Investigation of the Structure and Electrochemical Performance of Perovskite Oxide La1−xCaxCrO3 Utilized as Electrode Materials for Supercapacitors
Abstract
1. Introduction
2. Experimental Section
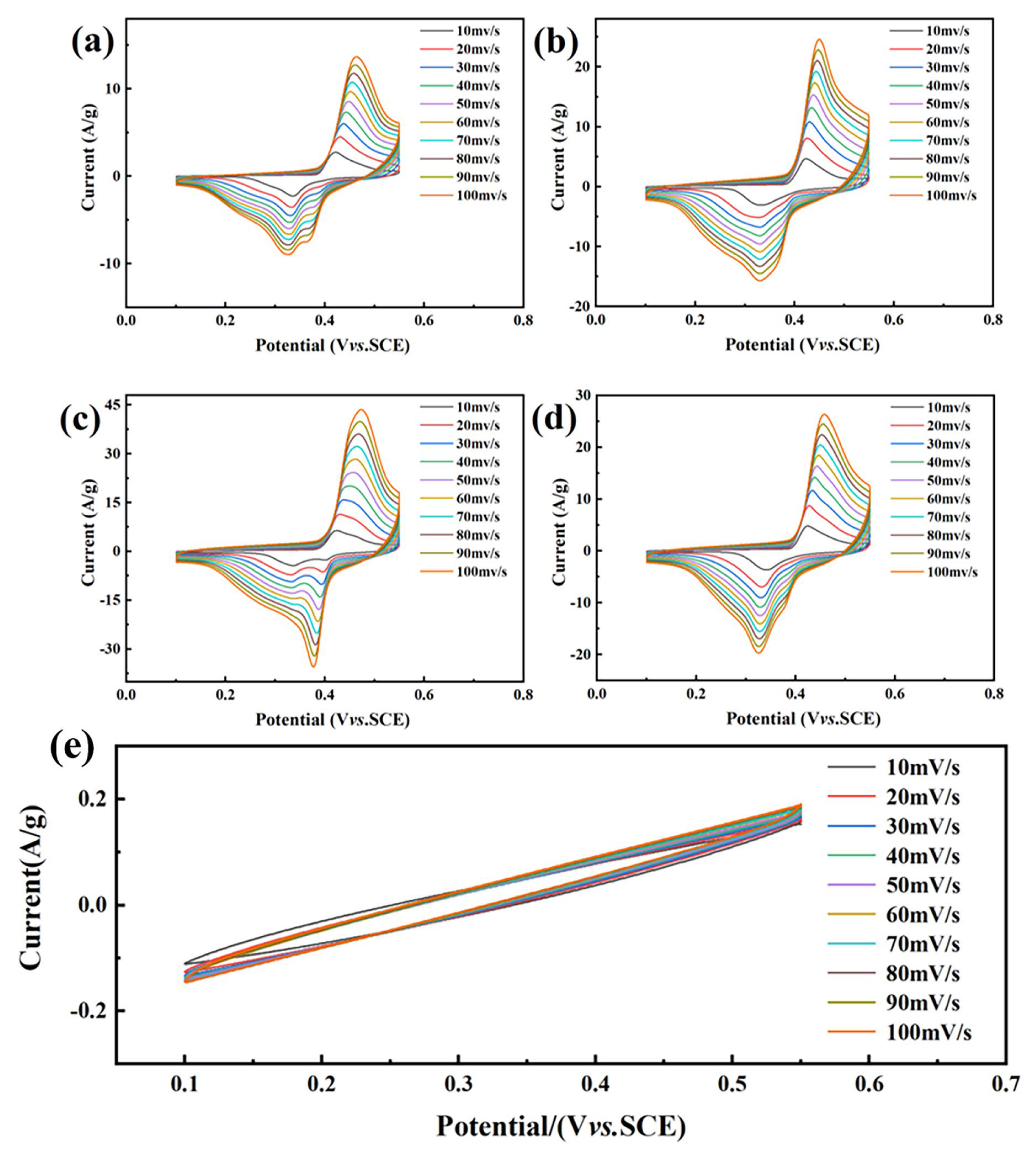
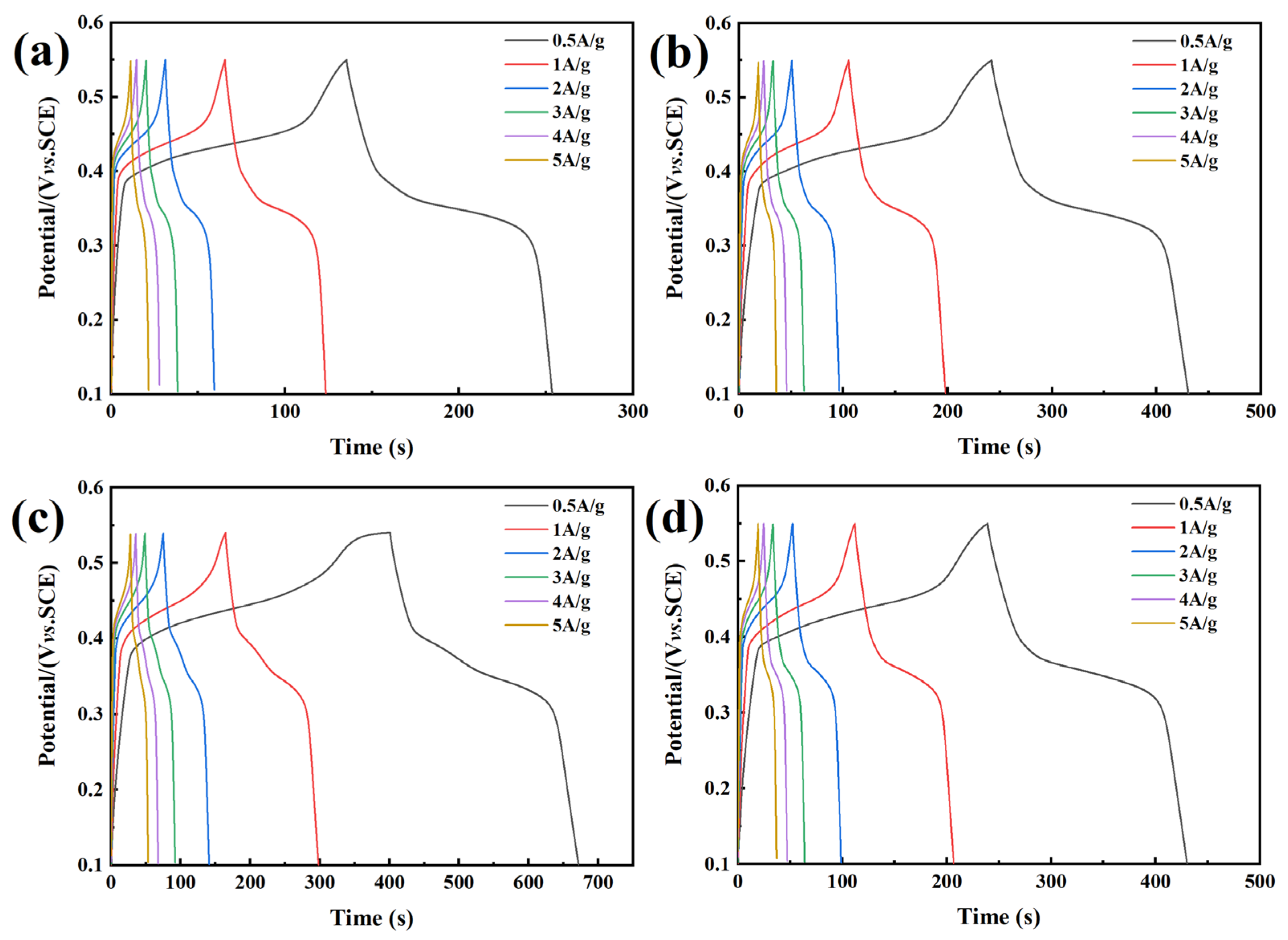
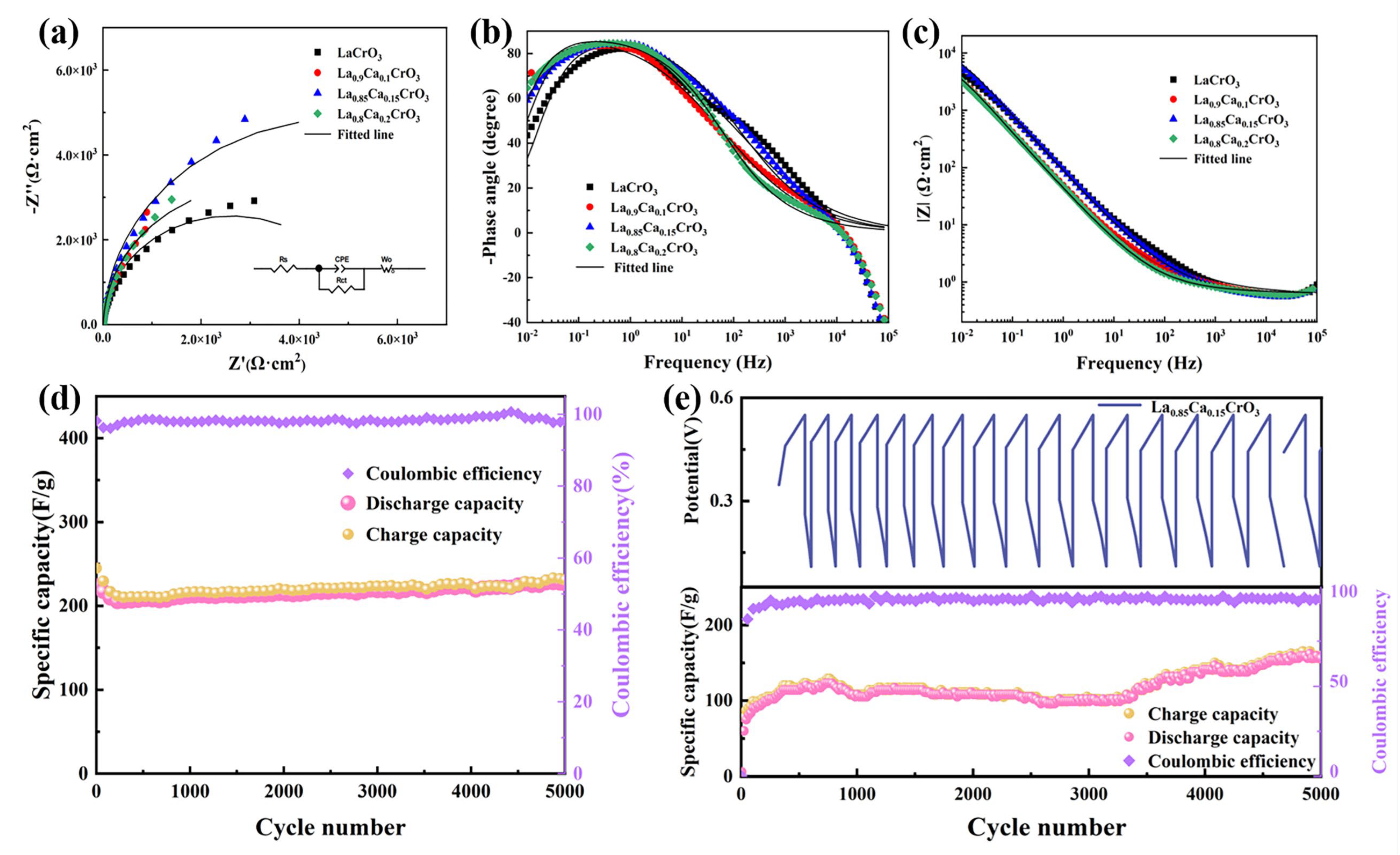
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anil, N.; Rao, P.K.; Sarkar, A.; Kubavat, J.; Vadivel, S.; Manwar, N.R.; Paul, B. Advancements in sustainable biodiesel production: A comprehensive review of bio-waste derived catalysts. Energy Convers. Manag. 2024, 318, 1188874. [Google Scholar] [CrossRef]
- Farghali, M.; Osman, A.I.; Mohamed, I.M.A.; Chen, Z.; Chen, L.; Ihara, I.; Yap, P.-S.; Rooney, D.W. Strategies to save energy in the context of the energy crisis: A review. Environ. Chem. Lett. 2023, 21, 2003–2039. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Li, J.; He, M.; Li, J.; Zhi, D.; Qin, F.; Zhang, C. Global evolution of research on green energy and environmental technologies: A bibliometric study. J. Environ. Manag. 2021, 297, 113382. [Google Scholar] [CrossRef] [PubMed]
- Soeder, D.J. Fossil fuels and climate change. In Fracking and the Environment; Springer International Publishing: Cham, Switzerland, 2021; pp. 155–185. [Google Scholar]
- Borowski, P.F. Mitigating Climate Change and the Development of Green Energy versus a Return to Fossil Fuels Due to the Energy Crisis in 2022. Energies 2022, 15, 9289. [Google Scholar] [CrossRef]
- Akalin, G.; Erdogan, S.; Sarkodie, S.A. Do dependence on fossil fuels and corruption spur ecological footprint. Environ. Impact Assess. Rev. 2021, 90, 106641. [Google Scholar] [CrossRef]
- Lu, Y.; Khan, Z.A.; Alvarez-Alvarado, M.S.; Zhang, Y.; Huang, Z.; Imran, M. A Critical Review of Sustainable Energy Policies for the Promotion of Renewable Energy Sources. Sustainability 2020, 12, 5078. [Google Scholar] [CrossRef]
- Yadav, G.; Ahmaruzzaman, M. Multi anion-based materials: Synthesis and catalytic applications. Mater. Res. Bull. 2022, 152, 111836. [Google Scholar] [CrossRef]
- Tan, K.M.; Babu, T.S.; Ramachandaramurthy, V.K.; Kasinathan, P.; Solanki, S.G.; Raveendran, S.K. Empowering smart grid: A comprehensive review of energy storage technology and application with renewable energy integration. J. Energy Storage 2021, 39, 102591. [Google Scholar] [CrossRef]
