Synthesis of ZnS Nano-Powders and Fabrication of ZnS Thin Films via Electron-Beam Evaporation: Structural and Optical Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Devices
- A hot plate with magnetic stirring (HS-3000, Camlab Ltd., Cambridge, UK) for heating and mixing as required.
- A four-digit analytical microbalance (Model SEJ-205, Taipei, Taiwan) for high-accuracy weighing.
- A pH meter (EUTEGH, Thermos Scientific, Singapore) for solution pH measurement.
- A scanning electron microscope (SEM) (Inspect F50-FEI company, Eindhoven, The Netherlands) for high-magnification and high-resolution imaging.
- An Agar sputter coater instrument (AGB7340 Agar Scientific, Essex, UK) for specimen coating.
- A vacuum oven (JEIO TECH, MODEL OV-11, Seoul, Republic of Korea) for drying under vacuum.
- A centrifuge system (HERMILE Labortechnik GmbH, Type Z 326 K, Wehingen, Germany) for powder separation.
- A tube furnace (Protherm alumina tube furnace, Model PTF 12/50/450, Protherm Inc., Ankara, Turkey) with a combined home-designed argon gas line and vacuum fitting for powder sintering.
- A digital caliper (Total, TMT 322001, Guangzhou, China) for high-accuracy dimension measurements.
- A milling machine with variable speed (Changsha Tiachuang, Powder Technology Co., Changsha, China) for the milling process.
- A compaction system consisting of a homemade stainless-steel die integrated with a CARVER press (model 4350. L, CARVER, Inc., Wabash, IN, USA) for powder compaction.
- An electron-beam evaporation system (SCT-1800, SCT, System Control Technologies, sctec.com, Battle Ground, WA 98604, USA) for thin-film deposition.
- A UV–Visible spectrophotometer (UV-1601 (PC) S, Shimadzu Corporation, Tokyo, Japan) for the investigation of optical properties.
- An optical reflectance spectrometer (FilmTek 3000, Scientific Computing International, Carlsbad, CA, USA) for thin-film thickness measurements.
- A Fourier Transform Infrared (FTIR, NEXUS, EPS-87, Thermo Fisher Scientific, Waltham, MA, USA) spectrometer in the wavenumber range of 400–4000 cm−1 for powder and thin-film FTIR tests.
