Abstract
The antifouling performance of a zwitterionic sulfobetaine-hydroxyethyl-containing polymethylmethacrylate ter-co-polymer (PSBM) is evaluated against three photosynthetic strains, namely Chlorella sp., Nannochloropsis sp., and Arthrospira maxima. PSBM-coated polymethylmethacrylate (PMMA) surfaces displayed a significantly reduced propensity for biofilm formation compared to rough and untreated controls, leaving clean surfaces after 7 days of exposure. A tribological approach was adopted to estimate the long-term durability of the PSBM coating. Repeated cycles of exposure to Chlorella sp., Nannochloropsis sp., and A. maxima biomass subject the coating to stress and continuous biofilm challenges. After several cycles, the PSBM coating maintains a higher antifouling efficacy than the untreated PMMA surface, suggesting stability and high potential in photobioreactor applications.
1. Introduction
The microalgae and cyanobacteria biomass cultivation are promising bio-factories because they can be used in food, feed, bioactive bulk substances, wastewater treatment, and fuel production [1]. They provide valuable natural antibiotics, pigments, vitamins, and bioactive compounds [2,3,4]. Microalgae use CO2 as a carbon source to generate biomass through a photosynthetic process. This makes them active materials in CO2 sequestration to mitigate global warming and for the sustainable production of bioenergy and other value-added products [5,6,7,8].
The most prevalent approaches for large-scale phototrophic biomass production are open systems like ponds and artificial basins or closed photobioreactors (PBRs) [9,10,11]. Open systems are cheaper to design, build, and maintain, while the closed ones, despite a higher initial investment, produce biomass more efficiently for fine chemical, nutraceutical, and pharmaceutical applications.
However, large-scale microalgae production presents several challenges, including selecting the correct algal strain and biomass-producing methods, long-term efficiency, and harvesting [1,12]. Furthermore, bioproducts and microorganisms, which adhere and multiply on the inner surfaces, cause a counterproductive process called biofouling [13,14,15]. This reduces solar/UV radiation penetration through the PBRs walls and biomass production, also causing cell pigmentation alterations, culture degradation, and invasive microbe contamination [16].
Biofilm growth on PBR surfaces depends on several factors, and its understanding requires a holistic approach, as it involves biological, engineering, and chemico-physical expertise. PBR material and geometry, algae typology, culture medium ionic strength, and fluid dynamics [14,15,16,17,18] are among the most demanding.
Biofouling on PBR surfaces begins with organic molecule adsorption, creating a conditioning film to which microalgae adhere and secrete exopolymeric substances (EPS), increasing the surface biofouling potential [17,18]. Environmental variables, including temperature, light intensity, and nutritional ratios, impact it. High temperatures and light, especially in outdoor culture, boost EPS synthesis and cell adhesion and biofouling [17].
PBR walls are often made of acrylate, polymethylmethacrylates, and polyethylene plastics due to their transparency and mechanical properties. These polymers are hydrophobic, and, over time, their wettability reduces while cell adherence increases [9,14]. Even surface roughness, often caused by aging, can promote cell adhesion and biofilm growth [13,14].
Recent studies highlight innovative surface modifications to reduce biofouling [17,18,19]. A very promising method is surface grafting of hydrophilic “fouling-resistant coatings” (FRCs). Examples include the pioneering polyethylene glycol (PEG), polyamide (PA) hydrogel, and zwitterionic (ZW) coatings [19,20,21,22,23,24]. These coatings are biomimetic [25] since, like peptides and glycoproteins, in water, they generate a surface hydration layer that prevents proteins, algae, and other generic foulants from settling [26].
Among these, zwitterionic polymers, a family of polyampholytes with high polarizable unit pairs of cations and anions, were extensively investigated in the context of marine, bio, and medical fields due to their excellent biocompatibility, stability, and minimal fouling [27,28]. A large variety of sulfo- and carboxy-betaine methylmethacrylate-based ZW polymers have been developed. These display a plethora of properties, such as conformational electrolyte responsiveness [29,30,31], AF activity retention in high salt concentrations [27,32], and composition-controlled surface activity, which make them suitable candidates for the engineering of AF coatings for PBR surface application [33,34]. In addition, ZW exhibits higher stability with respect to the deeply investigated PEG-based polymers. These latter are featured by a low chemical stability due to PEG chains, which can rapidly autoxidize and degrade during storage and handling at room temperature, especially by transition metal ions, which are present in most biologically related solutions [19,26,33].
Nevertheless, only a few examples are reported. These concern omo-polysulfobetaine and sulfobetaine copolymers grafted on chemically activated PE (polyethylene) and EVA (ethylene-vinyl acetate copolymer) substrates [35,36], which displayed satisfactory AF behavior against Chlorella sp. [35].
Biofouling and surface topography highly affect material surface performance and lifespan. Prevention methods included surface engineering and coatings design to inhibit biological material adherence and wear effects [37]. Tribology is a multidisciplinary science that studies friction effects, wear, and lubrication of surfaces under extreme environments. It is essential for assessing surface material life, mechanical contact efficiency, degradation properties of polymer coatings [37,38], and, at the same time, improving their biodegradability and end-of-cycle recycling [39]. Literature reports [40,41] emphasize the importance of using this technique to create tribosystems with coatings that function in the intended wear regime, enhancing surface life cycle.
