Composite Coating Enriched with Lemon Peel Extract for Enhancing the Postharvest Quality of Cherry Tomatoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit
2.2. Preparation of Lemon Peel Powder Extraction Process
2.3. Edible Coating Manufacture
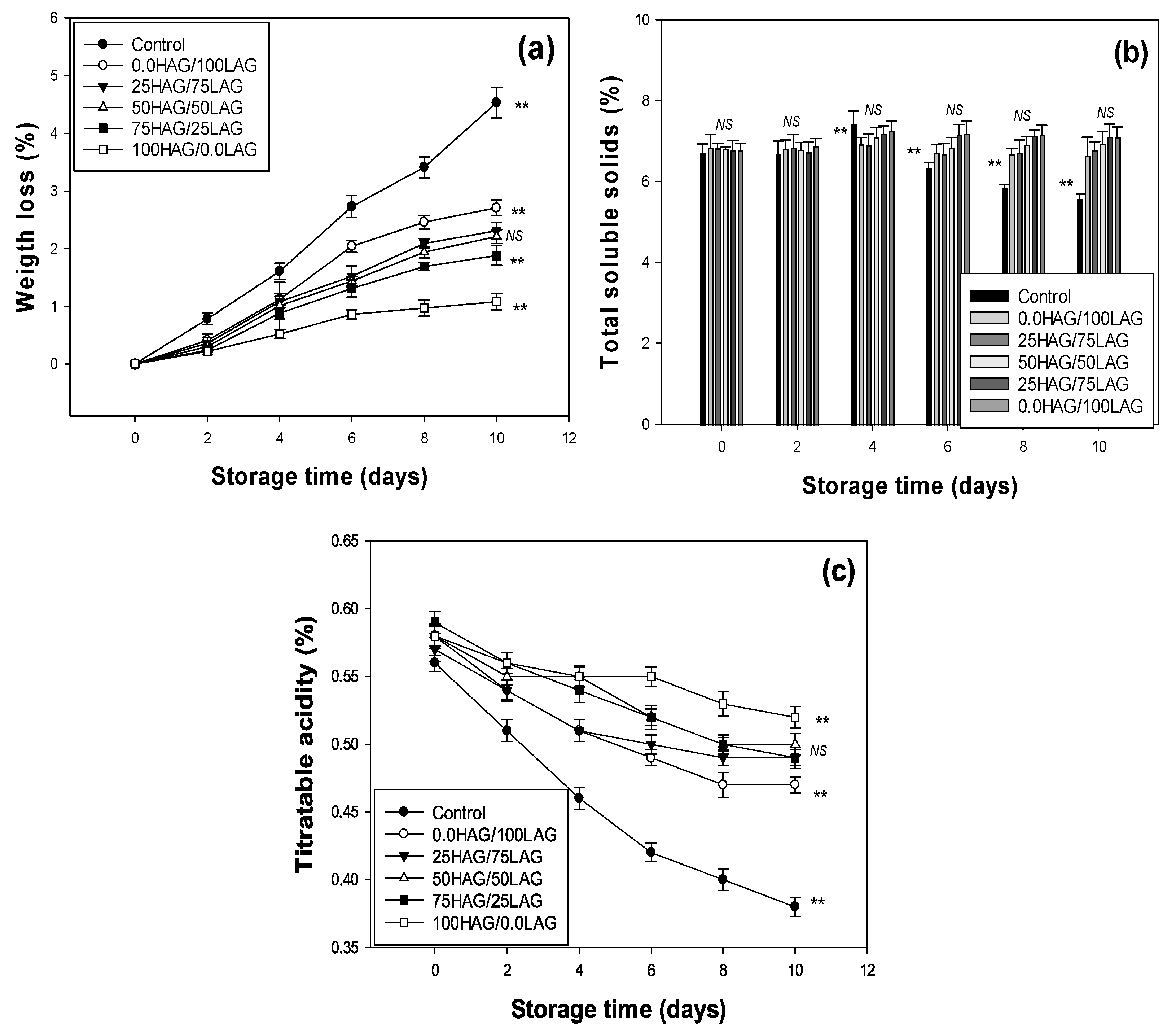
2.4. Weight Loss and Soluble Solids
2.5. Titratable Acidity
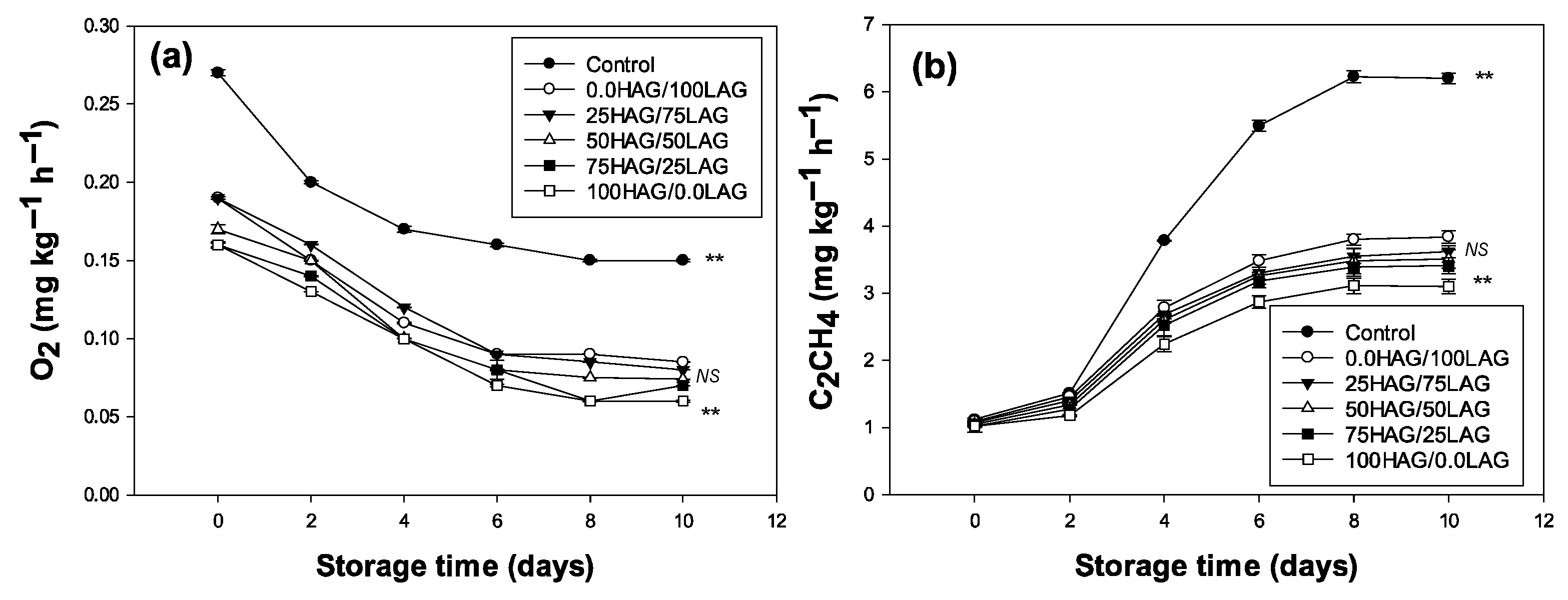
2.6. Consumption of O2 and Production of Ethylene (C2H4)
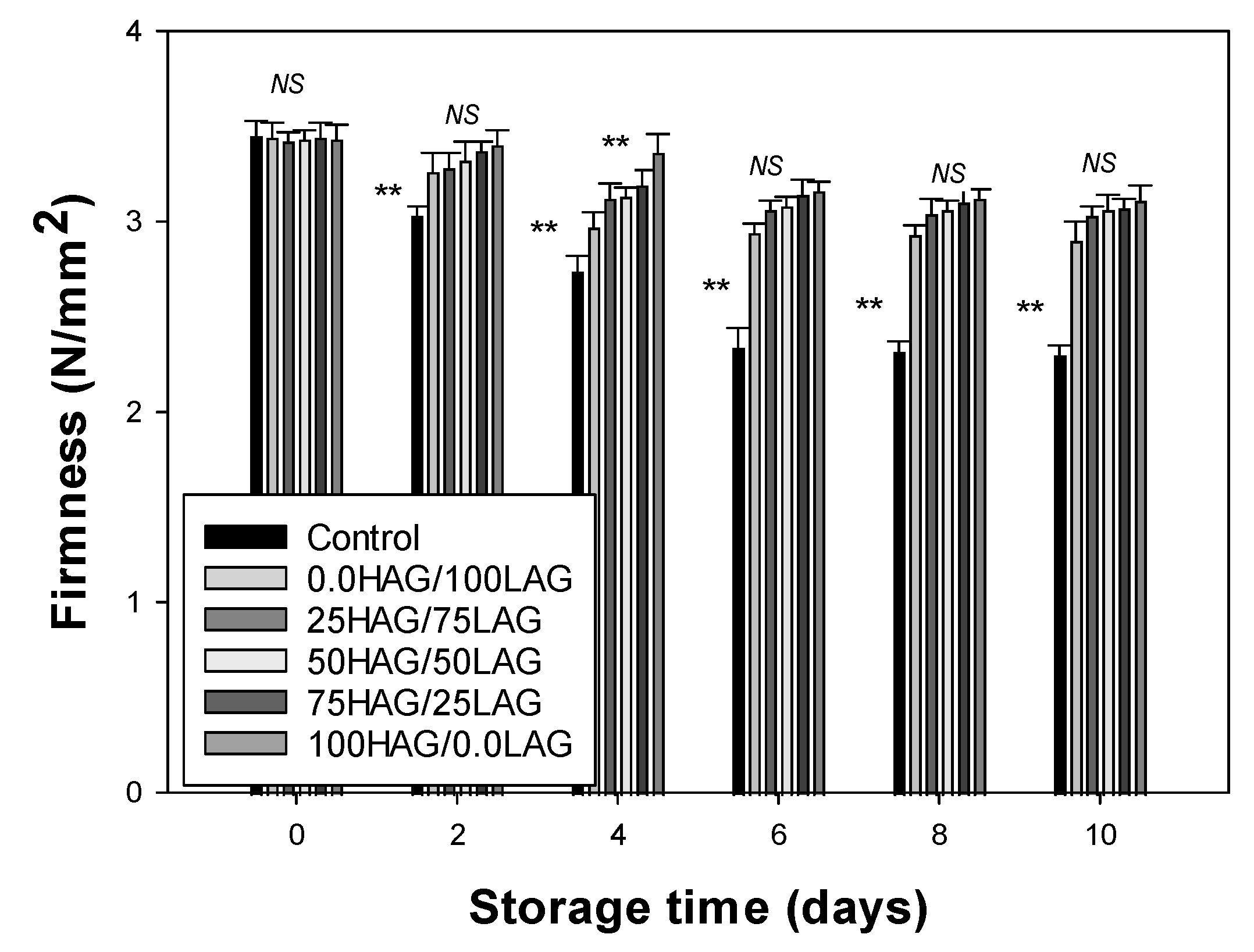
2.7. Firmness
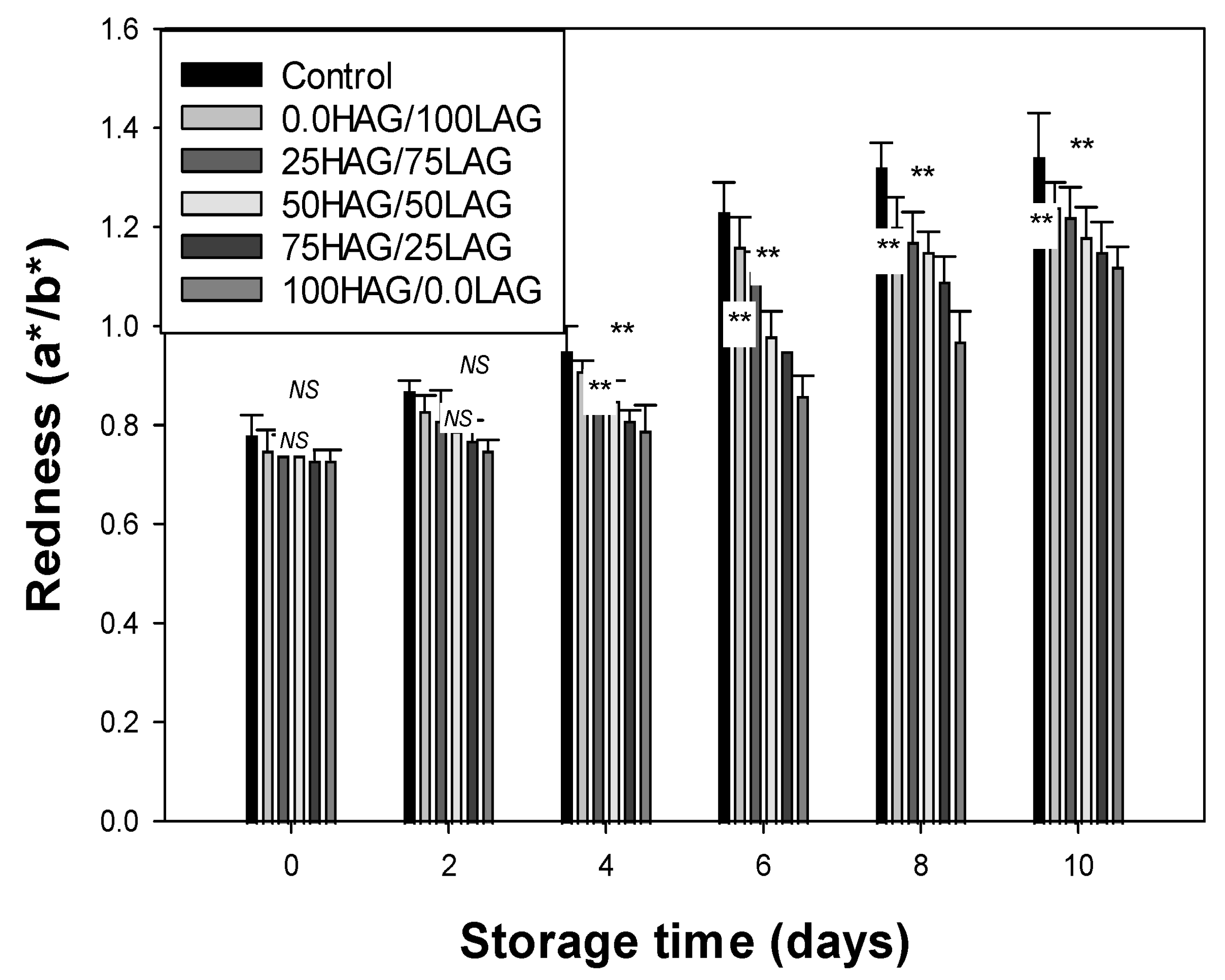
2.8. Redness
2.9. Decay Rate
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Quality
3.2. Oxygen Consumption and Ethylene Production
3.3. Firmness Analysis
3.4. Redness
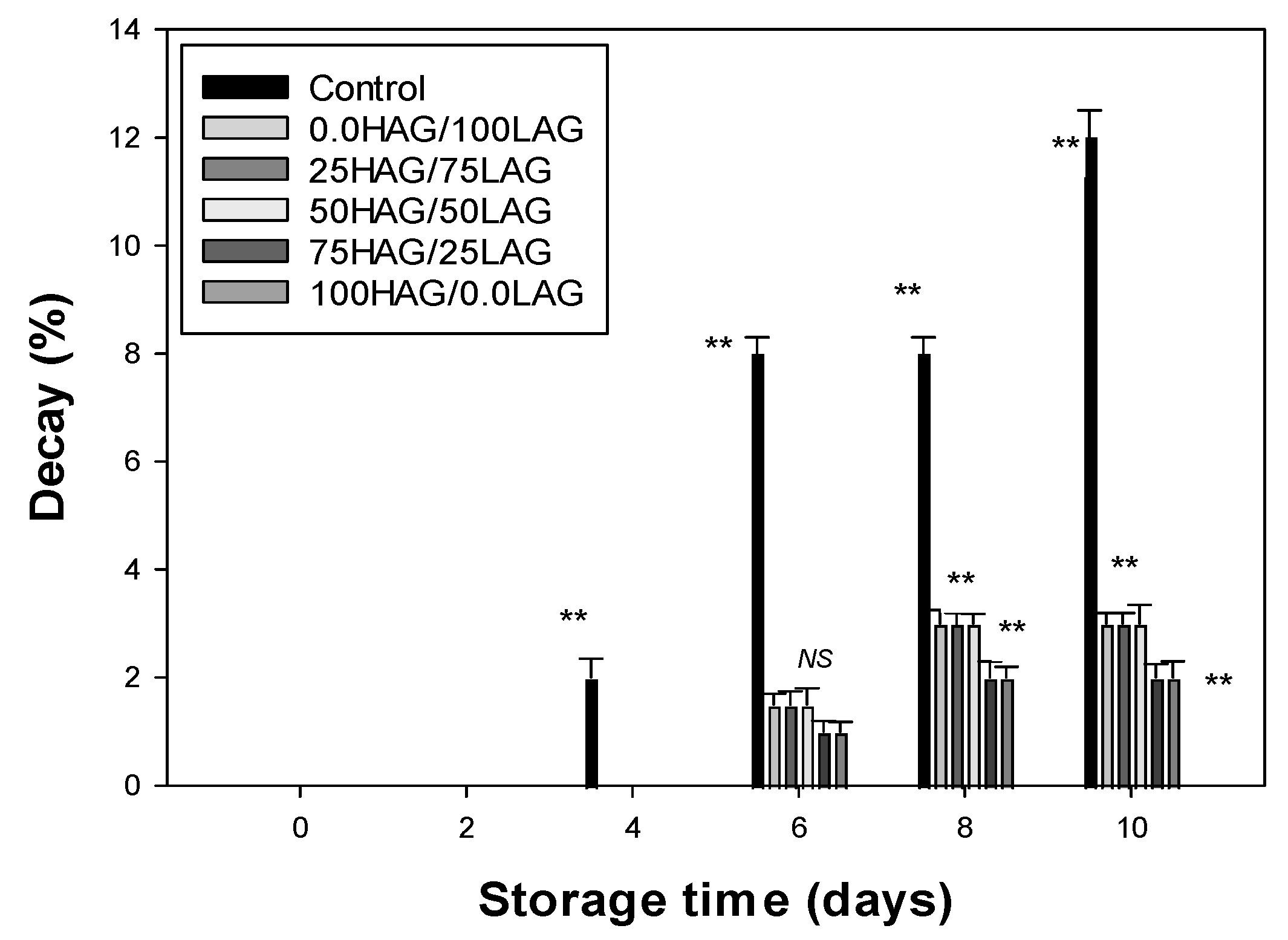
3.5. Decay Rate Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerra, I.C.D.; de Oliveira, P.D.L.; de Souza Pontes, A.L.; Lúcio, A.S.S.C.; Tavares, J.F.; Barbosa-Filho, J.M.; Madruga, M.S.; de Souza, E.L. Coatings Comprising Chitosan and Mentha piperita L. or Mentha × villosa Huds Essential Oils to Prevent Common Postharvest Mold Infections and Maintain the Quality of Cherry Tomato Fruit. Int. J. Food Microbiol. 2015, 214, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Agricultura y Desarrollo Rural de Colombia. Reporte: Área, Producción y Rendimiento Nacional por Cultivo (Tomate). Agronet 2024. Available online: https://www.agronet.gov.co/estadistica/Paginas/home.aspx?cod=1# (accessed on 24 July 2024).
- Manjarres Melo, J.J.; Alvarez, A.; Ramirez, C.; Bolivar, G. Antagonistic activity of lactic acid bacteria against phytopathogenic fungi isolated from cherry tomato (Solanum lycopersicum var. cerasiforme). Curr. Microbiol. 2021, 78, 1399–1408. [Google Scholar] [CrossRef]