- An X-ray diffraction (XRD) instrument (Malvern Panalytical, Aeris, monochromatic Cu kα1, 1.5406 Å, 0.02 step angle, with 2θ ranging from 10° to 60°, Almelo, The Netherlands) for phase and unit cell investigation.
2.2. Materials
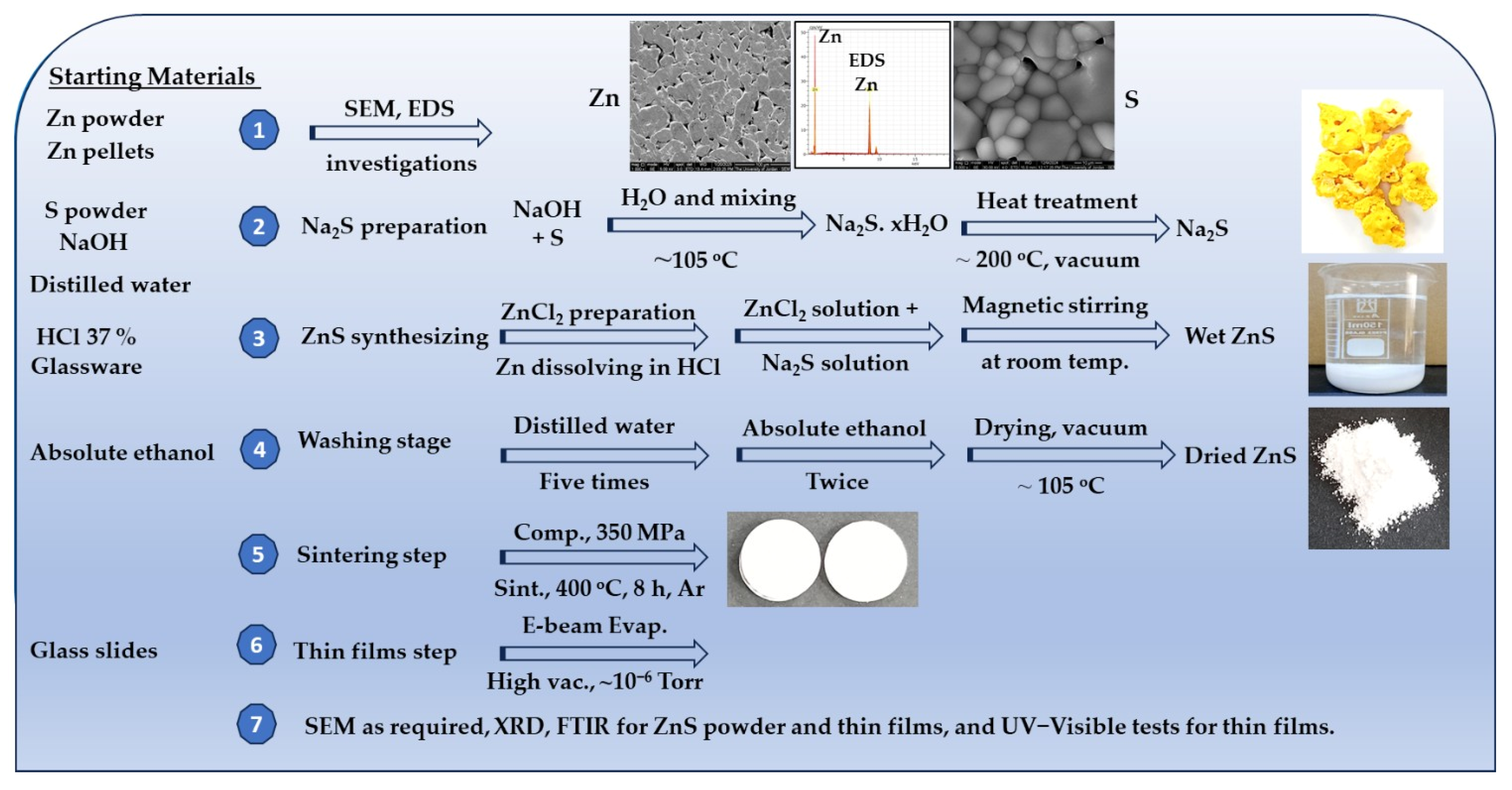
2.3. Production of Na2S Compound
2.4. Synthesis of ZnS
2.5. Sintering of ZnS Powder
2.6. Thin-Film Deposition and UV–Visible Spectrophotometry
2.7. Characterizations of the Starting Powders, Synthesized ZnS, and ZnS Thin Films
3. Results and Discussion
3.1. SEM Results of Zn Powder, S Powder, and Synthetized ZnS Investigations
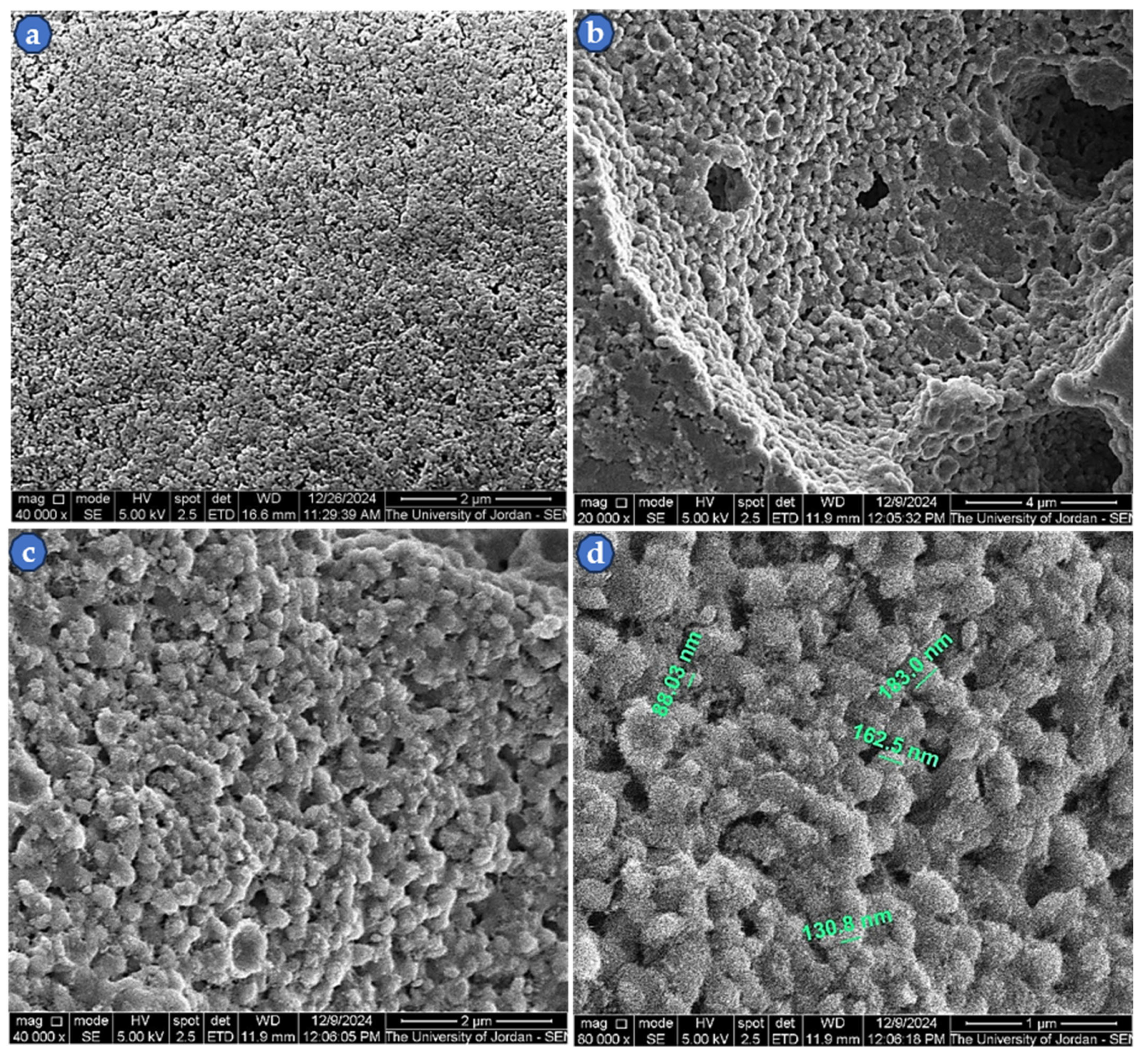
3.2. SEM Images of Sintered ZnS Powder
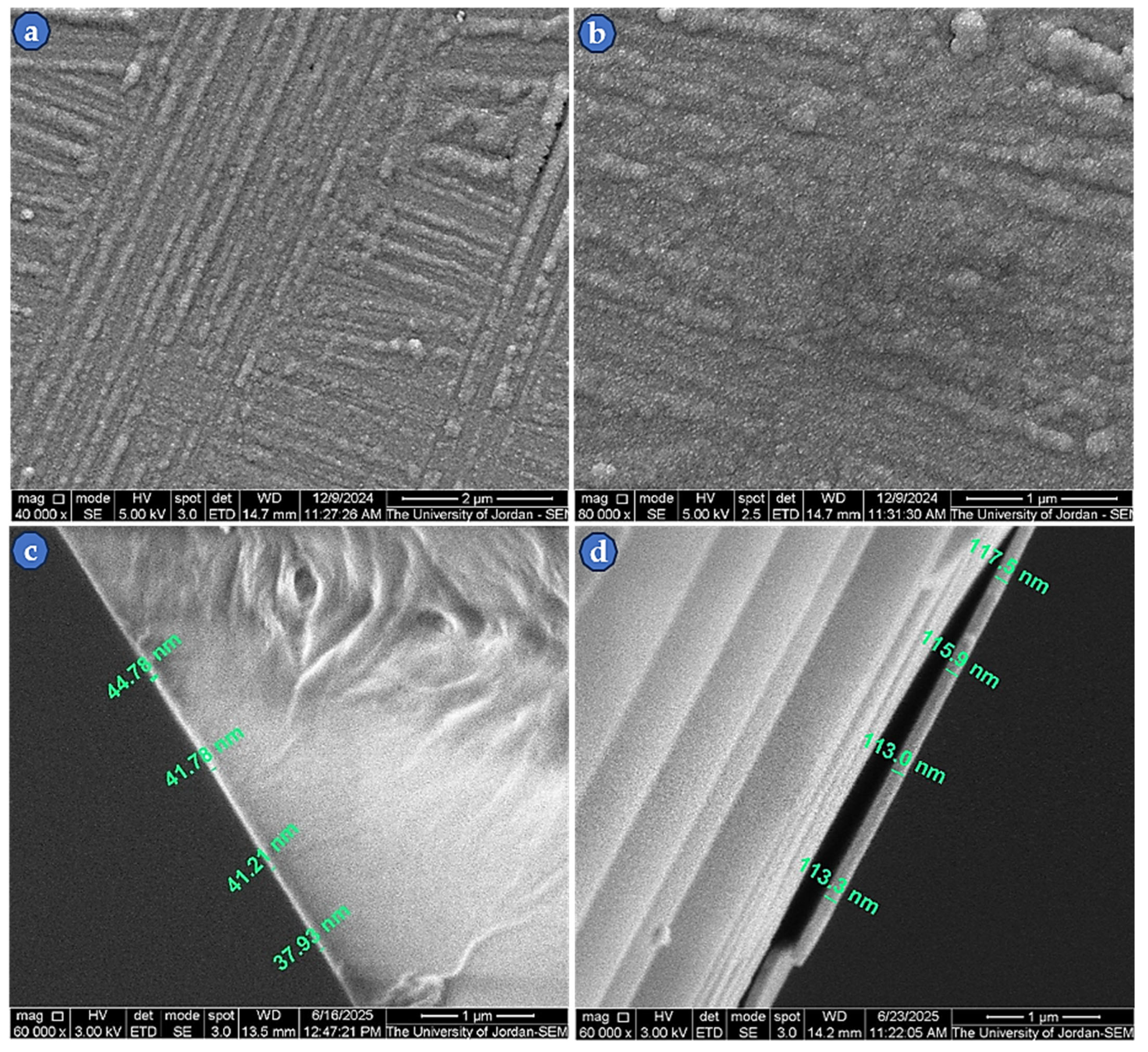
3.3. Thin-Film Microstructure at High Magnification
3.4. UV–Visible and FTIR Spectroscopy of the Produced ZnS
3.4.1. UV–Visible Spectroscopy of the Thin Film Samples
3.4.2. FTIR of ZnS Powder and Its Thin Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mana, P.M.; Bhujbal, P.K.; Pathan, H.M. Fabrication and Characterization of ZnS based Photoelectrochemical Solar Cell. ES Energy Environ. 2021, 12, 77–85. [Google Scholar] [CrossRef]
- Isac, L.; Enesca, A. Recent Developments in ZnS-Based Nanostructures Photocatalysts for Wastewater Treatment. Int. J. Mol. Sci. 2022, 23, 15668. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Tahir, K.; Albalawi, K.; Khalil, M.Y.; Almarhoon, Z.M.; Zaki, M.E.A.; Latif, S.; Hassan, H.M.A.; Refat, M.S.; Munshi, A.M. Synthesis of ZnO and ZnS nanoparticles and their structural, optical, and photocatalytic properties synthesized via the wet chemical method. Mater. Chem. Phys. 2022, 219, 126667. [Google Scholar] [CrossRef]
- Faita, F.L.; Ersching, K.; Poffo, C.M.; Benetti, L.C.; Trichês, D.M.; Souza, S.M.; Viegas, A.D.C.; de Lima, J.C. Structural, thermal, magnetic and optical characterization of undoped nanocrystalline ZnS prepared by solid state reaction. J. Alloys Compd. 2014, 590, 176–183. [Google Scholar] [CrossRef]