To overcome the issue of biofouling inside PBR walls, the zwitterionic sulfobetaine-hydroxyethyl-containing polymethylmethacrylate ter-co-polymer coating (PSBM) was designed (Figure 1) and characterized. It displayed the required prerequisites for PMMA PBRs application, such as electrolyte responsiveness both in solution and as a solid, easy handling, and a transparent and low-roughness surface when applied on a PMMA substrate. Preliminary antifouling tests carried out over 7 days against the Chlorella sp., commonly adopted as a model strain, were consistent with excellent activity of PSBM [42]. These results prompted us to deeply investigate PSBM AF behavior in terms of coating stability and the nature of strain adhesion.
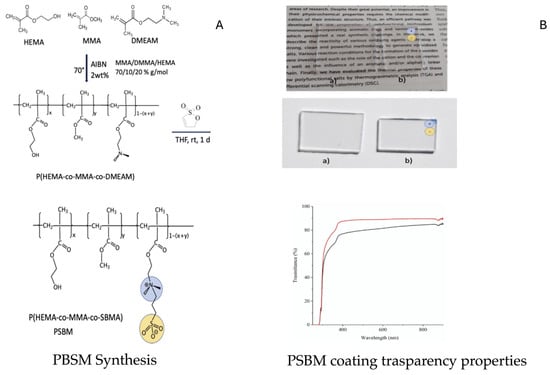
Figure 1.
Synthesis (A,B) transparency properties of PBSM coating seen under UV-vis transmittance spectra of PMMA before (a, black line) and after deposition of PSBM film (b, red line).
Therefore, here we report on PSBM antifouling characteristics compared to different photosynthetic strains, namely Chlorella sp., Nannochloropsis sp., and Arthrospira maxima. Antifouling tests were also performed on untreated PMMA and mechanically aged/rough coupons for 7 days for comparison. To test the long-term working of PSBM-coated PMMA coupons, a tribological approach is used here. To this aim, the PSBM coupons were repeatedly exposed to Chlorella sp., Nannochloropsis sp., and A. maxima, and their behaviors were compared to uncoated PMMA controls.
2. Materials and Methods
All the chemicals methylmethacrylate (stabilized for synthesis) (MMA), 2-hydroxyethyl-methacrylate (99%) (HEMA), dimethylaminoethylmethacrylate (stabilized for synthesis) (DMEAM), 1,3-propanesultone (98%), azoisobutyronitrile (98%) (AIBN), and solvents employed were purchased from Sigma-Aldrich and used as supplied. Commercially available polymethylmethacrylate (PMMA) was used to obtain coupons.
2.1. PSBM Synthesis
Synthesis of sulfobetaine-hydroxyethyl-containing polymethylmethacrylate ter-co-polymer (PSBM) was achieved as already reported by Lo Schiavo and coworkers [42] and summarized in Figure 1A. The procedure involved two steps: (a) the synthesis of the random terpolymer, P(MMA-co-DMEAM-co-HEMA), via a radical polymerization by using AIBN (azoisobutyronitrile) as a radical initiator; (b) the covalent attachment of sulfonate groups via ring opening of 1,3-sultone. (a) A mixture of MMA, DMMA, and HEMA in a 70/10/20 molar ratio was placed into a two-necked round-bottom flask equipped with a condenser under a nitrogen atmosphere. The resulting mixture was left to stir for ca. 15 min at room temperature. After this time, AIBN (2% w/w) was added as a radical initiator, and the resulting solution was left to stir for a further 15 min. Then, the temperature was raised to 70 °C, and the reaction stopped when a transparent solid was formed. This was crashed in the presence of ethanol, washed repeatedly with chloroform and diethyl ether, and dried. Yield about 95%. (b) The as-obtained terpolymer was dissolved in THF by ultrasonic treatment until a clear solution/suspension was formed. Furthermore, 1,3-sultone (in a 1.2:1 molar ratio with respect to DMEAM) was then added, and the mixture was left to stir for 1 day under nitrogen. During this time, a white solid, named PSBM, was obtained. The mother liquids were pipetted off, and the resulting residue was washed several times with diethyl ether and dried; the resulting yield was 90%.
2.2. Preparation of PSBM Drop-Casting Mixture
A transparent PSBM “suspension” (c = 2.1 mg/mL) used for drop-casting was obtained as follows: 76 mg of terpolymers were dissolved in 20 mL of H2O/IPA (2:3 in v/v) by using an ultrasonic bath. Then, a further 10 mL of IPA were added, and the mixture was left to stir for 20 min. The final addition of 4 mL of NaOH 10−5 M produced a low-viscosity and transparent suspension used for drop-casting procedures.
2.3. PMMA Coupons
PSBM-coated PMMA coupons were prepared by following a drop-casting procedure as described by Lo Schiavo et al. [42]: 0.070 mL of the as-prepared PSBM drop-casting mixture (2.2.2) was pipetted on the PMMA coupons. These were kept at a temperature of 40 °C for 12 h and then at 60° for further a 24 h (density = 39 mg/cm2) (Figure 1B).
Untreated PMMA (UT) and rough PMMA (R), obtained by using sandpaper of grit size P800, were used for comparative tests. The dimensions of all three types of coupons were 16 mm × 12 mm × 2 mm, and they were used in duplicate in each set of experiments. Each side of the coupons was sterilized under ultraviolet light (UV) for 10 min each. The coupons were glued on glass slides and sterilized under UV and then inserted in double into Erlenmeyer flasks containing the growth medium for the AF tests (Figure 2a). For the tribological experiments, glass Petri dishes Ø 110 mm were used (Figure 2b). Coupons were hydrated before experimentation by covering them with the appropriate medium for 24 h.
