Influence of Mg/Al Coating on the Ignition and Combustion Behavior of Boron Powder
Abstract
1. Introduction
2. Experiment
2.1. Raw Materials
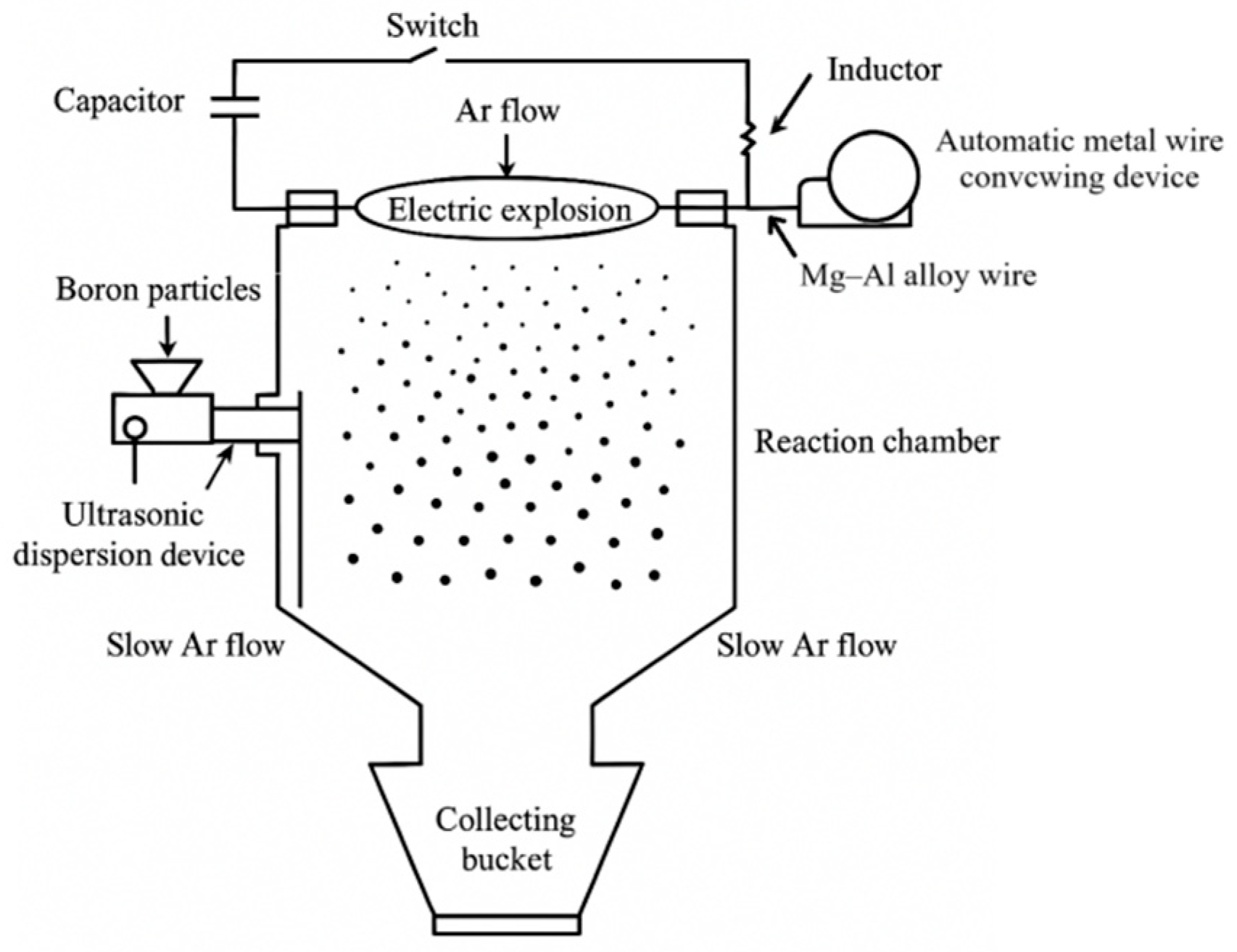
2.2. Mg/Al-Coated Boron Powder Preparation
2.2.1. Boron Powder Dispersion Treatment
2.2.2. Preparation
3. Results and Discussion
3.1. Basic Performance Analysis
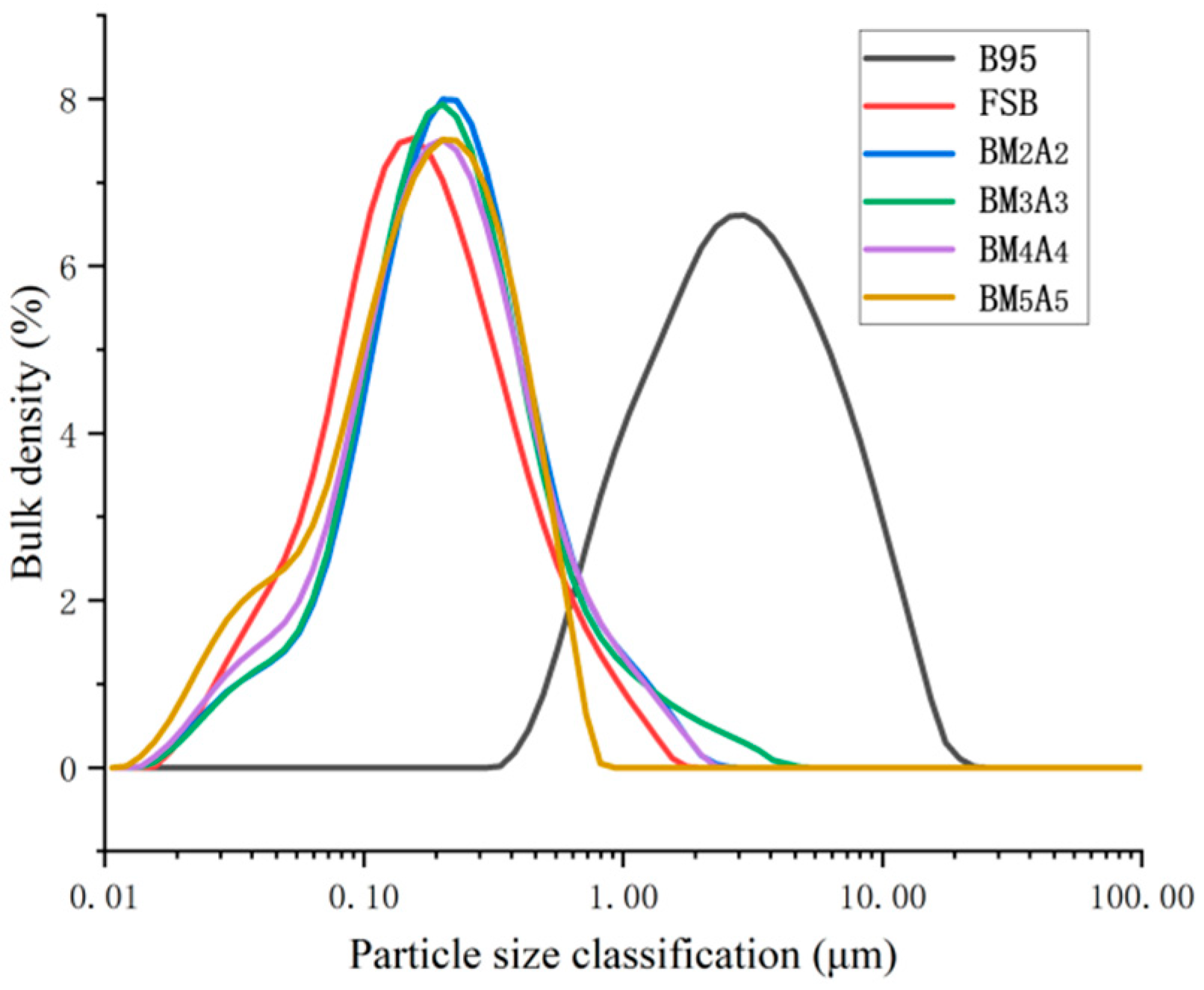
3.1.1. Powder Size Distribution
3.1.2. Specific Surface Area
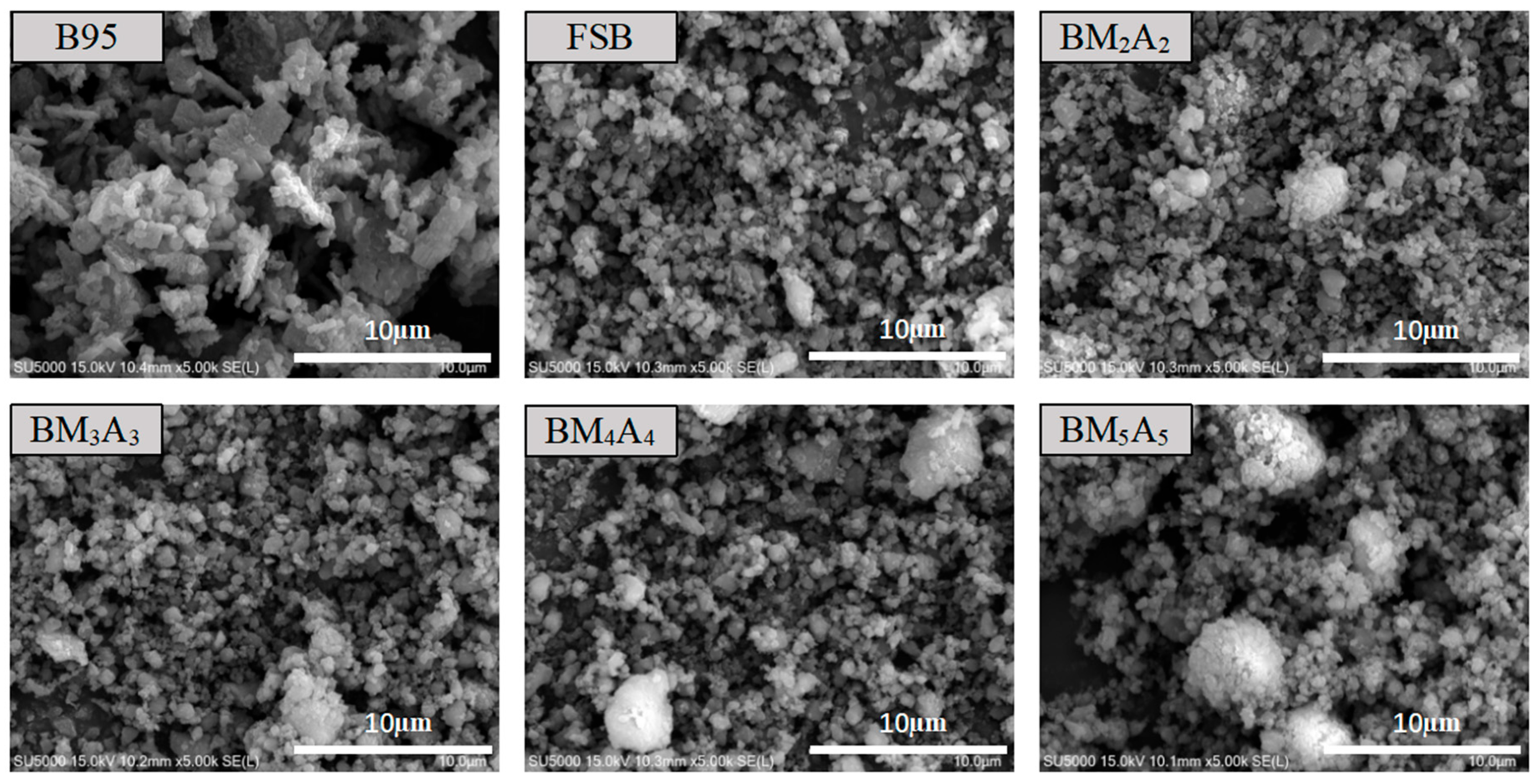
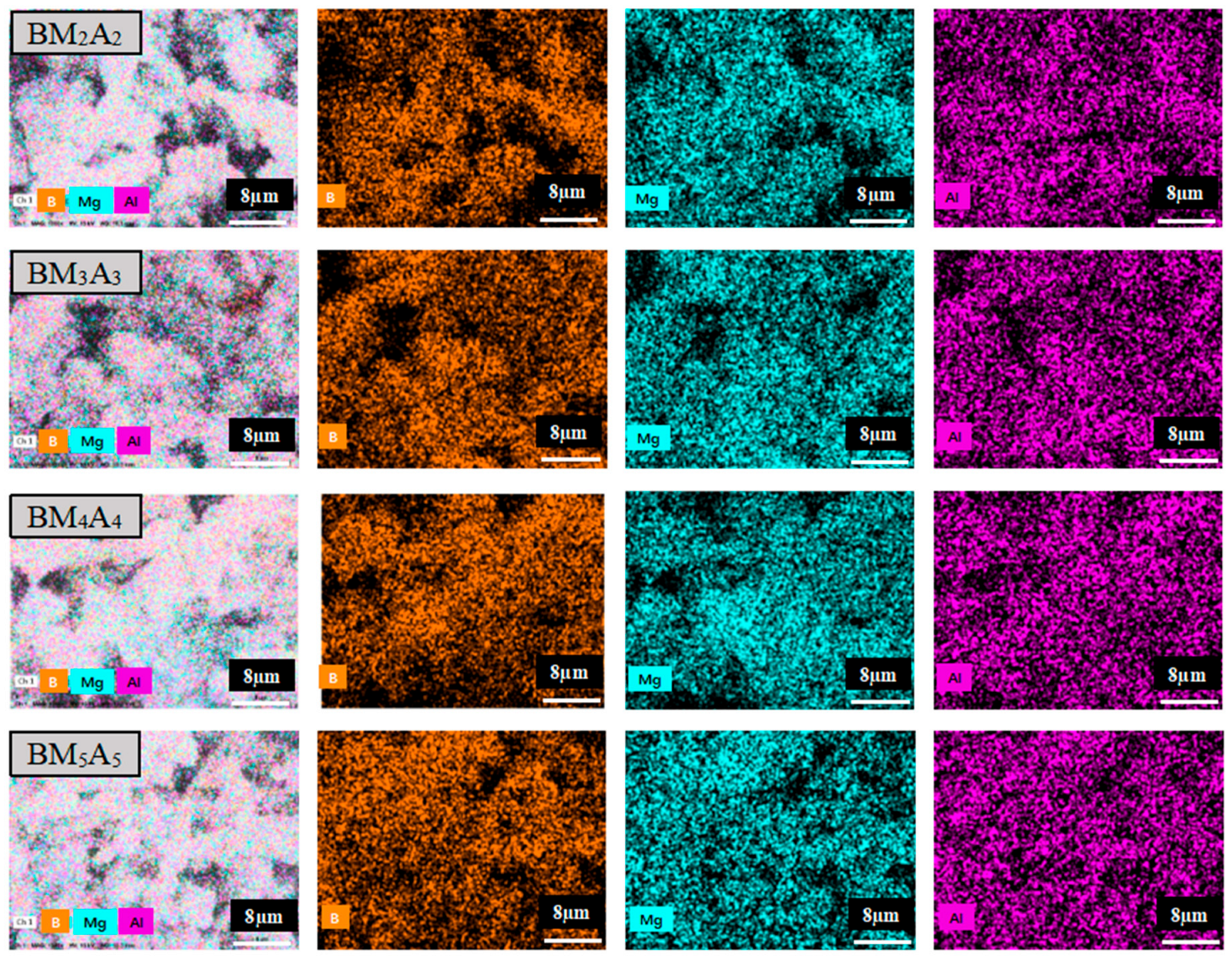
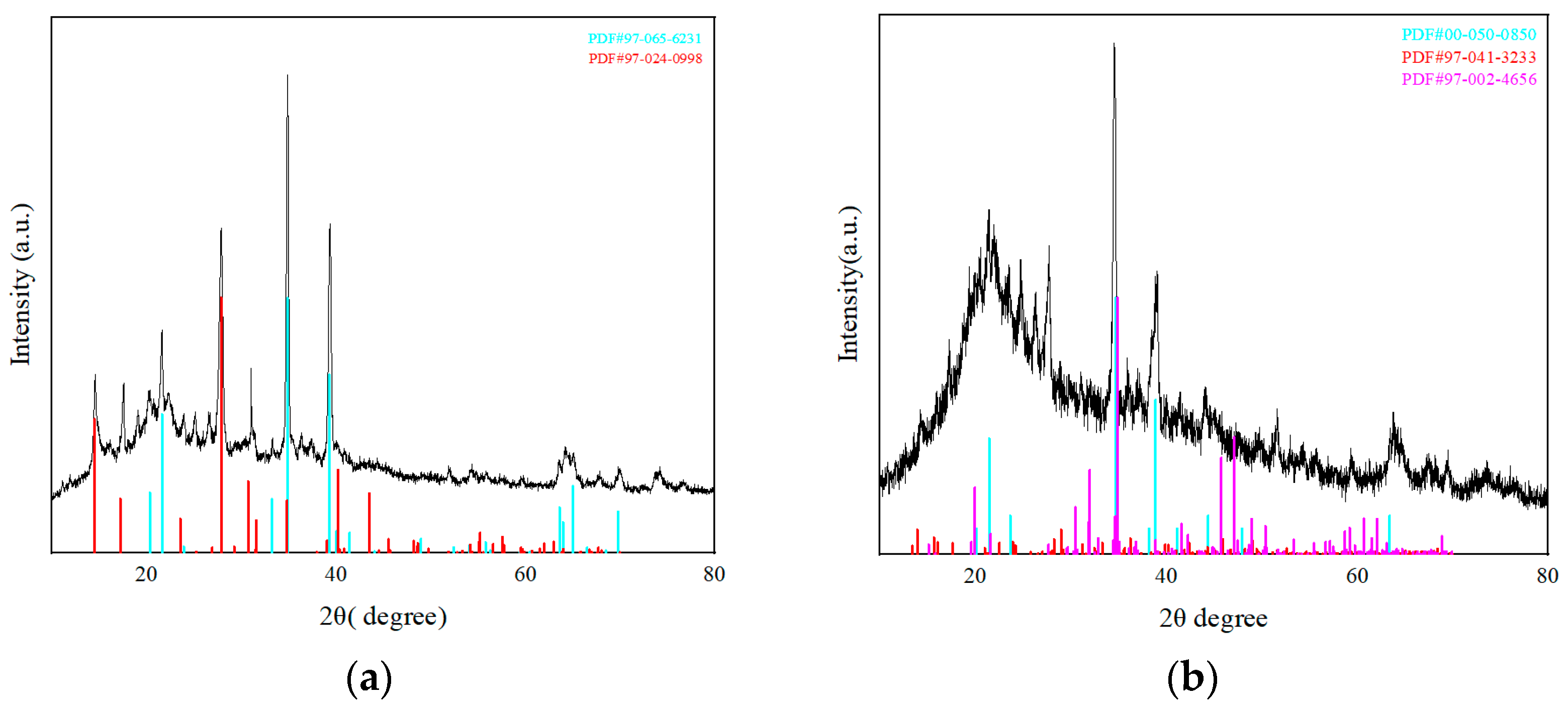
3.1.3. Microscopic Characterization
3.2. Combustion Performance
3.2.1. Ignition Delay Time
3.2.2. Combustion Rate
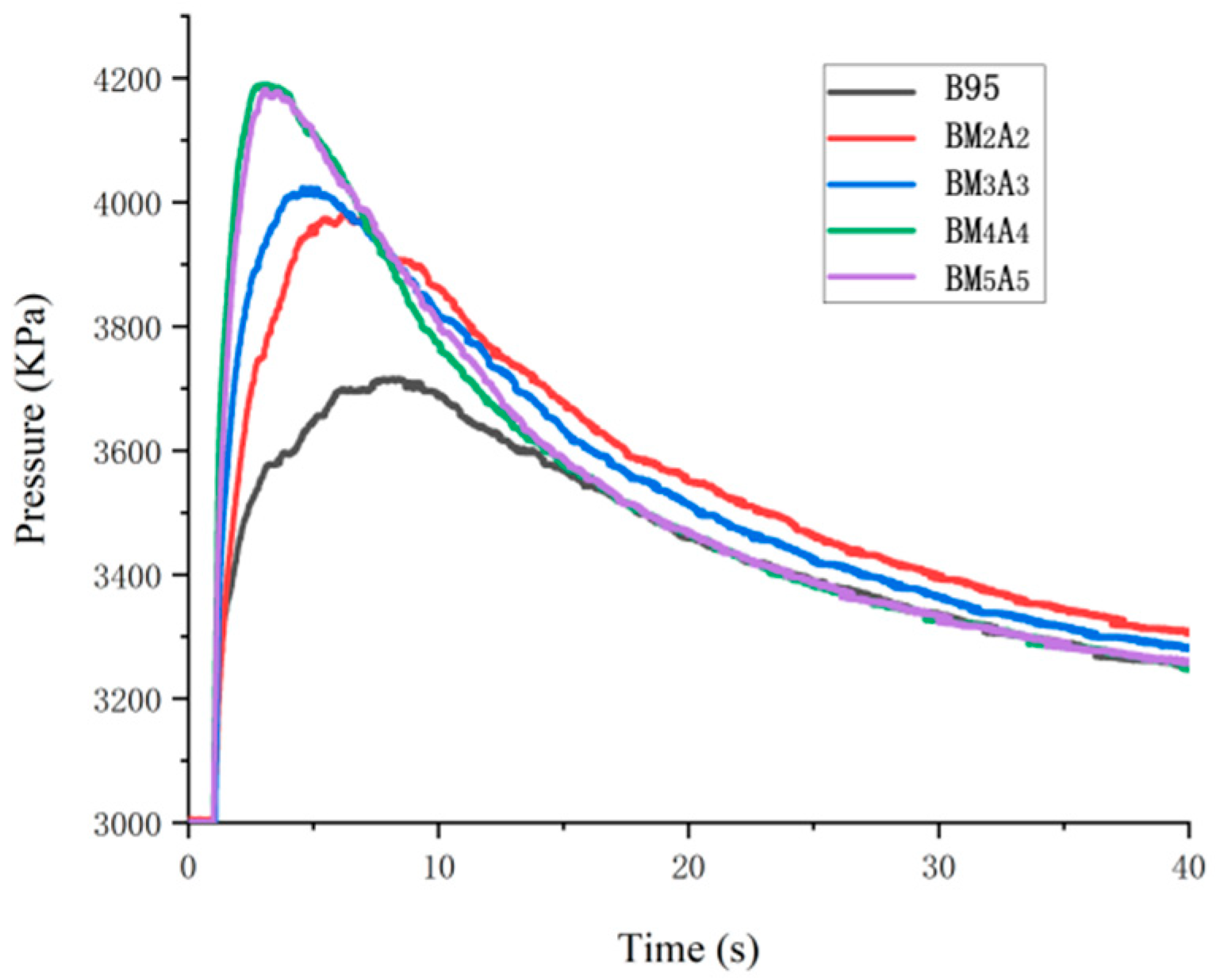
3.2.3. Combustion Pressure
3.2.4. Combustion Calorific Value
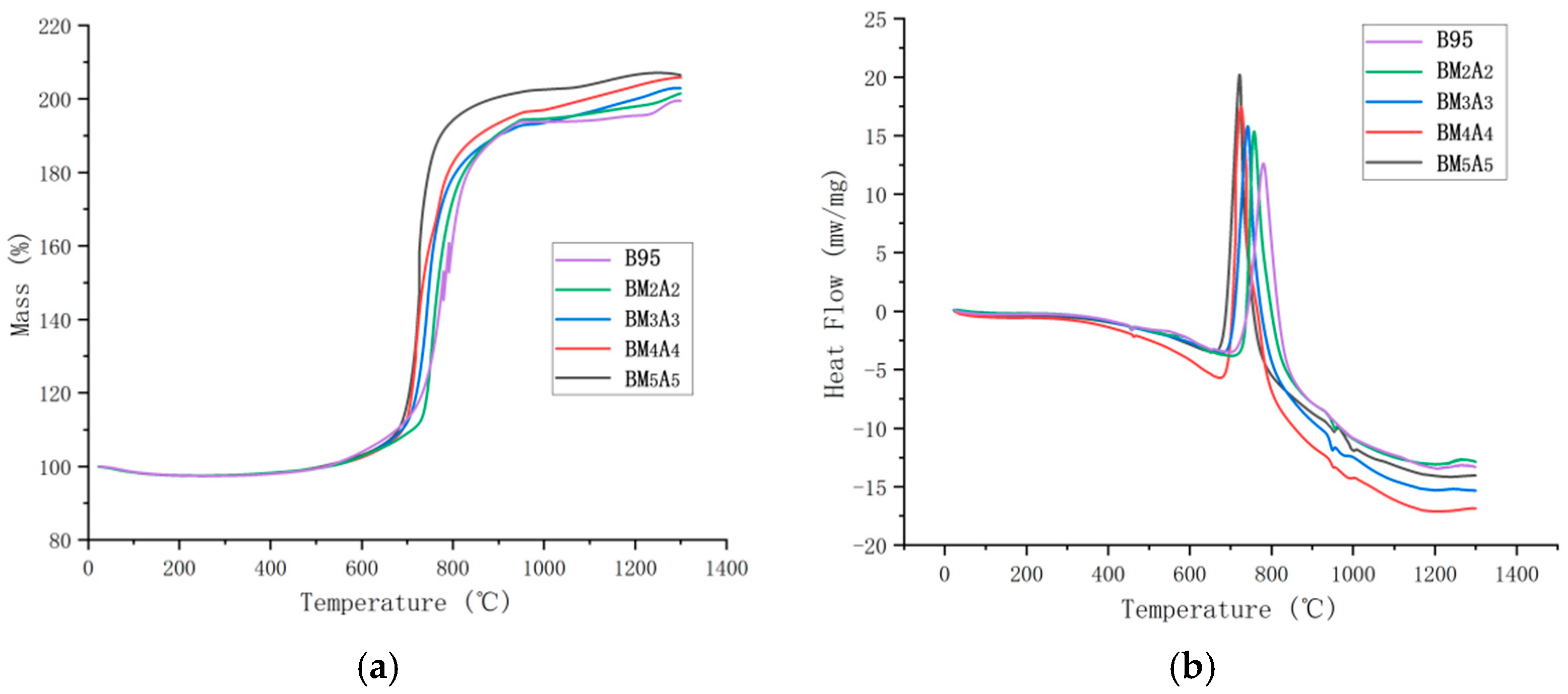
3.3. Thermal Performance Analysis
3.4. Phase Analysis of Combustion Residues
3.5. Analysis of Combustion Mechanism
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FESEM | Field Emission Scanning Electron Microscope |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| BET | Brunauer–Emmett–Teller |
| TGA | Thermogravimetric Analysis |