- Wei, Z.; Lu, Y.; Zhao, J.; Zhao, S.; Wang, R.; Fu, N.; Li, X.; Guan, L.; Teng, F. Synthesis and Luminescent Modulation of ZnS Crystallite by a Hydrothermal Method. ACS Omega 2018, 3, 137–143. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, S.; Kumar, P.; Ghfar, A.A.; Bouzid, G. Tailoring ZnS nanostructures through precipitation-cum-hydrothermal synthesis for enhanced wastewater purification and antibacterial treatment. Environ. Res. 2024, 259, 119534. [Google Scholar] [CrossRef]
- Lee, G.J.; Wu, J.J. Recent developments in ZnS photocatalysts from synthesis to photocatalytic applications—A review. Powder Technol. 2017, 318, 8–22. [Google Scholar] [CrossRef]
- Sonawane, H.; Deore, J.; Rajshri, S.; Chavan, P. Synthesis of ZnS Nanomaterials and Their Applications via Green Approaches: An Overview. BioNanoScience 2023, 13, 879–890. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Alessandri, E. Syntheses, Properties, and Applications of ZnS-Based Nanomaterials. Appl. Nano 2024, 5, 116–142. [Google Scholar] [CrossRef]
- Elsayed, I.A.; Afify, A.S. Controlling the Surface Morphology of ZnO Nano-Thin Film Using the Spin Coating Technique. Materials 2022, 15, 6178. [Google Scholar] [CrossRef]
- Lee, K.T.; Choi, B.H.; Woo, J.U.; Kang, J.S.; Paik, J.H.; Byoung-Uck Chu, B.U.; Nahm, S. Microstructural and optical properties of the ZnS ceramics sintered by vacuum hot-pressing using hydrothermally synthesized ZnS powders. J. Eur. Ceram. Soc. 2018, 38, 4237–4244. [Google Scholar] [CrossRef]
- Sadovnikov, S.I.; Ishchenko, A.V.; Weinstein, I.A. Synthesis and optical properties of nanostructured ZnS and heteronanostructures based on zinc and silver sulfides. J. Alloys Compd. 2020, 831, 154846. [Google Scholar] [CrossRef]
- Gao, X.D.; Li, X.M.; Yu, W.D. Morphology and optical properties of amorphous ZnS films deposited by ultrasonic-assisted successive ionic layer adsorption and reaction method. Thin Solid Film. 2004, 468, 43–47. [Google Scholar] [CrossRef]
- Khan, J.A.; Ahamad, S.; Ansari, M.A.H.; Tauqeer, M.; Park, C.H.; Park, J.P.; Choi, C.H.; Mohammad, A. State-of-the-art in ZnS-based nanoarchitects for visible-light photocatalytic degradation of antibiotics and organic dyes. J. Water Process Eng. 2024, 67, 106151. [Google Scholar] [CrossRef]