- Sousa, A.R.; Oliveira, J.C.; Sousa-Gallagher, M.J. Determination of the Respiration Rate Parameters of Cherry Tomatoes and Their Joint Confidence Regions Using Closed Systems. J. Food Eng. 2017, 206, 13–22. [Google Scholar] [CrossRef]
- Lukasse, L.J.S.; Schouten, R.E.; Castelein, R.B.; Lawton, R.; Paillart, M.J.M.; Guo, X.; Woltering, E.J.; Tromp, S.; Snels, J.C.M.A.; Defraeye, T. Perspectives on the Evolution of Reefer Containers for Transporting Fresh Produce. Trends Food Sci. Technol. 2023, 140, 104147. [Google Scholar] [CrossRef]
- Ghidelli, C.; Perez-Gago, M.B. Recent Advances in Modified Atmosphere Packaging and Edible Coatings to Maintain Quality of Fresh-Cut Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2018, 58, 662–679. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, N.; Upadhyay, A.; Sethi, S.; Singh, A. Edible Coating as Postharvest Management Strategy for Shelf-Life Extension of Fresh Tomato (Solanum lycopersicum L.): An Overview. J. Food Sci. 2022, 87, 2256–2290. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, X.; Song, X.; Yu, T.; Lu, H.; Wang, P.; Wang, J.; Zheng, X.D. Control of Postharvest Decay on Cherry Tomatoes by Marine Yeast Rhodosporidium paludigenum and Calcium Chloride. J. Appl. Microbiol. 2010, 109, 651–656. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, M.; Tong, H.; Zhang, J.; Li, Q.; Gao, X.; Qi, W.; Lam, H.L.; Huang, R.; Su, R. Multi-Functional Edible Coatings Tailored with Nanocellulose for Perishable Fruits. Carbohydr. Polym. 2025, 358, 123520. [Google Scholar] [CrossRef]
- Martinez, C.; Hertog, M.; Raemdonck, G.V.; Baggerman, G.; Nicolai, B.M. Omics Analysis of the Ethylene Signal Transduction in Tomato as a Function of Storage Temperature. Postharvest Biol. Technol. 2019, 155, 1–10. [Google Scholar] [CrossRef]
