Studies on the Coating Formation and Structure Property for Plasma Electrolytic Oxidation of AZ31 Magnesium Alloy
Abstract
1. Introduction
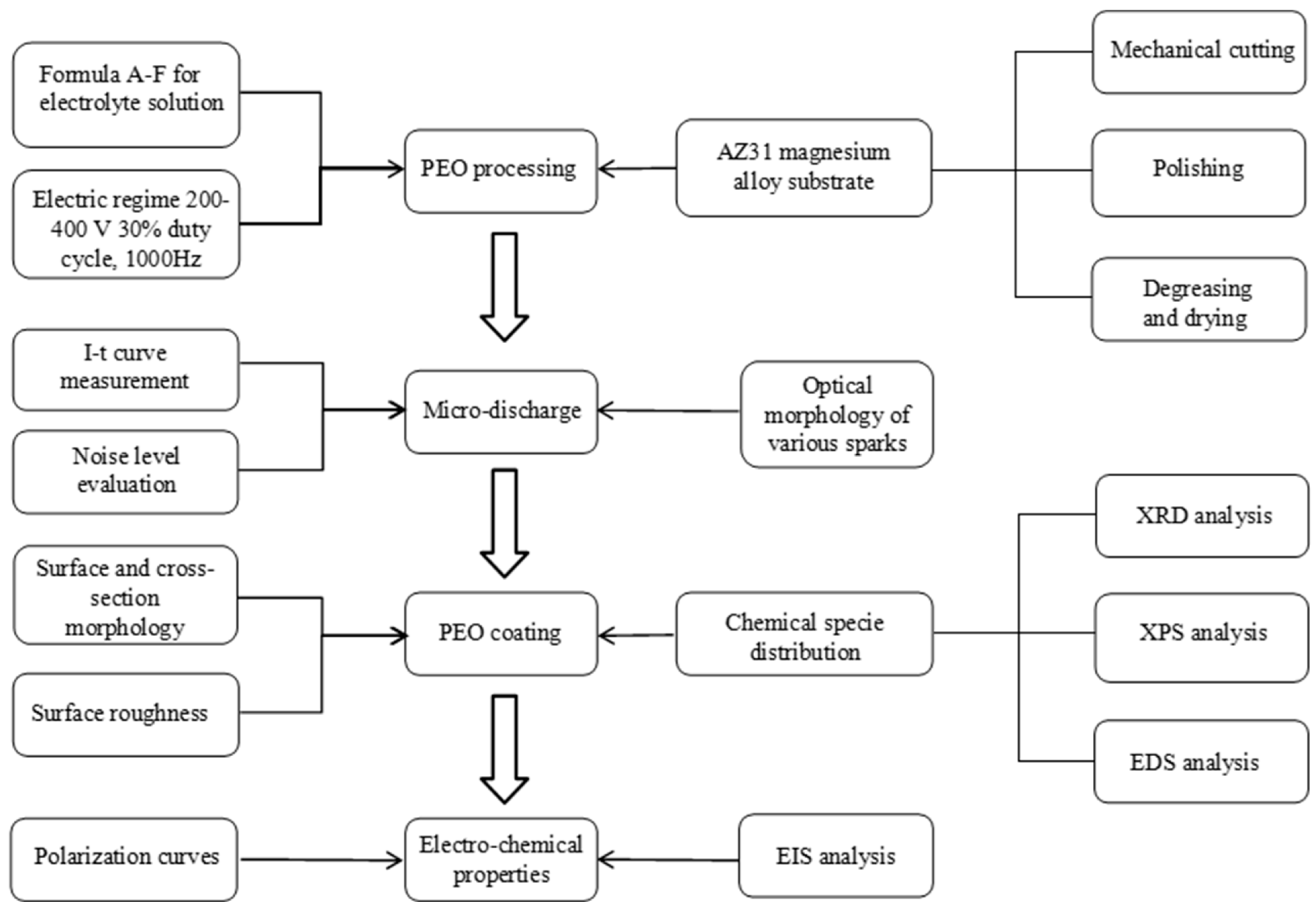
2. Materials and Methods
3. Results and Discussions
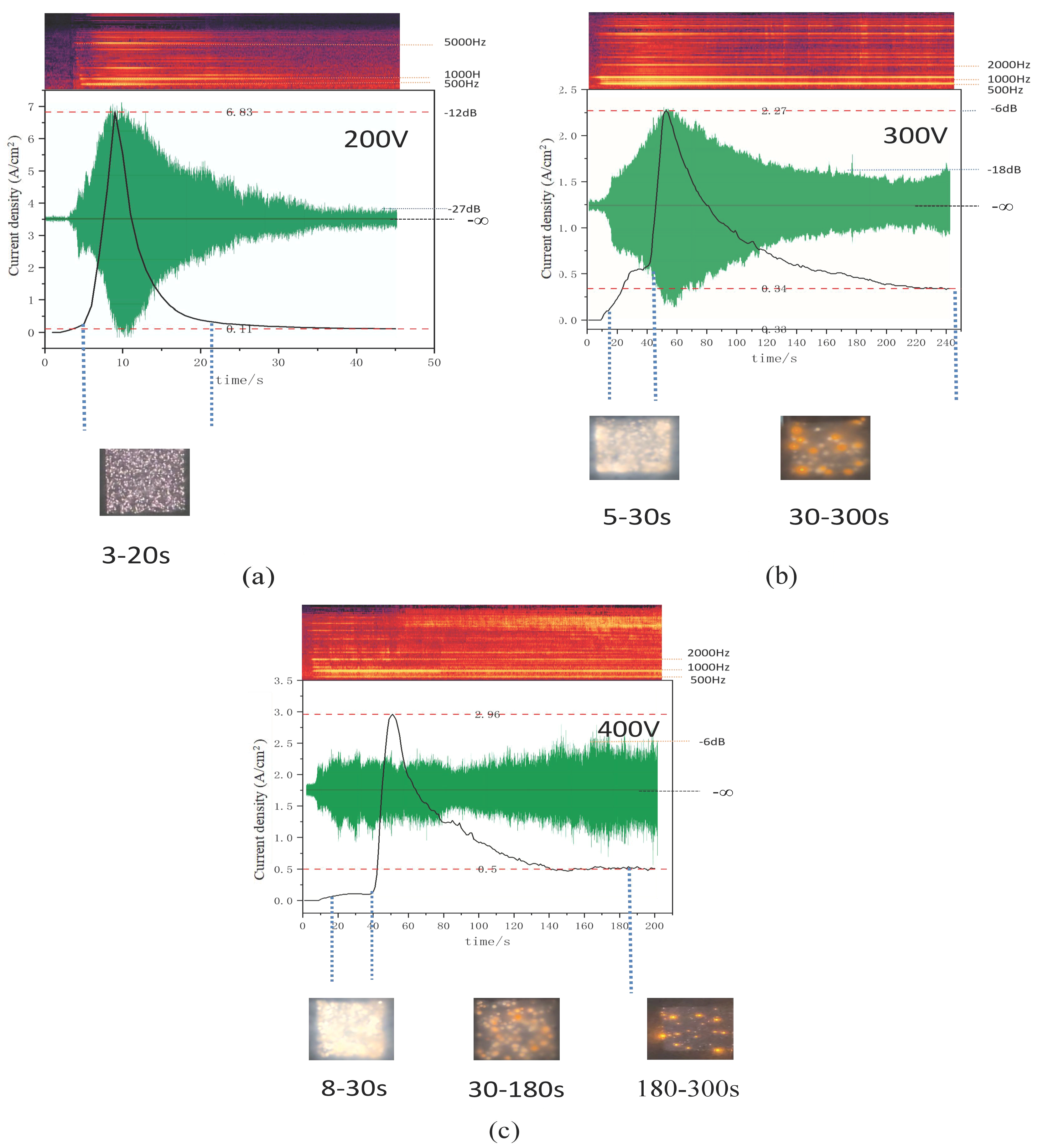
3.1. Monitoring of the PEO Process for AZ31
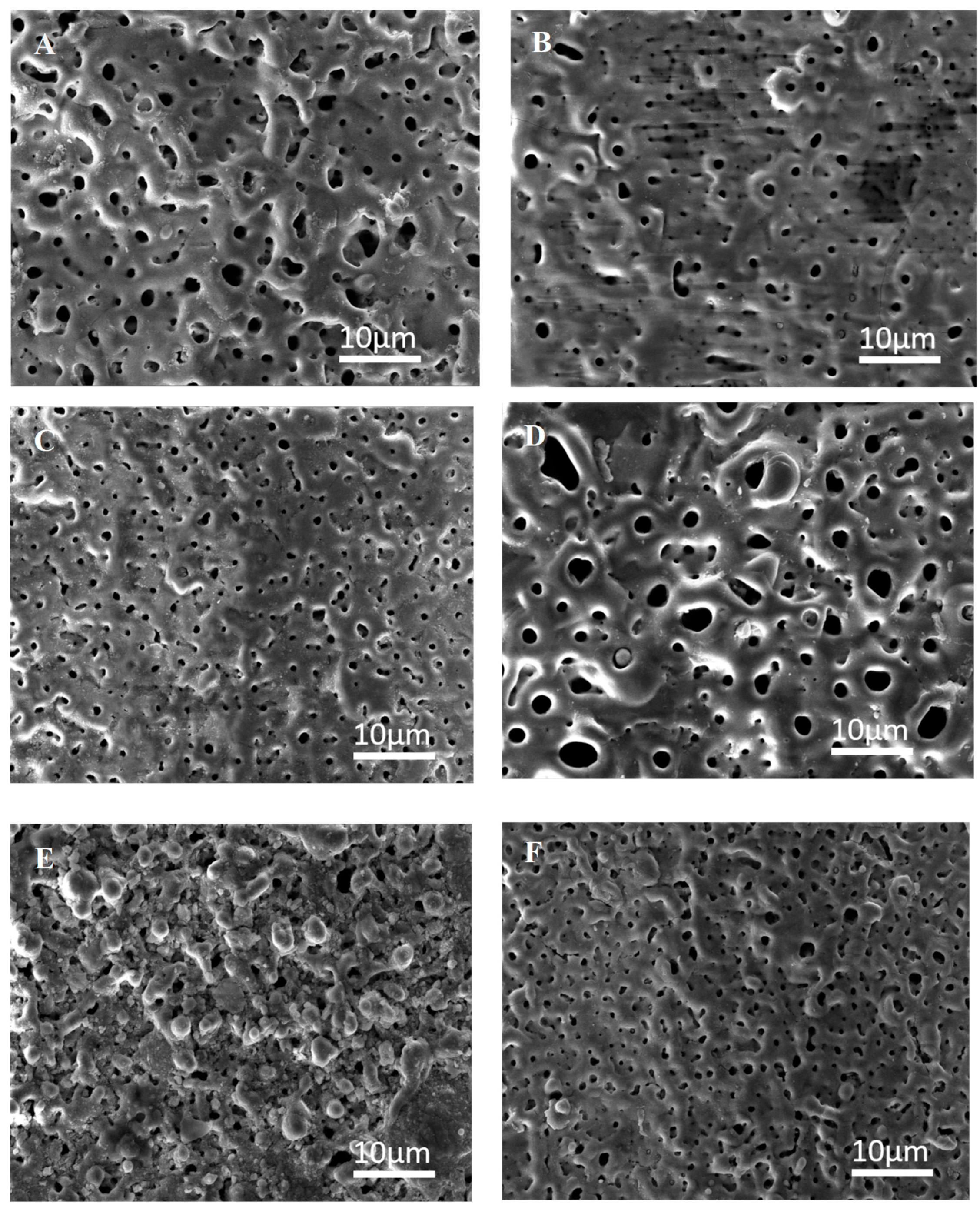
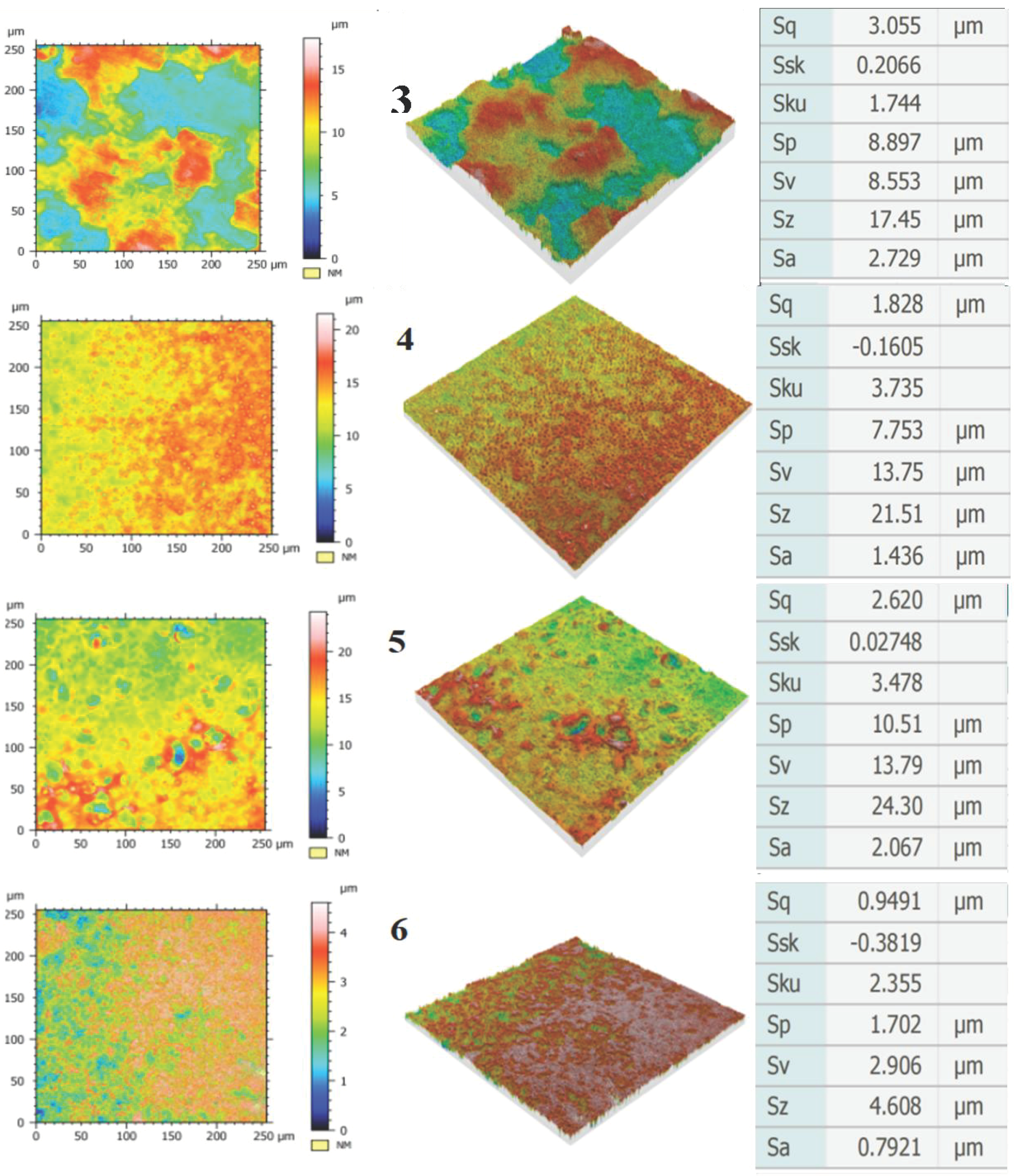
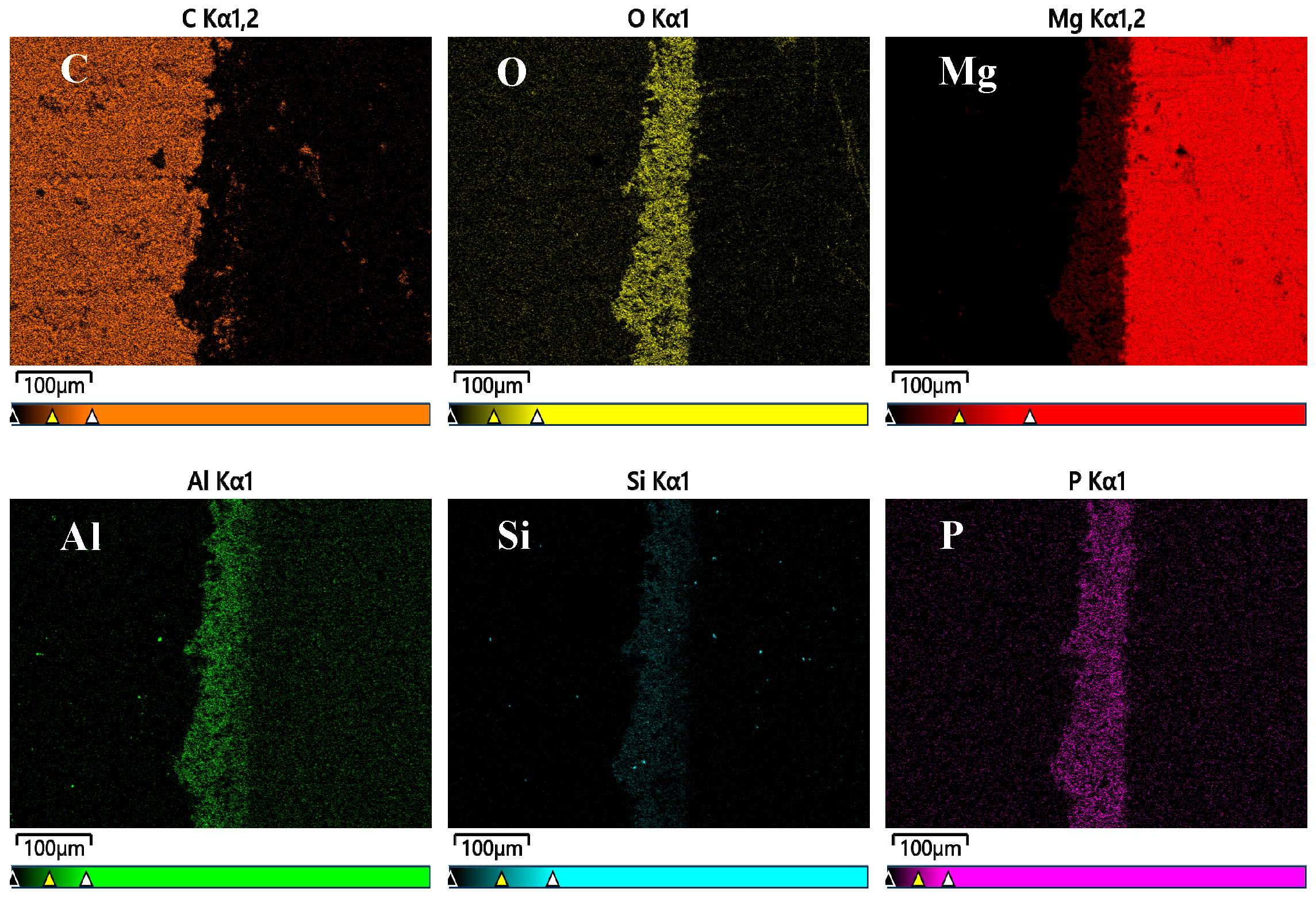
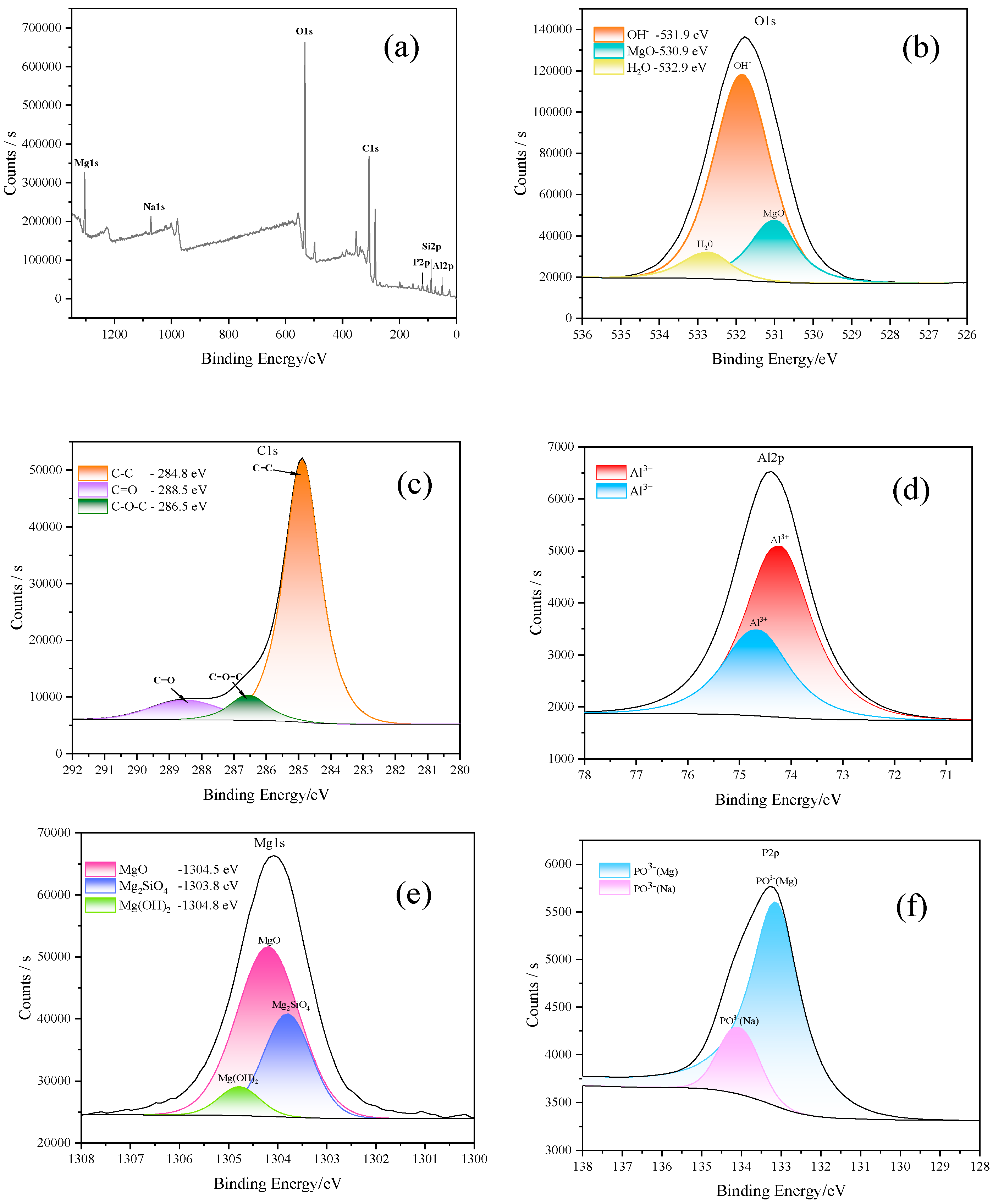
3.2. Characteristics of PEO Coating
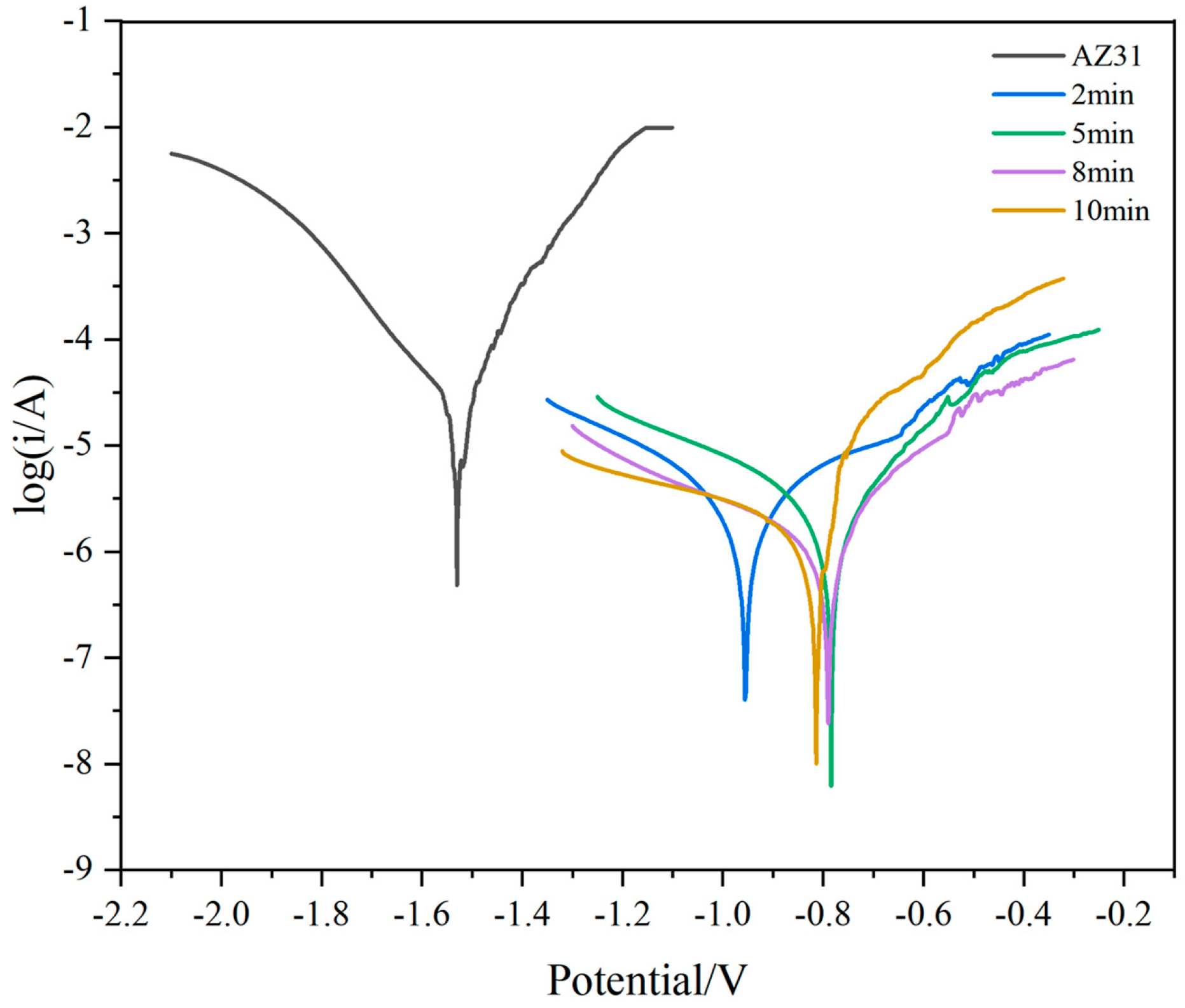
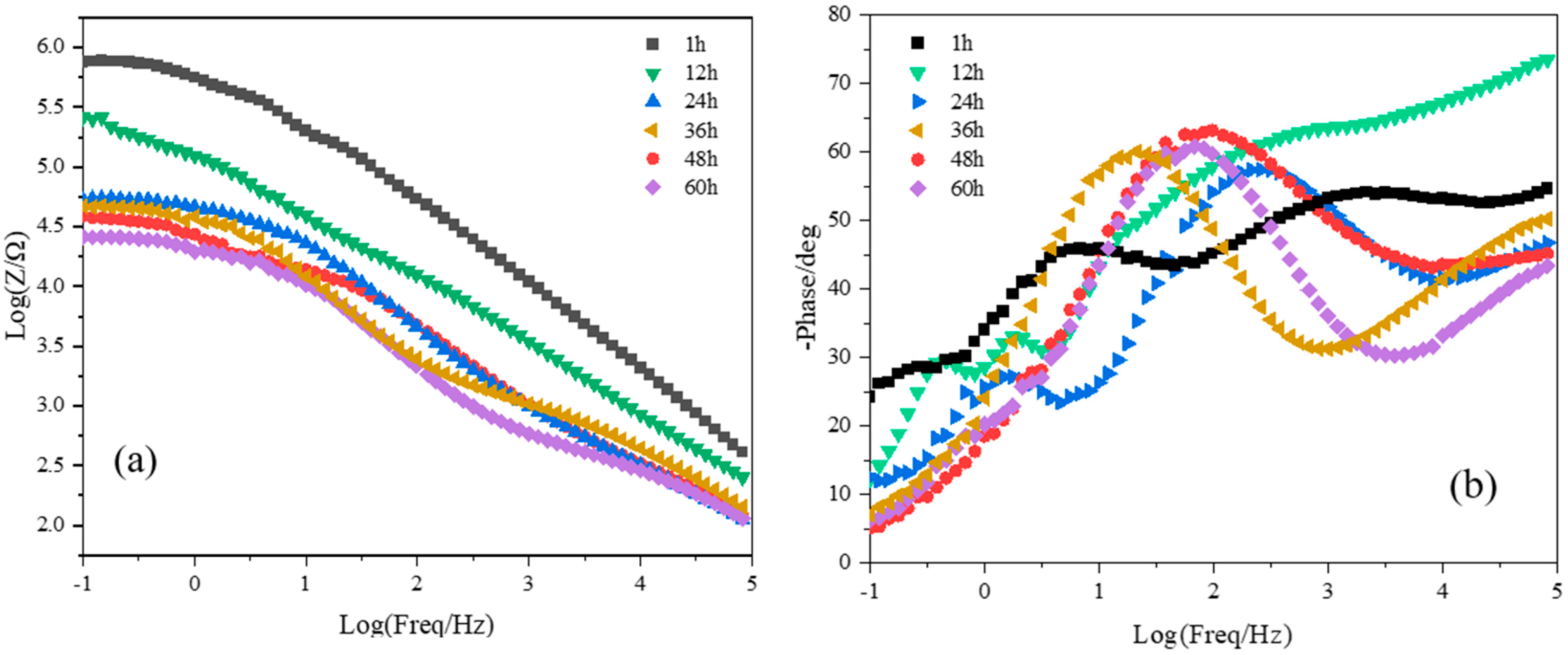
3.3. Corrosion Resistance of PEO Coating
3.4. Discussion on the Mechanism of PEO
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orlov, D.; Ralston, K.D.; Birbilis, N.; Estrin, Y. Enhanced corrosion resistance of Mg alloy ZK60 after processing by integrated extrusion and equal channel angular pressing. Acta Mater. 2011, 59, 6176–6186. [Google Scholar] [CrossRef]