- Sharma, M.; Chauhan, A.; Vaish, R. Energy harvesting using piezoelectric cementitious composites for water cleaning applications. Mater. Res. Bull. 2021, 137, 111205. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Narenthiran, B.; Sivanantham, A.; Bhatlu, L.D.; Maridurai, T. Supercapacitor: Evolution and review. Mater. Today Proc. 2021, 46, 3984–3988. [Google Scholar] [CrossRef]
- Karabacak, K. Towards sustainable solar energy solutions: Harnessing supercapacitors in PV systems. Energy Storage Convers. 2024, 2, 436. [Google Scholar] [CrossRef]
- Karunakaran, S.K.; Arumugam, G.M.; Yang, W.; Ge, S.; Khan, S.N.; Lin, X.; Yang, G. Research Progress on the Application of Lanthanide-Ion-Doped Phosphor Materials in Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2021, 9, 1035–1060. [Google Scholar] [CrossRef]
- Arumugam, G.M.; Karunakaran, S.K.; Galian, R.E.; Pérez-Prieto, J. Recent Progress in Lanthanide-Doped Inorganic Perovskite Nanocrystals and Nanoheterostructures: A Future Vision of Bioimaging. Nanomaterials 2022, 12, 2130. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Guo, X.; Tian, Z.; Wang, H.; Ye, X.; Wang, L.; Dong, S. Effect of Sr Doping at the A Site on the Electrochemical Properties of LaCoO3 Used for Supercapacitor Applications. J. Electron. Mater. 2025, 54, 3851–3862. [Google Scholar] [CrossRef]
- Sun, X.; Pei, Z.; Guo, X.; Ye, X.; Wang, L.; Zhang, Y.; Dong, S. Impact of Ca ions substitution at A-site on LaCoO3 perovskite energy applications. Mater. Sci. Eng. 2025, 319, 118343. [Google Scholar] [CrossRef]
- Guo, X.; Tian, Z.; Qu, J.; Ye, X.; Wang, L.; Zhang, Y.; Dong, S.; Ju, H. Enhanced the electrochemical performance in Sr-doped LaCoO3 nanofibers. J. Alloys Compd. 2025, 1031, 181003. [Google Scholar] [CrossRef]
- Ashok C, S.; Vazhayil, A.; Vinaykumar, R.; Thomas, J.; Jeffery, A.A.; Hasan, I.; Thomas, N.; Yadav, A.K.; Ahn, Y.-H. The effect of B–site cation on the supercapacitive properties of LaBO3 (B = Cr, Mn, Fe and Co) porous perovskites. J. Energy Storage 2024, 86, 111145. [Google Scholar] [CrossRef]
- Dutta, S. Investigation of Photocatalytic Properties of Molybdenum Disulfide Incorporated Lanthanum Ferrite Nanocomposites. Master’s Thesis, Bangladesh University of Engineering and Technology, Dhaka, Bangladesh, February 2020. [Google Scholar]
- Sun, H.; Song, J.; Shi, P.; Cheng, Z.; Tian, L.; Zhou, M.; Qi, T. Selective detection of NO using the perovskite-type oxide LaMO3 (M = Cr, Mn, Fe, Co, and Ni) as the electrode material for yttrium-stabilized zirconia-based electrochemical sensors. J. Mater. Chem. A 2024, 12, 18234–18247. [Google Scholar] [CrossRef]