- Navaneethan, M.; Archana, J.; Nisha, K.D.; Hayakawa, Y.; Ponnusamy, S.; Muthamizhchelvan, C. Temperature dependence of morphology, structural and optical properties of ZnS nanostructures synthesized by wet chemical route. J. Alloys Compd. 2010, 506, 249–252. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, D.; Wang, D.; Kong, X.; Huang, J.; Wang, F.; Wu, Y. Size dependent photocatalytic activity of ZnS nanostructures prepared by a facile precipitation method. Mater. Sci. Eng. B 2016, 208, 15–21. [Google Scholar] [CrossRef]
- Shobana, T.; Venkatesan, T.; Kathirvel, D. A Comprehensive Review on Zinc Sulphide Thin Film by Chemical Bath Deposition Techniques. J. Environ. Nanotechnol. 2020, 9, 50–59. [Google Scholar] [CrossRef]
- Arsad, A.Z.; Zuhdi, A.W.M.; Abdullah, F.A.; Chau, C.F.; Ghazali, A.; Ahmad, I.; Abdullah, W.S.W. Effect of Chemical Bath Deposition Variables on the Properties of Zinc Sulfide Thin Films: A Review. Molecules 2023, 28, 2780. [Google Scholar] [CrossRef]
- Ali, A.H.; Hashem, H.A.; Elfalaky, A. Preparation, Properties, and Characterization of ZnS Nanoparticles. Eng. Proc. 2023, 31, 74. [Google Scholar] [CrossRef]
- Shahjahan, M.; Kabir, R.; Hossain, M.S.; Haque, M.A.; Prasad Paul, D. Effect of pH on Optical Properties of Nanostructured Cu-Doped ZnS Thin Films for Photovoltaic Applications. Eur. J. Mater. Sci. Eng. 2024, 8, 226–239. [Google Scholar] [CrossRef]
- AlShamaileh, E.; Lahlouh, B.; AL-Masri, A.N.; Al-Qderat, M.; Wadah Mahmoud, W.; Alrbaihat, M.; Moosa, I.S. Comparative Microstructure Characteristics of Synthesized PbS Nanocrystals and Galena. Sci 2024, 6, 61. [Google Scholar] [CrossRef]
- Ebnelwaled, A.A.; Elsheikh, H.H.A.; Hamed, Z.H. Synthesis and characterization of ZnS nanoparticles by chemical precipitation method. Aswan Univ. J. Environ. Stud. 2021, 2, 147–154. [Google Scholar] [CrossRef]
- Nagamani, K.; Prathap, P.; Lingappa, Y.; Miles, R.W.; Reddy, K.T.R. Properties of Al-doped ZnS Films Grown by Chemical Bath Deposition. Phys. Procedia 2012, 25, 137–142. [Google Scholar] [CrossRef]
