Effects of Pulse Ion Source Arc Voltage on the Structure and Friction Properties of Ta-C Thin Films on NBR Surface
Abstract
1. Introduction
2. Experiment Design
2.1. Sample Material
2.2. Sample Preparation
- (1)
- the cleaned nitrile rubber substrates were mounted onto a rotating support inside the vacuum chamber. The chamber was sealed and evacuated to a base pressure of 5 × 10−3 Pa. High-purity argon gas was introduced into the vacuum chamber at a pressure of 0.13 Pa. A Hall ion source was employed to excite the argon gas, generating argon ions to bombard the substrate surface. This ion cleaning process removed residual contaminants and enhanced film-substrate adhesion. The ion source was operated at a current of 60 mA, with an etching duration of 5 min.
- (2)
- A high-purity graphite target (99.999%) was used as the cathode. The ta-C deposition processes were carried out with fixed pulse frequency and pulse count. The arc voltage was varied to investigate its effect on the deposited film. Starting from the equipment’s minimum arc voltage, which is 150 V, the voltage was increased in 50 V increments up to 350 V. Five sets of ta-C films were prepared. The detailed deposition parameters are shown in Table 1.
2.3. Structural Characterization and Performance Testing
3. Results and Discussion
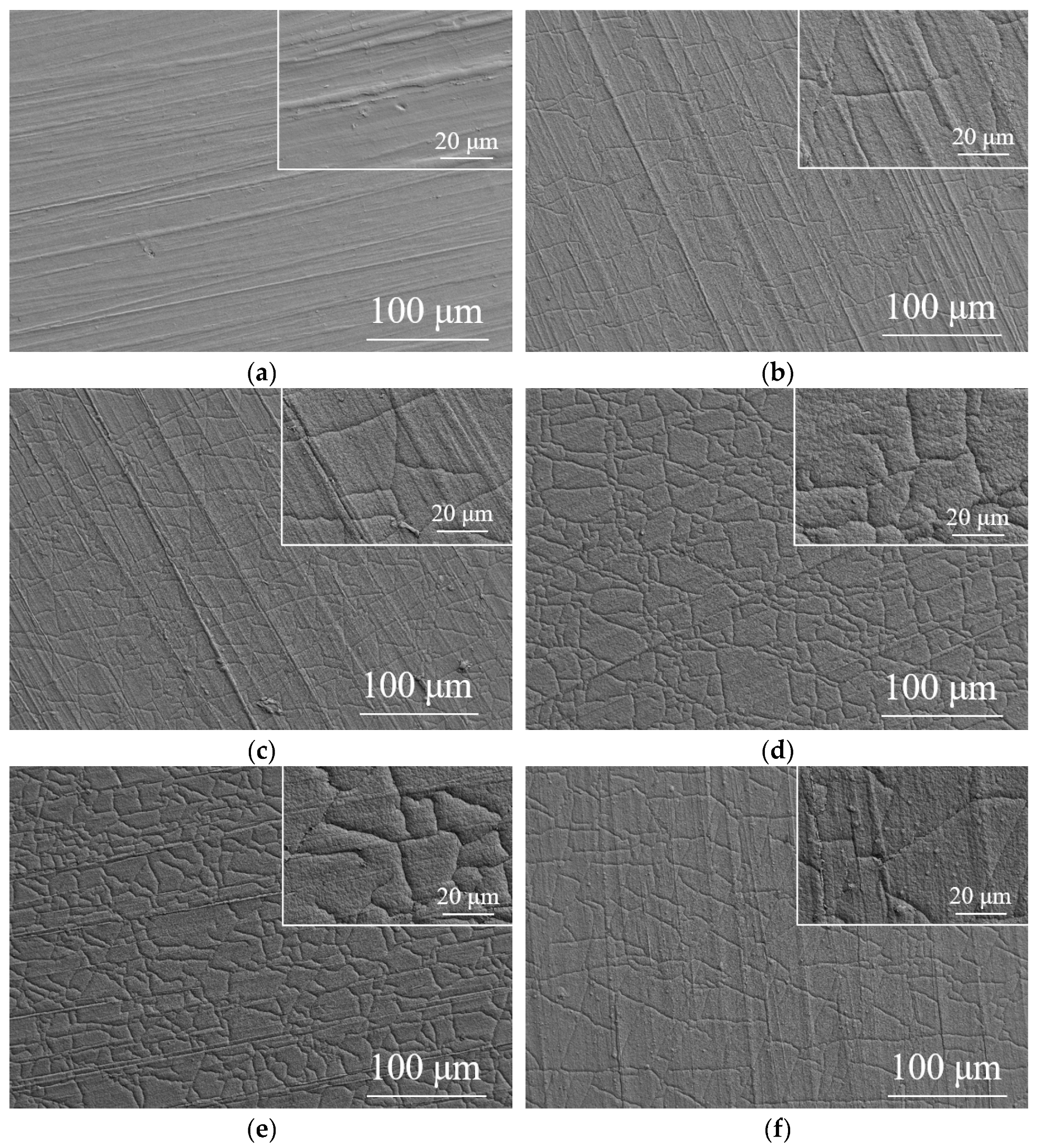
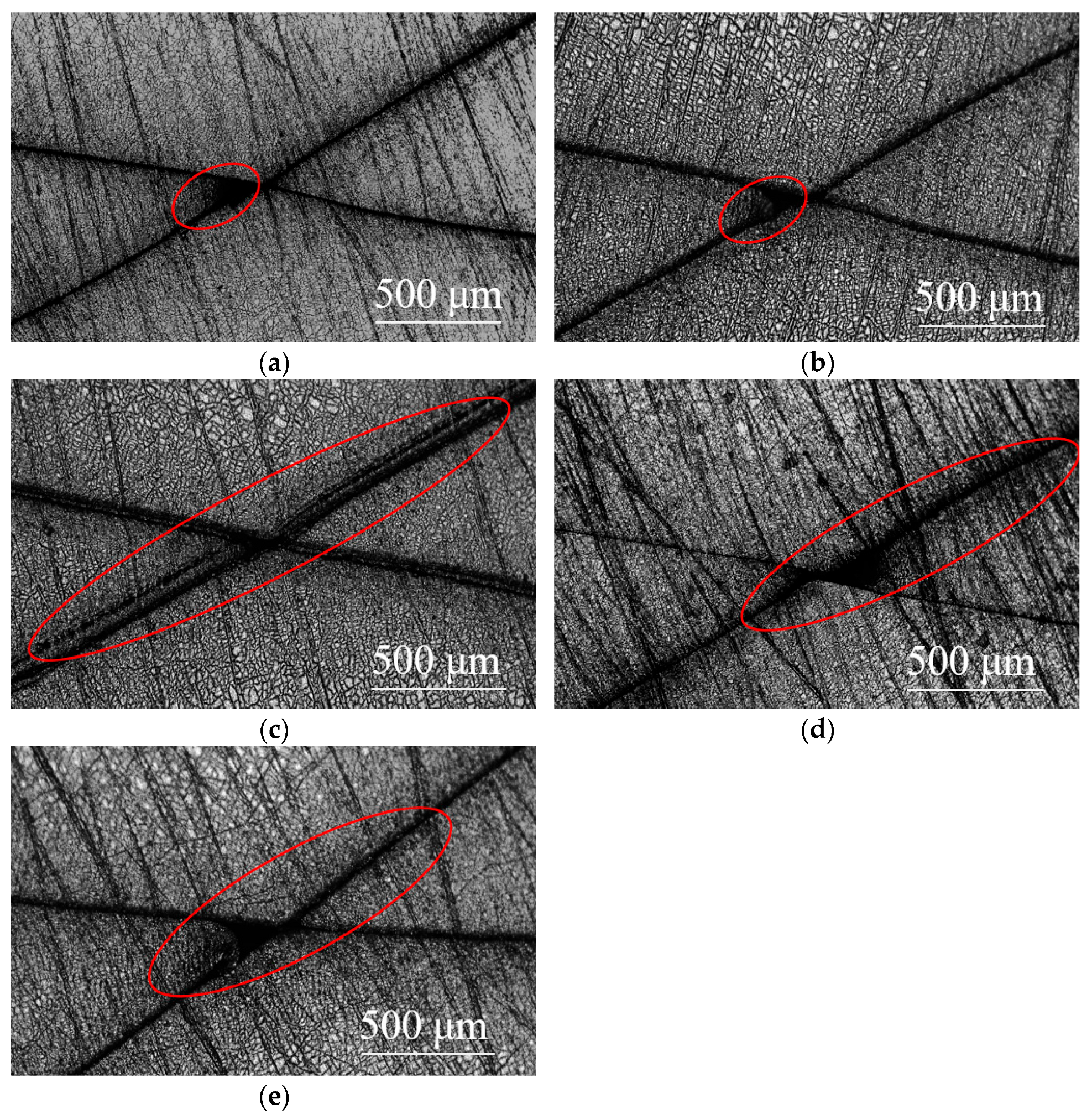