| DSC | Differential Scanning Calorimetry |
| XRD | X-ray Diffraction |
| SSA | Specific Surface Area |
| TGA/DSC | Thermogravimetric and Differential Scanning Calorimetry |
| SDT | Simultaneous Differential Thermal Analyzer |
References
- King, M.K. Ignition and Combustion of Boron Particles and Clouds. J. Spacecr. Rocket. 1982, 19, 294–306. [Google Scholar] [CrossRef]
- Zheng, J. Research status of combustion performance of boron based fuel rich propellant. Winged Missile 2003, 4, 50–57. [Google Scholar]
- Zang, L. A investigation on boron used as a component of solid propellant. Propuls. Technol. 1990, 79, 56–62. [Google Scholar]
- Sullivan, K.; Young, G.; Zachariah, M.R.; Yu, K. Combustion characteristics of boron nanoparticles. Combust. Flame 2009, 156, 322–333. [Google Scholar] [CrossRef]
- Zhang, C.-M.; Zhang, X.; Fang, Z.-S.; Wang, Y.-J.; Peng, H.-R. Study on electroless nickel plating on the surface of agglomerated boron powder. Nonferrous Met. Eng. 2014, 4, 17–19. [Google Scholar]
- Zhang, S.-Y.; Zhang, X.; Peng, H.-R.; Wang, Y.-J.; Yin, Y.; Zhang, K. Study on Preparation of High Purity Nano Amorphous Boron Powder. Therm. Spray Technol. 2022, 14, 23–29. [Google Scholar]