- Ncama, K.; Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z. Plant-Based Edible Coatings for Managing Postharvest Quality of Fresh Horticultural Produce: A Review. Food Packag. Shelf Life 2018, 16, 157–167. [Google Scholar] [CrossRef]
- La, B.; Ac, A.; Rbab, C.; Aaa, B. Active Packaging from Triticale Flour Films for Prolonging Storage Life of Cherry Tomato. Food Packag. Shelf Life 2020, 25, 100520. [Google Scholar] [CrossRef]
- González, R.; Ramos, G.; Cruz, A.; Salazar, A. Rheological Characterization and Activation Energy Values of Binary Mixtures of Gellan. Eur. Food Res. Technol. 2012, 234, 305–313. [Google Scholar] [CrossRef]
- Huang, H.; Yan, W.; Tan, S.; Zhao, Y.; Dong, H.; Liao, W.; Shi, P.; Yang, X.; He, Q. Frontier in Gellan Gum-Based Micro-Capsules Obtained by Emulsification: Core-Shell Structure, Interaction Mechanism, Intervention Strategies. Int. J. Biol. Macromol. 2024, 272, 132697. [Google Scholar] [CrossRef] [PubMed]
- González-Cuello, R.E.; Mendoza-Nova, L.; Rodríguez-Rodríguez, V.C.; Hernández-Fernández, J.; Ortega-Toro, R. Composite Coatings of Gellan Gum and Inulin with Lactobacillus casei: Enhancing the Post-Harvest Quality of Guava. J. Compos. Sci. 2024, 8, 353. [Google Scholar] [CrossRef]
- Gomes, D.; Batista-Silva, J.; Sousa, A.; Passarinha, L. Progress and Opportunities in Gellan Gum-Based Materials: A Review of Preparation, Characterization and Emerging Applications. Carbohydr. Polym. 2023, 311, 120782. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of Hydrophobic and Low-Soluble Food Bioactive Compounds Within Different Nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- González-Cuello, R.; Fuentes, L.G.; Castellanos, H.M.; Hernández-Fernández, J.; Ortega-Toro, R. Composite Coatings with Liposomes of Melissa officinalis Extract for Extending Tomato Shelf Life. J. Compos. Sci. 2024, 8, 283. [Google Scholar] [CrossRef]
