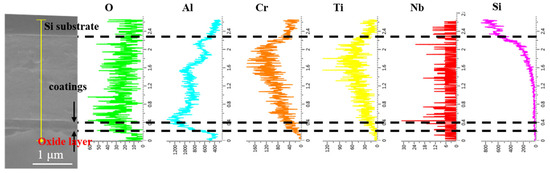
Figure 7.
EDS line scan results of the AlCrNbSiTiN coating cross-section after oxidation.
As shown in Figure 8, for the O-30 coating, a delaminated layer approximately 400 nm thick can be seen at the top of the FIB-prepared cross-section. According to the EDS line scan results, this layer shows enrichment of O and Al, indicating that the top layer is mainly Al2O3. For the O-100 coating, the morphology of the FIB-prepared cross-section and the EDS line scan results show no enrichment phenomenon for any element. The reason is that the oxygen concentration of the O-100 coating is already near saturation. The coating exhibits excellent stability, and elemental diffusion is difficult in a high-temperature oxidizing environment.
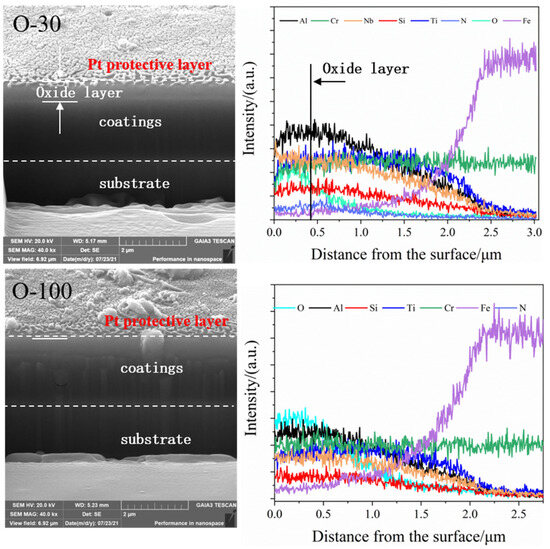
Figure 8.
Cross-sectional morphology and EDS line scan analysis results for N, O-30, and O-100 coatings after oxidation at 1000 °C.
To study the structural changes during coating oxidation, XRD tests were performed on the coatings after oxidation at different temperatures, with the results shown in Figure 9. For the AlCrNbSiTiN coating, XRD after oxidation at 800–900 °C only detected a decrease in the intensity of the (200) diffraction peak, with no new phases appearing. At 1000 °C, weak diffraction peaks of Al2O3 were detected in the coatings. The grain size calculated by the Scherrer formula changes at different oxidation temperatures. The grain size of the coatings remained stable up to 1000 °C without coarsening. The diffraction peak intensity of the O-30 coating gradually decreased with increasing oxidation temperature and shifted towards the positions of the AlCrNbSiTiO diffraction peaks. The O-60 coating remained amorphous after oxidation at 800–900 °C. When the oxidation temperature increased to 1000 °C, diffraction peaks of AlCrNbSiTiO appeared in the coating. For the O-100 coating, the phase structure showed no significant change after oxidation at 800–900 °C. After oxidation at 1000 °C, diffraction peaks of Al2O3 appeared in the coating. As the temperature rises, the diffraction peaks of the coating shift towards the position of the AlCrNbSiTiO diffraction peaks, and the angle of the diffraction peaks increases. Therefore, the interplanar spacing decreases, and the lattice parameters decrease. Similar to the nitride coating, the oxygen-incorporated coatings also maintained their nanostructure at high temperatures without significant coarsening.
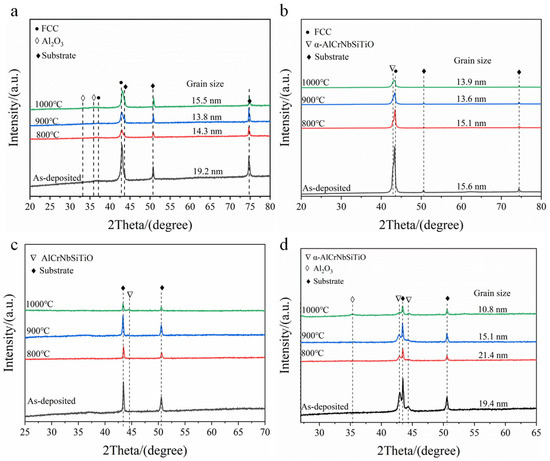
Figure 9.
XRD patterns of N, O-30, O-60, and O-100 coatings after oxidation. (a) N, (b) O-30, (c) O-60, (d) O-100.
