Preparation of S-Doped Ni-Mn-Fe Layered Hydroxide for High-Performance of Oxygen Evolution Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
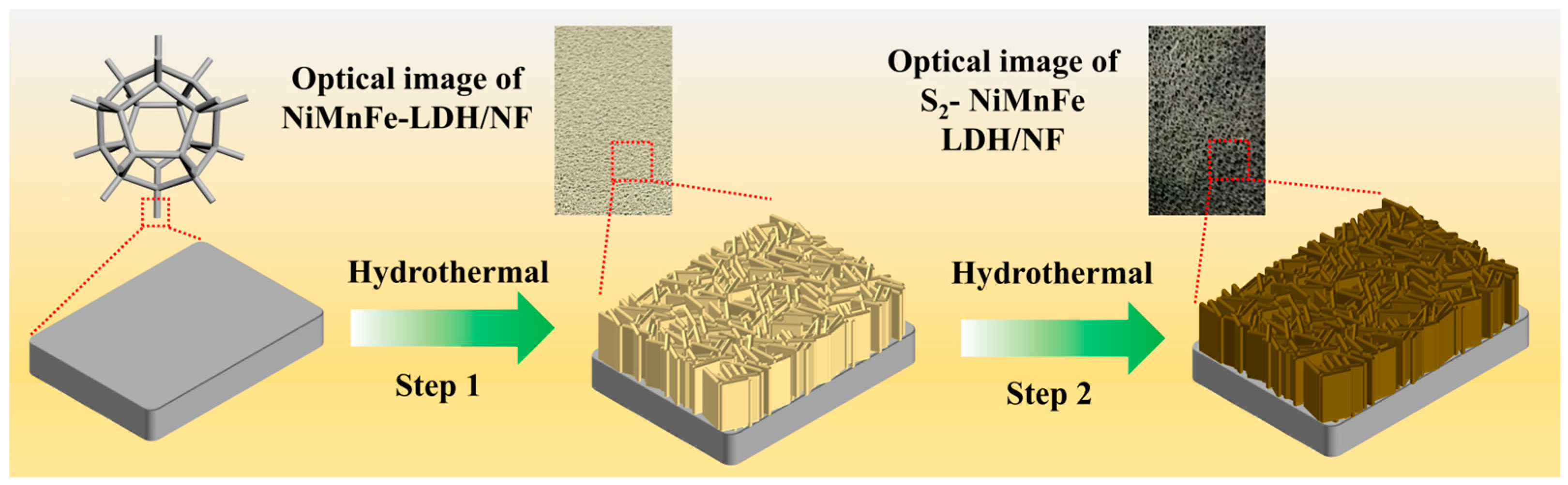
2.2. Preparation of NiMnFe LDH/NF Catalysts
2.3. Preparation of S-NiMnFe LDH/NF Catalysts
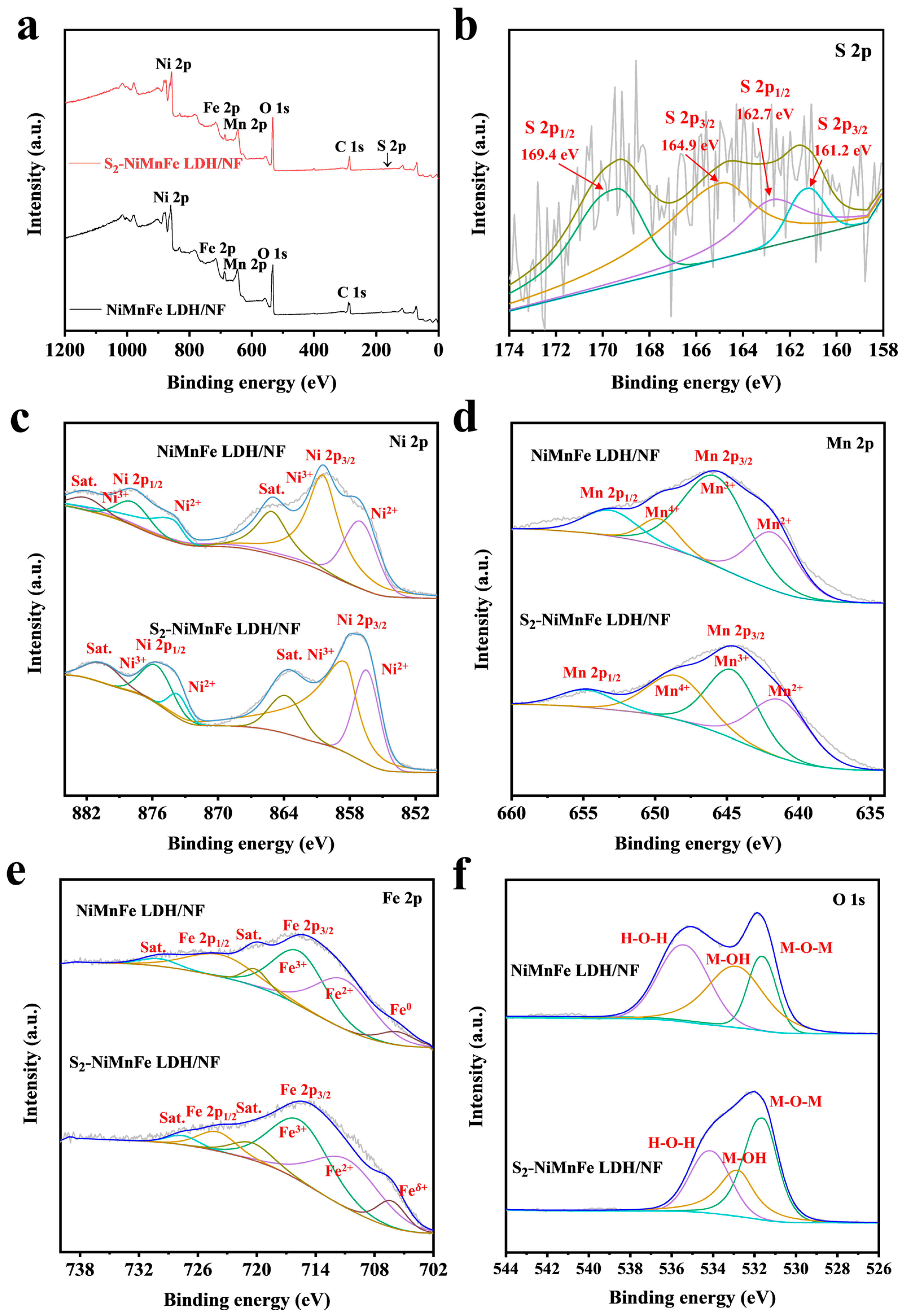
2.4. Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
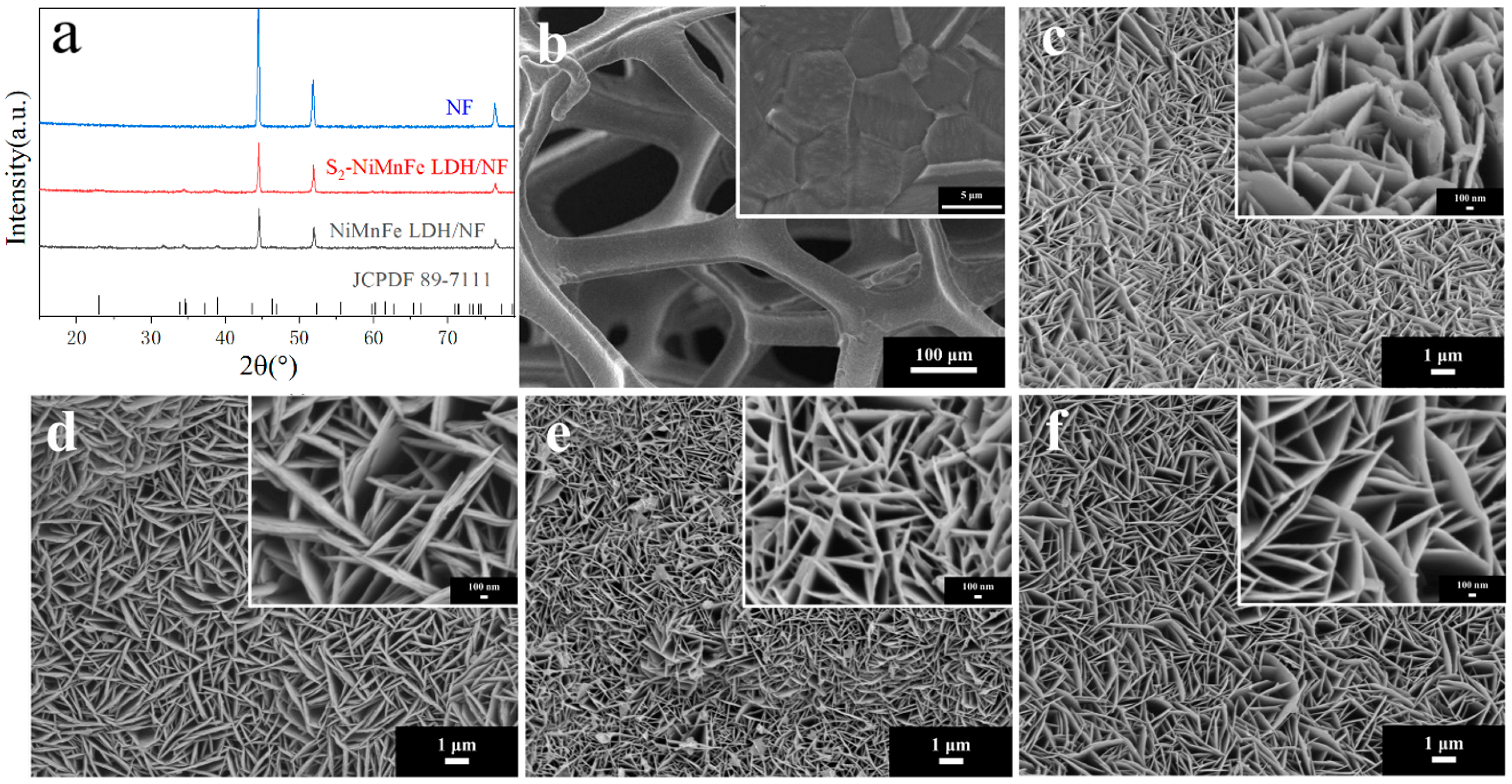
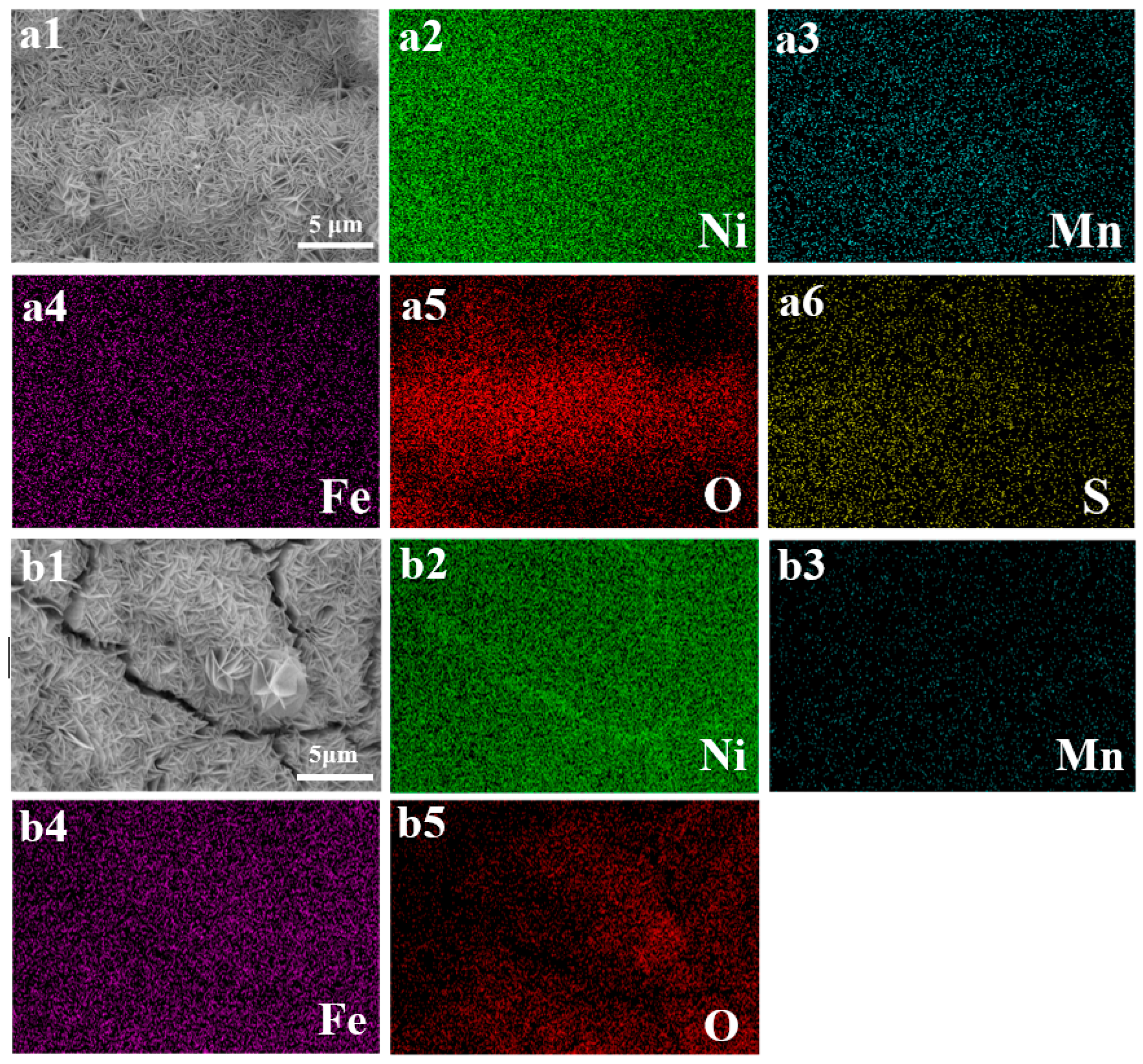
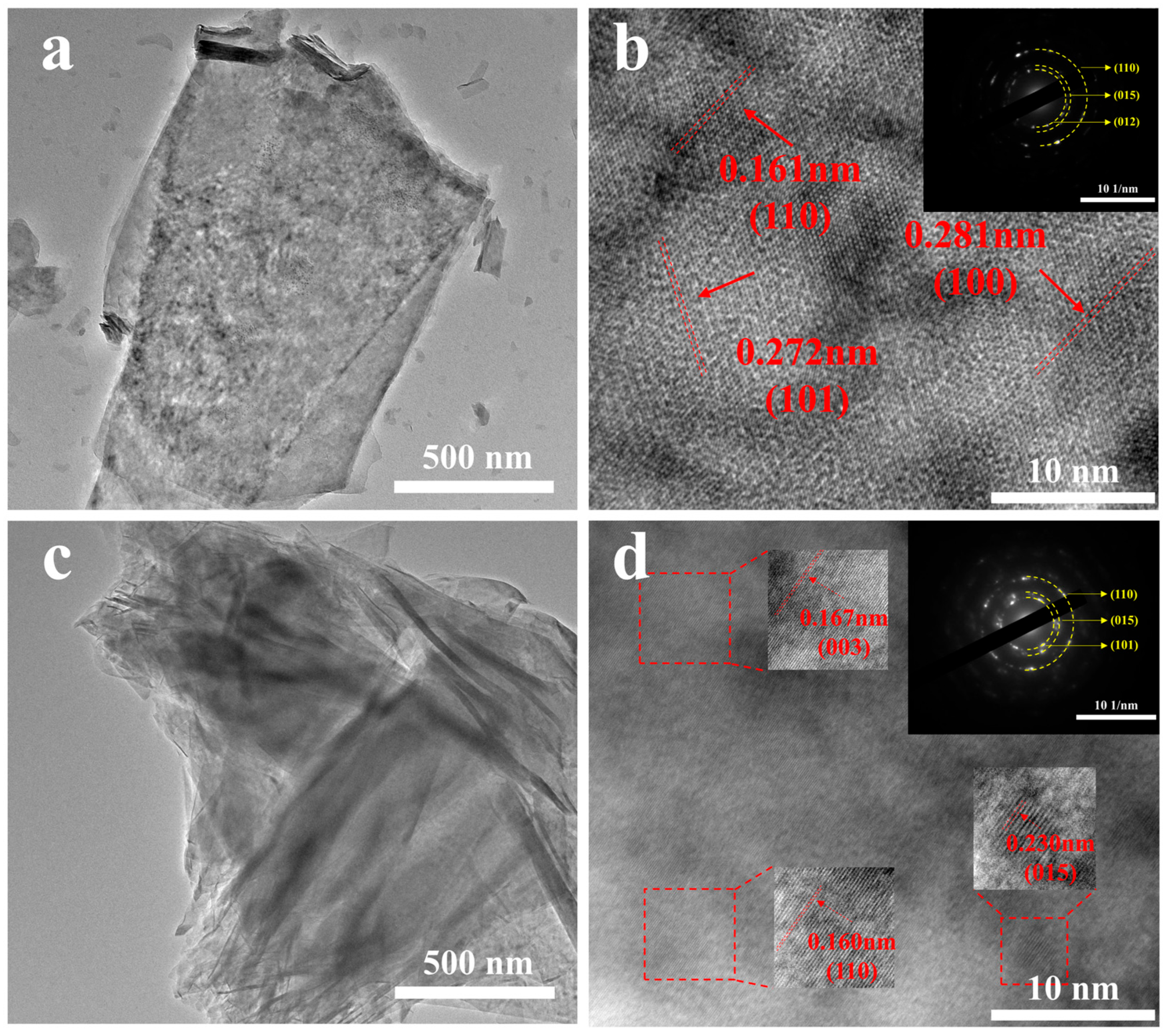
3.1. Morphological and Structural Analysis of S-NiMnFe LDH/NF
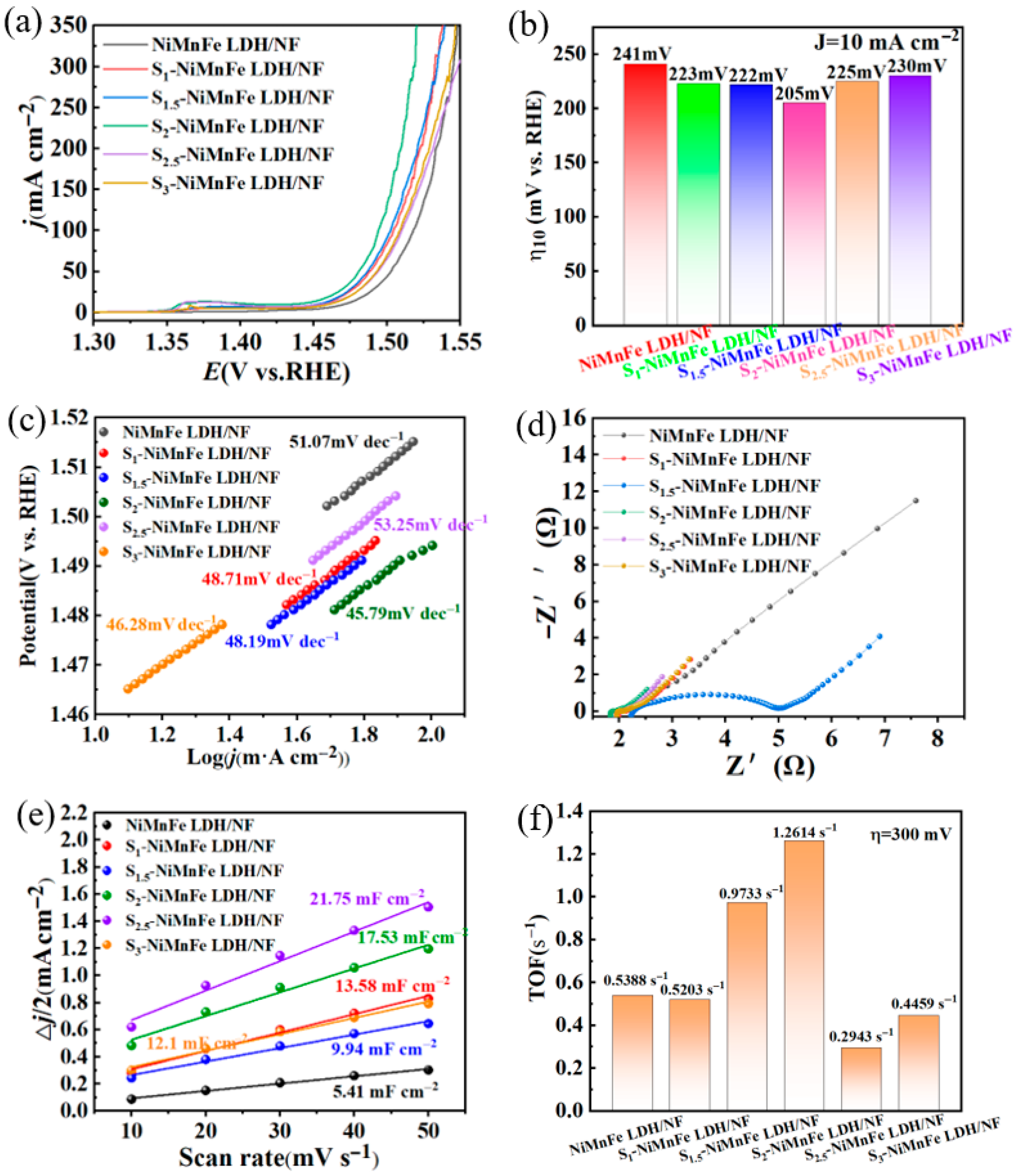
3.2. Oxygen Evolution Reaction Performance Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, B.; Gao, R.; Zou, J.; Yang, H. Surface Design Strategy of Catalysts for Water Electrolysis. Small 2022, 18, 2202336. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yao, R.; You, J.; Liu, L.; Yi, B.; Zhao, Y.; Li, Y.; Zhang, H. Non-Precious Metal High-Entropy Electrocatalysts (Al0.5NiCoCr-X0.5) for OER Application. Mater. Today Commun. 2024, 39, 109052. [Google Scholar] [CrossRef]
- Hou, C.; Xue, L.; Li, J.; Ma, W.; Wang, J.; Dai, Y.; Chen, C.; Dang, J. Unlocking the Potential of Lattice Oxygen Evolution in Stainless Steel to Achieve Efficient OER Catalytic Performance. Acta Mater. 2024, 277, 120176. [Google Scholar] [CrossRef]
- Qi, M.; Zheng, X.; Tong, H.; Liu, Y.; Li, D.; Yan, Z.; Jiang, D. Synergizing Ruthenium Oxide with Bimetallic Co2CrO4 for Highly Efficient Oxygen Evolution Reaction. J. Colloid Interface Sci. 2025, 677, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Qiu, Z.; Li, J.; Hu, L.; Li, Q.; Lv, X.; Dang, J. Interfacial Electronic Coupling of V-Doped Co2P with High-Entropy MXene Reduces Kinetic Energy Barrier for Efficient Overall Water Splitting. J. Energy Chem. 2023, 85, 301–309. [Google Scholar] [CrossRef]
- Zhao, H.; Yuan, Z. Progress and Perspectives for Solar-Driven Water Electrolysis to Produce Green Hydrogen. Adv. Energy Mater. 2023, 13, 2300254. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Yu, J.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Water Splitting: From Electrode to Green Energy System. Nano-Micro Lett. 2020, 12, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Liu, Z.; Zhang, X.; Xue, Y.; Huang, H.; Jiang, Q.; Tang, J. Enhanced OER Electrocatalyst Performance by Sulfur Doping Trimetallic Compounds Hybrid Catalyst Supported on Reduced Graphene Oxide. J. Alloys Compd. 2024, 991, 174238. [Google Scholar] [CrossRef]