- Yetter, R.A.; Zhou, W.; Dryer, F.L.; Rabitz, H.; Brown, R.C.; Kolb, C.E. Multi-phase model for ignition and combustion of boron particles. Combust. Flame 1999, 117, 227–243. [Google Scholar] [CrossRef]
- Chintersingh, K.L.; Schoenitz, M.; Dreizin, E.L. Oxidation kinetics and combustion of boron particles with modified surface. Combust. Flame 2016, 173, 288–295. [Google Scholar] [CrossRef]
- Chintersingh, K.L.; Sun, Y.; Schoenitz, M.; Dreizin, E.L. Heterogeneous reaction kinetics for oxidation and combustion of boron. Thermochim. Acta 2019, 682, 178415. [Google Scholar] [CrossRef]
- Wang, X.; Wu, T.; Wang, H.; DeLisio, J.B.; Yang, Y.; Zachariah, M.R. Boron ignition and combustion with doped δ-Bi2O3: Bond energy/oxygen vacancy relationships. Combust. Flame 2018, 197, 127–133. [Google Scholar] [CrossRef]
- Macek, A.; Semple, J.M. Combustion of Boron Particles at Atmospheric Pressure. Combust. Sci. Technol. 1969, 1, 181–191. [Google Scholar] [CrossRef]
- Chintersingh, K.-L.; Schoenitz, M.; Dreizin, E.L. Effect of purity, sur-face modification and iron coating on ignition and combus-tion of boron in air. Combust. Sience Technol. 2021, 193, 1567–1586. [Google Scholar] [CrossRef]