- Yadagiri, K.; Anvay, Y.; Dinakar, D.; Narasaiah, N.; Sathe, V.G.; Kumar, K.; Haranath, D. Structural transformation and magnetic properties of Fe-substituted nano CuCr2O4 spinel structure. Ceram. Int. 2023, 50, 4987–4993. [Google Scholar] [CrossRef]
- Qahtan, A.A.; Zarrin, N.; Fatema, M.; Al-Shamiri, H.A.; Khan, W.; Husain, S. A brief review on rare-earth chromites: Synthesis, properties and applications. Phys. Scr. 2024, 99, 072001. [Google Scholar] [CrossRef]
- Al-Awadi, A.S.; Al-Zahrani, S.M.; El-Toni, A.M.; Abasaeed, A.E. Dehydrogenation of Ethane to Ethylene by CO2 over Highly Dispersed Cr on Large-Pore Mesoporous Silica Catalysts. Catalysts 2020, 10, 97. [Google Scholar] [CrossRef]
- Bachankar, S.; Malavekar, D.; Lokhande, V.; Ji, T. Empowering supercapacitors: Strategic B-site modulations of transition metal atoms in perovskite oxide based electrodes. J. Alloys Compd. 2024, 1003, 175708. [Google Scholar] [CrossRef]
- Li, S.; Irvine, J.T. Non-stoichiometry, structure and properties of proton-conducting perovskite oxides. Solid State Ion. 2021, 361, 115571. [Google Scholar] [CrossRef]
- Sun, X.; Meng, Z.; Hao, Z.; Du, Z.; Xu, J.; Nan, H.; Shi, W.; Zeng, F.; Hu, X.; Tian, H. Efficient fabrication of flower-like core–shell nanochip arrays of lanthanum manganate and nickel cobaltate for high-performance supercapacitors. J. Colloid Interface Sci. 2022, 630, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Nan, H.; Lang, X.; Liu, S.; Qiao, L.; Hu, X.; Tian, H. Influence of calcium doping on performance of LaMnO3 supercapacitors. Ceram. Int. 2018, 44, 9733–9741. [Google Scholar] [CrossRef]
- Shafi, P.M.; Mohapatra, D.; Reddy, V.P.; Dhakal, G.; Kumar, D.R.; Tuma, D.; Brousse, T.; Shim, J.-J. Sr- and Fe-substituted LaMnO3 Perovskite: Fundamental insight and possible use in asymmetric hybrid supercapacitor. Energy Storage Mater. 2022, 45, 119–129. [Google Scholar] [CrossRef]
- Dong, S.; Jin, X.; Ye, X.; Wei, J.; Wang, L.; Zhang, Y. Effects of Calcium Substitution for La on the Electrochemical Performance of LaMnO3 Nanoparticles. ChemistrySelect 2023, 8, e202203977. [Google Scholar] [CrossRef]
- Dong, S.-T.; Ye, X.; Fu, Z.; Jin, X.; Wei, J.; Wang, L.; Zhang, Y.-M. Effects of strontium substitution for La on the electrochemical performance of LaAlO3 perovskite nanotubes. J. Mater. Res. Technol. 2022, 19, 91–100. [Google Scholar] [CrossRef]