- Qiao, W.; Ding, P.; Pan, J.; Yan, S.; Wang, D.; Xu, X. Thermal-Driven Coordination Adaption of Metal Sites in Layered Double Hydroxides towards High-Performance Water Oxidation. Mater. Today Commun. 2021, 29, 102836. [Google Scholar] [CrossRef]
- Liu, X.; Shao, Y.; Guo, S.; Song, Y.; Li, X.; Song, B.; Ni, J. Enhancement of Electrocatalytic Oxygen Evolution Performance for FeCrNiCoTi Alloys via Powder Modification. J. Alloys Compd. 2024, 1007, 176427. [Google Scholar] [CrossRef]
- Kim, H.; Min, K.; Song, G.; Kim, J.; Ham, H.C.; Baeck, S.-H. Hollow-Structured Cobalt Sulfide Electrocatalyst for Alkaline Oxygen Evolution Reaction: Rational Tuning of Electronic Structure Using Iron and Fluorine Dual-Doping Strategy. J. Colloid Interface Sci. 2024, 665, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shao, X.; Umair, M.M.; Hou, F.; Li, S.; Tang, Y.; Cao, L.; Yang, J. Superhydrophilic Cobalt-Doped NiFe LDH Graphite Felt with Enriched Oxygen Vacancy as an Efficient Oxygen Evolution Electrocatalyst in Alkaline Media. Int. J. Hydrogen Energy 2024, 80, 11–21. [Google Scholar] [CrossRef]
- Zhao, Y.; You, J.; Wang, Z.; Liu, G.; Huang, X.; Duan, M.; Zhang, H. High-Entropy FeCoNiCuAlV Sulfide as an Efficient and Reliable Electrocatalyst for Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2024, 70, 599–605. [Google Scholar] [CrossRef]
- Cao, K.L.A.; Ogi, T. Advanced Carbon Sphere-based Hybrid Materials Produced by Innovative Aerosol Process for High-Efficiency Rechargeable Batteries. Energy Storage Mater. 2025, 74, 103901. [Google Scholar] [CrossRef]
- Guo, T.; Li, L.; Wang, Z. Recent Development and Future Perspectives of Amorphous Transition Metal-Based Electrocata lysts for Oxygen Evolution Reaction. Adv. Energy Mater. 2022, 12, 2200827. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, X.; Wei, W.; Wang, S.; Ni, B. Recent advances in transition metal-based electrocatalysts for alkaline hydrogen evolution. J. Mater. Chem. A 2019, 25, 14971–15005. [Google Scholar] [CrossRef]
- Vedanarayanan, M.; Chen, C.-M.; Sethuraman, M.G. Efficient Hydrogen and Oxygen Evolution: Dual-Functional Electrocatalyst of Zinc Iron Layered Double Hydroxides and Nickel Cobalt Sulfides on Nickel Foam for Seawater Splitting. ACS Appl. Energy Mater. 2024, 7, 7260–7271. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Anandha Babu, G.; Harish, S.; Kumar, E.S.; Navaneethan, M. Interface Engineering of Heterogeneous NiMn Layered Double Hydroxide/Vertically Aligned NiCo2S4 Nanosheet as Highly Efficient Hybrid Electrocatalyst for Overall Seawater Splitting. Chemosphere 2024, 350, 141016. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Ruan, Y.; Lv, L.; Ji, X.; Xu, K.; Miao, L.; Jiang, J. Hierarchical NiCo2S4@NiFe LDH Heterostructures Supported on Nickel Foam for Enhanced Overall-Water-Splitting Activity. ACS Appl. Mater. Interfaces 2017, 9, 15364–15372. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, M.; Wu, Y.; Chen, R.; Guo, D.; Liu, L. Rational Design of Bifunctional Hydroxide/Sulfide Heterostructured Catalyst for Efficient Electrochemical Seawater Splitting. J. Colloid Interface Sci. 2023, 647, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Li, H.; Liu, Y.; Huang, J.; Li, Y. Amorphous FeOOH Decorated Hierarchy Capillary-Liked CoAl LDH Catalysts for Efficient Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2021, 46, 21289–21297. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Fang, Z.; Xu, L.; Lu, C.; Hou, W. Ultrafast Room-Temperature Synthesis of Self-Supported NiFe-Layered Double Hydroxide as Large-Current–Density Oxygen Evolution Electrocatalyst. Small 2022, 18, 2104354. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Kong, L.; Zhang, X.; Wei, T.; Yin, J.; Cheng, K.; Ye, K.; Zhu, K.; Yan, J.; Cao, D.; et al. Vertical Nickel-Iron Layered Double Hydroxide Nanosheets Grown on Hills-like Nickel Framework for Efficient Water Oxidation and Splitting. Int. J. Hydrogen Energy 2020, 45, 3986–3994. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Duan, S.; Wang, T.; Li, Q. Tuning the Oxygen Evolution Electrocatalysis on NiFe-Layered Double Hydroxides via Sulfur Doping. Chin. J. Catal. 2020, 41, 847–852. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, Z.; Lin, J.; Zhang, K.; Li, N.; Zhao, C. Hydrolysis Assisted in-Situ Growth of 3D Hierarchical FeS/NiS/Nickel Foam Electrode for Overall Water Splitting. Electrochim. Acta 2020, 332, 135534. [Google Scholar] [CrossRef]
- Ganesan, P.; Sivanantham, A.; Shanmugam, S. Inexpensive Electrochemical Synthesis of Nickel Iron Sulphides on Nickel Foam: Super Active and Ultra-Durable Electrocatalysts for Alkaline Electrolyte Membrane Water Electrolysis. J. Mater. Chem. A 2016, 4, 16394–16402. [Google Scholar] [CrossRef]
- Guo, X.L.; Liu, X.Y.; Hao, X.D.; Zhu, S.J.; Dong, F.; Wen, Z.Q.; Zhang, Y.X. Nickel-Manganese Layered Double Hydroxide Nanosheets Supported on Nickel Foam for High-Performance Supercapacitor Electrode Materials. Electrochim. Acta 2016, 194, 179–186. [Google Scholar] [CrossRef]