- Lin, M.C.; Tsai, C.Y.; Uan, J.Y. Electrochemical behaviour and corrosion performance of Mg–Li–Al–Zn anodes with high Al composition. Corros. Sci. 2009, 51, 2463–2472. [Google Scholar] [CrossRef]
- Song, J.F.; She, J.; Chen, D.L.; Pan, F.S. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloys 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.J.; Zhang, K.; Wang, C.S.; Li, H.W.; Ma, M.L.; Zhang, B.D. Corrosion and electrochemical behavior of Mg–Y alloys in 3.5% NaCl solution. Trans. Nonferrous Met. Soc. China 2013, 23, 1226–1236. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, H.; Northwood, D.; Nie, X. Optimization of the electrolytic plasma oxidation processes for corrosion protection of magnesium alloy AM50 using the Taguchi method. J. Mater. Process. Technol. 2007, 182, 58–64. [Google Scholar] [CrossRef]
- Song, D.; Jing, X.; Wang, J.; Lu, S.; Yang, P.; Wang, Y.; Zhang, M. Microwave-assisted synthesis of lanthanum conversion coating on Mg-Li alloy and its corrosion resistance. Corros. Sci. 2011, 53, 3651–3656. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Sharifi-Asl, S.; Mozafari, M. Improved corrosion performance of biodegradable magnesium in simulated inflammatory condition via drug-loaded plasma electrolytic oxidation coatings. Mater. Chem. Phys. 2019, 239, 122003. [Google Scholar] [CrossRef]
- Boinet, M.; Verdier, S.; Maximovitch, S.; Dalard, F. Application of acoustic emission technique for in situ study of plasma anodising. NDTE Int. 2004, 37, 213–219. [Google Scholar] [CrossRef]
- Boinet, M.; Verdier, S.; Maximovitch, S.; Dalard, F. Plasma electrolytic oxidation of AM60 magnesium alloy: Monitoring by acoustic emission technique. Electrochemical properties of coatings. Surf. Coat. Technol. 2005, 199, 141–149. [Google Scholar] [CrossRef]
- Bao, F.; Li, F.; Bashkov, O.; Wang, Z.; Sund, L. Stage division and discharge mechanism characterization of micro-arc oxidation based on acoustic emission. Surf. Coat. Technol. 2025, 502, 131964. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Yan, Z.; Fu, W. Optical emission spectroscopy studies of discharge mechanism and plasma characteristics during plasma electrolytic oxidation of magnesium in different electrolytes. Surf. Coat. Technol. 2010, 205, 1651–1658. [Google Scholar] [CrossRef]
- Yang, X.; Chen, L.; Qu, Y.; Liu, R.; Wei, K.; Xue, W. Optical emission spectroscopy of plasma electrolytic oxidation process on 7075 aluminum alloy. Surf. Coat. Technol. 2017, 324, 18–25. [Google Scholar] [CrossRef]
- Dunleavy, C.S. Development of Quantitative Techniques for the Study of Discharge Events During Plasma Electrolytic Oxidation Processes. Ph.D. Thesis, Department of Materials Science and Metallurgy, University of Cambridge, Cambridge, UK, 2010. [Google Scholar]
- Rogov, A.B.; Yerokhin, A.; Matthews, A. The Role of Cathodic Current in Plasma Electrolytic Oxidation of Aluminum: Phenomenological Concepts of the “Soft Sparking” Mode. Langmuir 2017, 33, 11059–11069. [Google Scholar] [CrossRef] [PubMed]
- Kamil, M.P.; Kaseem, M.; Ko, Y.G. Soft plasma electrolysis with complex ions for optimizing electrochemical performance. Sci. Rep. 2017, 7, 44458. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Ma, L.; Wu, L. Effect of the gas layer evolution on electrolytic plasma polishing of stainless steel. Sci. Rep. 2024, 14, 22099. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, H.; Yang, D.; Ji, G.; Duan, H.; Xiang, Y. Local surface smoothness on TC4 alloy during microbubbles flow electrolytic plasma polishing: Prototype rig, phenomena, and mechanism. Int. J. Adv. Manuf. Technol. 2024, 134, 1879–1892. [Google Scholar] [CrossRef]
- Nikoomanzari, E.; Fattah-alhosseini, A. Maximizing the potential applications of plasma electrolytic oxidation coatings produced on Mg-based alloys in anti-corrosion, antibacterial, and photocatalytic targeting through harnessing the LDH/PEO dual structure. J. Magnes. Alloys 2024, 12, 2674–2694. [Google Scholar] [CrossRef]