- Shakil, M.A.; Das, S.; Rahman, M.A.; Akther, U.S.; Majumdar, M.K.H.; Rahman, M.K. A Review on Zinc Sulphide Thin Film Fabrication for Various Applications Based on Doping Elements. Mater. Sci. Appl. 2018, 9, 751–778. [Google Scholar] [CrossRef]
- Ahn, H.; Um, Y. Post-annealing Effects on ZnS Thin Films Grown by Using the CBD Method. J. Korean Phys. Soc. 2015, 67, 1045–1050. [Google Scholar] [CrossRef]
- Karimi, M.; Mirkazemi, S.M.; Vahidshad, Y.; Javadpour, J. Preparation and characterization of zinc sulfide thin film by electrostatic spray deposition of nano-colloid. Thin Solid Film. 2021, 737, 138929. [Google Scholar] [CrossRef]
- Krystýnová, M.; Doležal, P.; Fintová, S.; Březina, M.; Zapletal, J.; Wasserbauer, J. Preparation and Characterization of Zinc Materials Prepared by Powder Metallurgy. Metals 2017, 7, 396. [Google Scholar] [CrossRef]
- Petersen, H.; Reichle, S.; Leiting, S.; Losch, P.; Kersten, W.; Rathmann, T.; Tseng, J.; Etter, M.; Schmidt, W.; Weidenthaler, C. In Situ Synchrotron X-ray Diffraction Studies of the Mechanochemical Synthesis of ZnS from its Elements. Chem. Eur. J. 2021, 27, 12558–12565. [Google Scholar] [CrossRef]
- Moosa, I.S.; Kazem, H.A.; Al-Iessi, L.M.R. Production of Hydrogen via Renewable Energy and Investigation of Water Molecular Changes During Electrolysis Process. JREE 2021, 8, 19–28. [Google Scholar] [CrossRef]
- Labiadh, H.; Moualhi, Y.; Moualhi, K.; Abdelhak Othmani, A.; Zouaoui, M. Synthesis of ZnS nanoparticles and the investigation of their structural, optical and electrical properties. Euro-Mediterr. J. Environ. Integr. 2025, 10, 119–130. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Watcharakitti, J.; Piyavarakorn, V.; Johnson, B.; Bushby, R.J.; Chuaicham, C.; Sasaki, K.; Nijpanich, S.; Nakajima, H.; Chanlek, N.; et al. ZnO/ZnS photocatalyst from thermal treatment of ZnS: Influence of calcination temperature on development of heterojunction structure and photocatalytic performance. J. Phys. Chem. Solids 2023, 179, 111393. [Google Scholar] [CrossRef]