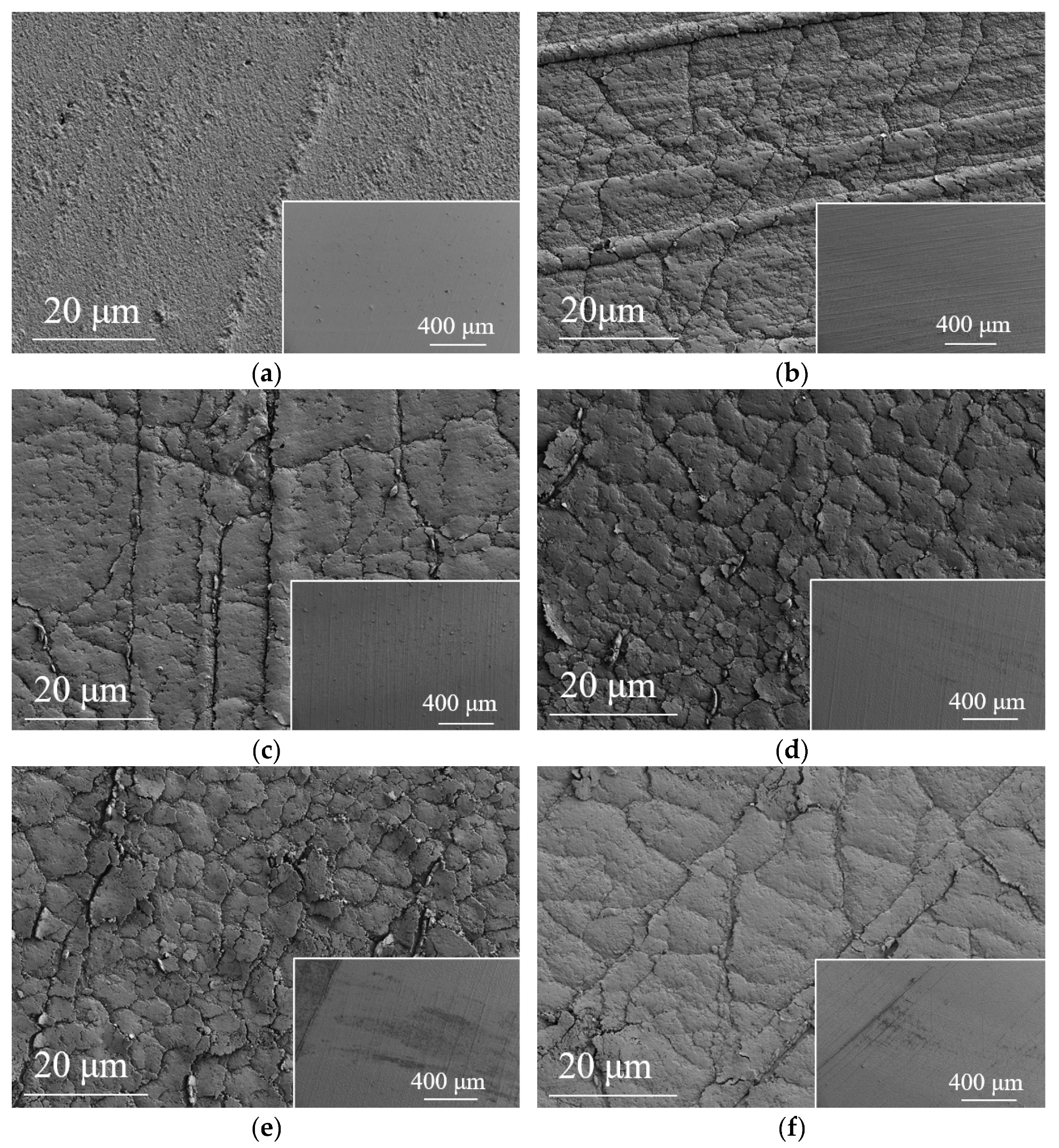
3.1. Substrate and Film Morphology
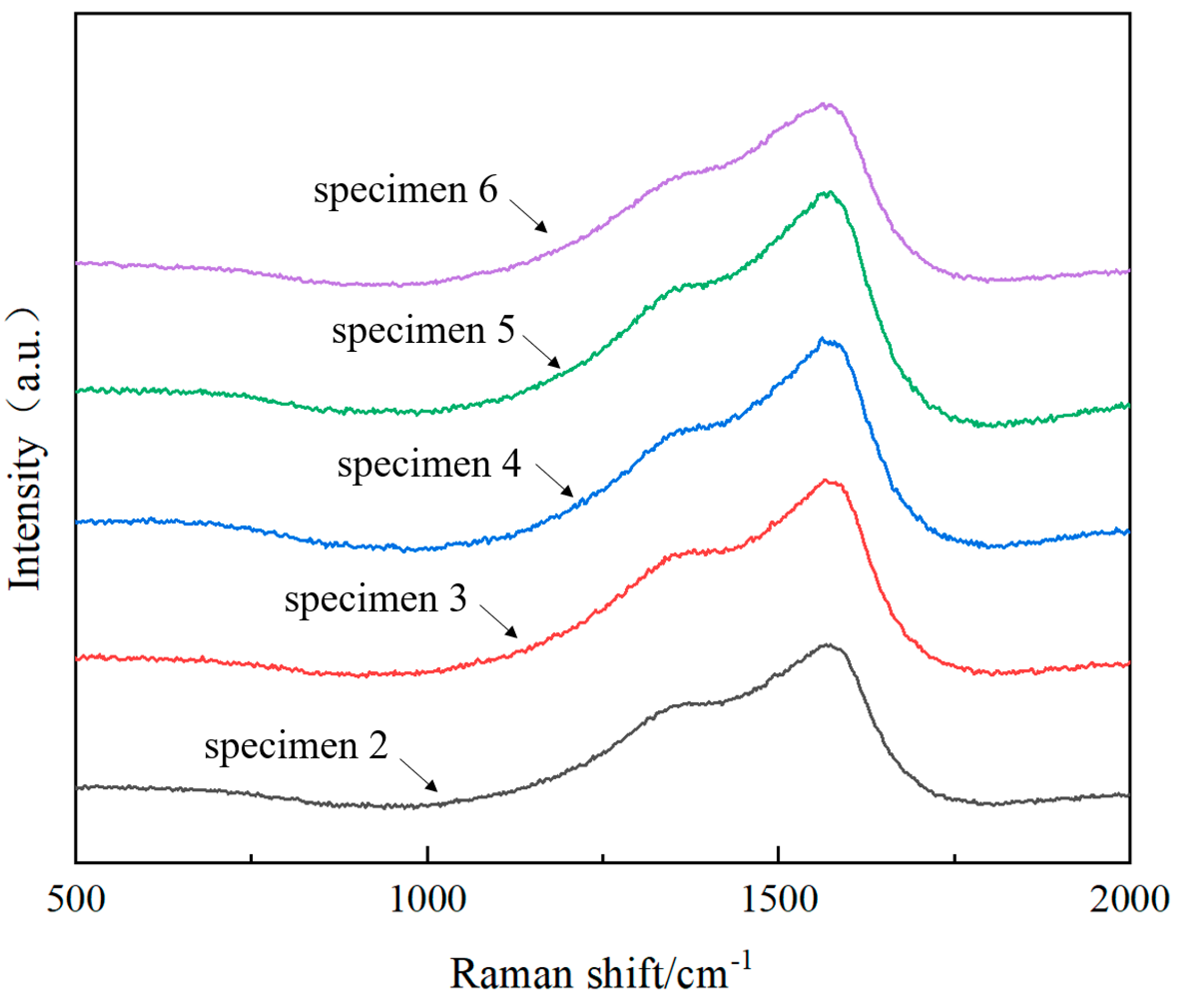
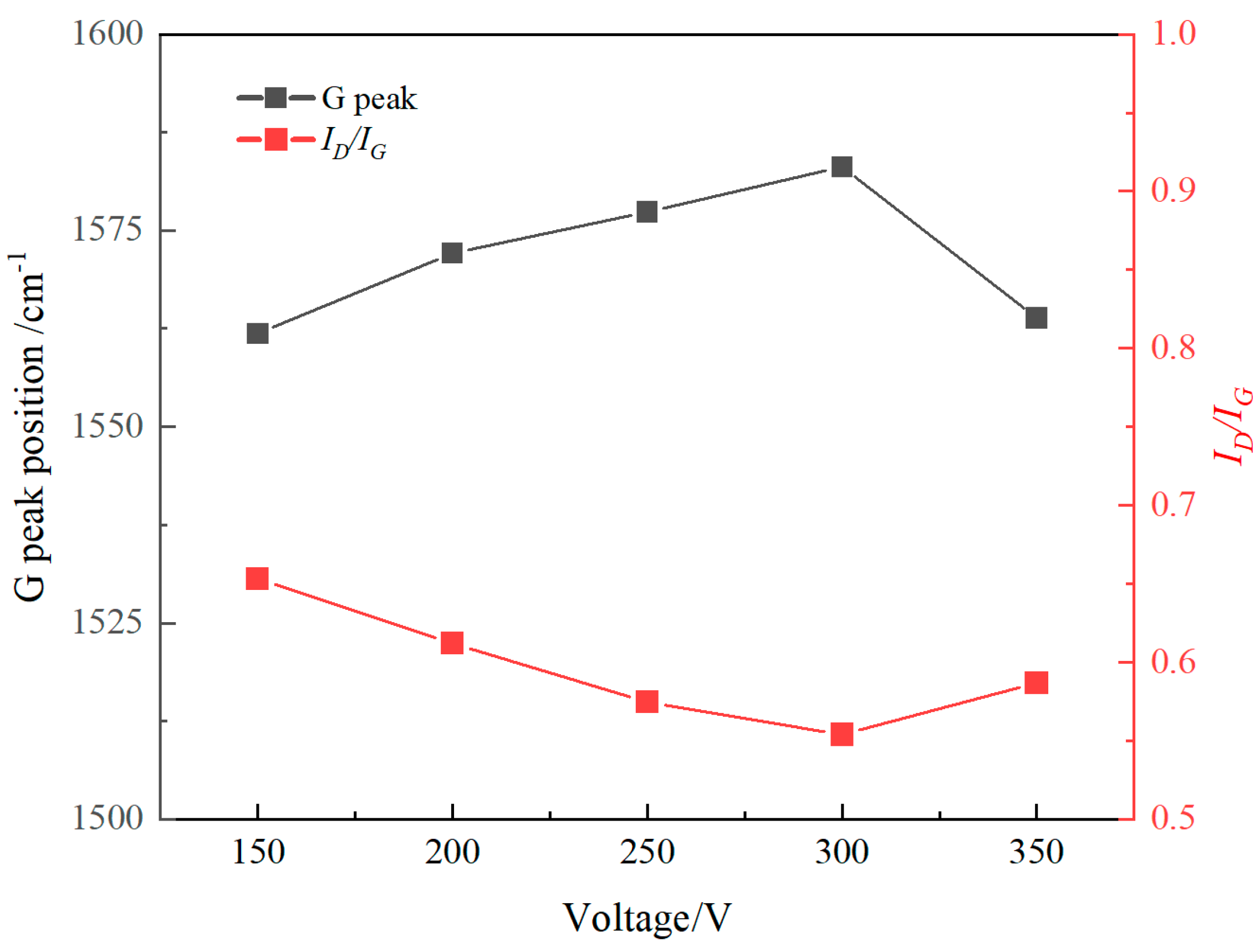
3.2. Raman Spectrum
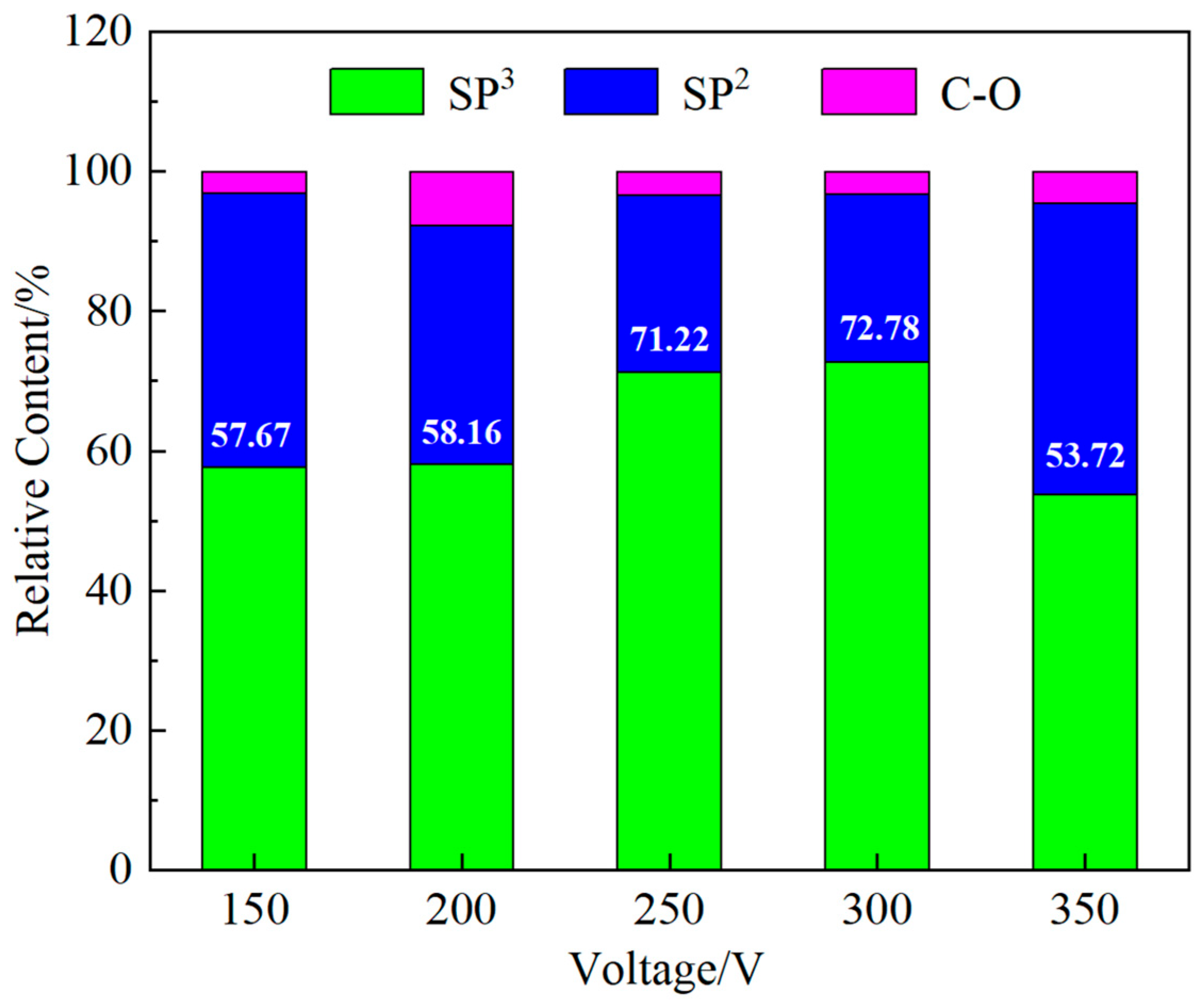
3.3. Chemical State of Thin Film Surface
3.4. Film Adhesion
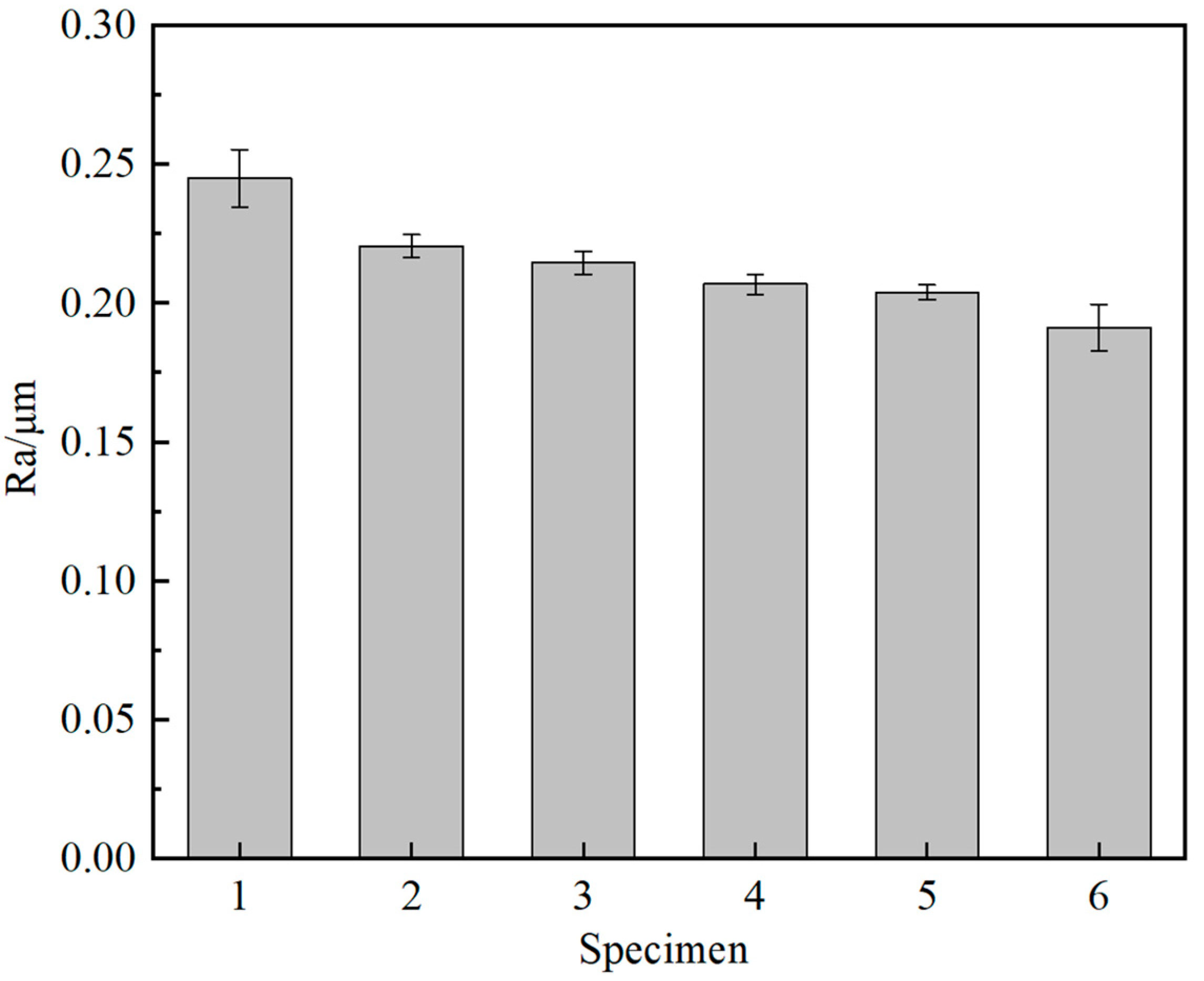
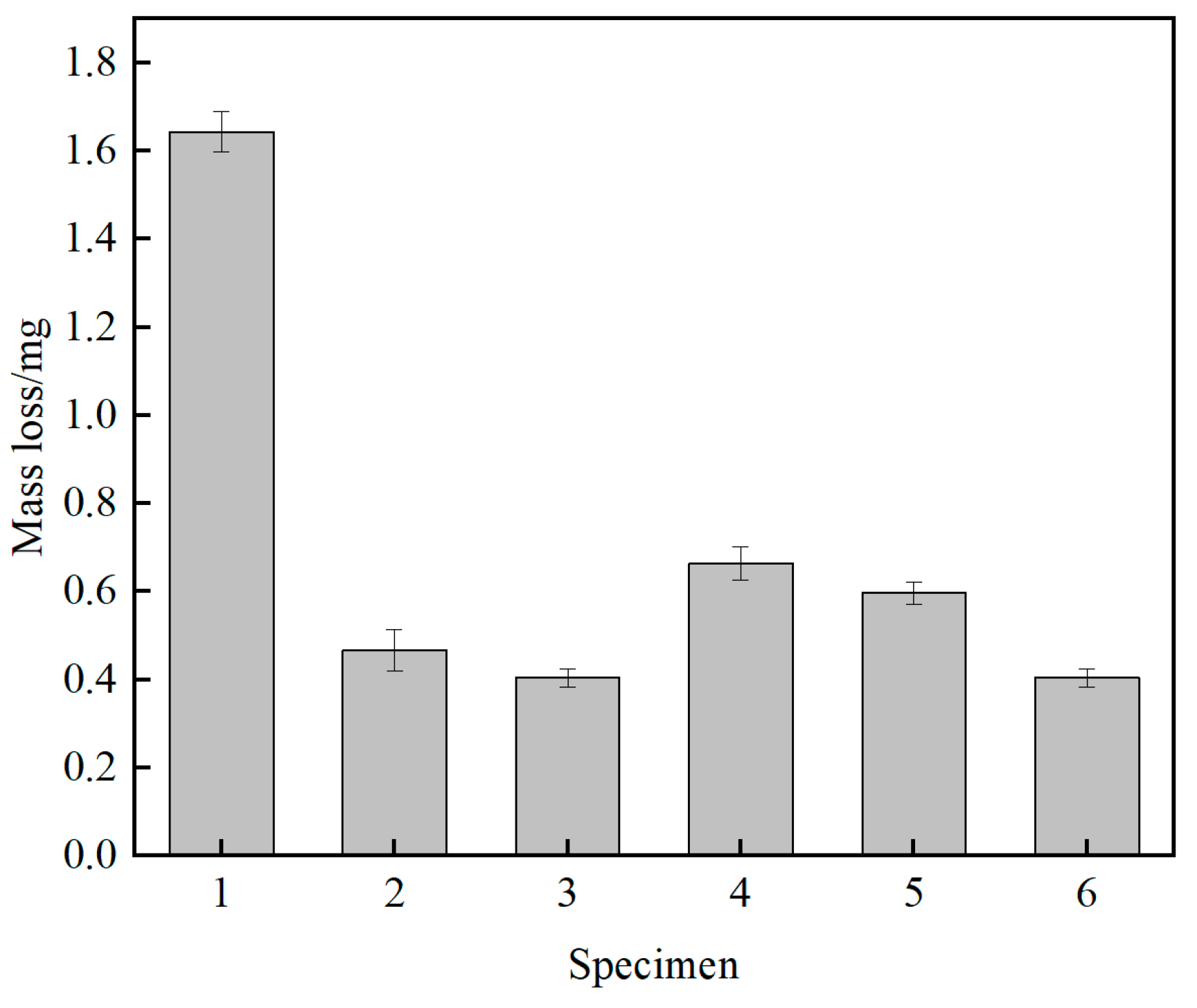
3.5. Film Friction Performance
4. Conclusions
- (1)
- Ta-C films were successfully deposited on the surface of nitrile rubber using the pulsed arc ion plating technique. The resulting films were uniform and dense, exhibiting typical network-like crack patterns. The coating significantly reduced surface roughness. The sp3 content in the ta-C films varied with different arc voltages, ranging from a minimum of 53.72% at an arc voltage of 350 V to a maximum of 72.78% at an arc voltage of 300 V.
- (2)
- As the arc voltage increases, the sp3 hybridized bond in the films initially increases and then decreases. Higher sp3 content led to increased internal stress and brittleness, adversely affected adhesion and friction stability. At 200 V, the sp3 content was 58.16%, with good film-substrate adhesion and a stable friction coefficient of approximately 0.38. In contrast, at 300 V, although the sp3 content peaked at 72.78%, the adhesion deteriorated, leading to film delamination and increased friction due to abrasive wear.