- Sisarwal, V.; Dong, S.; Toh, R.J.; Gamaleldin, K.; Kulkarni, S.; Li, H.; Cole, I.S.; Dong, J.; Chen, X. Plasma electrolytic oxidation upon Mg alloys: Fundamentals, state-of-the-art progress and challenges. In Conversion Coatings for Magnesium and Its Alloys; Saji, V.S., Narayanan, T.S.N.S., Chen, X., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 445–465. [Google Scholar]
- Zozulin, A.J.; Bartak, D.E. Anodized coatings for magnesium alloys. Met. Finish. 1994, 29, 39–44. [Google Scholar]
- Liu, B.; Zhang, P.L.; Wang, J.Q.; Zhang, D.; Zhou, J.F.; Yang, S.R. AZ91D Micro-arc oxidation behavior of magnesium alloys in different electrolyte systems. J. Shanghai Jiao Tong Univ. 2010, 44, 16–20. [Google Scholar]
- Wang, S.; Yan, F.Y. Preparation of light green ceramic film by microarc oxidation of magnesium alloy. Nonferrous Met. Eng. 2014, 4, 36–38. [Google Scholar]
- Luo, H.H.; Cai, Q.Z.; Wei, B.C. AZ91D Optimization of microarc oxidation process parameters for magnesium alloy. Spec. Cast. Nonferrous Alloys 2007, 27, 554–556. [Google Scholar]
