Preparation of CF-NiO-PANI Electrodes and Study on the Efficiency of MFC in Recovering Potato Starch Wastewater
Abstract
1. Introduction
2. Preparation and Methods
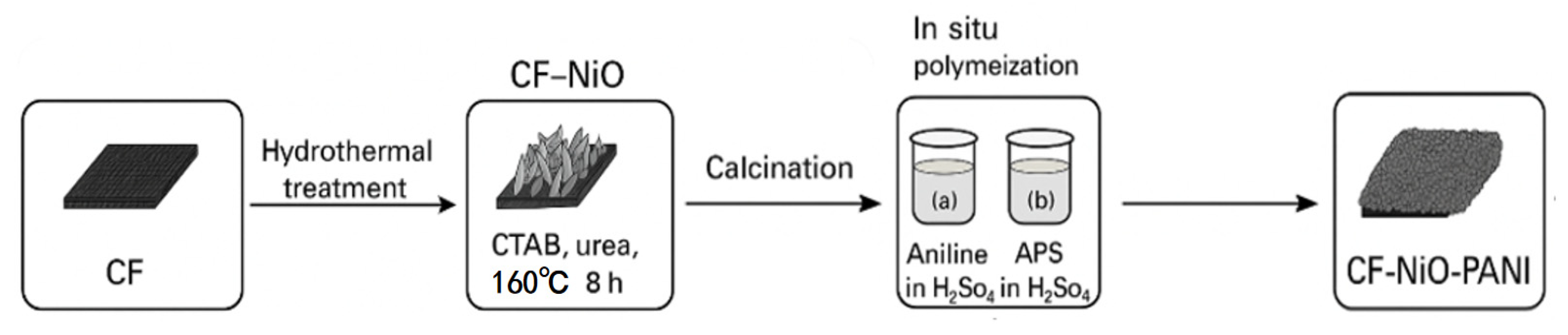