- Alexander, C.T.; Mefford, J.T.; Saunders, J.; Forslund, R.P.; Johnston, K.P.; Stevenson, K.J. Anion-Based Pseudocapacitance of the Perovskite Library La1−xSrxBO3−δ (B = Fe, Mn, Co). ACS Appl. Mater. Interfaces 2019, 11, 5084–5094. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.; Rehman, M.O.U.; Ahmad, F.; Khan, M.A.; Ramay, S.M.; Atiq, S. Performance optimization of Nd-doped LaNiO3 as an electrode material in supercapacitors. Solid State Ion. 2023, 395, 116227. [Google Scholar] [CrossRef]
- Liu, P.; Weng, X.; Liu, Z.; Zhang, Y.; Qiu, Q.; Wang, W.; Zhou, M.; Cai, W.; Ni, M.; Liu, M.; et al. High-Performance Quasi-Solid-State Supercapacitor Based on CuO Nanoparticles with Commercial-Level Mass Loading on Ceramic Material La1−xSrxCoO3−δ as Cathode. ACS Appl. Energy Mater. 2019, 2, 1480–1488. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, Q.; Ye, X.; Wang, L.; Dong, S.-T. Electrochemical Properties of LaMO3 (M= Cr, Mn, and Co) Perovskite Materials. Coatings 2024, 14, 147. [Google Scholar] [CrossRef]
- Jiang, S.P.; Liu, L.; Ong, K.P.; Wu, P.; Li, J.; Pu, J. Electrical conductivity and performance of doped LaCrO3 perovskite oxides for solid oxide fuel cells. J. Power Sources 2008, 176, 82–89. [Google Scholar] [CrossRef]
- Rashtchi, H.; Sani, M.A.F.; Dayaghi, A.M. Effect of Sr and Ca dopants on oxidation and electrical properties of lanthanum chromite-coated AISI 430 stainless steel for solid oxide fuel cell interconnect application. Ceram. Int. 2013, 39, 8123–8131. [Google Scholar] [CrossRef]
- Deshmukh, V.; Tejashwini, D.; Nagaswarupa, H.; Naik, R.; Al-Kahtani, A.A.; Kumar, Y.A. Sr and Fe substituted LaCoO3 nano perovskites: Electrochemical energy storage and sensing applications. J. Energy Storage 2024, 89, 111724. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total. Environ. 2021, 763, 144204. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Feng, Z.; Deng, D.; Sun, D.; Chen, M.; Han, K.; Ye, H.; Wang, H.; Tang, Y. Optimization of Sr-doping boosting the structural stability for single crystalline LiNi0.8Co0.1Mn0.1O2 cathode to enhance its electrochemical performance at elevated voltage and temperature. J. Power Sources 2022, 545, 231915. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Z.; Guo, Z.; Wang, H.; Qaid, S.M.H.; Yang, K.; Zang, Z. Phosphonate Diacid Molecule Induced Crystallization Manipulation and Defect Passivation for High-Performance Inverted MA-Free Perovskite Solar Cells. Adv. Energy Mater. 2024, 14, 2402249. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Yang, L. Optimization of dielectric loss in calcium copper titanate based on different doping modification strategies. Ceram. Int. 2023, 49, 38399–38419. [Google Scholar] [CrossRef]