- Xue, W.B.; Lai, Y.C.; Deng, Z.W.; Chen, R.Y.; Liu, G. The properties of coating formed by microplasma oxidation on magnesium alloy. Mater. Sci. Technol. 1997, 5, 89–92. [Google Scholar]
- Wang, Y.; Huang, Z.; Yan, Q.; Liu, C.; Liu, P.; Zhang, Y.; Guo, C.; Jian, G.; Shen, D. Corrosion behaviors and effects of corrosion products of plasma electrolytic oxidation coated AZ31 magnesium alloy under the salt spray corrosion test. Appl. Surf. Sci. 2016, 378, 435–442. [Google Scholar] [CrossRef]



| Al | Zn | Mn | Si | Cu | Ca | Mg |
|---|---|---|---|---|---|---|
| 2.9% | 1.1% | 0.3% | 0.07% | 0.01% | 0.04% | Margin |
| Formula | NaSiO3 | NaOH | Na3PO4 | NaAlO2 |
|---|---|---|---|---|
| A | 30 | 0 | 0 | 0 |
| B | 0 | 0 | 30 | 0 |
| C | 0 | 0 | 0 | 12 |
| D | 15 | 7.5 | 0 | 0 |
| E | 15 | 0 | 15 | 0 |
| F | 10 | 5 | 0 | 4 |
| Sample | OCP/V | Ecorr/V | Icorr/(A/cm2) |
|---|---|---|---|
| Initial | −1.604 | −1.5247 | 2.712 × 10−5 |
| 400 V–2 min | −0.951 | −0.942 | 1.738 × 10−6 |
| 400 V–5 min | −0.8239 | −0.799 | 1.601 × 10−6 |
| 400 V–8 min | −0.8205 | −0.793 | 1.172 × 10−6 |
| 400 V–10 min | −0.8041 | −0.825 | 1.415 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Wang, L.; Hu, X.; Bu, Z. Studies on the Coating Formation and Structure Property for Plasma Electrolytic Oxidation of AZ31 Magnesium Alloy. Coatings 2025, 15, 846. https://doi.org/10.3390/coatings15070846
Ye Y, Wang L, Hu X, Bu Z. Studies on the Coating Formation and Structure Property for Plasma Electrolytic Oxidation of AZ31 Magnesium Alloy. Coatings. 2025; 15(7):846. https://doi.org/10.3390/coatings15070846
Chicago/Turabian StyleYe, Yingting, Lishi Wang, Xinbin Hu, and Zhixiang Bu. 2025. "Studies on the Coating Formation and Structure Property for Plasma Electrolytic Oxidation of AZ31 Magnesium Alloy" Coatings 15, no. 7: 846. https://doi.org/10.3390/coatings15070846
APA StyleYe, Y., Wang, L., Hu, X., & Bu, Z. (2025). Studies on the Coating Formation and Structure Property for Plasma Electrolytic Oxidation of AZ31 Magnesium Alloy. Coatings, 15(7), 846. https://doi.org/10.3390/coatings15070846