2.1. Preparation of the CF-NiO-PANI Electrode
2.2. Electrodes’ Electrochemical Tests
2.3. CF-NiO-PANI Electrode Characterization
2.4. MFC Construction and Performance Testing
2.4.1. Pretreatment of Carbon Felt
2.4.2. Proton Exchange Membrane Pretreatment
2.4.3. Start-Up of Microbial Fuel Cells
2.4.4. MFC Performance Testing
- (1)
- Polarization curve
- (2)
- Power density curve
- (3)
- Anodic polarization curve
- (4)
- MFC cyclic voltammetry test
- (5)
- MFC AC impedance test
- (6)
- Chronograph current test (CA)
2.5. MFC High-Throughput Sequencing
3. Experimental Results and Discussion
3.1. The Characteristics of CF-NiO-PANI Electrode Structures
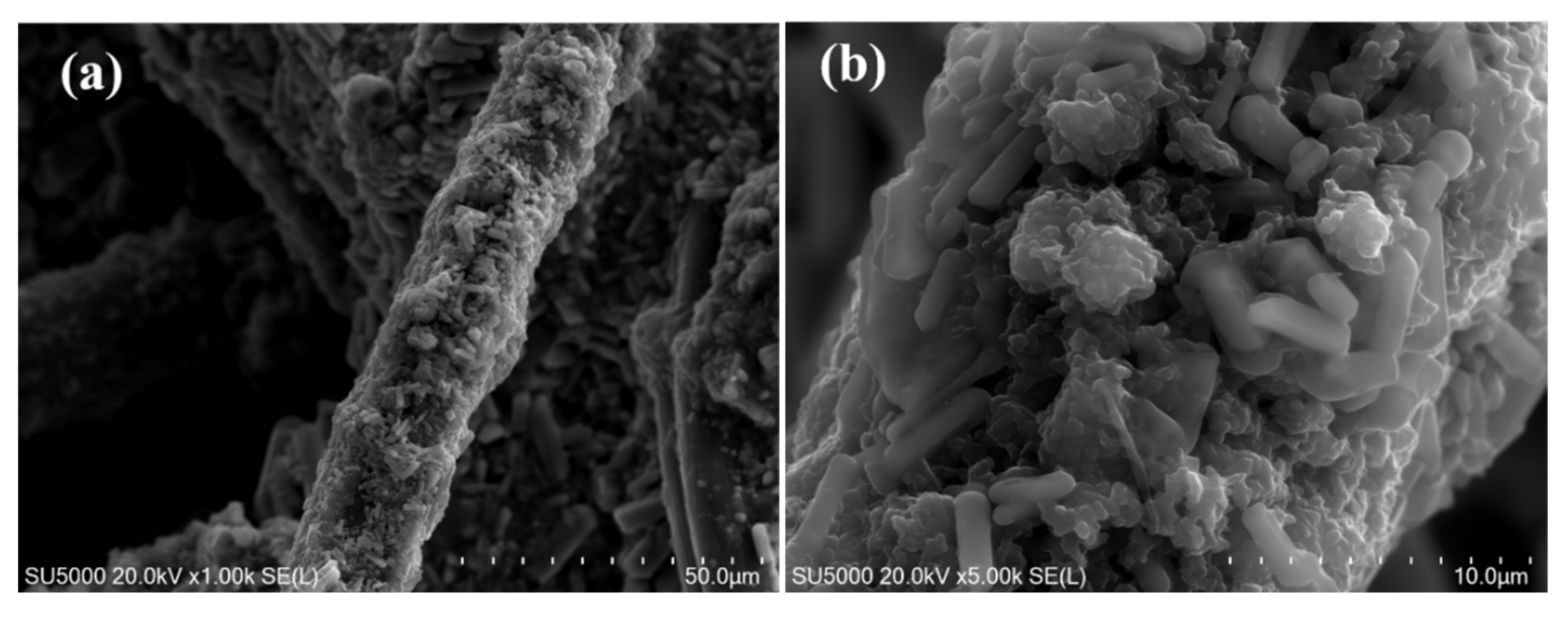
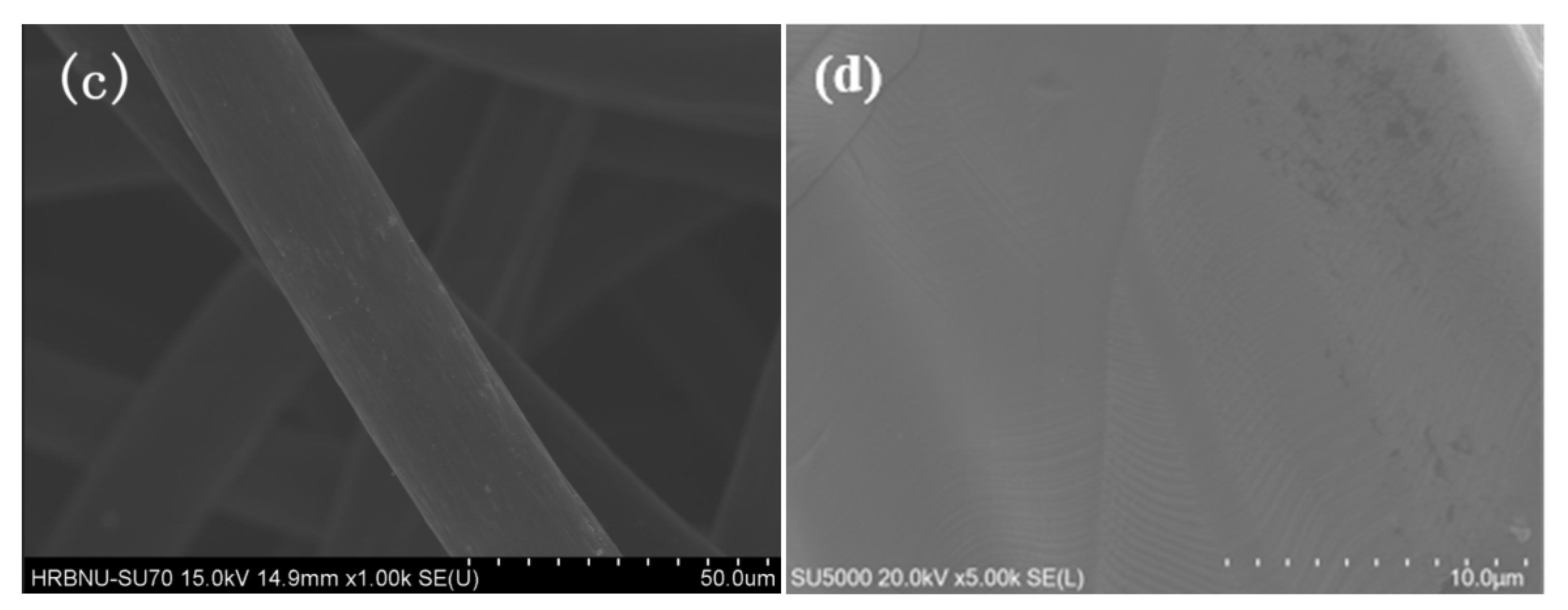
3.1.1. SEM: Scanning Electron Microscopy Analysis
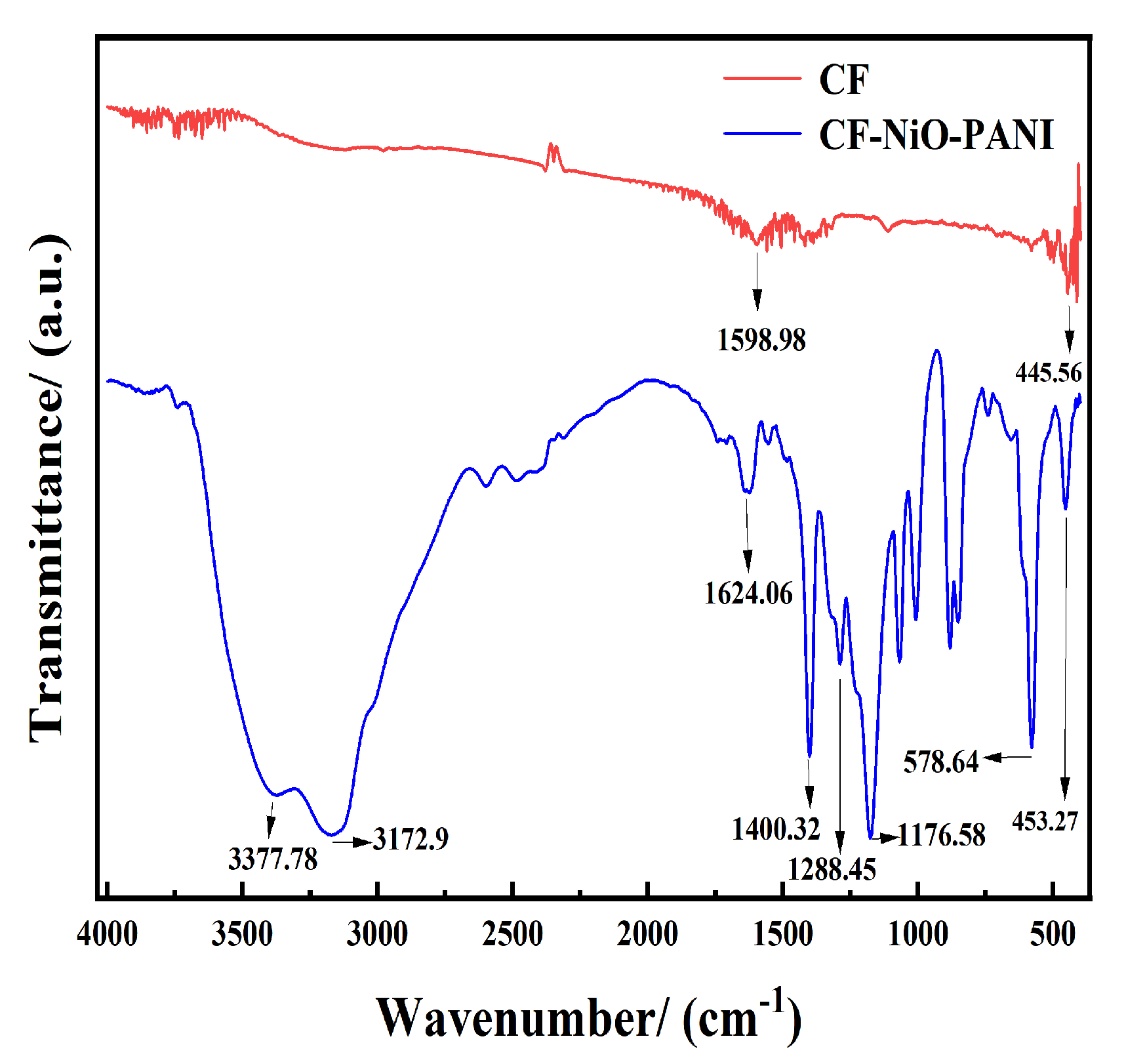