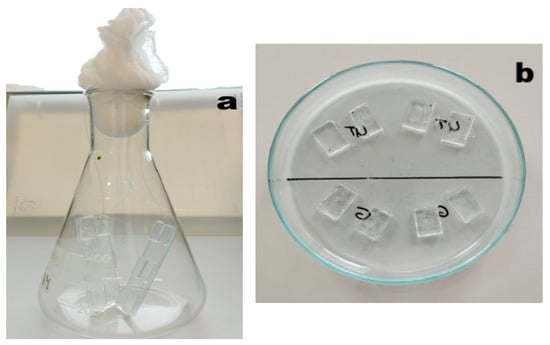
Figure 2.
Erlenmeyer flask (a) and glass Petri dish (b) with coupons before the addition of algal suspension.
2.4. Photosynthetic Strains
The three photosynthetic strains used to test the AF coating performance were as follows: Chlorella sp. strain coming from the culture collection of the Laboratory of Environment Microbiology and Cultural Heritage (Micro ABC); Nannochloropsis sp.; and the filamentous cyanobacterium A. maxima from the culture collection of the Laboratory of Microbial Ecology (Figure 3).
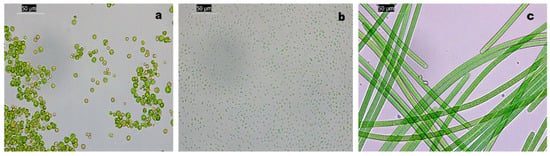
Figure 3.
Light microscopy images of the 3 strains used. (a) Chlorella sp.; (b) Nannochloropsis sp.; and (c) A. maxima. Bars are 50 µm.
Chlorella sp. and Nannochloropsis sp. microalgal strains were grown in BG 11 medium [43], while the cyanobacterium A. maxima was grown in Zarrouk’s medium [44]. For all experiments, fresh biomass was obtained by growing each strain in the respective liquid medium for 7 days under a constant light intensity of 3000 lux and an agitation at 100 rpm at room temperature of about 24 °C. Biomass was then collected by centrifugation at 10,000 rpm for 30 min at 10 °C, rinsed and centrifuged three times with sterile physiological solution, and the pellet wet weight was then determined. The biomass amount inoculated into the flasks or into the Petri dishes was adjusted to be about ≥1 g/L for all the strains.
2.5. Antifouling Assays
The AF experiments for each strain were carried out in duplicate in Erlenmeyer flasks, each containing two sets of glass slides with attached coupons (PSBM, UT, and R), 400 mL of liquid medium, and biomass adjusted to a final value > 1 g/L (Figure 2a). Then, the flasks were placed on the orbital shaker for 7 days under a continuous light exposure of 3000 lux and agitation (100 rpm) at room temperature (~24 °C). At the end of the 7-day cycle, the biomass was collected by centrifugation as previously described, and the wet weight obtained was calculated and compared to the starter amount to determine the biomass production. The coupons were detached from the glass slide, rinsed with sterile distilled water, and observed under a light microscope (Leica DM750, Leica Microsystems GmbH, Switzerland) and a fluorescent microscope (Leica DMRE, Leica Microsystems GmbH, Switzerland). Each image, representative of the general behavior of algal cells, was analyzed by Leica LAS EZ Application Version 3.4.0, (Leica Microsystems GmbH, Switzerland) and further processed by ImageJ software (Version 1.46r). Cell counts, area coverage/mm2, and percentage of coverage of the coupons were determined.
2.6. Tribological Tests
For running tribological cycles, untreated (UT) and PSBM PMMA coupons coming from the antifouling tests described in Section 2.4 were employed (this cycle was considered as cycle 1). The experiments were carried out using glass Petri plates Ø 110 mm instead of Erlenmeyer flasks (Figure 2b). For each cycle, the same coupons were placed at the bottom of sterile glass Petri plates and sterilized by UV radiation for 10 min. The starting biomass was obtained as described in Section 2.3 and adjusted to ≥1 g/L when inoculated onto Petri plates with hydrated PMMA coupons. The inoculated Petri dishes were placed on an orbital agitator at 80 rpm to evenly distribute biomass on all surfaces. Light intensity was 3000 lux, and temperature was 25 °C. After each 7-day experimental cycle, biomass was collected and weighed as indicated in Section 2.4, and the coupons were rinsed with sterile deionized water to eliminate loose biomass. Then, they were observed under the light microscope, and ImageJ software (ver. 1.46) was used to assess biofouling and performance over time. Finally, the coupons were gently washed and rinsed and used for the next cycle.
2.7. Statistical Analysis
The experimental results are presented as the mean ± standard deviation (SD), and they were repeated at least three times. Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons using GraphPad Prism software version 9.5.1 (GraphPad Software, San Diego, CA, USA). p < 0.01 was considered to indicate a statistically significant difference.
3. Results and Discussion
3.1. Biomass Production
The biomass production was not affected by the presence of the coupons, and it reached very satisfactory values for all three strains. Biomass production doubled after 7 days of incubation (from 1.3 g/L to 2.65 g/L for Chlorella sp.; from 0.85 g/L to 1.7 g/L for Nannochloropsis sp.; and from 1.0 g/L to 2.0 g/L for A. maxima). This indicates a proportional biomass increase for all strains under the given incubation conditions.