- Lv, X.; Chen, L.; Min, X.; Lin, X.; Ni, Y. Flower-like MnNi2O4-MnNi2S4 Core@shell Composite Electrode as Battery-Type Supercapacitors. J. Energy Storage 2022, 55, 105792. [Google Scholar] [CrossRef]
- Jiang, M.; Li, L.; Fu, L.; Zuo, Y.; Wang, S.; Zhang, L.; Zhang, G. Scalable Room-Temperature Synthesis of NiFe Oxyhydroxide Tailored with S Atoms for Enhanced Oxygen Evolution in Alkaline Seawater and Domestic Sewage. Int. J. Hydrogen Energy 2024, 51, 31–43. [Google Scholar] [CrossRef]
- Tang, T.; Zeng, L.; Liang, Y.; Jiang, S.; Dong, R.; Xu, X.; Wang, F. NiMn Layered Double Hydroxide Nanoarrays with High Mass-Loading Prepared via a Differentiated Deposition Strategy for High-Performance Supercapacitor Electrode. J. Energy Storage 2023, 73, 109132. [Google Scholar] [CrossRef]
- Li, D.; Wan, W.; Wang, Z.; Wu, H.; Wu, S.; Jiang, T.; Cai, G.; Jiang, C.; Ren, F. Self-Derivation and Surface Reconstruction of Fe-Doped Ni3S2 Electrode Realizing High-Efficient and Stable Overall Water and Urea Electrolysis. Adv. Energy Mater. 2022, 12, 2201913. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, Z.; Xing, H.; Fei, S.; Li, J.; Ma, L.; Li, Y.; Liu, D. Sulfur-Doping/Leaching Induced Structural Transformation toward Boosting Electrocatalytic Water Splitting. Appl. Catal. B 2022, 305, 121030. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, H.; Hong, X.; Tang, J. Non-Noble Metal High Entropy Sulfides for Efficient Oxygen Evolution Reaction Catalysis. Appl. Surf. Sci. 2024, 642, 158598. [Google Scholar] [CrossRef]
- Lei, H.; Ma, L.; Wan, Q.; Tan, S.; Yang, B.; Wang, Z.; Mai, W.; Fan, H. Promoting Surface Reconstruction of NiFe Layered Double Hydroxide for Enhanced Oxygen Evolution. Adv. Energy Mater. 2022, 12, 2202522. [Google Scholar] [CrossRef]
- Liu, Y.; Vijayakumar, P.; Liu, Q.; Sakthivel, T.; Chen, F.; Dai, Z. Shining Light on Anion-Mixed Nanocatalysts for Efficient Water Electrolysis: Fundamentals, Progress, and Perspectives. Nano-Micro Lett. 2022, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Wen, J.; Chong, X.; Dai, X.; Yang, Y.; Zhao, J. Sulfur Doping and Heterostructure on NiSe@Co(OH)2 with Facilitated Surface Reconstruction and Interfacial Electron Regulation to Boost Oxygen Evolution Reaction. Fuel 2025, 384, 133978. [Google Scholar] [CrossRef]