- Ashfaq, A.; Sabugaa, M.M.; Ben Moussa, M.; Almousa, N.; Shokralla, E.A.; Capangpangan, R.Y.; Alguno, A.C.; Hossain, A.; Alanazi, A.M.; Abboud, M. High thermoelectric power factor of Sr doped Bi2Te3 thin film through energy filtering effect. Int. Commun. Heat Mass Transf. 2023, 143, 106719. [Google Scholar] [CrossRef]
- Zhang, W.; Li, F.; Zhang, W.; Wang, Y.; Liu, S.; Mo, X.; Li, K. B-site cation synergy in Mn doped LaCoO3 for enhanced electrocatalysts for electrochemical reduction of nitrate. Sep. Purif. Technol. 2023, 317, 123775. [Google Scholar] [CrossRef]
- Xu, C.; Lei, C.; Wang, Y.; Yu, C. Dendritic Mesoporous Nanoparticles: Structure, Synthesis and Properties. Angew. Chem. Int. Ed. Engl. 2021, 61, e202112752. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Dong, S.; Jin, X.; Wei, J.; Wang, L.; Zhang, Y. Enhancement in the Electrochemical Performance of Strontium (Sr)-Doped LaMnO3 as Supercapacitor Materials. Coatings 2022, 12, 1739. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, W.; Wu, S.; Lu, G.; Zhang, Y.; Zheng, K.; Wang, Y.-J.; Li, K. Rational engineering of meso-macroporous structured carbon materials for revealing capacitive mechanism. J. Energy Storage 2024, 104, 114478. [Google Scholar] [CrossRef]
- Takegami, D.; Fujinuma, K.; Nakamura, R.; Yoshimura, M.; Tsuei, K.-D.; Wang, G.; Wang, N.N.; Cheng, J.-G.; Uwatoko, Y.; Mizokawa, T. Absence of Ni2+/Ni3+ charge disproportionation and possible roles of O 2p holes in La3Ni2O7−δ revealed by hard x-ray photoemission spectroscopy. Phys. Rev. B Condens. Matter. 2024, 109, 125119. [Google Scholar] [CrossRef]
- Mena, J.A.; Mendoza-Estrada, V.; Sabolsky, E.M.; Sierros, K.A.; Sabolsky, K.; González-Hernández, R.; Idhaiam, K.S.V. Exploring the impact of multivalent substitution on high-temperature electrical conductivity in doped lanthanum chromite perovskites: An experimental and ab-initio study. J. Eur. Ceram. Soc. 2024, 44, 7040–7058. [Google Scholar] [CrossRef]
- Silva, R.S., Jr.; Ferreira, N.; Fontes, J.F.D.; da Costa, M.E.H.M.; Barrozo, P. Toward the stabilization of perovskite phase at low temperature and decrease of the magnetic ordering by Sr2+-doping in LaCrO3. Chem. Phys. Lett. 2022, 787, 139278. [Google Scholar] [CrossRef]