3.2. Antifouling Performance
The antifouling performance of R, UT, and PSBM PMMA surfaces was evaluated by the image analysis obtained under fluorescent microscopy. The results are reported in Figure 4 and Figure 5 and Table 1. These clearly show that after a 7-day cycle, rough PMMA allowed the highest adhesion of cells for all three strains. For Chlorella sp., the average number of cells/mm2 was 4780 cells/mm2, corresponding to an area coverage of 9.3%, while, for Nannochloropsis sp., an average of 5144 cells/mm2 was observed with 2.8% of occupied area. For A. maxima, only the area coverage was analyzed, due to the poor persistence of the filaments on R and UT surfaces, as shown in Figure 5g,h. The value of 34.1% also considers the presence of biofilm and cell debris. The UT coupons showed for Chlorella sp. a cell adhesion of 3369 cells/mm2 and a surface coverage of 7%, while for Nannochloropsis sp. the average cell adhesion was 3415 cells/mm2 with 2% area coverage. For A. maxima, no significant presence of filaments could be detected on the surfaces (Figure 5i), but the biofouling observed was approximately 5% per mm2 of surface. In contrast, microscopic observations showed that PSBM-coated PMMA surfaces were characterized by a very low cell adhesion of Chlorella sp. and Nannochloropsis sp. cells, being 3 cells/mm2 and 218 cells/mm2, respectively. PSBM surfaces showed negligible area coverage, being 0.006% for Chlorella, 0.2% for Nannochloropsis, and 0.1% for A. maxima, respectively. Therefore, it was evident that PSBM-coated surfaces display remarkable antifouling properties against all the model strains tested here.
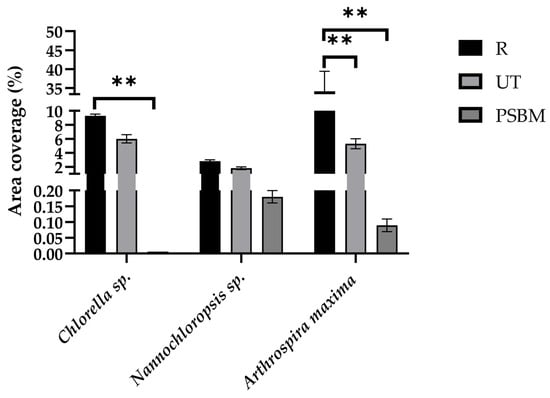
Figure 4.
Graphical representation of area coverage/mm2 for each strain on three different types of PMMA surfaces. Data are represented as the mean ± SD of three independent experiments. ** p ≤ 0.01 indicated significant differences among R, UT, and PSBM surfaces, as reported by Tukey’s post hoc test.
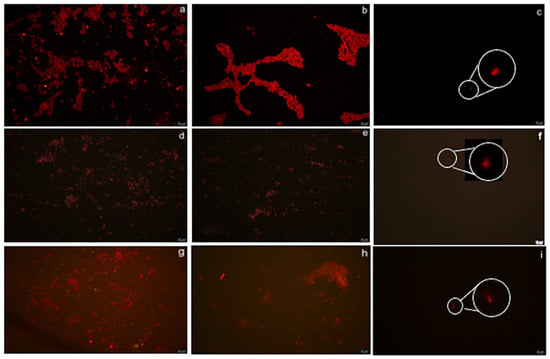
Figure 5.
Antifouling performance of different PMMA surfaces (R on the left, UT in the center, and PSBM on the right) against the three strains as seen under fluorescent microscopy (FM). (a–c) Chlorella strain adhesion; (d–f) Nannochloropsis strain adhesion; (g–i) A. maxima strain biofilm. The R surfaces are mostly prone to fouling, showing a uniform distribution of adhering cell patterns, while UT PMMA presented typical aggregates for both microalgae. In the case of A. maxima, the FM images show mostly debris and fingerprints of filaments but no attached filaments. The bar is 20 µm. The few cells seen on PSBM-coated PMMA (c,f,i) are shown by magnifying indication.

Table 1.
Average of adhering cell/mm2 and area coverage (%/mm2) of the three algal strains (Chlorella sp., Nannochloropsis, and Arthrospira maxima (only area coverage)) on rough (R), untreated (UT), and PSBM-coated (PSBM) as determined under fluorescent microscopy and ImageJ analysis. N.A., data are not available. The data are expressed as averages ± standard deviations.
Fluorescent microscopy (FM) images (Figure 5) showed, for both the microalgae, a scattered adhesion pattern on R surfaces (Figure 5a,d), while, on UT surfaces, typical aggregates due to liquid agitation were observed (Figure 5b,e). In the case of A. maxima, the images showed mainly debris traces of filament attachment on the surfaces (Figure 5g,h). Extremely low cell adhesion and almost no biofilm coverage for all tested strains were observed on the PSBM surface (Figure 5c,f,i).
Rough surfaces, as expected, were those that showed the most intense fouling, especially in the case of A. maxima, with biofilm observed on most of the surface. These findings agreed with previously reported studies, which suggest that roughness or other irregularities promote microbial attachment [45]. Moreover, the microscopic images of A. maxima show general debris and biofilm coverage and, sometimes, fingerprints of the attached filaments, but never real filaments, as already reported [46,47,48,49]. A comparison between R and UT coupons shows that the latter, in early stages of use, is less prone to fouling and that cell aggregations depend on liquid movement. Very low cell adhesion and almost no biofilm coverage for all tested strains were observed on the PSBM surfaces. These data support, once more, the fact that zwitterionic-based coatings can provide a non-fouling environment due to their ability to maintain a hydration layer, which prevents protein adsorption reduction and microbial adhesion [50,51].
3.3. Long-Term Effectiveness of the PSBM
3.3.1. Biomass Production During Each Cycle
For all the tested strains, Chlorella sp., Nannochloropsis sp., and A. maxima, biomass yield was doubled or even more with respect to the initial weight of ≥1 g/L. This increase was constant in each cycle. The average and standard deviations were calculated over nine successive cycles. For Chlorella sp., the gain in biomass across tribological cycles was found to be 2.45 ± 0.40 g/L, and for Nannochloropsis sp., the gain was 2.25 ± 0.38 g/L, while for A. maxima, it was 2.09 ± 0.35 g/L.