- Magalhães, D.; Vilas-Boas, A.A.; Teixeira, P.; Pintado, M. Functional Ingredients and Additives from Lemon By-Products and Their Applications in Food Preservation: A Review. Foods 2023, 12, 1095. [Google Scholar] [CrossRef]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of Phenolic Profile and Antioxidant Capacity of Different Fruit Parts from Lemon (Citrus limon Burm.) Cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Xu, Y.; Chen, L.; Cao, J.; Jiang, W. An Advance on Nutritional Profile, Phytochemical Profile, Nutraceutical Properties, and Potential Industrial Applications of Lemon Peels: A Comprehensive Review. Trends Food Sci. Technol. 2022, 124, 219–236. [Google Scholar] [CrossRef]
- Novita, Z.D.; Cahya, P.E.; Yelliantty; Garnida, Y. Effect of a Pectin Edible Coating with Lemon Peel Extract to Maintain Strawberry Fruit’s Quality During Cold Storage. Food Hum. 2025, 4, 100541. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Chen, K.; Tian, R.; Xu, G.; Wu, K.; Liu, Y.; Jiang, F. Characterizations of Konjac Glucomannan/Curdlan Edible Coatings and the Preservation Effect on Cherry Tomatoes. Int. J. Biol. Macromol. 2023, 232, 123359. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Varghese, E. Influence of Edible Coatings on Physiological and Biochemical Attributes of Japanese Plum (Prunus salicina Lindell cv. Santa Rosa). Fruits 2018, 73, 31–38. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, B.; Cui, K.; Cao, J.; Jiang, W. Improving Postharvest Quality and Antioxidant Capacity of Sweet Cherry Fruit by Storage at Near-Freezing Temperature. Sci. Hortic. 2019, 246, 68–78. [Google Scholar] [CrossRef]