- Mazza, A.R.; Skoropata, E.; Lapano, J.; Zhang, J.; Sharma, Y.; Musico, B.L.; Keppens, V.; Gai, Z.; Brahlek, M.J.; Moreo, A. Charge doping effects on magnetic properties of single-crystal La1−xSrx (Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)O3 (0≤ x ≤ 0.5) high-entropy perovskite oxides. Phys. Rev. B Condens. Matter. 2021, 104, 094204. [Google Scholar] [CrossRef]
- Hu, M.; Jing, Y.; Zhang, X. Low thermal conductivity of graphyne nanotubes from molecular dynamics study. Phys. Rev. 2015, 91, 155408. [Google Scholar] [CrossRef]
- Dhandapani, P.; Nayak, P.K.; Maruthapillai, A. Improved electrochemical performance and charge storage mechanism of NiMnCoO4 by XPS study. Mater. Chem. Phys. 2023, 297, 127287. [Google Scholar] [CrossRef]
- Tapia-P, J.; Gallego, J.; Gamba, O.; Espinal, J. Insight into the Interaction of Perovskite-Like Surfaces (LaMnO3 and LaCoO3) with Ar, H2, CO, and O2 through NAP-XPS Analysis. Catal. Lett. 2024, 154, 6133–6142. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, N.; Wang, L.; Zhao, H.; Zhao, G. In Situ Production of Hydroxyl Radicals via Three-Electron Oxygen Reduction: Opportunities for Water Treatment. Angew. Chem. 2024, 63, e202407628. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.-Y.; Akande, S.O.; Schwingenschlögl, U. Anisotropic O vacancy formation and diffusion in LaMnO3. J. Mater. Chem. 2014, 2, 19733–19737. [Google Scholar] [CrossRef]
- Xia, W.; Leng, K.; Tang, Q.; Yang, L.; Xie, Y.; Wu, Z.; Yi, K.; Zhu, X. Structural characterization, magnetic and optical properties of perovskite (La1−xLnx)0.67Ca0.33MnO3 (Ln= Nd and Sm; x= 0.0–0.5) nanoparticles synthesized via the sol-gel process. J. Alloys Compd. 2021, 867, 158808. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Guan, X.; Liu, J.; Luo, J.; Xia, Y. Redox mediators as charge agents for changing electrochemical reactions. Chem. Soc. Rev. 2020, 49, 7454–7478. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, J.; Lin, L.; Bai, W.; Wu, S.; Zheng, P.; Zhang, J.; Zhai, J. Superior Energy Storage Capability and Stability in Lead-Free Relaxors for Dielectric Capacitors Utilizing Nanoscale Polarization Heterogeneous Regions. Small 2023, 19, e2206662. [Google Scholar] [CrossRef] [PubMed]
- Coslop, G. Experimental Investigation of La(0.6)Sr(0.4)Mn(0.6)Cr(0.4)O3 Perovskite Oxidation Kinetics for Thermochemical Carbon Dioxide Splitting Redox Cycles. Master’s Thesis, The Politecnico di Torino, Torino, Italy, March 2023. [Google Scholar]