- Liu, S.; Han, L.; Liu, H.; Song, Y.; Liu, L.-L.; Hu, S. Experimental and numerical study on ignition and combustion characteristics of boron-magnesium composite powders. Particuology 2024, 84, 12–29. [Google Scholar] [CrossRef]
- Mursalat, M.; Schoenitz, M.; Dreizin, E.L. Effect of particle morphology on reactivity, ignition and combustion of boron powders. Fuel 2022, 324, 124538. [Google Scholar] [CrossRef]
- Valluri, S.K.; Schoenitz, M.; Dreizin, E. Boron-metal fluoride reactive composites: Preparation and reaction sleading to their ignition. J. Porpulsion Power 2019, 35, 802–810. [Google Scholar] [CrossRef]
- Panz, W.; Feng, X.-S.; Zhang, K. Effect of fluorine containing binder on the completeness of boron powder reactionin explosion process. Initiat. Pyrotech. 2022, 5, 41–45. [Google Scholar]
- Yu, R.-T.; Ma, M.-M.; Zhang, R.; Qin, Z.; Liu, D. Ignition and combustion characteristics of single particles of boron-based composite metal fuels. Chin. J. Explos. Propellants (Huozhayao Xuebao) 2022, 45, 862–869. [Google Scholar]
- Pang, W.-Q. New progressin the research of boron based composite energetic fuels. Chin. J. Explos. Propellants (Huozhayao Xuebao) 2023, 46, 85–88. [Google Scholar]