- Flores-López, M.L.; Vieira, J.M.; Rocha, C.M.R.; Lagarón, J.M.; Cerqueira, M.A.; Jasso De Rodríguez, D.; Vicente, A.A. Postharvest Quality Improvement of Tomato (Solanum lycopersicum L.) Fruit Using a Nanomultilayer Coating Containing Aloe Vera. Foods 2023, 13, 83. [Google Scholar] [CrossRef]
- Khaliq, G.; Ramzan, M.; Baloch, A.H. Effect of Aloe vera Gel Coating Enriched with Fagonia indica Plant Extract on Physicochemical and Antioxidant Activity of Sapodilla Fruit During Postharvest Storage. Food Chem. 2019, 286, 346–353. [Google Scholar] [CrossRef]
- Fagundes, C.; Palou, L.; Monteiro, A.R.; Pérez-Gago, M.B. Effect of Antifungal Hydroxypropyl Methylcellulose-Beeswax Edible Coatings on Gray Mold Development and Quality Attributes of Cold-Stored Cherry Tomato Fruit. Postharvest Biol. Technol. 2014, 92, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Wang, Z.; Cai, R.; Tian, L.; Cui, L. Preparation and Characterization of Chitosan–Nano-ZnO Composite Films for Preservation of Cherry Tomatoes. Foods 2021, 10, 3135. [Google Scholar] [CrossRef]
- Álvarez, A.; Manjarres, J.J.; Ramírez, C.; Bolívar, G. Use of an Exopolysaccharide-Based Edible Coating and Lactic Acid Bacteria with Antifungal Activity to Preserve the Postharvest Quality of Cherry Tomato. LWT 2021, 151, 112225. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Zhang, H.; Dong, M.; Li, L.; Jia, P.; Bu, T.; Wang, X.; Wang, L. Development of Functional Gelatin-Based Composite Films Incorporating Oil-in-Water Lavender Essential Oil Nano-Emulsions: Effects on Physicochemical Properties and Cherry Tomatoes Preservation. LWT 2021, 142, 110987. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; Zhang, P.; Lai, S.; Yang, H. Influence of Rice Bran Wax Coating on the Physicochemical Properties and Pectin Nanostructure of Cherry Tomatoes. Food Bioprocess. Technol. 2017, 10, 349–357. [Google Scholar] [CrossRef]