- (3)
- To improve the wear resistance of nitrile rubber with ta-C coatings, it is important to keep the sp3 content within a moderate range. This can be accomplished by choosing a lower arc voltage, which enhances interfacial compatibility and mechanical performance between the coating and the elastomer substrate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.S.; Shuai, C.G.; Lu, G.; Yang, X.; Hu, X.Y. Tribological characteristics and mechanism of nitrile butadiene rubber coated with typical liquid lubricants. Polym. Test. 2022, 115, 107724. [Google Scholar] [CrossRef]
- Liu, Y.H.; Xu, P.H.; Zhu, X.H.; Liu, L.G.; Li, B.; Li, Q.Q. Interfacial friction evolution between DLC film and sealing NBR on the plunger of fracturing pump based on molecular dynamics simulation. Ind. Lubr. Tribol. 2024; ahead of print. [Google Scholar] [CrossRef]
- Vasconcelos, B.; Serra, R.; Oliveira, J.C.; Fonseca, C. Durable electroless deposited Ni-P films on NBR for dynamic contacts. Characterization and tribological performance. Surf. Coat. Technol. 2021, 423, 127579. [Google Scholar] [CrossRef]
- Wu, Y.M.; Liu, J.Q.; Cao, H.T.; Wu, Z.Y.; Wang, Q.; Ma, Y.P.; Jiang, H.; Wen, F.; Pei, Y.T. On the adhesion and wear resistance of DLC films deposited on nitrile butadiene rubber: A Ti-C interlayer. Diam. Relat. Mater. 2020, 101, 107563. [Google Scholar] [CrossRef]
- Yang, T.; Han, C.X.; Deng, Q.Y.; Wen, F. Optimized Tribological Performance of Nitrogen-Doped Diamond-like Carbon Films on NBR: Influence of Bias Voltage of DC Magnetron Sputtering. Nanomaterials 2024, 14, 564. [Google Scholar] [CrossRef] [PubMed]
- Thirumalai, S.; Hausberger, A.; Lackner, J.M.; Waldhauser, W.; Schwarz, T. Anode layer source plasma-assisted hybrid deposition and characterization of diamond-like carbon coatings deposited on flexible substrates. Thin Solid Film 2018, 655, 54–61. [Google Scholar] [CrossRef]
- Lubwama, M.; Corcoran, B.; Sayers, K.; Kirabira, J.B.; Sebbit, A.; McDonnell, K.A.; Dowling, D. Adhesion and composite micro-hardness of DLC and Si-DLC films deposited on nitrile rubber. Surf. Coat. Technol. 2012, 206, 4881–4886. [Google Scholar] [CrossRef]
- Nakahigashi, T.; Tanaka; Miyake, K.; Oohara, H. Properties of flexible DLC film deposited by amplitude-modulated RF P-CVD. Tribol. Int. 2004, 37, 907–912. [Google Scholar] [CrossRef]
- Martinez, L.; Nevshupa, R.; Alvarez, L.; Huttel, Y.; Mendez, J.; Roman, E.; Mozas, E.; Valdes, J.R.; Jimenez, M.A.; Gachon, Y.; et al. Application of diamond-like carbon coatings to elastomers frictional surfaces. Tribol. Int. 2009, 42, 584–590. [Google Scholar] [CrossRef]
- Mangolini, F.; Krick, B.A.; Jacobs, T.D.B.; Khanal, S.R.; Streller, F.; McClimon, J.B.; Hilbert, J.; Prasad, S.V.; Scharf, T.W.; Ohlhausen, J.A.; et al. Effect of silicon and oxygen dopants on the stability of hydrogenated amorphous carbon under harsh environmental conditions. Carbon 2018, 130, 127–136. [Google Scholar] [CrossRef]
- Kang, M.C.; Tak, H.S.; Jeong, Y.K.; Lee, H.W.; Kim, J.S. Properties and tool performance of ta-C films deposited by double-bend filtered cathodic vacuum arc for micro drilling applications. Diam. Relat. Mater. 2010, 19, 866–869. [Google Scholar] [CrossRef]
- Harigai, T.; Miyamoto, Y.; Yamano, M.; Tanimoto, T.; Suda, Y.; Takikawa, H.; Kawano, T.; Nishiuchi, M.; Sakaki, H.; Kondo, K.; et al. Self-supporting tetrahedral amorphous carbon films consisting of multilayered structure prepared using filtered arc deposition. Thin Solid Films 2019, 675, 123–127. [Google Scholar] [CrossRef]
- Zhang, L.J.; Zong, X.M.; Yuan, X.M.; Xing, X.L. Effects of Polyurethane Substrate Pre-Treatment on Pulsed Cathodic Arc Deposited DLC Films. Coatings 2020, 10, 545. [Google Scholar] [CrossRef]
- Zhao, D.C.; Ma, Z.J.; Liu, X.G.; Zheng, J.; Zhang, Z.Y. Study on Mechanical Properties of Nitrogen-doped ta-C Thin Films Prepared by Multi-stage Triggered Pulsed Arc Source Technology. J. Mech. Eng. 2023, 59, 108–117. [Google Scholar] [CrossRef]
- Li, Z.D.; Zhan, H.; Wang, Y.Q.; Wang, R.J.; Wang, W.P. Effects of Plasma Etching Pretreatment on Adhesion of Carbon-based Film. Surf. Technol. 2017, 46, 64–68. [Google Scholar] [CrossRef]
- Song, Y.Q.; Han, C.X.; Zhen, N.N.; Wang, Y.K.; Leng, Y.X.; Wu, Z.Y.; Deng, Q.Y.; Wen, F. Nitrogen-Doped Diamond-like Carbon Buffer Layer Enhances the Mechanical and Tribological Properties of Diamond-like Carbon Films Deposited on Nitrile Rubber Substrate. Coatings 2024, 14, 515. [Google Scholar] [CrossRef]
- Zhang, T.F.; Wan, Z.X.; Ding, J.C.; Zhang, S.H.; Wang, Q.M.; Kim, K.H. Microstructure and high-temperature tribological properties of Si-doped hydrogenated diamond-like carbon films. Appl. Surf. Sci. 2018, 435, 963–973. [Google Scholar] [CrossRef]