- Glotov, O.G. Screening of metal fuels for use in composite propellants for ramjets. Prog. Aerosp. Sci. 2023, 143, 100954. [Google Scholar] [CrossRef]
- Qin, Z.; Yi, J.; Pang, W.; Wang, C.; Li, H.; Xu, H.; Qu, B.; Zhao, F.; Hao, N.; Huang, X.-F. Effect of spherical Al-Mg-Zr on the combustion characteristics of composite propellants. FirePhysChem 2022, 2, 14–19. [Google Scholar] [CrossRef]
- Song, N.; Liu, J.; Zhang, G.; Yan, Z.; Gao, H.; Yang, L. Catalytic action of submicrometer spherical Ta/Ph-Fe on combustion of AP/HTPB propellant. Propellants Explos. Pyrotech. 2018, 43, 637–641. [Google Scholar] [CrossRef]
- Li, R.-X.; Pang, W.-Q.; Yu, H.-J. Progresson Ignition and Combustion Performance of Boron-based Metal Composites. Chin. J. Explos. Propellants 2014, 47, 197–207. [Google Scholar]
- Qin, Z.; Paravan, C.; Colombo, G.; Deluca, L.T.; Shen, R.; Ye, Y. Ignition temperature of metal fuel in different atmosphere. Initiat. Pyrotech. 2014, 4, 24–27. [Google Scholar]