3.3.2. Antifouling Performance of Strains Through Tribological Approach
The antifouling performance of the PSBM-coated surface was analyzed over multiple (nine) cycles and compared to that of UT PMMA. The results obtained clearly show that the PSBM led to a remarkable reduction in cell adhesion with respect to UT PMMA. Furthermore, along the cycles, only a light increment of attached cells was observed, which, on the contrary, was consistent on UT. Surface resistance to biofouling was slightly variable when compared against the three different strains, i.e., Chlorella sp., Nannochloropsis sp., and A. maxima. Results are reported in Table 2 and Table 3 and Figure 6a,b and Figure 7. As shown in Table 2, on UT PMMA, the number of cells increased along the cycles. In fact, cell count averages were 763 cells/mm2 and 526 cells/mm2 at the beginning, respectively, for Chlorella and Nannochloropsis; cell count averages were 3800 and 3769 cells/mm2 at the end, respectively. On the other hand, PSBM-coated PMMA demonstrated good antifouling behavior with low and consistent cell counts from cycle 2 (7 and 10 cells/mm2) to cycle 10 (22 and 102 cells/mm2).

Table 2.
Antifouling performance of UT vs. PSBM surface for Chlorella sp. and Nannochloropsis sp. expressed as No. of cells/mm2. The data are expressed as averages ± standard deviations.

Table 3.
Comparison of percentage of area coverage/mm2 for Chlorella, Nannochloropsis, and A. maxima on UT PMMA and PSBM surfaces. Data are expressed as averages of four replicates and standard deviation (±SD).
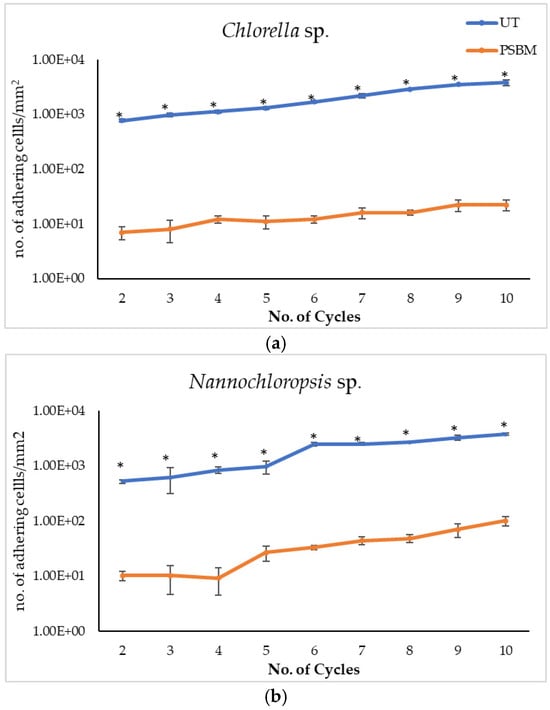
Figure 6.
Log of adhering cells/mm2 of Chlorella sp. (a) and Nannochloropsis (b) on the UT surface (control) vs. the PSBM surface along nine tribological cycles. Data shown are the means of four replicates; the vertical bar represents ± standard error. Asterisks above each point denote significant differences (p ≤ 0.01) between UT and PSBM treatment by one-way ANOVA.
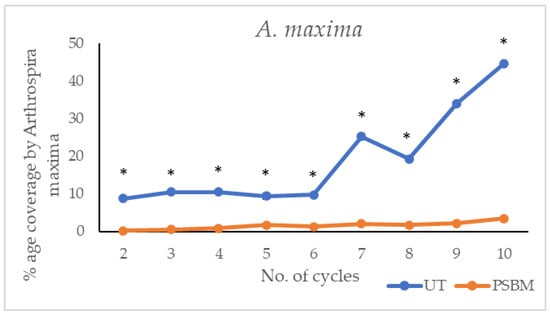
Figure 7.
Graphical representation of area covered by A. maxima on UT PMMA surface vs. PSBM surface. Data shown are the means of four replicates; the vertical bar represents ± standard error. Asterisks above each point denote significant differences (p ≤ 0.01) between UT and PSBM treatment by one-way ANOVA.
The logarithmic trends in biofouling on UT PMMA and PSBM surfaces for the two strains, along nine cycles, are reported in Figure 6a,b. These clearly show that the PMMA is more prone to fouling than the PSBM-treated PMMA coupon. Furthermore, PSBM displays remarkable chemical stability in algal media (pH 7–9, ionic strength ~0.5 M), aligning with the known resistance to hydrolysis resistance of zwitterionic polymers [26].
Regarding the extent of biofouling observed on UT PMMA vs. PSBM, as summarized in Table 3 and Figure 6, it is evident that the PSBM coating significantly reduced cell adhesion across all cycles. This finding aligns with the antifouling performance observed in the previous sections, highlighting the superior resistance of PSBM against biofouling, particularly with Chlorella sp. and Nannochloropsis sp. The consistency of these results across multiple cycles further supports the long-term effectiveness of PSBM coatings in preventing biofouling.
The comparison of surface covered by debris and biofilm due to A. maxima growth on untreated PMMA surfaces vs. those coated with PSBM is reported in Figure 7. The results show that the PSBM coating effectively resisted biofouling for nine cycles, maintaining minimal coverage by A. maxima. In contrast, the untreated PMMA surface experienced significant biofouling, with a high area covered by A. maxima.