- Cai, C.L.; Wang, J.M.; Mi, Q.; Hang, L.X.; Yan, Y.X.; Xu, J.Q. Study on the Deposition Ratio of Pulsed Vacuum Arc Ion Deposition. Surf. Technol. 2002, 31, 27–29. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.G.; Zhang, G.A.; Chen, T.J.; Jia, J.H. Effect of Rubber Surface Roughness on the Tribological Properties of DLC Film. Surf. Technol. 2023, 52, 225–232. [Google Scholar] [CrossRef]
- Wang, M.L.; Cheng, W.J.; Lin, G.Q. Influence of Arc Current on Structure and Properties of the Super-Hard ta-C Film Deposited by Arc Ion Plating. Rare Met. Mater. Eng. 2022, 51, 3095–3102. [Google Scholar]
- He, M.Y.; Yeo, C.D. Evaluation of Thermal Degradation of DLC Film Using a Novel Raman Spectroscopy Technique. Coatings 2018, 8, 143. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.T.; Xie, H.; Hing, P. A phenomenological approach for the Id/Ig ratio and sp3 fraction of magnetron sputtered a-C films. Surf. Coat. Technol. 2000, 123, 256–260. [Google Scholar] [CrossRef]
- Xu, S.P.; Chen, W.Q.; Li, Y.H.; Li, X.W. Effect of Arc Current on Structure and Properties of Ultrathin Tetrahedral Amorphous Carbon Film. Chin. J. Mater. Res. 2016, 30, 11. [Google Scholar] [CrossRef]
- Mabuchi, Y.; Higuchi, T.; Yoshimura, D.; Murashima, M.; Kousaka, H.; Umehara, N. Influence of carbon black in engine oil on wear of H-free diamond-like carbon coatings. Tribol. Int. 2014, 73, 138–147. [Google Scholar] [CrossRef]
- Chen, R.F.; Ye, C.C.; Sun, X.H.; Li, F.F.; Chen, H.B.; Miao, Y.L.; Zhao, Z.S.; Zhao, Y.H. Effect of Bias Substrate on the Structure and Corrosion Resistance of Tetrahedral Amorphous Carbon Thin Films. J. Mater. Eng. Perform. 2025. [Google Scholar] [CrossRef]
- Walter, M.; Mangolini, F.; McClimon, J.B.; Carpick, R.W.; Moseler, M. Origin of C(1s) binding energy shifts in amorphous carbon materials. Phys. Rev. Mater. 2025, 9, 035601. [Google Scholar] [CrossRef]
- Yudhanto, A.; Li, X.L.; Tao, R.; Melentiev, R.; Lubineau, G. Identifying adhesion characteristics of metal-polymer interfaces: Recent.advances in the case of electroplated acrylonitrile butadiene styrene. Mater. Today Commun. 2023, 35, 106218. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, J.H.; Zhang, G.A.; Li, H.C.; Chen, T.J. Effect of rubber substrates on the flexibility and tribological properties of diamond-like carbon coatings. Surf. Coat. Technol. 2021, 422, 127526. [Google Scholar] [CrossRef]
- Bai, C.N.; Qiang, L.; Zhang, B.; Gao, K.X.; Zhang, J.Y. Optimizing the tribological performance of DLC-coated NBR rubber: The role of hydrogen in films. Friction 2022, 10, 866–877. [Google Scholar] [CrossRef]
- Si, Y.J.; Wei, Y.J. Semi-analytical solutions of kinked edge cracks. Eng. Fract. Mech. 2024, 309, 110392. [Google Scholar] [CrossRef]
- Xing, Y.Q.; Wang, X.S.; Du, Z.H.; Zhu, Z.W.; Wu, Z.; Liu, L. Synergistic effect of surface textures and DLC coatings for enhancing friction and wear performances of Si3N4/TiC ceramic. Ceram. Int. 2021, 48, 514–524. [Google Scholar] [CrossRef]
- Wang, L.J.; Zhang, Z.L.; Chen, H.; Wang, H.Y.; Liu, Y.; Wang, J.X.; Wang, M.C. Friction behavior of diamond-like carbon coatings with different sp3 contents by atomistic-scale friction dynamics. Surf. Coat. Technol. 2023, 464, 129580. [Google Scholar] [CrossRef]
- Shen, M.X.; Peng, X.D.; Meng, X.K.; Zheng, J.P.; Zhu, M.H. Fretting wear behavior of acrylonitrile-butadiene rubber (NBR) for mechanical seal applications. Tribol. Int. 2016, 93, 419–428. [Google Scholar] [CrossRef]
- Suzuki, A.; Aiyama, Y.; Tokoro, M.; Sekiguchi, H.; Masuko, M. Friction and wear characteristics of hydrogenated diamond-like carbon films formed on the roughened stainless steel surface. Wear 2010, 269, 118–124. [Google Scholar] [CrossRef]




| Sample No. | Voltage/V | Working Pressure/Pa | Pulse Frequency/Hz | Pulse Count | Film Thickness/nm |
|---|---|---|---|---|---|
| 1 | / | / | / | / | / |
| 2 | 150 | 5 × 10−3 | 3 | 12,000 | 58 |
| 3 | 200 | 5 × 10−3 | 3 | 12,000 | 100 |
| 4 | 250 | 5 × 10−3 | 3 | 12,000 | 156 |
| 5 | 300 | 5 × 10−3 | 3 | 12,000 | 225 |
| 6 | 350 | 5 × 10−3 | 3 | 12,000 | 310 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Lu, W.; Guo, F.; Wang, C.; Zou, L. Effects of Pulse Ion Source Arc Voltage on the Structure and Friction Properties of Ta-C Thin Films on NBR Surface. Coatings 2025, 15, 809. https://doi.org/10.3390/coatings15070809
Feng S, Lu W, Guo F, Wang C, Zou L. Effects of Pulse Ion Source Arc Voltage on the Structure and Friction Properties of Ta-C Thin Films on NBR Surface. Coatings. 2025; 15(7):809. https://doi.org/10.3390/coatings15070809
Chicago/Turabian StyleFeng, Sen, Wenzhuang Lu, Fei Guo, Can Wang, and Liang Zou. 2025. "Effects of Pulse Ion Source Arc Voltage on the Structure and Friction Properties of Ta-C Thin Films on NBR Surface" Coatings 15, no. 7: 809. https://doi.org/10.3390/coatings15070809
APA StyleFeng, S., Lu, W., Guo, F., Wang, C., & Zou, L. (2025). Effects of Pulse Ion Source Arc Voltage on the Structure and Friction Properties of Ta-C Thin Films on NBR Surface. Coatings, 15(7), 809. https://doi.org/10.3390/coatings15070809