- Yang, K.; Li, B.; Zeng, G. Effects of temperature on properties of ZnS thin films deposited by pulsed laser deposition. Superlattices Microstruct. 2019, 130, 409–415. [Google Scholar] [CrossRef]
- Al-Saqarat, B.S.; Al-Mobydeen, A.; Al-Dalahmeh, Y.; AL-Masri, A.N.; Altwaiq, A.M.; Hamadneh, I.; Abu-Afifeh, Q.; Zoubi, M.M.; Esaifan, M.; Moosa, I.S. Study of Galena Ore Powder Sintering and Its Microstructure. Metals 2024, 14, 439. [Google Scholar] [CrossRef]
- William, D.P.; Sangmoon, P.; Tom, V.; Douglas, J.B. Electron Beam-Induced Fragmentation and Dispersion of Bi-Ni Nanoparticles. J. Phys. Chem. C 2007, 111, 10824–10828. [Google Scholar] [CrossRef]
- Grayeli, A.; Sadeghi, M.; Shakoury, R.; Matos, R.S.; Filho, H.D.F.; Arman, A. Structural, morphological, and optical bandgap properties of ZnS thin films: A case study on thickness dependence. Opt. Quantum Electron. 2024, 56, 1142. [Google Scholar] [CrossRef]
- Jafarov, M.A.; Nasirov, E.F.; Jahangirova, S.A.; Jafarli, R. Nano-ZnS thin films for solar cell. Nanosyst. Phys. Chem. Math. 2015, 6, 644–649. [Google Scholar] [CrossRef][Green Version]
- Choudapura, V.H.; Kapatkara, S.B.; Rajub, A.B. Structural and Optoelectronic Properties of Zinc Sulfide Thin Films Synthesized by Co-Precipitation Method. Acta Chem. IASI 2019, 27, 287–302. [Google Scholar] [CrossRef]
- Djelloul, A.; Adnane, M.; Larbah, Y.; Sahraoui, T.; Zegadi, C.; Maha, A.; Rahal, B. Properties Study of ZnS Thin Films Prepared by Spray Pyrolysis Method. J. Nano-Electron. Phys. 2015, 7, 04045. [Google Scholar]
- Nadeem, M.Y.; Ahmed, W. Optical Properties of ZnS Thin Films. Turk. J. Phys. 2000, 24, 651–659. [Google Scholar]
- Alzaid, M.; Mohamed, W.S.; El-Hagary, M.; Shaaban, E.R.; Hadia, N.M.A. Optical properties upon ZnS film thickness in ZnS/ITO/glass multilayer films by ellipsometric and spectrophotometric investigations for solar cell and optoelectronic applications. Opt. Mater. 2021, 118, 111228. [Google Scholar] [CrossRef]