- Kaur, K.; Dhillon, W.S. Influence of Maturity and Storage Period on Physical and Biochemical Characteristics of Pear During Post Cold Storage at Ambient Conditions. J. Food Sci. Technol. 2015, 52, 5352–5356. [Google Scholar] [CrossRef] [PubMed]
- Dave, R.K.; Ramana Rao, T.V.; Nandane, A.S. Improvement of Post-Harvest Quality of Pear Fruit with Optimized Composite Edible Coating Formulations. J. Food Sci. Technol. 2017, 54, 3917–3927. [Google Scholar] [CrossRef] [PubMed]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent Advances on Polysaccharides, Lipids and Protein Based Edible Films and Coatings: A Review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Fagundes, C.; Moraes, K.; Pérez-Gago, M.; Palou, L.; Maraschin, M.; Monteiro, A. Effect of Active Modified Atmosphere and Cold Storage on the Postharvest Quality of Cherry Tomatoes. Postharvest Biol. Technol. 2015, 109, 73–81. [Google Scholar] [CrossRef]
- Liplap, P.; Vigneault, C.; Toivonen, P.; Charles, M.T.; Raghavan, G.S.V. Effect of Hyperbaric Pressure and Temperature on Respiration Rates and Quality Attributes of Tomato. Postharvest Biol. Technol. 2013, 86, 240–248. [Google Scholar] [CrossRef]
- Obadina, A.; Ibrahim, J.; Adekoya, I. Influence of Drying Temperature and Storage Period on the Quality of Cherry and Plum Tomato Powder. Food Sci. Nutr. 2018, 6, 1146–1153. [Google Scholar] [CrossRef]