- Huang, L.; Cheng, L.; Pan, S.; He, Y.; Tian, C.; Yu, J.; Zhou, H. Effects of Sr doping on the structure, magnetic properties and microwave absorption properties of LaFeO3 nanoparticles. Ceram. Int. 2020, 46, 27352–27361. [Google Scholar] [CrossRef]
- Yadlapalli, R.T.; Alla, R.R.; Kandipati, R.; Kotapati, A. Super capacitors for energy storage: Progress, applications and challenges. J. Energy Storage 2022, 49, 104194. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, J.; Simon, P.; Xu, B. Two-dimensional MXenes for electrochemical capacitor applications: Progress, challenges and perspectives. Energy Storage Mater. 2021, 35, 630–660. [Google Scholar] [CrossRef]
- Guo, X.; Sun, X.; Ju, H.; Jin, L.; Wang, L.; Dong, S. Facile synthesis of perovskite La2CoMnO6 nanoparticles for high-performance supercapacitor electrodes. J. Energy Storage 2025, 130, 117444. [Google Scholar] [CrossRef]
- Pu, L.; Zhang, J.; Jiresse, N.K.L.; Gao, Y.; Zhou, H.; Naik, N.; Gao, P.; Guo, Z. N-doped MXene derived from chitosan for the highly effective electrochemical properties as supercapacitor. Adv. Compos. Hybrid Mater. 2021, 5, 356–369. [Google Scholar] [CrossRef]
- Singh, N.; Tanwar, S.; Kumar, P.; Sharma, A.; Yadav, B. Advanced sustainable solid state energy storage devices based on FeOOH nanorod loaded carbon@PANI electrode: GCD cycling and TEM correlation. J. Alloys Compd. 2023, 947, 169580. [Google Scholar] [CrossRef]
- An, S.; Gao, X.; Zheng, M.; Zhang, M.; Liu, P.; Zhu, M.; Hou, Y. Boosting power output performance of Ba0.85Ca0.15SnZr0.1-Ti0.9O3 piezoelectric nanogenerators via internal resistance regulation strategy. Nano Energy 2024, 123, 109420. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y. Energy harvesting performance of an elastically mounted semi-circular cylinder. Renew. Energy 2024, 229, 120692. [Google Scholar] [CrossRef]
- Fujiwara, K.; Sriram, R.; Kontis, K. Experimental investigations on the sharp leading-edge separation over a flat plate at zero incidence using particle image velocimetry. Exp. Fluids 2020, 61, 205. [Google Scholar] [CrossRef]
- Sreematha, B.; Arundhathi, N.; Ravinder, D. Influence of Cerium substitution on structural, optical, and electrical transport properties of Ni-Nano ferrites prepared by Citrate gel auto combustion method. Results Chem. 2023, 5, 100929. [Google Scholar] [CrossRef]