3.1.2. FTIR: Fourier Transform Infrared Spectroscopy
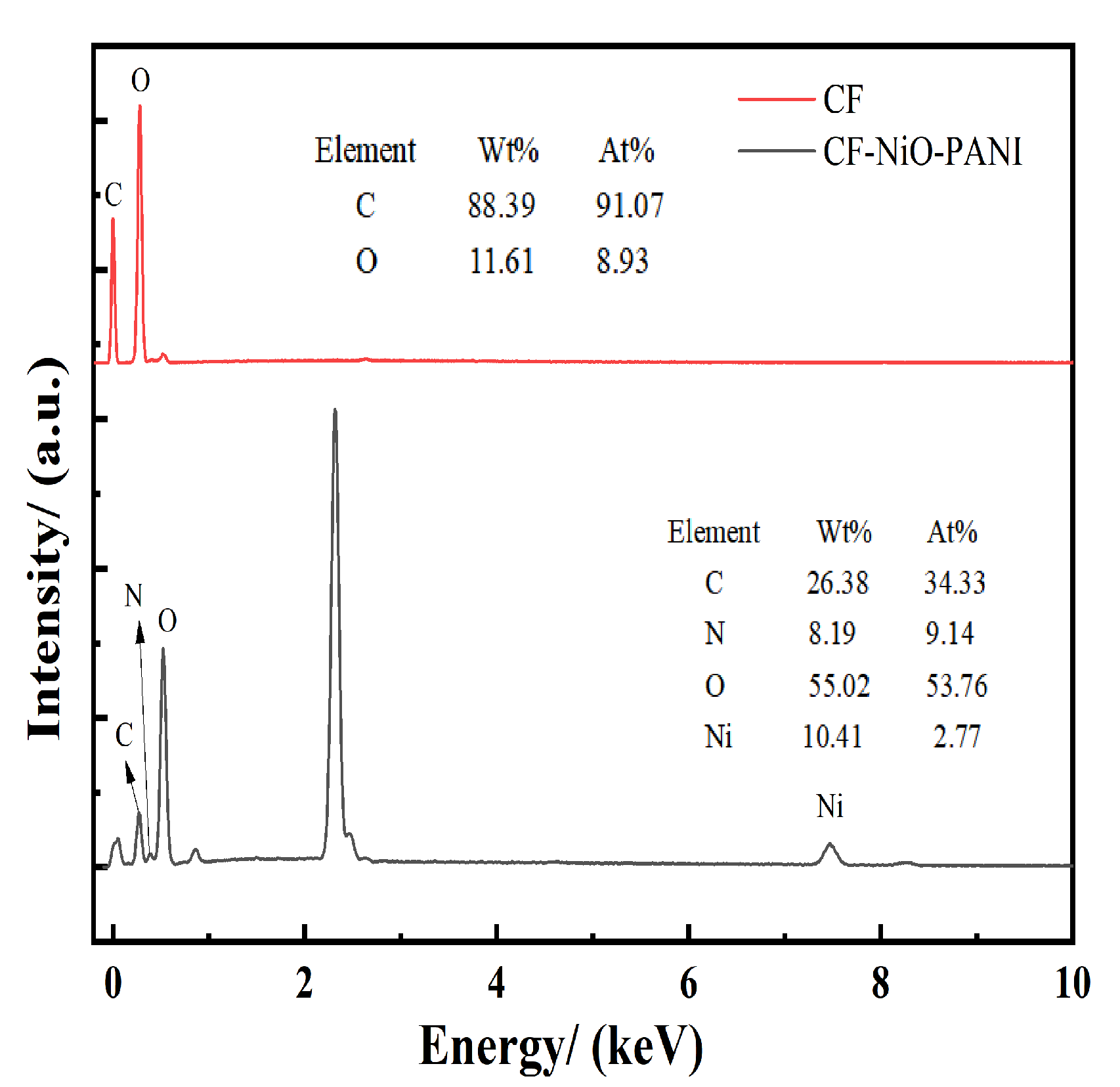
3.1.3. EDS: Energy Diffusion Spectrometer
3.2. MFC Performance Test of the CF-NiO-PANI Anode