The data obtained show that advanced surface coating technologies, such as the PSBM antifouling coating on PMMA, can significantly enhance the wear resistance and durability of a given material. The tribological approach shows the importance of testing antifouling coatings under environmental and prolonged exposure circumstances to determine the coating durability and performance over multiple utilization cycles [50,51]. In our opinion, this study represents an important step in this direction, showing the need to test the durability and performance of PSBM surfaces in repeated biofouling cycles to assess coating integrity and antifouling efficacy during multiple mechanical and environmental challenges [52]. PSBM forms a strong hydration layer in salt medium, has no toxicity, gave excellent antifouling results against all tested strains, and maintained excellent optical properties when it was compared to control PMMA [40]. The long-term performance of PSBM over cycles combining both biofouling resistance and durability, maintaining optical properties, is critical for photobioreactor applications.
This study confirms, once more, that “covering” the PBR’s inner walls with FRC coatings, capable of generating hydration layers on the surface, represents a useful strategy to infer/increase their antifouling activities. On the other hand, hydrogel technology has been widely applied to impart hydrophilic properties to hydrophobic surfaces such as polydimethylsiloxane (PDMS), a well-known and widely applied highly hydrophobic material [13]. In this context, Soriano-Jerez [12] has demonstrated that PEG-containing PDMS coating exhibits a higher AF activity than PDMS products used as controls.
4. Conclusions
In conclusion, our study demonstrates that the sulfobetaine-zwitterionic PMMA (PSBM) was effective in the following:
- mitigate the biofouling due to the three photosynthetic strains considered as models, i.e., Chlorella sp., Nannochloropsis sp., and A. maxima;
- maintenance of optical transparency;
- surface protection against wear.
Thus, the use of PSBM coating can contribute to lowering maintenance costs, increasing reactor lifespan, and increasing overall productivity by reducing biofouling and maintaining reactor efficiency.
This study provides significant findings to advance the development of more sustainable and biocompatible alternatives to conventional antifouling treatments in industrial and biotechnological applications against biofouling, addressing an important lack of data in the literature within the long-term assessment of antifouling coatings.
5. Patents
No patents resulted from the work reported in this manuscript.
Author Contributions
Conceptualization, S.L.S. and C.U.; methodology, S.L.S., C.U., and R.H.A.; software, R.H.A.; validation, S.L.S. and C.U.; formal analysis, S.L.S., C.U., and R.H.A.; investigation, R.H.A., M.N., and V.Z.; resources S.L.S., F.D.L., and C.U.; data curation, S.L.S. and C.U.; writing—original draft preparation, R.H.A.; writing—review and editing, S.L.S., C.U., and R.H.A.; visualization, R.H.A.; supervision, S.L.S. and C.U.; project administration, S.L.S. and C.U.; funding acquisition, S.L.S., F.D.L., and C.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially carried out with the financial contribution from FFABR 2022 (Italian fund for basic research activities). R.H.A. carried out his doctorate work with a scholarship in the frame of RISORSE PON “RICERCA E INNOVAZIONE” 2014–2020.
Institutional Review Board Statement
Our research did not involve humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article.
Acknowledgments
We thank Luciano Falqui from Di Bella Costruzioni srl, Catania, for his valuable suggestions throughout the project.
Conflicts of Interest
The authors declare no conflicts of interest and the funders had no role in the design of this study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| PMMA | Polymethylmethacrylate |
| PSBM | Polysulfobetaine methacrylate/zwitterionic sulfobetaine-hydroxyethyl-containing polymethylmethacrylate ter-co-polymer |
| AF | Antifouling |
| UT | Untreated |
| R | Rough |
References
- Joy, S.R.; Anju, T.R. Microalgal Biomass: Introduction and Production Methods. In Handbook of Biomass; Thomas, S., Hosur, M., Pasquini, D., Jose Chirayil, C., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- López-Hernández, J.-F.; Kean-Meng, T.; Asencio-Alcudia, G.-G.; Asyraf-Kassim, M.; Alvarez-González, C.-A.; Márquez-Rocha, F.-J. Sustainable Microalgae and Cyanobacteria Biotechnology. Appl. Sci. 2022, 12, 6887. [Google Scholar] [CrossRef]
- Mobin, S.M.A.; Chowdhury, H.; Alam, F. Commercially important bioproducts from microalgae and their current applications—A review. Energy Procedia 2019, 160, 752–760. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
- Eze, C.N.; Onyejiaka, C.K.; Ihim, S.A.; Ayoka, T.O.; Aduba, C.C.; Ndukwe, J.K.; Nwaiwu, O.; Onyeaka, H. Bioactive compounds by microalgae and potentials for the management of some human disease conditions. AIMS Microbiol. 2023, 7, 55–74. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, Y.; Liao, Q.; Fu, Q.; Zhu, X. Enhancement of microalgae production by embedding hollow light guides to a flat-plate photobioreactor. Bioresour. Technol. 2016, 207, 31–38. [Google Scholar] [CrossRef]