- Prathap, P.; Revathi, N.; Venkata Subbaiah, Y.P.; Ramakrishna Reddy, K.T. Thickness effect on the microstructure, morphology and optoelectronic properties of ZnS films. J. Phys. Condens. Matter 2008, 20, 035205. [Google Scholar] [CrossRef]
- Singh, M.; Monika Goyal, M.; Devlal, K. Size and shape effects on the band gap of semiconductor compound nanomaterials. J. Taibah Univ. Sci. 2018, 12, 470–475. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Hadia, N.M.A.; Awada, M.A.; Shaaban, E.R. Effects of Thickness and Ag Layer Addition on the Properties of ZnS Thin Films. Acta Phys. Pol. A 2019, 135, 420–425. [Google Scholar] [CrossRef]
- Jothibas, M.; Jeyakumar, S.J.; Manoharan, C.; Punithavathy, I.K.; Praveen, P.; Richard, J.P. Structural and optical properties of zinc sulphide nanoparticles synthesized via solid state reaction method. J. Mater. Sci. Mater. Electron. 2017, 28, 1889–1894. [Google Scholar] [CrossRef]
- Mani, S.K.; Manickam, S.; Muthusamy, V.; Thangaraj, R. Antimicrobial Activity and Photocatalytic Degradation Properties of Zinc Sulfide Nanoparticles Synthesized by Using Plant Extracts. J. Nanostructures 2018, 8, 107–118. [Google Scholar] [CrossRef]
- Tounsi, A.; Talantikite-Touati, D.; Merzouk, H.; Haddad, H.; Khalfi, R. Study of the properties of La-doped ZnS thin Films synthesized by Sol-gel method. Adv. Mater. Lett. 2018, 9, 216–219. [Google Scholar] [CrossRef]
- Włodarski, M.; Putkonen, M.; Norek, M. Infrared Absorption Study of Zn–S Hybrid and ZnS Ultrathin Films Deposited on Porous AAO Ceramic Support. Coatings 2020, 10, 459. [Google Scholar] [CrossRef]
- Hameed, O.M. Synthesis of Zinc Sulfide Nanocrystals by Chemical Bath Deposition Methods. E3S Web Conf. 2023, 391, 01134. [Google Scholar] [CrossRef]
- Khosravi, S.; Chaibakhsh, N.; Jafari, S.; Nilkar, M. Enhanced photocatalytic activity of ZnS/TiO2 nanocomposite by nitrogen and tetrafluoromethane plasma treatments. Sci. Rep. 2024, 14, 28385. [Google Scholar] [CrossRef]



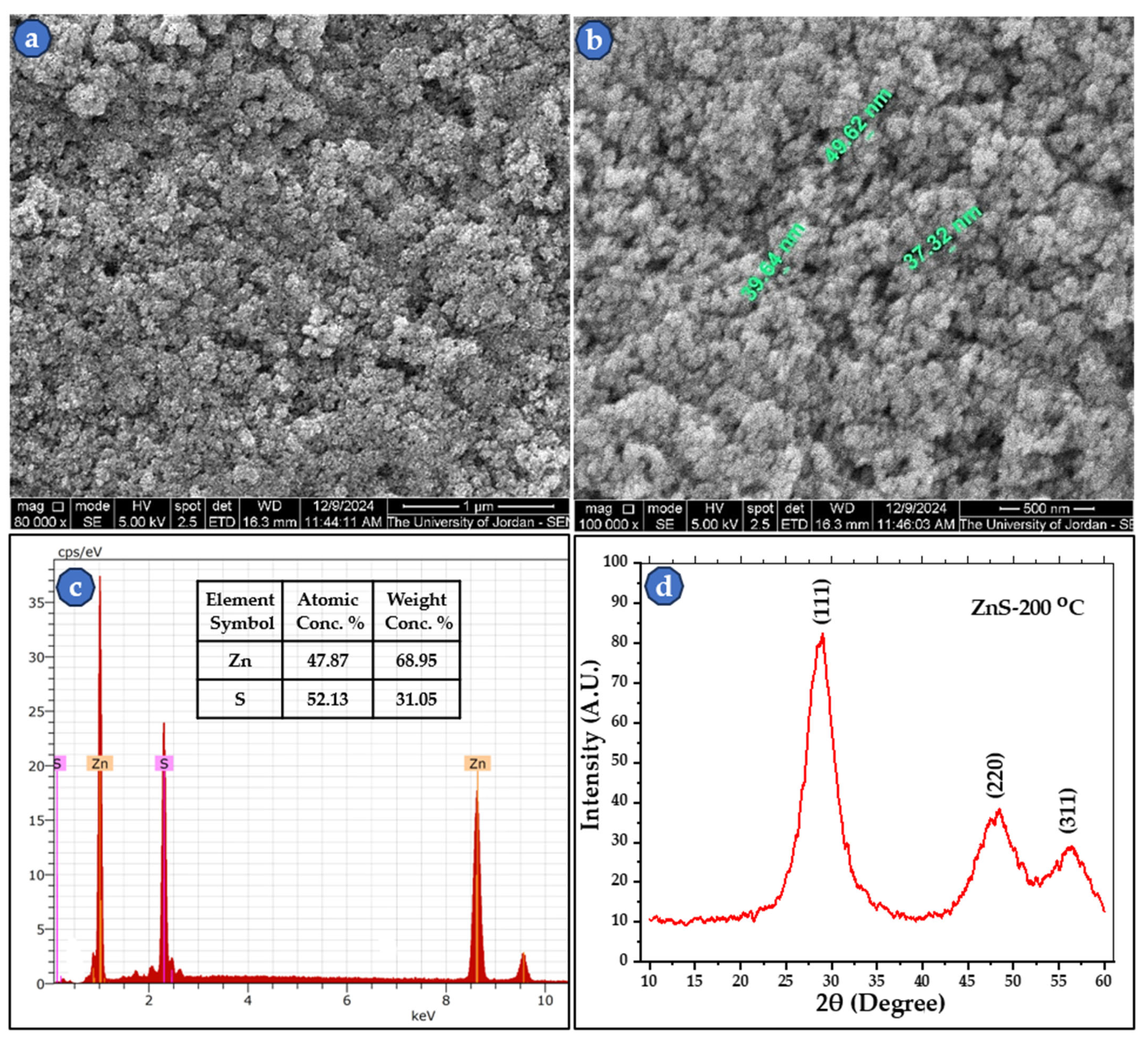
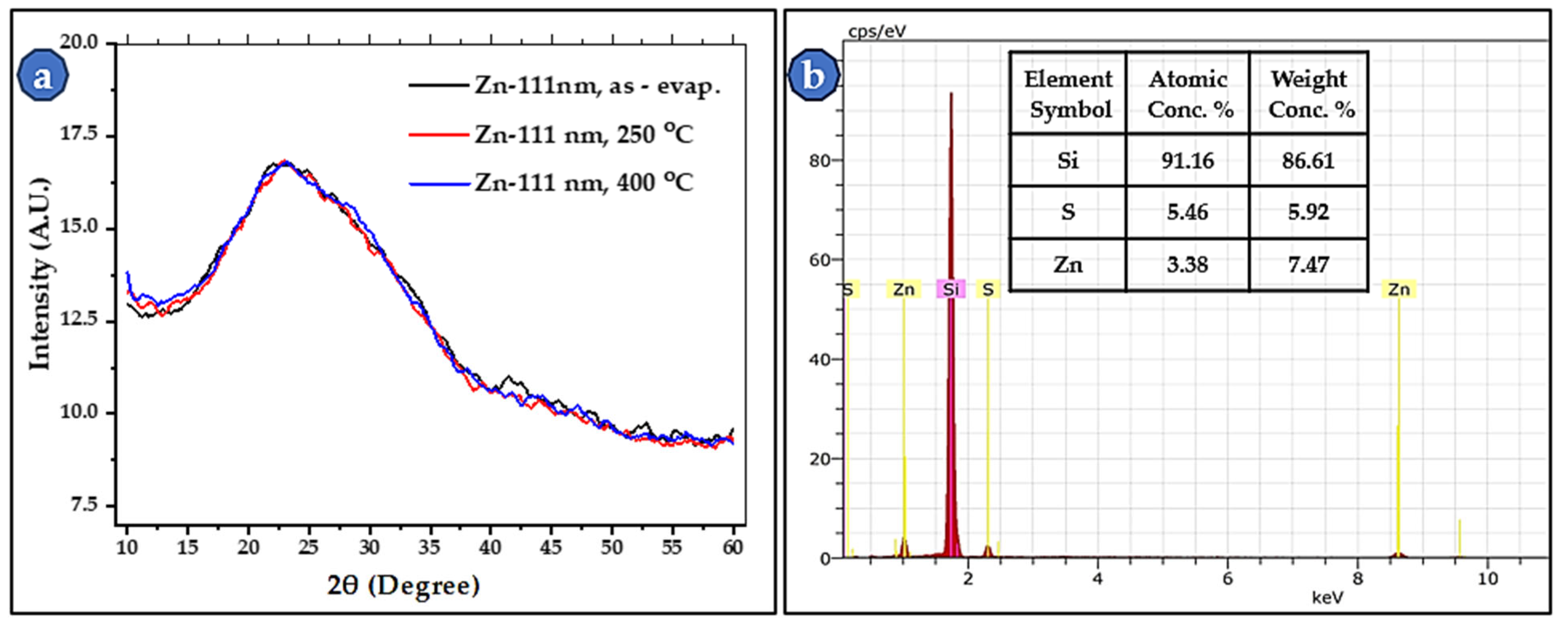
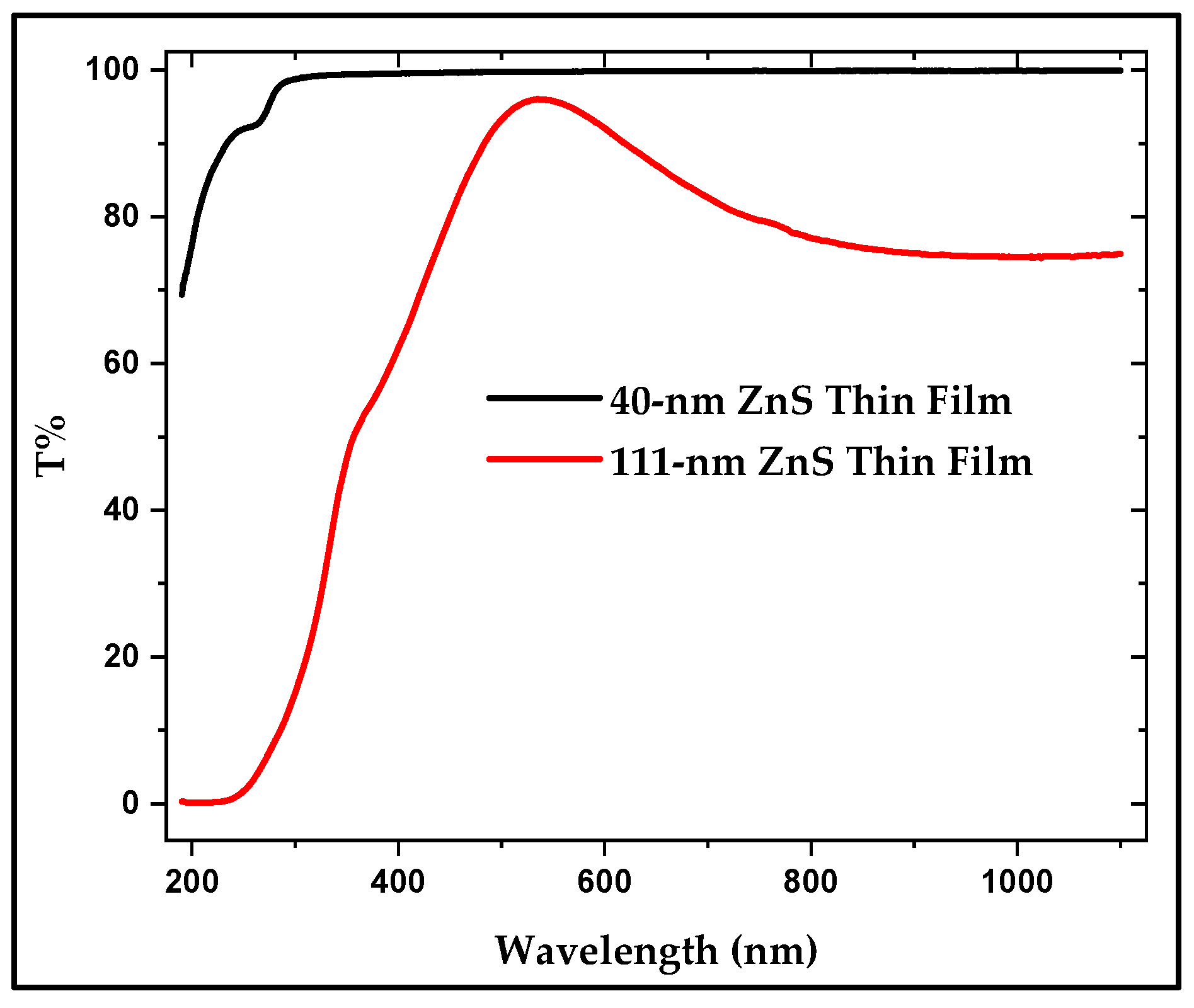
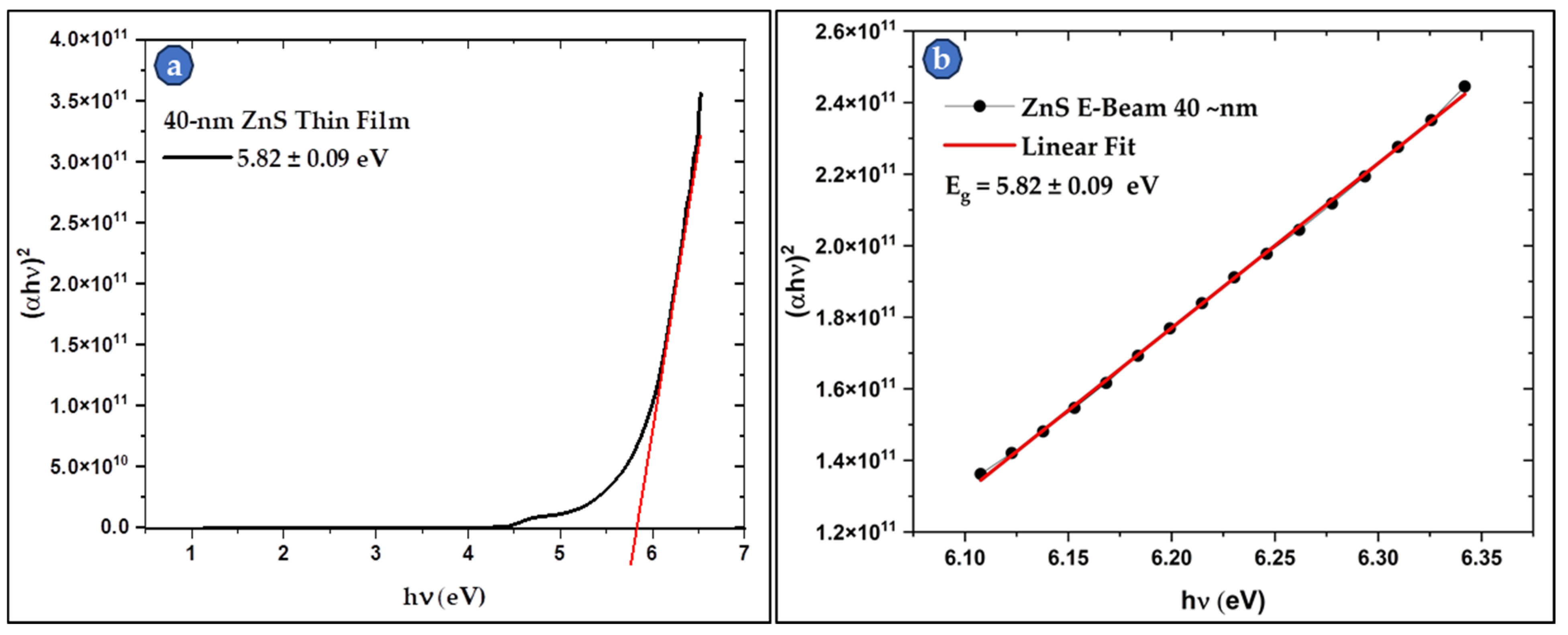
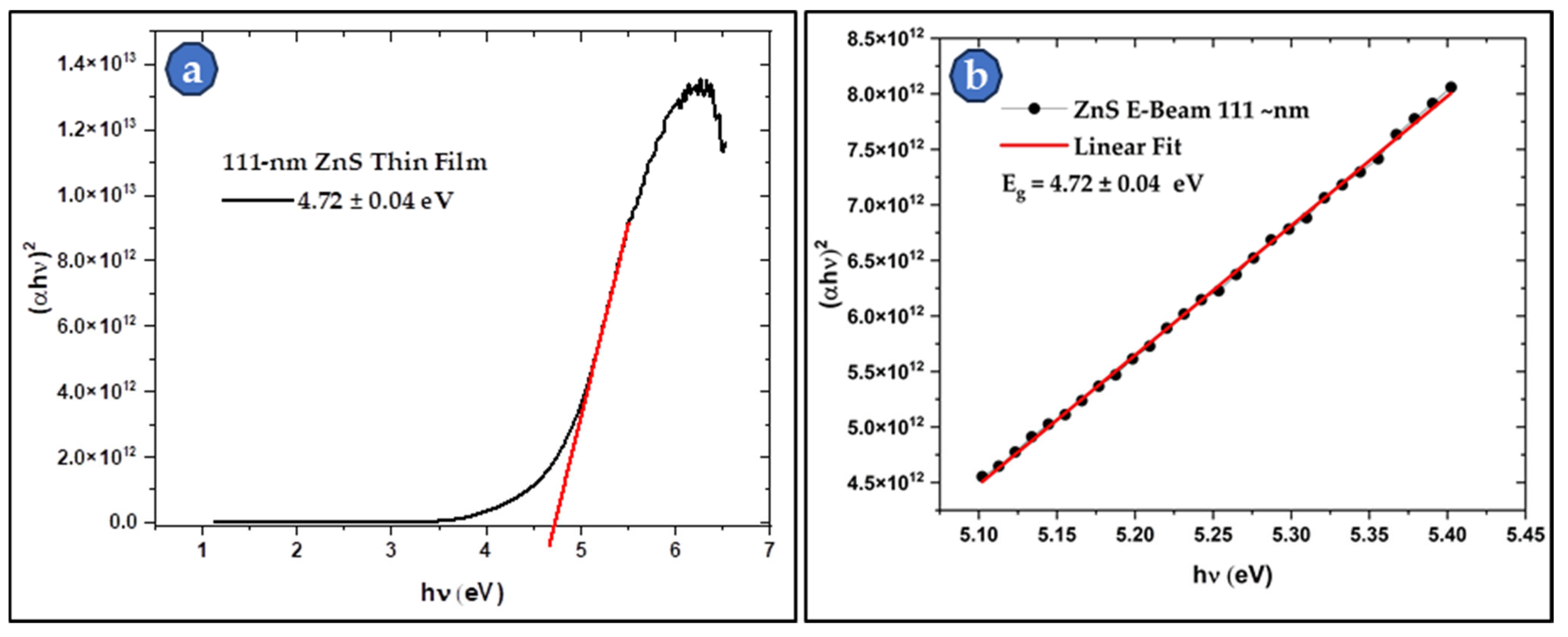