- Oh, S.; Roh, H.; Im, H.; Seo, O.; Takagi, Y.; Watanabe, T.; Han, J.W.; Yong, K. Mn Doped Hierarchical Water Splitting Electrocatalyst: Synthesis, Surface Analysis and Application to AEMWE. Chem. Eng. J. 2024, 500, 157106. [Google Scholar] [CrossRef]
- Gao, G.; Sun, Z.; Chen, X.; Zhu, G.; Sun, B.; Yamauchi, Y.; Liu, S. Recent Advances in Ru/Ir-Based Electrocatalysts for Acidic Oxygen Evolution Reaction. Appl. Catal. B 2024, 343, 123584. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Chavan, H.S.; Jo, Y.; Im, H.; Kim, H. Nonprecious Bimetallic NiFe -layered Hydroxide Nanosheets as a Catalyst for Highly Efficient Electrochemical Water Splitting. Int. J. Energy Res. 2021, 45, 16963–16972. [Google Scholar] [CrossRef]
- Hu, R.; Jiang, H.; Xian, J.; Mi, S.; Wei, L.; Fang, G.; Guo, J.; Xu, S.; Liu, Z.; Jin, H.; et al. Pearson’s Principle-Inspired Robust 2D Amorphous Ni-Fe-Co Ternary Hydroxides on Carbon Textile for High-Performance Electrocatalytic Water Splitting. Nanomaterials 2022, 12, 2416. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.I. Chorkendorff, Identification of active edge sites for electro chemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Qayum, A.; Peng, X.; Yuan, J.; Qu, Y.; Zhou, J.; Huang, Z.; Xia, H.; Liu, Z.; Tan, D.; Chu, P.; et al. Highly durable and efficient Ni-FeOx/FeNi3 electrocatalysts synthesized by a facile in situ combustion-based method for overall water splitting with large current densities. ACS Appl. Mater. Interfaces 2022, 14, 27842–27853. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Baldwin, M.J.; Lowery, M.D. Electronic structures of active sites in copper proteins: Contributions to reactivity. Chem. Rev. 1992, 92, 521–542. [Google Scholar] [CrossRef]
- Conesa, J.C. Electronic structure of the (undoped and Fe-doped) NiOOH O2 evolution electrocatalyst. J. Phys. Chem. C 2016, 120, 18999–19010. [Google Scholar] [CrossRef]
- Du, X.; Huang, J.; Zhang, J.; Yan, Y.; Wu, C.; Hu, Y.; Yan, C.; Lei, T.; Chen, W.; Fan, C.; et al. Modulating electronic structures of inorganic nanomaterials for efficient electrocatalytic water splitting. Angew. Chem. Int. Ed. 2019, 58, 4484–4502. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, M.; Geng, H.; Dong, H.; Wu, P.; Li, X.; Guan, G.; Wang, T. Synergistically tuning electronic structure of porous β-Mo2C spheres by Co doping and Mo-vacancies defect engineering for optimizing hydrogen evolution reaction activity. Adv. Funct. Mater. 2020, 30, 2000561. [Google Scholar] [CrossRef]