- Ali, A.; Maqbool, M.; Ramachandran, S.; Alderson, P.G. Gum Arabic as a Novel Edible Coating for Enhancing Shelf-Life and Improving Postharvest Quality of Tomato (Solanum lycopersicum L.) Fruit. Postharvest Biol. Technol. 2010, 58, 42–47. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández-Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical Impregnation of Olive Leaf Extract to Obtain Bioactive Films Effective in Cherry Tomato Preservation. Food Packag. Shelf Life 2019, 21, 100338. [Google Scholar] [CrossRef]
- Wu, S.; Lu, M.; Wang, S. Effect of oligosaccharides derived from Laminaria japonica-incorporated pullulan coatings on preservation of cherry tomatoes. Food Chem. 2016, 199, 296–300. [Google Scholar] [CrossRef]
- Barreto, T.A.; Andrade, S.C.A.; Maciel, J.F.; Arcanjo, N.M.O.; Madruga, M.S.; Meireles, B.; Cordeiro, A.M.T.; Souza, E.L.; Magnani, M. A Chitosan Coating Containing Essential Oil from Origanum vulgare L. to Control Postharvest Mold Infections and Keep the Quality of Cherry Tomato Fruit. Front. Microbiol. 2016, 7, 1724. [Google Scholar] [CrossRef] [PubMed]
- Yun, X.; Qi, X.; Zhang, Y.; Song, S.; Dong, T. Application of SiOx-Coated Poly (ε-caprolactone) Film for Preservation of Cherry Tomato. Polym. Polym. Compos. 2019, 28, 309–319. [Google Scholar] [CrossRef]
- Carrillo-Lomelí, D.A.; Cerqueira, M.A.; Moo-Huchin, V.; Bourbon, A.I.; Souza, V.G.L.; Lestido-Cardama, A.; Pastrana, L.M.; Ochoa-Fuentes, Y.M.; Hernandez-Castillo, F.D.; Villarreal-Quintanilla, J.Á.; et al. Influence of Edible Multilayer Coatings with Opuntia stenopetala Polysaccharides and Flourensia microphylla Extract on the Shelf-Life of Cherry Tomato (Solanum lycopersicum L.). Sci. Hortic. 2024, 332, 113224. [Google Scholar] [CrossRef]