- Kaur, M.; Bhatia, S.; Gupta, U.; Decker, E.; Tak, Y.; Bali, M.; Gupta, V.K.; Dar, R.A.; Bala, S. Microalgal bioactive metabolites as promising implements in nutraceuticals and pharmaceuticals: Inspiring therapy for health benefits. Phytochem. Rev. 2023, 22, 903–933. [Google Scholar] [CrossRef]
- Chanquia, S.N.; Vernet, G.; Kara, S. Photobioreactors for cultivation and synthesis: Specifications, challenges, and perspectives. Eng. Life Sci. 2022, 12, 712–724. [Google Scholar] [CrossRef]
- Razzak, S.A.; Bahar, K.; Islam, K.M.O.; Abdul Khaleel Haniffa, A.K.; Faruque, M.O.; Hossain, K.M.O.; Hossain, M.M. Microalgae cultivation in photobioreactors: Sustainable solutions for a greener future. Green Chem. Eng. 2024, 5, 418–439. [Google Scholar] [CrossRef]
- Soriano-Jerez, Y.; Gallardo-Rodríguez, J.J.; López-Rosales, L.; García-Camacho, F.; Cerón-García, M.C. Preventing biofouling in microalgal photobioreactors. Bioresour. Technol. 2024, 407, 131125. [Google Scholar] [CrossRef]
- Genin, S.N.; Aitchison, J.S.; Allen, D.G. Design of algal film photobioreactors: Material surface energy effects on algal film productivity, colonization and lipid content. Bioresour. Technol. 2014, 155, 136–143. [Google Scholar] [CrossRef]
- Soriano-Jerez, Y.; Macías-de la Rosa, A.; García-Abad, L.; López-Rosales, L.; Maza-Márquez, P.; García-Camacho, F.; Bressy, C.; Cerón-García, M.C.; Molina-Grima, E. Transparent antibiofouling coating to improve the efficiency of Nannochloropsis gaditana and Chlorella sorokiniana culture photobioreactors at the pilot-plant scale. Chemosphere 2024, 347, 140669. [Google Scholar] [CrossRef] [PubMed]
- Zeriouh, O.; Reinoso-Moreno, J.V.; López-Rosales, L.; Cerón-García, M.C.; Sánchez-Mirón, A.; García-Camacho, F.; Molina-Grima, E. Biofouling in photobioreactors for marine microalgae. Crit. Rev. Biotechnol. 2017, 37, 1006–1023. [Google Scholar] [CrossRef]
- Ouassim, Z.; Reinoso-Moreno, J.V.; López-Rosales, L.; Cerón-García, M.C.; Mirón, A.S.; García-Camacho, F.; Molina-Grima, E. Assessment of a photobioreactor-coupled modified Robbins device to compare the adhesion of Nannochloropsis gaditana on different materials. Algal Res. 2019, 37, 277–287. [Google Scholar] [CrossRef]
- Talluri, S.N.L.; Winter, R.M.; Salem, D.R. Conditioning film formation and its influence on the initial adhesion and biofilm formation by a cyanobacterium on photobioreactor materials. Biofouling 2020, 36, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef]
- García-Abad, L.; López-Rosales, L.; Cerón-García, M.D.C.; Fernández-García, M.; García-Camacho, F.; Molina-Grima, E. Influence of abiotic conditions on the biofouling formation of flagellated microalgae culture. Biofouling 2022, 38, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Maan, A.M.C.; Hofman, A.H.; de Vos, W.M.; Kamperman, M. Recent developments and practical feasibility of polymer-based antifouling coatings. Adv. Funct. Mater. 2020, 30, 2000936. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Soriano-Jerez, Y.; López-Rosales, L.; Cerón-García, M.C.; Sánchez-Mirón, A.; Gallardo-Rodríguez, J.J.; García-Camacho, F.; Molina-Grima, E. Long-term biofouling formation mediated by extracellular proteins in Nannochloropsis gaditana microalga cultures at different medium N/P ratios. Biotechnol. Bioeng. 2021, 118, 1152–1165. [Google Scholar] [CrossRef]
- Borucinska, E.; Zamojski, P.; Grodzki, W.; Blaszczak, U.; Zglobicka, I.; Zielinski, M.; Kurzydlowski, K.J. Degradation of polymethylmethacrylate (PMMA) bioreactors used for algal cultivation. Materials 2023, 16, 4873. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, B.; Gao, L.; Wang, W.; Liu, T.; Su, G. Impacts of surface wettability and roughness of styrene-acrylic resin films on adhesion behavior of microalgae Chlorella sp. Colloids Surf. B Biointerfaces 2021, 199, 111522. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.Y.; Derek, C.J.C. Membrane surface roughness promotes rapid initial cell adhesion and long-term microalgal biofilm stability. Environ. Res. 2022, 206, 112602. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, F.; Guo, Z. The antifouling mechanism and application of bio-inspired superwetting surfaces with effective antifouling performance. Adv. Colloid Interface Sci. 2024, 325, 103097. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Sundaram, H.S.; Wei, Z.; Li, C.; Yuan, Z. Applications of zwitterionic polymers, reactive and functional polymers. React. Funct. Polym. 2017, 118, 51–61. [Google Scholar] [CrossRef]
- Li, M.; Zhuang, B.; Yu, J. Functional zwitterionic polymers on surface: Structures and applications. Chem. Asian J. 2020, 15, 2060–2075. [Google Scholar] [CrossRef]
- Qu, K.; Yuan, Z.; Wang, Y.; Song, Z.; Gong, X.; Zhao, Y.; Mu, Q.; Zhan, Q.; Xu, W.; Wang, L. Structures, properties, and applications of zwitterionic polymers. ChemPhysMater 2022, 1, 294–309. [Google Scholar] [CrossRef]