- Xiong, L.; Qiu, Y.; Peng, X.; Liu, Z.; Chu, P.K. Electronic structural engineering of transition metal-based electrocatalysts for the hydrogen evolution reaction. Nano Energy 2022, 104, 107882. [Google Scholar] [CrossRef]
- Chen, P.; Xu, K.; Tao, S.; Zhou, T.; Tong, Y.; Ding, H.; Zhang, L.; Chu, W.; Wu, C.; Xie, Y. Phase-transformation engineering in cobalt diselenide realizing enhanced catalytic activity for hydrogen evolution in an alkaline medium. Adv. Mater. 2016, 28, 7527–7532. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, F.; Chen, W.; Xiang, Q.; Ma, Y.; Zhu, H.; Tao, P.; Song, C.; Shang, W.; Deng, T.; et al. Coupling interface constructions of MoS2/Fe5Ni4S8 heterostructures for efficient electrochemical water splitting. Adv. Mater. 2018, 30, 1803151. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guan, J.; Yang, S.; Yu, Y.; Shao, R.; Zhang, Z.; Dou, M.; Wang, F.; Xu, Q. Metal-organic-framework-derived Co2P nanopar ticle/multi-doped porous carbon as a trifunctional electrocatalyst. Adv. Mater. 2020, 32, 2003649. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.R.; Kang, Z.Y.; Luo, S.X.; Li, J.; Deng, P.L.; Wang, C.T.; Hua, Y.J.; Zhong, S.K.; Tian, X.L. Boosting NiFe-LDH by Ru- thenium Dioxide for Effective Overall Water Splitting. Int. J. Hydrogen Energy 2021, 46, 8888–8897. [Google Scholar]
- Gao, T.Q.; Zhou, Y.Q.; Zhao, X.J.; Liu, Z.H.; Chen, Y. Borate Anion-Intercalated NiV-LDH Nanoflakes/NiCoP Nanowires Het erostructures for Enhanced Oxygen Evolution Selectivity in Seawater Splitting. Adv. Funct. Mater. 2024, 23, 15949. [Google Scholar]
- Zhang, R.-L.; Duan, J.-J.; Feng, J.-J.; Mei, L.-P.; Zhang, Q.-L.; Wang, A.-J. Walnut Kernel-like Iron-Cobalt-Nickel Sulfide Nanosheets Directly Grown on Nickel Foam: A Binder-Free Electrocatalyst for High-Efficiency Oxygen Evolution Reaction. J. Colloid Interface Sci. 2021, 587, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Ahmad, I.; Rashid, N.; Hussain, S.; Zairov, R.; Alsaiari, M.; Alkorbi, A.S.; Ullah, Z.; urRehman, H.; Nazar, M.F. Effective CuO/CuS Heterostructures Catalyst for OER Performances. Int. J. Hydrogen Energy 2023, 48, 31142–31151. [Google Scholar] [CrossRef]
- Chang, J.; Zang, S.; Song, F.; Wang, W.; Wu, D.; Xu, F.; Jiang, K.; Gao, Z. Heterostructured Nickel, Iron Sulfide@nitrogen, Sulfur Co-Doped Carbon Hybrid with Efficient Interfacial Charge Redistribution as Bifunctional Catalyst for Water Electrolysis. Appl. Catal. A 2022, 630, 118459. [Google Scholar] [CrossRef]