- Pace, K.K.; Jmymowycz, T.A.; Yang, V. Effect of Magnesium-Coated Boron Particles on Burning Characteristics of Solid Fuels in High-Speed Crossflows. Int. J. Energetic Mater. Chem. Propuls. 1993, 2, 332–347. [Google Scholar] [CrossRef]
- Zhang, B.-Y.; Huang, C.; Yan, S.; Li, Y.-C.; Cheng, Y. Enhanced reactivity of boron, through adding nano-aluminum and wet ball milling. Appl. Surf. Sci. 2013, 286, 91–98. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.-L.; Liu, Y.-Z.; Zhao, B.-D.; Liu, W.-X.; Yan, Q.-L.; Fu, X.-L. High Calorific Values Boron Powder: Ignition and Combustion Mechanism, Surface Modification Strategies and Properties. Molecules 2023, 28, 3209. [Google Scholar] [CrossRef] [PubMed]
- Bgrimm Advanced Materials Science & Technology Co., Ltd. A Method for Preparing Active Metal Composite Boron Powder. CN105458276A, 15 December 2017. [Google Scholar]
- Wang, D.-H.; Lin, G.-Z.; Gao, D.-Y.; Li, X.-L.; Song, Q.-G.; Zheng, B.-H. Application of B/Al Compound Powde in Energetic Materials. J. Ordnance Equip. Eng. 2018, 39, 157–163. [Google Scholar]
- Korotkikh, A.G.; Sorokin, I.V.; Selikhova, E.A.; Arkhipov, V.A. Effect of B, Fe, Ti, Cu nanopowders on the laser ignition of Al-based high-energy materials. Combust. Flame 2020, 222, 103–110. [Google Scholar] [CrossRef]