It can be seen that both the AlCrNbSiTiN coating and the oxygen-incorporated coatings exhibit excellent oxidation resistance. The reasons for the nitride coating’s performance are mainly the following aspects:
- 1.
- The lattice distortion effect and sluggish diffusion effect hinder the diffusion of oxygen into the coating interior and the diffusion of coating elements towards the surface;
- 2.
- The formation of a dense Al2O3 layer on the coating surface protects the underlying coating from oxidation.
The oxygen-incorporated coatings also exhibit excellent oxidation resistance. This pre-oxidation approach reduces the driving force for the coating to react with oxygen, thereby maintaining the composition and structural stability of the coating in high-temperature oxidizing environments.
4. Conclusions
The structure and properties of the AlCrNbSiTiN coatings were optimized by introducing oxygen doping. By varying the oxygen flow rate, a transition from nitride to oxynitride and finally to oxide can be achieved. Coatings with low oxygen incorporation can maintain their FCC structure, while at high oxygen flow rates, the FCC structure is disrupted, and the coating transforms into an amorphous or spinel structure. Oxygen doping causes significant changes in the electronic structure and physical properties of the coating, resulting in stress relaxation. The transformation of the bonding state, along with the generation of interstitial anions and cation vacancies, leads to a decrease in coating hardness. When the coating transforms into an oxide, its hardness drops to 12 GPa. Oxygen-doped coatings exhibit excellent oxidation resistance; this is particularly the case for the oxide coating, whose structure remains stable up to 900 °C in an oxidizing environment.
Author Contributions
Data Curation, J.Z. and Y.Z.; Formal Analysis, H.H.; Funding Acquisition, B.Y.; Investigation, Y.Z. and L.C.; Methodology, H.H. and X.M.; Project Administration, J.Z.; Validation, J.L. and X.M.; Visualization, J.Z.; Writing—Original Draft, H.H.; Writing—Review and Editing, J.L. and B.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Plan of Jiangsu Provincial Market Regulation Bureau (KJ2024031).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cegla, F.; Cawley, P.; Allin, J.; Davies, J. High-Temperature (>500 °C) Wall Thickness Monitoring Using Dry-Coupled Ultrasonic Waveguide Transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2011, 58, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Kazys, R.; Vaskeliene, V. High Temperature Ultrasonic Transducers: A Review. Sensors 2021, 21, 3200. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, J.; Gong, H.; Deng, X. A comprehensive review of loosening detection methods for threaded fasteners. Mech. Syst. Signal Process. 2022, 168, 108652. [Google Scholar] [CrossRef]
- Hou, R.; Hutson, D.; Kirk, K. Development of sputtered AlN thin-film ultrasonic transducers for durable high-temperature applications. Insight 2013, 55, 302–307. [Google Scholar] [CrossRef]
- Xu, T.; Wu, G.; ZHang, G.; Hao, Y. The compatibility of ZnO piezoelectric film with micromachining process. Sens. Actuators A-Phys. 2003, 104, 61–67. [Google Scholar] [CrossRef]
- Kazys, R.; Voleisis, A.; Sliteris, L.; Mazeika, L.; Van Nieuwenhove, R.; Kupschus, P.; Abderrahim, H. High temperature ultrasonic transducers for imaging and measurements in a liquid Pb/Bi eutectic alloy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 525–537. [Google Scholar] [CrossRef]
- Kim, T.; Kim, J.; Dalmau, R.; Schlesser, R.; Preble, E.; Jiang, X. High-Temperature Electromechanical Characterization of AlN Single Crystals. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 1880–1887. [Google Scholar] [CrossRef]
- Jiang, Y.; Jing, X.; Li, J.; Zeng, X.; Pelenovich, V.; Xu, C.; Xiong, Z.; Zhang, J.; Yang, B. Extreme high temperature tolerant AlN thin film ultrasonic transducer and its application for bolt preload measurement. J. Alloys Compd. 2025, 1027, 180498. [Google Scholar] [CrossRef]