- Naderi, A.; Yong, X.; Karamad, M.; Cai, J.; Zang, Y.; Gates, I.; Siahrostami, S.; Wang, G. Ternary Cobalt-Iron Sulfide as a Robust Electrocatalyst for Water Oxidation: A Dual Effect from Surface Evolution and Metal Doping. Appl. Surf. Sci. 2021, 542, 148681. [Google Scholar] [CrossRef]
- Hou, J.; Zhou, X.; Yang, Z.; Nie, H. The Electrochemical Synthesis of CNTs/N-Cu2S Composites as Efficient Electrocatalysts for Water Oxidation. J. Nanoparticle Res. Interdiscip. Forum Nanoscale Sci. Technol. 2020, 22, 1–12. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, K.; Li, X.; Yang, K.; Zha, Q.; Ni, Y. Amorphous Mo-Fe-Ni-S Nanospheres Electrochemically Deposited on Ni Foam for Boosting Water Oxygen Performances. Mater. Res. Bull. 2023, 165, 112302. [Google Scholar] [CrossRef]
- Wen, F.; Pang, L.; Zhang, T.; Huang, X.; Li, C.; Liu, H. Conductive Nitrogen-Doped Carbon Armored MOF-Derived Fe Doped Nickel Sulfide for Efficient Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2024, 57, 263–272. [Google Scholar] [CrossRef]
- Huang, R.; Chen, W.; Zhang, Y.; Huang, Z.; Dai, H.; Zhou, Y.; Wu, Y.; Lv, X. Well-Designed Cobalt-Nickel Sulfide Microspheres with Unique Peapod-like Structure for Overall Water Splitting. J. Colloid Interface Sci. 2019, 556, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, D.; Srinivas, K.; Yu, H.; Ma, F.; Zhang, Z.; Wu, Y.; Li, X.; Wang, Y.; Chen, Y. Ni3S4/FeNi2S4 Heterostructure-Embedded Metal-Organic Framework-Derived Nanosheets Interconnected by Carbon Nanotubes for Boosting Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2024, 51, 820–827. [Google Scholar] [CrossRef]
- Khan, M.A.; Li, H.; Zeng, Y.; Muhammed, A.Y.A.; Lu, F.; Zhou, M. The Synthesis of Highly Efficient NiFe hydroxide@CoS Electrocatalyst for Oxygen Evolution Reaction. J. Mater. Sci. 2022, 57, 7804–7813. [Google Scholar] [CrossRef]
- Li, Q.; Tang, S.; Tang, Z.; Zhang, Q.; Yang, W. Microwave-Assisted Synthesis of FeCoS2/XC-72 for Oxygen Evolution Reaction. Solid State Sci. 2019, 96, 105968. [Google Scholar] [CrossRef]
- Zhan, C.; Liu, Z.; Zhou, Y.; Guo, M.; Zhang, X.; Tu, J.; Ding, L.; Cao, Y. Triple Hierarchy and Double Synergies of NiFe/Co9S8/Carbon Cloth: A New and Efficient Electrocatalyst for the Oxygen Evolution Reaction. Nanoscale 2019, 11, 3378–3385. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, L.; Zhu, Q.; Huang, C.; Yu, Y. Defective and Ultrathin NiFe LDH Nanosheets Decorated on V-Doped Ni3S2 Nanorod Arrays: A 3D Core-Shell Electrocatalyst for Efficient Water Oxidation. J. Mater. Chem. A 2019, 7, 18118–18125. [Google Scholar] [CrossRef]