| Parameters | 2θ Peaks (in °) | β (FWHM) (in °) | D (in nm) | Mean D (in nm) | |
|---|---|---|---|---|---|
| K | λ (Å) | 28.688 | 3.139 | 2.73 | 2.26 |
| 0.94 | 1.54178 | 48.187 | 4.612 | 1.97 | |
| 56.174 | 4.513 | 2.09 | |||
| Sample No. | ρg (g/cm3) | ρs (g/cm3) | Compaction and Sintering Conditions |
|---|---|---|---|
| 1 | 2.64 | 3.19 | Compaction pressure 400 MPa and sintering temp. 400 °C for 8 h in an argon atmosphere with a flow rate of approximately 1 L/min, heating rates of 5 °C/min, and furnace cooling at a rate of 2.5 °C/min. |
| 2 | 2.61 | 3.15 | |
| 3 | 2.69 | 3.32 | |
| 4 | 2.61 | 3.18 | |
| Mean value | 2.64 | 3.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mobydeen, A.; AlShamaileh, E.; Lahlouh, B.; Al-Qderat, M.; AL-Masri, A.N.; Mahmoud, W.; Hamadneh, I.; Esaifan, M.; Moosa, I.S. Synthesis of ZnS Nano-Powders and Fabrication of ZnS Thin Films via Electron-Beam Evaporation: Structural and Optical Characterization. Coatings 2025, 15, 796. https://doi.org/10.3390/coatings15070796
Al-Mobydeen A, AlShamaileh E, Lahlouh B, Al-Qderat M, AL-Masri AN, Mahmoud W, Hamadneh I, Esaifan M, Moosa IS. Synthesis of ZnS Nano-Powders and Fabrication of ZnS Thin Films via Electron-Beam Evaporation: Structural and Optical Characterization. Coatings. 2025; 15(7):796. https://doi.org/10.3390/coatings15070796
Chicago/Turabian StyleAl-Mobydeen, Ahmed, Ehab AlShamaileh, Bashar Lahlouh, Mariam Al-Qderat, Ahmed N. AL-Masri, Wadah Mahmoud, Imad Hamadneh, Muayad Esaifan, and Iessa Sabbe Moosa. 2025. "Synthesis of ZnS Nano-Powders and Fabrication of ZnS Thin Films via Electron-Beam Evaporation: Structural and Optical Characterization" Coatings 15, no. 7: 796. https://doi.org/10.3390/coatings15070796
APA StyleAl-Mobydeen, A., AlShamaileh, E., Lahlouh, B., Al-Qderat, M., AL-Masri, A. N., Mahmoud, W., Hamadneh, I., Esaifan, M., & Moosa, I. S. (2025). Synthesis of ZnS Nano-Powders and Fabrication of ZnS Thin Films via Electron-Beam Evaporation: Structural and Optical Characterization. Coatings, 15(7), 796. https://doi.org/10.3390/coatings15070796