- de Sá, M.; Pereira, C.M. The relevance of the initial conditions in glassy carbon electrode sensing applications: The ferri/ferrocyanide redox reaction model system in aqueous solution. Electrochim. Acta 2024, 489, 141158. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Reddy, G.R.; Ghanem, M.A.; Dillip, G.; Joo, S. Insights of oxygen vacancies engineered structural, morphological, and electrochemical attributes of Cr-doped Co3O4 nanoparticles as redox active battery-type electrodes for hybrid supercapacitors. J. Energy Storage 2024, 100, 113751. [Google Scholar] [CrossRef]
- Pfeiffer, N.; Wachter, T.; Frickel, J.; Halima, H.; Hofmann, C.; Errachid, A.; Heuberger, A. Determination of Charge Transfer Resistance from Randles Circuit Spectra Using Elliptical Fitting. In Biomedical Engineering Systems and Technologies, Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021), Vienna, Austria, 11–13 February 2021; Springer: Berlin, Germany, 2023; pp. 61–79. [Google Scholar]
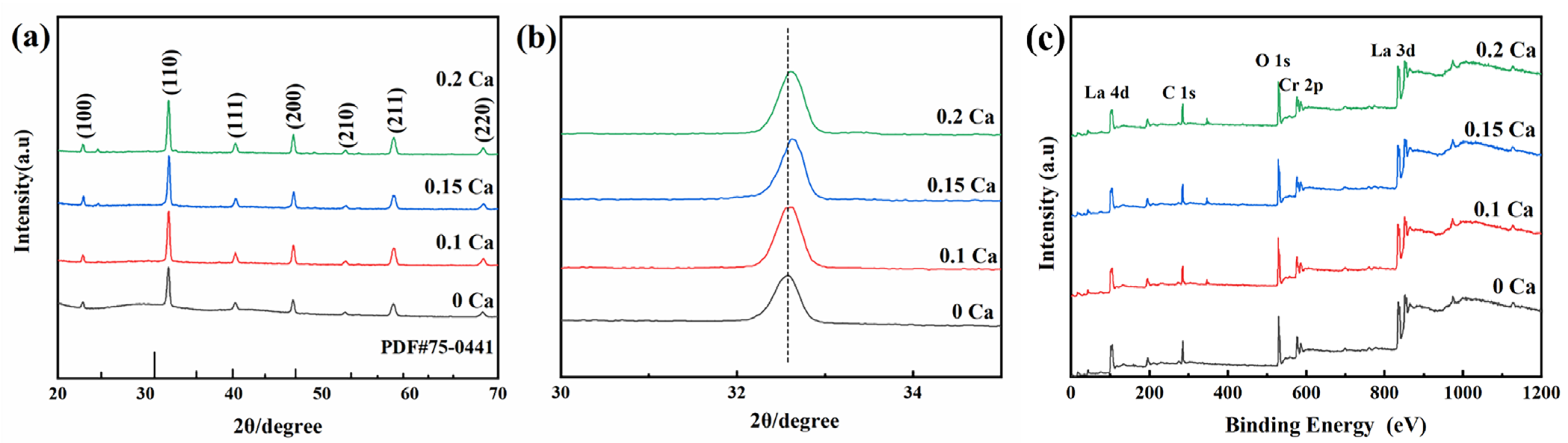
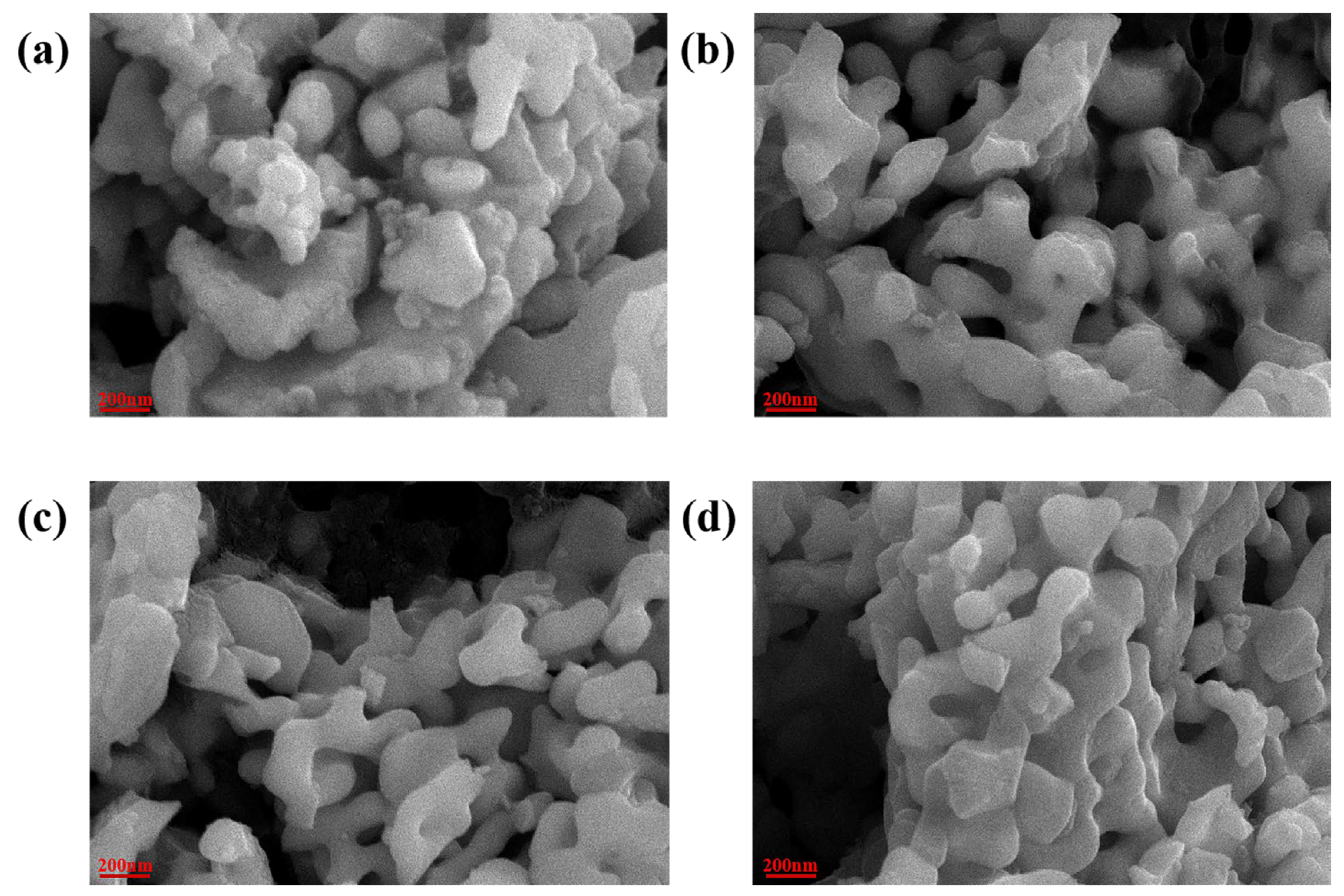
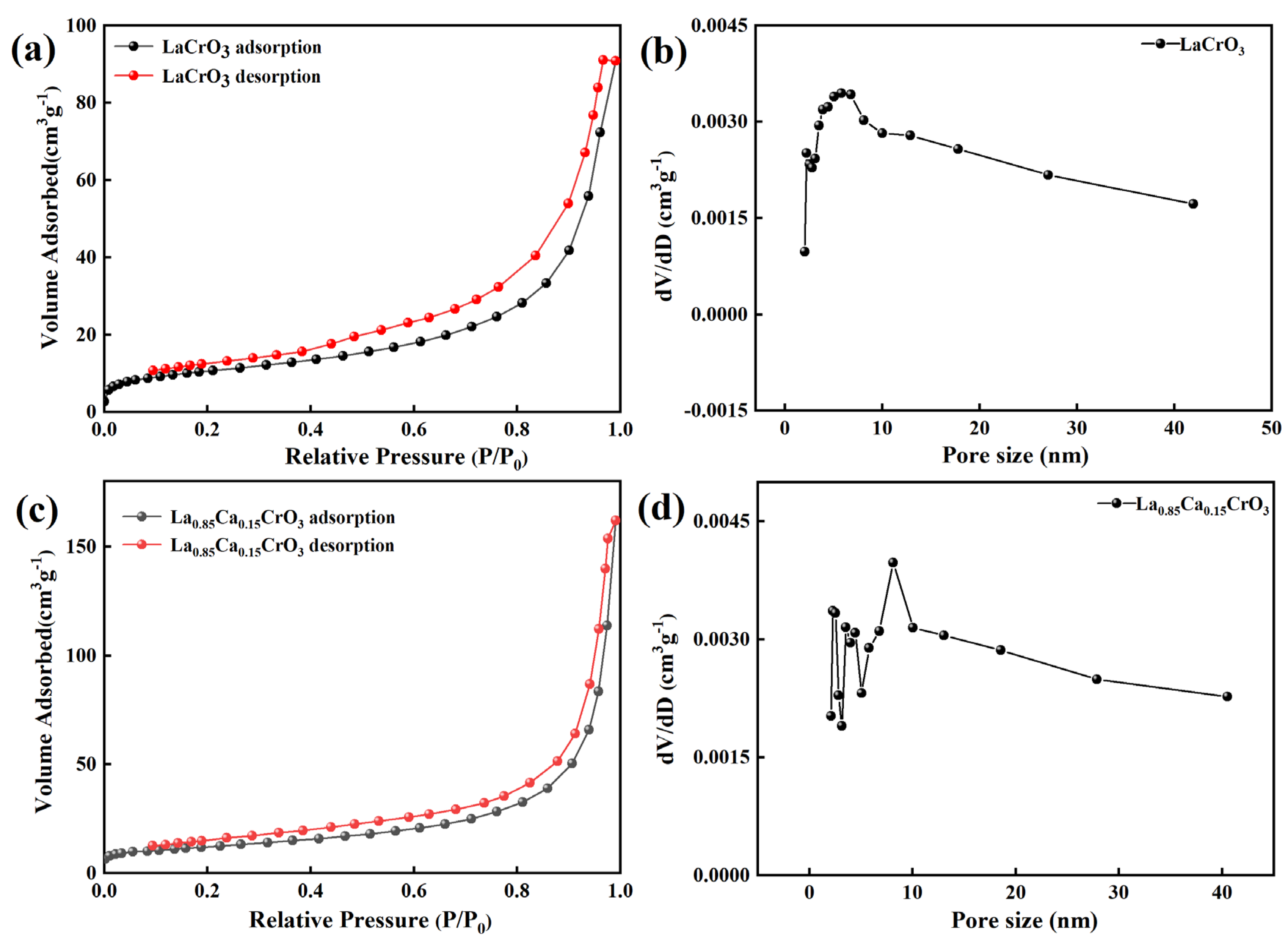
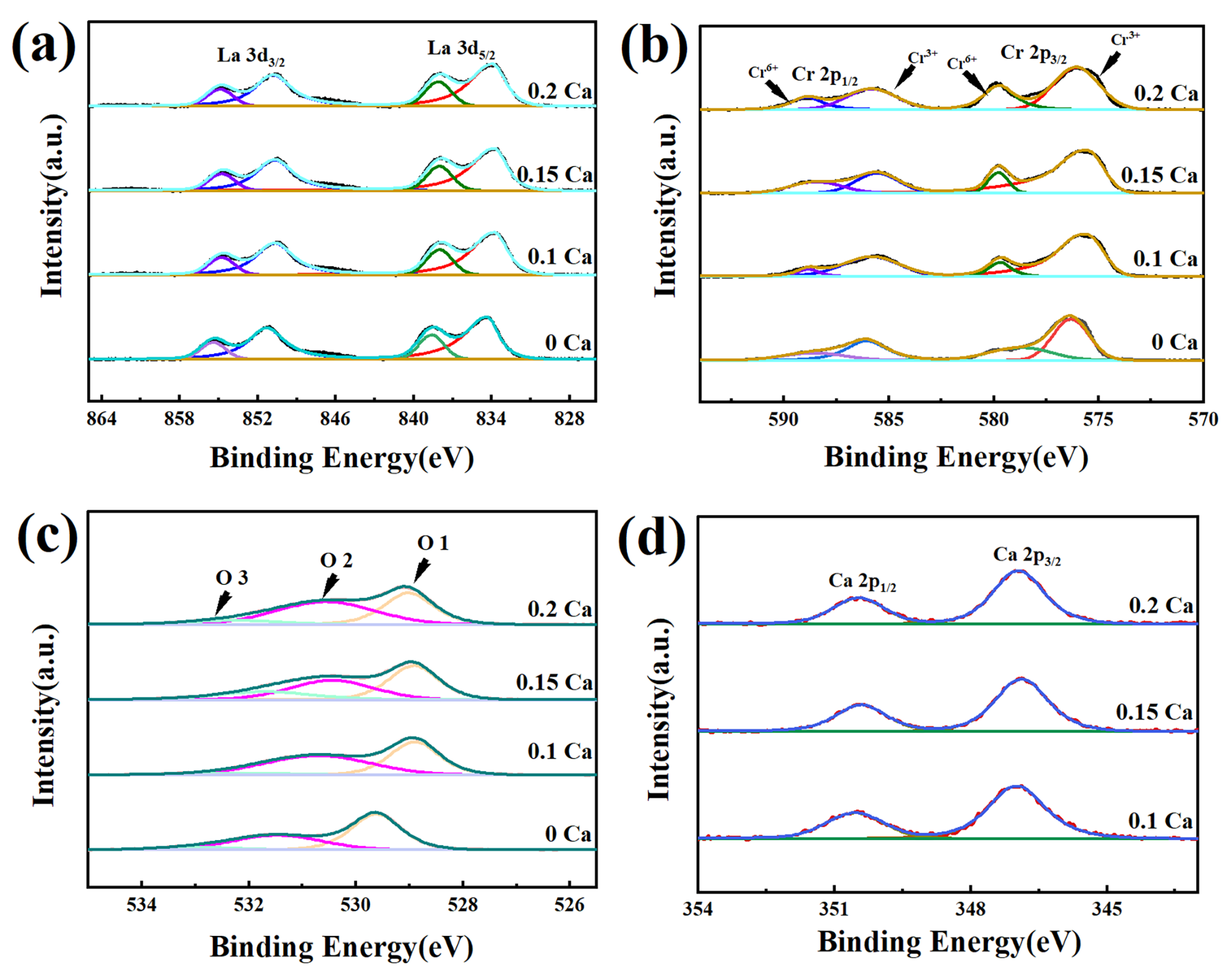
| LaCrO3 | La0.9Ca0.1CrO3 | La0.85Ca0.15CrO3 | La0.8Ca0.2CrO3 | |
|---|---|---|---|---|
| O 1(%) | 45.8 | 44.7 | 42.1 | 43.9 |
| O 2(%) | 47.6 | 49.6 | 55.6 | 49.2 |
| O 3(%) | 6.6 | 5.7 | 2.3 | 6.9 |
| O 2/O 1 | 1.04 | 1.11 | 1.32 | 1.12 |
| Coulombic Efficiency (%) | Active Site | |
|---|---|---|
| LaCrO3 | 88.4 | 0.072 |
| La0.9Ca0.1CrO3 | 87.7 | 0.083 |
| La0.85Ca0.15CrO3 | 91.4 | 0.118 |
| La0.8Ca0.2CrO3 | 91.1 | 0.094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Sun, X.; Wang, L.; Qiao, Y.; Dong, S. Investigation of the Structure and Electrochemical Performance of Perovskite Oxide La1−xCaxCrO3 Utilized as Electrode Materials for Supercapacitors. Coatings 2025, 15, 837. https://doi.org/10.3390/coatings15070837
Guo X, Sun X, Wang L, Qiao Y, Dong S. Investigation of the Structure and Electrochemical Performance of Perovskite Oxide La1−xCaxCrO3 Utilized as Electrode Materials for Supercapacitors. Coatings. 2025; 15(7):837. https://doi.org/10.3390/coatings15070837
Chicago/Turabian StyleGuo, Xu, Xin Sun, Lei Wang, Yanxin Qiao, and Songtao Dong. 2025. "Investigation of the Structure and Electrochemical Performance of Perovskite Oxide La1−xCaxCrO3 Utilized as Electrode Materials for Supercapacitors" Coatings 15, no. 7: 837. https://doi.org/10.3390/coatings15070837
APA StyleGuo, X., Sun, X., Wang, L., Qiao, Y., & Dong, S. (2025). Investigation of the Structure and Electrochemical Performance of Perovskite Oxide La1−xCaxCrO3 Utilized as Electrode Materials for Supercapacitors. Coatings, 15(7), 837. https://doi.org/10.3390/coatings15070837