| Sample | Average Pores Volume (cm3/g) | Average Specific Surface Area (m2/g) |
|---|---|---|
| NiMnFe LDH/NF | 0.042 | 6.382 |
| S1-NiMnFe LDH/NF | 0.041 | 6.271 |
| S2-NiMnFe LDH/NF | 0.050 | 8.189 |
| S3-NiMnFe LDH/NF | 0.034 | 5.412 |
| Sample | Overpotential (mV) at 10 mA cm−2 | Tafel (mV dec−1) | TOF (S−1) |
|---|---|---|---|
| NiMnFe LDH/NF | 241 | 51.07 | 0.5388 |
| S1-NiMnFe LDH/NF | 223 | 48.71 | 0.5203 |
| S1.5-NiMnFe LDH/NF | 222 | 48.19 | 0.9733 |
| S2-NiMnFe LDH/NF | 205 | 45.79 | 1.2614 |
| S2.5-NiMnFe LDH/NF | 225 | 53.25 | 0.2943 |
| S3-NiMnFe LDH/NF | 230 | 46.28 | 0.4459 |
| Catalyst | Current Density (mA cm−2) | Overpotential (mV) | Reference |
|---|---|---|---|
| Co3-Ni7Fe3 LDH/TGF | 10 | 252 | [12] |
| S-FeCoNiCuAlV | 20 | 362 | [13] |
| ZnFe LDH@NiCoS/NF | 10 | 285 | [17] |
| NM/NCS/NS/NF | 30 | 282 | [18] |
| NiCo2S4@NiFe LDH | 60 | 201 | [19] |
| (FeCoNi)OH-S/NF | 10 | 195 | [20] |
| FeCoNiSx/NF | 10 | 231 | [53] |
| CuO/CuS | 10 | 270 | [54] |
| Ni2FeS@NSC | 10 | 247 | [55] |
| CoxFe1-x-AO | 10 | 267 | [56] |
| CNTs/N-Cu2S | 10 | 280 | [57] |
| Mo-Fe-Ni-S/NF | 10 | 230 | [58] |
| Fe-NiS2@NC | 10 | 255 | [59] |
| CoNi2S4@CoS2/NF | 10 | 259 | [60] |
| NiFe-S@CNT | 10 | 246 | [61] |
| NiFe LDH@CoS | 10 | 259 | [62] |
| FeCoS2/XC-72 | 10 | 230 | [63] |
| NiFe/Co9S8/CC | 10 | 219 | [64] |
| V-Ni3S2@NiFe LDH | 10 | 209 | [65] |
| S2-NiMnFe LDH/NF | 10 | 205 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, S.; Guo, Y.; Ding, J.; Lu, Z. Preparation of S-Doped Ni-Mn-Fe Layered Hydroxide for High-Performance of Oxygen Evolution Reaction. Coatings 2025, 15, 825. https://doi.org/10.3390/coatings15070825
Wang J, Li S, Guo Y, Ding J, Lu Z. Preparation of S-Doped Ni-Mn-Fe Layered Hydroxide for High-Performance of Oxygen Evolution Reaction. Coatings. 2025; 15(7):825. https://doi.org/10.3390/coatings15070825
Chicago/Turabian StyleWang, Jiefeng, Shilin Li, Yifan Guo, Jiaqi Ding, and Zhi Lu. 2025. "Preparation of S-Doped Ni-Mn-Fe Layered Hydroxide for High-Performance of Oxygen Evolution Reaction" Coatings 15, no. 7: 825. https://doi.org/10.3390/coatings15070825
APA StyleWang, J., Li, S., Guo, Y., Ding, J., & Lu, Z. (2025). Preparation of S-Doped Ni-Mn-Fe Layered Hydroxide for High-Performance of Oxygen Evolution Reaction. Coatings, 15(7), 825. https://doi.org/10.3390/coatings15070825