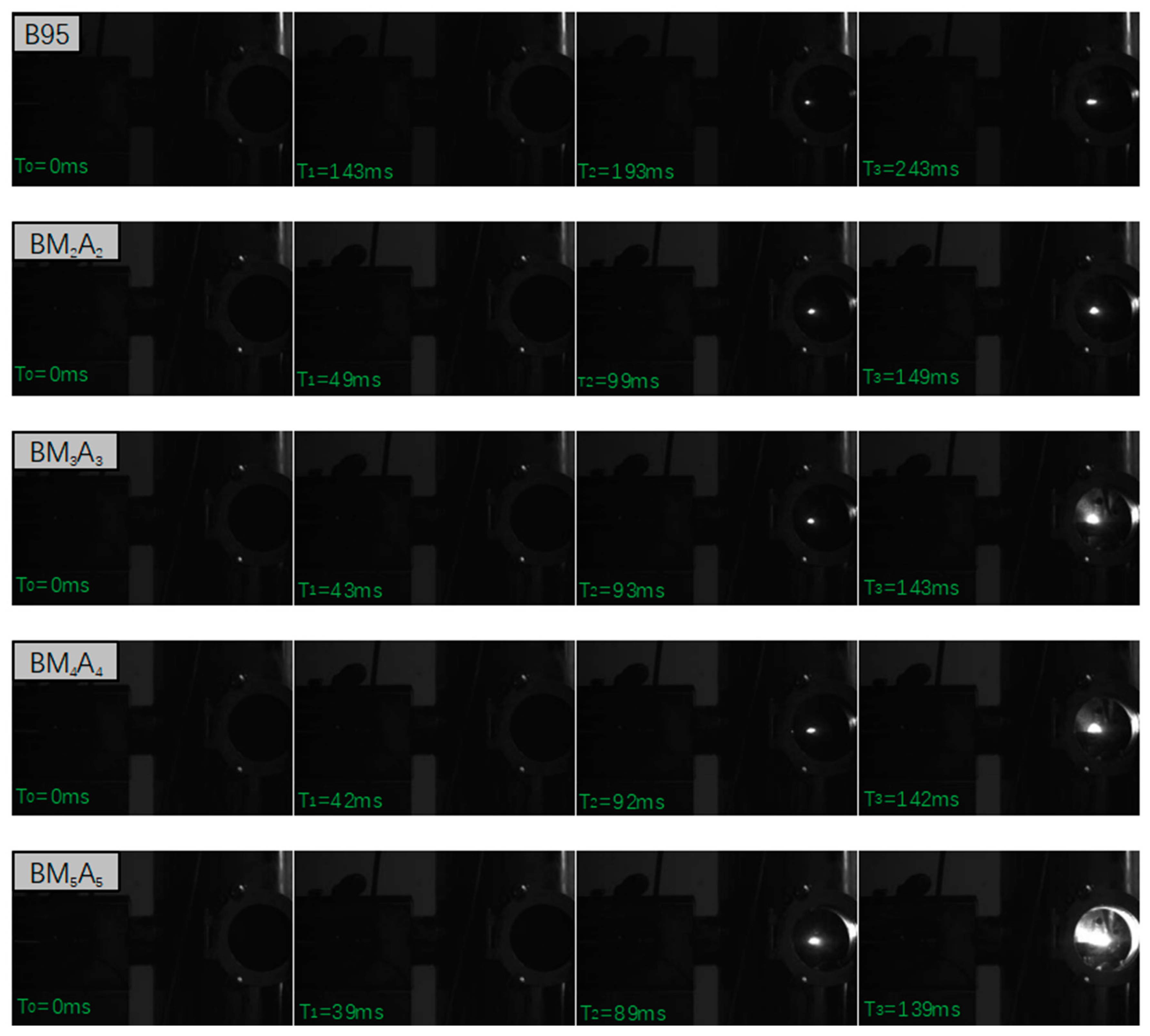
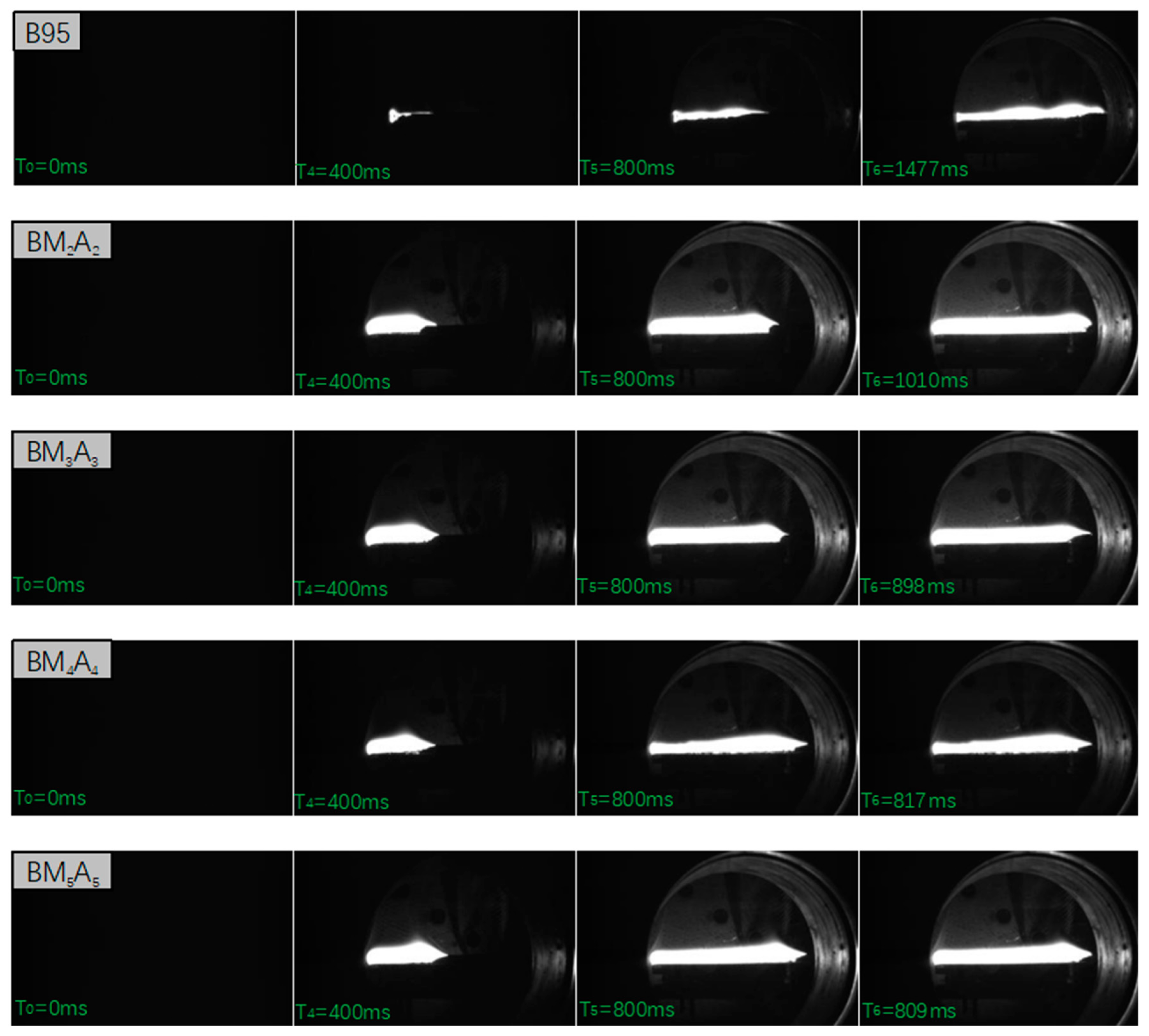
| Adhesive Type | B95 | BM2A2 | BM3A3 | BM4A4 | BM5A5 |
|---|---|---|---|---|---|
| Ignition delay time (ms) | 143 | 49 | 43 | 42 | 39 |
| Adhesive Type | Sample Length (mm) | Spread Time (ms) | Combustion Rate (m/s) |
|---|---|---|---|
| B95 | 100 | 1477 | 0.068 |
| BM2A2 | 100 | 1010 | 0.099 |
| BM3A3 | 100 | 898 | 0.112 |
| BM4A4 | 100 | 817 | 0.122 |
| BM5A5 | 100 | 809 | 0.124 |
| Adhesive Type | Tburn (s) | Ppeak (Kpa) | R (KPa/s) |
|---|---|---|---|
| B95 | 8.212 | 3716.58 | 452.58 |
| BM2A2 | 6.263 | 3983.48 | 636.03 |
| BM3A3 | 4.554 | 4023.49 | 883.51 |
| BM4A4 | 3.139 | 4190.29 | 1334.91 |
| BM5A5 | 3.094 | 4182.44 | 1351.79 |
| Adhesive Type | Theoretical Calorific Value (KJ/kg) | Measured Calorific Value (KJ/kg) | Combustion Efficiency (%) |
|---|---|---|---|
| B95 | 55,803.00 | 15,600 | 27.96 |
| FSB | 55,803.00 | 15,952 | 28.59 |
| BM2A2 | 54,686.68 | 17,057 | 31.19 |
| BM3A3 | 54,128.52 | 19,565 | 36.15 |
| BM4A4 | 53,570.36 | 21,103 | 39.39 |
| BM5A5 | 53,012.20 | 20,072 | 37.86 |
| Adhesive Type | Mass (%) | * Segregation Point Temperature/Ts (°C) | Peak Temperature/Tp (°C) | Peak Heat Release Enthalpy (J/g) |