- Xiao, S.; Ren, B.; Huang, L.; Shen, M.; Zhang, Y.; Zhong, M.; Yang, J.; Zheng, J. Salt-responsive zwitterionic polymer brushes with anti-polyelectrolyte property. Curr. Opin. Chem. Eng. 2018, 19, 86–93. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Y.; Fu, H.; Liang, Z.; Huang, B.; Jiang, R.; Wu, J.; Zhao, Y. Recent advances of multifunctional zwitterionic polymers for biomedical application. Acta Biomater. 2024, 181, 19–45. [Google Scholar] [CrossRef]
- Zheng, K.; Ouyang, X.; Xie, H.; Peng, S. Responsive Zwitterionic Materials for Enhanced Drug Delivery. Langmuir 2025, 41, 3744–3756. [Google Scholar] [CrossRef]
- Higaki, Y.; Nishida, J.; Takenaka, A.; Yoshimatsu, R.; Kobayash, M.; Takahara, A.A. Versatile inhibition of marine organism settlement by zwitterionic polymer brushes. Polym. J. 2015, 47, 811–818. [Google Scholar] [CrossRef]
- Mkpuma, V.O.; Moheimani, N.R.; Fischer, K.; Schulze, A.; Ennaceri, H. Membrane surface zwitterionization for an efficient microalgal harvesting: A review. Algal Res. 2022, 66, 102797. [Google Scholar] [CrossRef]
- Zeriouh, O.; Marco-Rocamora, A.; Reinoso-Moreno, J.V.; López-Rosales, L.; García-Camacho, F.; Molina-Grima, E. New insights into developing antibiofouling surfaces for industrial photobioreactors. Biotechnol. Bioeng. 2019, 116, 2212–2222. [Google Scholar] [CrossRef]
- Wang, D.; Wu, X.; Long, L.; Yuan, X.; Zhang, Q.; Xue, S.; Wen, S.; Yan, C.; Wange, J.; Cong, W. Improved antifouling properties of photobioreactors by surface grafted sulfobetaine polymers. Biofouling 2017, 33, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, C.; Wu, X.; Wang, Z.; Wen, S.; Yu, J.; Yan, C.; Cong, W. Improved antibiofouling properties of photobioreactor with amphiphilic sulfobetaine copolymer coatings. Prog. Org. Coat. 2020, 144, 105666. [Google Scholar] [CrossRef]
- Akhaia, S.; Wadhwa, A.S. Recent advances in bio-tribology from joint lubrication to medical implants: A review. J. Mater. Eng. 2024, 2, 125–135. [Google Scholar] [CrossRef]
- Simson, D.; Subbu, S.K. Investigating the tribological performance of bioimplants. In Bioimplants Manufacturing; eBook; CRC Press: Boca Raton, FL, USA, 2024; pp. 258–283. ISBN 9781003509943. [Google Scholar]
- Prabhu, A.; Raghavan, S.; Kumar, S. Recent review of tribology, rheology of biodegradable and FDM compatible polymers. J. Mater. Res. Technol. 2020, 9, 12345–12367. [Google Scholar] [CrossRef]
- Chen, M.; Song, Z.; Yang, X.; Song, Z.; Luo, X. Antifouling strategies for protecting bioelectronic devices. APL Mater. 2021, 9, 020701. [Google Scholar] [CrossRef]
- Jakovljević, J.; Rakić, D.; Vuković, M. Special issue: Tribological coatings—Properties, mechanisms, and applications in surface engineering. Materials 2023, 16, 451. [Google Scholar] [CrossRef]
- Lo Schiavo, S.; Gulino, A.; Fragalà, M.E.; Mineo, P.; Nicosia, A.; Ali, R.H.; Calorenni, P.; Ferlazzo, A.; Nicolò, M.S.; De Leo, F.; et al. A sulfobetaine containing-polymethylmethacrylate surface coating as an excellent antifouling agent against Chlorella sp. Prog. Org. Coat. 2025, 199, 108940. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.C.B.G.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Madkour, F.F.; Kamil, A.E.-W.; Nasr, H.S. Production and nutritive value of Spirulina platensis in reduced-cost media. Egypt. J. Aquat. Res. 2012, 38, 51–57. [Google Scholar] [CrossRef]
- Meireles, A.; Gonçalves, A.L.; Gomes, I.B.; Simões, L.C.; Simões, M. Methods to study microbial adhesion on abiotic surfaces. AIMS Bioeng. 2015, 2, 297–309. [Google Scholar] [CrossRef]
- Liao, Y.; Fatehi, P.; Liao, B. Microalgae cell adhesions on hydrophobic membrane substrates using quartz crystal microbalance with dissipation. Colloids Surf. B Biointerfaces 2023, 230, 113514. [Google Scholar] [CrossRef]
- El-Sapagh, S.; El-Shenody, R.; Pereira, L.; Elshobary, M. Unveiling the potential of algal extracts as promising antibacterial and antibiofilm agents against multidrug-resistant Pseudomonas aeruginosa: In vitro and in silico studies including molecular docking. Plants 2023, 12, 3324. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, S.; Yang, J.; Ma, A. Advancing strategies of biofouling control in water-treated polymeric membranes. Polymers 2022, 14, 1167. [Google Scholar] [CrossRef] [PubMed]
- Passos, L.F.; Berneira, L.M.; Poletti, T.; Mariotti, K.D.C.; Carreño, N.L.; Hartwig, C.A.; Pereira, C.M. Evaluation and characterization of algal biomass applied to the development of fingermarks on glass surfaces. Aust. J. Forensic Sci. 2021, 53, 337–346. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef]
- Murali, S.; Agirre, A.; Arrizabalaga, J.; Rafaniello, I.; Schäfer, T.; Tomovska, R. Zwitterionic stabilized water-borne polymer colloids for antifouling coatings. React. Funct. Polym. 2024, 196, 105843. [Google Scholar] [CrossRef]
- Xue, Y.J.; Zhang, Y.Z. Surface coatings in tribological and wear-resistant applications. Int. Heat Treat. Surf. Eng. 2009, 3, 17–25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).