- Chang, Y.; Weng, S.; Chen, C.; Fu, F. High temperature oxidation and cutting performance of AlCrN, TiVN and multilayered AlCrN/TiVN hard coatings. Surf. Coat. Technol. 2017, 332, 494–503. [Google Scholar] [CrossRef]
- Xiao, B.; Li, H.; Mei, H.; Dai, W.; Zuo, F.; Wu, Z.; Wang, Q. A study of oxidation behavior of AlTiN-and AlCrN-based multilayer coatings. Surf. Coat. Technol. 2018, 333, 229–237. [Google Scholar] [CrossRef]
- Song, J.; Sun, A.; Zhang, P.; Li, J. Microstructure, mechanical performance, thermal stability, and oxidation resistance of AlCrN/AlCrBN nano-multilayer coating. Surf. Coat. Technol. 2024, 493, 180498. [Google Scholar] [CrossRef]
- Du, G.; Li, C.; Li, J.; Wu, G.; Huang, Z.; Mao, A.; Ma, M.; Guo, Z.; Chen, Z. Research progress on high entropy oxide ceramics: Principles, preparation, and properties. J. Mater. Res. Technol. 2025, 35, 265–288. [Google Scholar] [CrossRef]
- Fracchia, M.; Coduri, M.; Ghigna, P.; Anselmi-Tamburini, U. Phase stability of high entropy oxides: A critical review. J. Eur. Ceram. Soc. 2024, 44, 585–594. [Google Scholar] [CrossRef]
- Kirnbauer, A.; Spadt, C.; Koller, C.M.; Kolozsvári, S.; Mayrhofer, P.H. High-entropy oxide thin films based on Al–Cr–Nb–Ta–Ti. Vacuum 2019, 168, 108850. [Google Scholar] [CrossRef]
- Chen, T.K.; Wong, M.S. Structure and properties of reactively-sputtered AlxCoCrCuFeNi oxide films. Thin Solid Film. 2007, 516, 141–146. [Google Scholar] [CrossRef]
- Chen, T.-K.; Wong, M.-S. Thermal stability of hard transparent AlxCoCrCuFeNi oxide thin films. Surf. Coat. Technol. 2008, 203, 495–500. [Google Scholar] [CrossRef]
- Castaldi, L.; Kurapov, D.; Reiter, A.; Shkover, V.; Schwaller, P.; Patscheider, J. Effect of the oxygen content on the structure, morphology and oxidation resistance of Cr-O-N coatings. Surf. Coat. Technol. 2008, 203, 545–549. [Google Scholar] [CrossRef]
- Khatibi, A.; Sjölen, J.; Greczynski, G.; Jensen, J.; Eklund, P.; Hultman, L. Structural and mechanical properties of Cr-Al-O-N thin films grown by cathodic arc deposition. ACTA Mater. 2012, 60, 6494–6507. [Google Scholar] [CrossRef]
- Ahmad, F.; Zhang, L.; Zheng, J.; Sidra, I.; Cai, F.; Zhang, S. Structural evolution and high-temperature tribological properties of AlCrON coatings deposited by multi-arc ion plating. Ceram. Int. 2020, 46, 24281–24289. [Google Scholar] [CrossRef]
- Stüber, M.; Albers, U.; Leiste, H.; Seemann, K.; Ziebert, C.; Ulrich, S. Magnetron sputtering of hard Cr-Al-N-O thin films. Surf. Coat. Technol. 2008, 203, 661–665. [Google Scholar] [CrossRef]
- Najafi, H.; Karimi, A.; Dessarzin, P.; Morstein, M. Correlation between anionic substitution and structural properties in AlCr(OxN1-x) coatings deposited by lateral rotating cathode arc PVD. Thin Solid Film. 2011, 520, 1597–1602. [Google Scholar] [CrossRef]
- Shen, Y.; Mai, Y. Effect of oxygen on residual stress and structural properties of tungsten nitride films grown by reactive magnetron sputtering. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2000, 76, 107–115. [Google Scholar] [CrossRef]
- Ye, J.; Ulrich, S.; Zlebert, C.; Stüber, M. Stress reduction of cubic boron nitride films by oxygen addition. Thin Solid Film. 2008, 517, 1151–1155. [Google Scholar] [CrossRef]
- Ebbinghaus, S.; Abicht, H.; Dronskowski, R.; Müller, T.; Reller, A.; Weidenkaff, A. Perovskite-related oxynitrides—Recent developments in synthesis, characterisation and investigations of physical properties. Prog. Solid State Chem. 2009, 37, 173–205. [Google Scholar] [CrossRef]
- Khatibi, A.; Palisaitis, J.; Höglund, C.; Eriksson, A.; Persson, P.; Jensen, J.; Birch, J.; Eklund, P.; Hultman, L. Face-centered cubic (Al1-xCrx)2O3. Thin Solid Film. 2011, 519, 2426–2429. [Google Scholar] [CrossRef]
- Shaha, K.; Ruess, H.; Rotert, S.; Baben, M.; Music, D.; Schneider, J. Nonmetal sublattice population induced defect structure in transition metal aluminum oxynitrides. Appl. Phys. Lett. 2013, 103, 221905. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).