|---|---|---|---|---|
| B95 | 99.46 | 746.65 | 779.55 | 11,230 |
| BM2A2 | 101.48 | 734.16 | 757.05 | 11,484 |
| BM3A3 | 102.93 | 703.88 | 741.55 | 11,811 |
| BM4A4 | 105.87 | 694.01 | 725.19 | 12,632 |
| BM5A5 | 107.08 | 686.18 | 721.81 | 11,962 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yu, Y.; Zhang, X.; Zhang, S. Influence of Mg/Al Coating on the Ignition and Combustion Behavior of Boron Powder. Coatings 2025, 15, 828. https://doi.org/10.3390/coatings15070828
Wang Y, Yu Y, Zhang X, Zhang S. Influence of Mg/Al Coating on the Ignition and Combustion Behavior of Boron Powder. Coatings. 2025; 15(7):828. https://doi.org/10.3390/coatings15070828
Chicago/Turabian StyleWang, Yanjun, Yueguang Yu, Xin Zhang, and Siyuan Zhang. 2025. "Influence of Mg/Al Coating on the Ignition and Combustion Behavior of Boron Powder" Coatings 15, no. 7: 828. https://doi.org/10.3390/coatings15070828
APA StyleWang, Y., Yu, Y., Zhang, X., & Zhang, S. (2025). Influence of Mg/Al Coating on the Ignition and Combustion Behavior of Boron Powder. Coatings, 15(7), 828. https://doi.org/10.3390/coatings15070828