- Salas-Méndez, E.D.J.; Vicente, A.; Cristina, A.; Fernanda, L.; Silva, P.; Rodríguez-García, R.; Hernández-Castillo, F.D.; Lourdes, M.D.; Díaz-Jiménez, V.; Flores-López, M.L.; et al. Application of Edible Nanolaminate Coatings with Antimicrobial Extract of Flourensia cernua to Extend the Shelf-Life of Tomato (Solanum lycopersicum L.) Fruit. Postharvest Biol. Technol. 2019, 150, 19–27. [Google Scholar] [CrossRef]
- Park, H.H.; Min, S.C.; Won, J.S.; Lee, S.J.; Song, K.B. Edible Coating Using a Chitosan-Based Colloid Incorporating Grapefruit Seed Extract for Cherry Tomato Safety and Preservation. J. Food Sci. 2017, 83, 138–146. [Google Scholar] [CrossRef]
- Mansourbahmani, S.; Ghareyazie, B.; Zarinnia, V.; Kalatejari, S.; Mohammadi, R.S. Study on the Efficiency of Ethylene Scavengers on the Maintenance of Postharvest Quality of Tomato Fruit. J. Food Meas. Charact. 2018, 12, 691–701. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Cuello, R.; Hernández-Fernández, J.; Ortega-Toro, R. Composite Coating Enriched with Lemon Peel Extract for Enhancing the Postharvest Quality of Cherry Tomatoes. Coatings 2025, 15, 810. https://doi.org/10.3390/coatings15070810
González-Cuello R, Hernández-Fernández J, Ortega-Toro R. Composite Coating Enriched with Lemon Peel Extract for Enhancing the Postharvest Quality of Cherry Tomatoes. Coatings. 2025; 15(7):810. https://doi.org/10.3390/coatings15070810
Chicago/Turabian StyleGonzález-Cuello, Rafael, Joaquín Hernández-Fernández, and Rodrigo Ortega-Toro. 2025. "Composite Coating Enriched with Lemon Peel Extract for Enhancing the Postharvest Quality of Cherry Tomatoes" Coatings 15, no. 7: 810. https://doi.org/10.3390/coatings15070810
APA StyleGonzález-Cuello, R., Hernández-Fernández, J., & Ortega-Toro, R. (2025). Composite Coating Enriched with Lemon Peel Extract for Enhancing the Postharvest Quality of Cherry Tomatoes. Coatings, 15(7), 810. https://doi.org/10.3390/coatings15070810