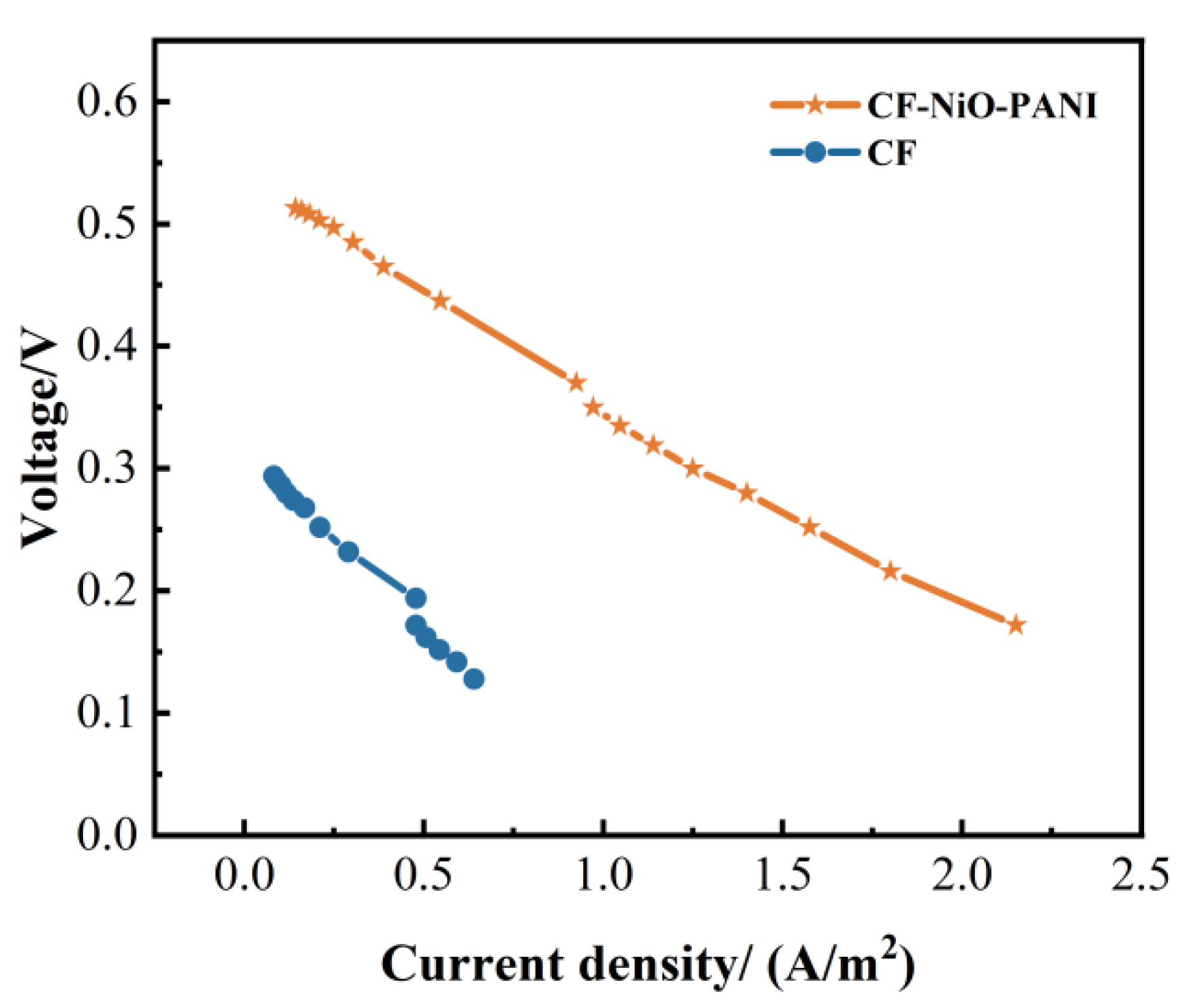
3.2.1. Polarization Curve
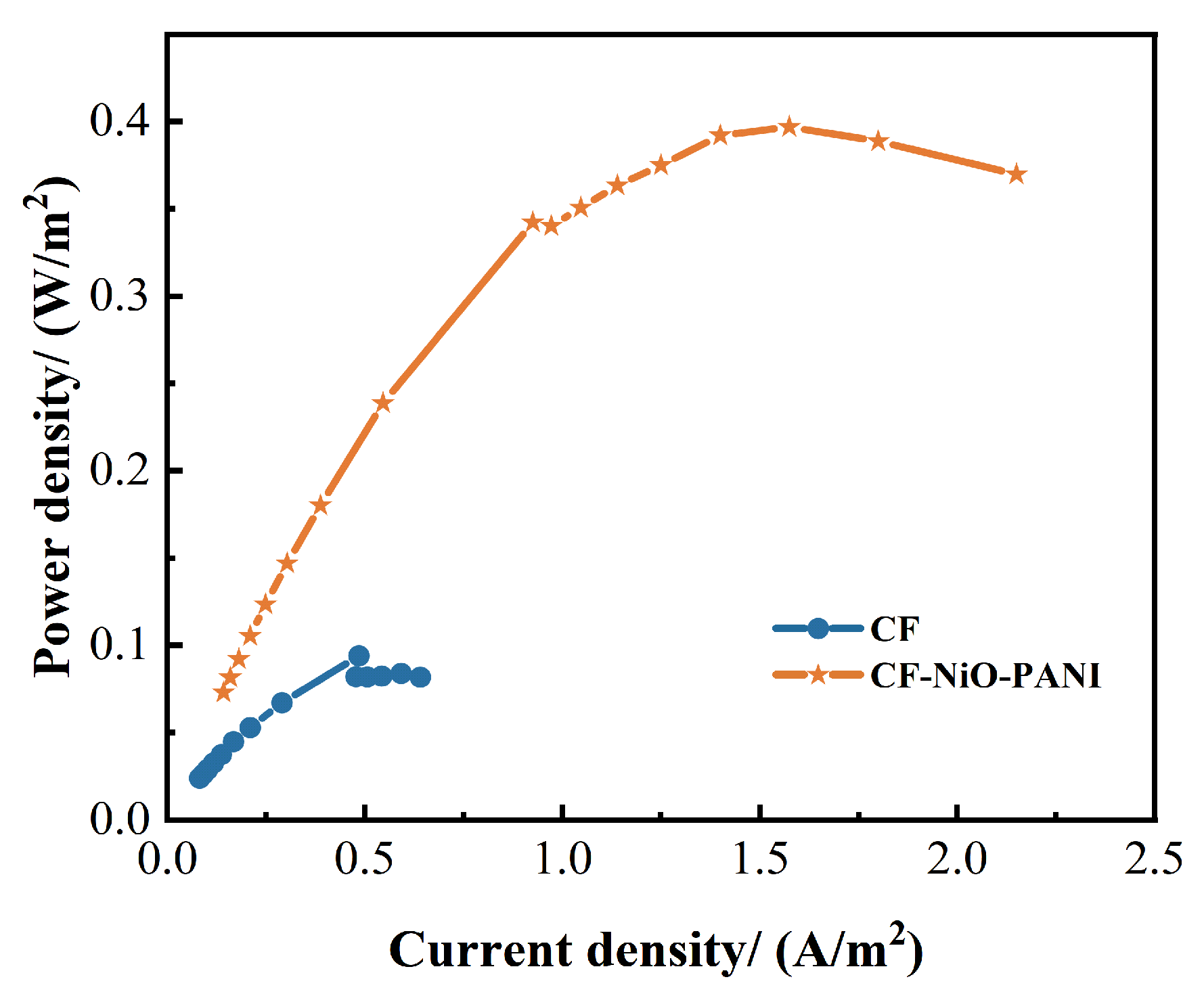
3.2.2. Power Density Curve
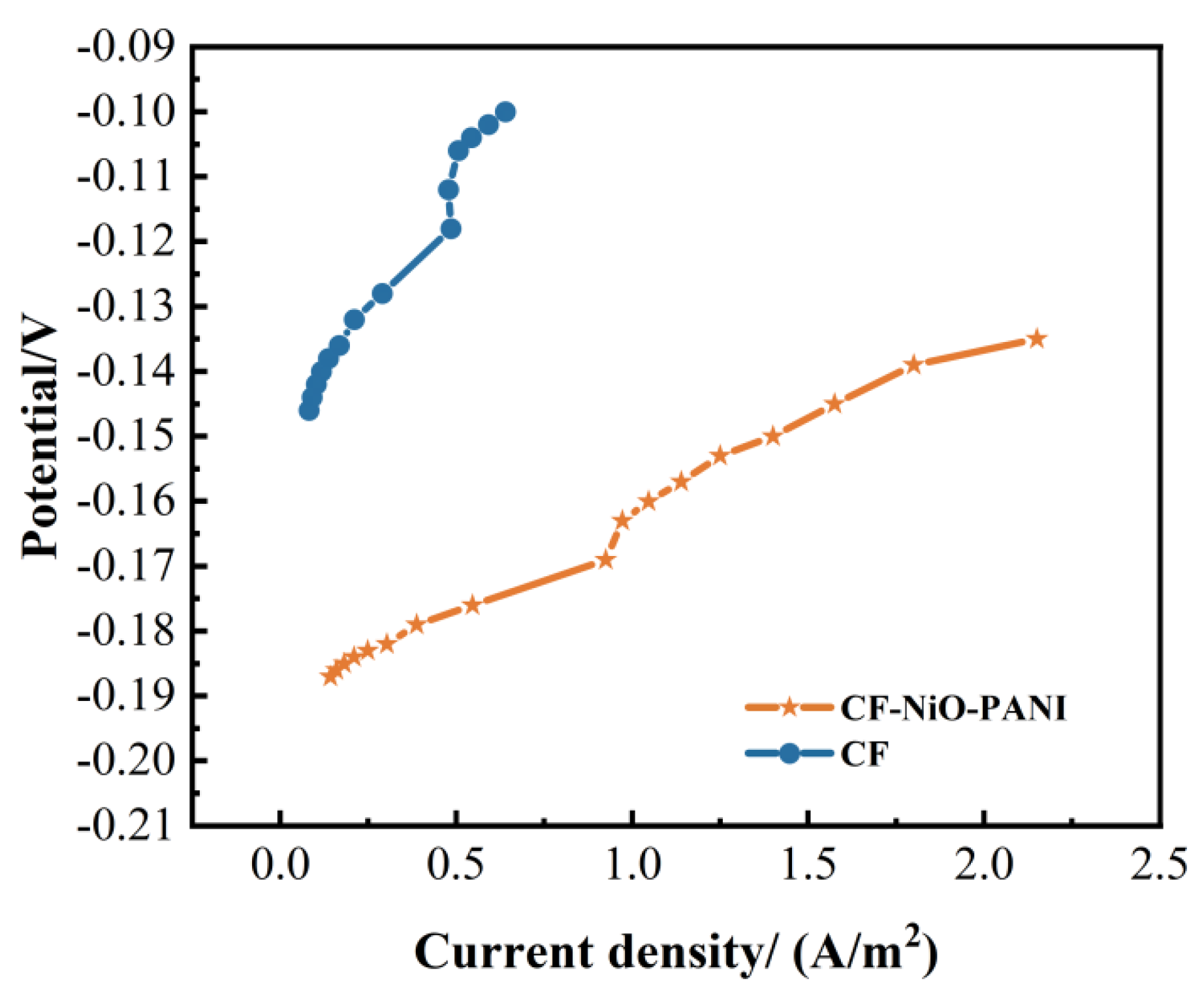
3.2.3. Anode Polarization Curve
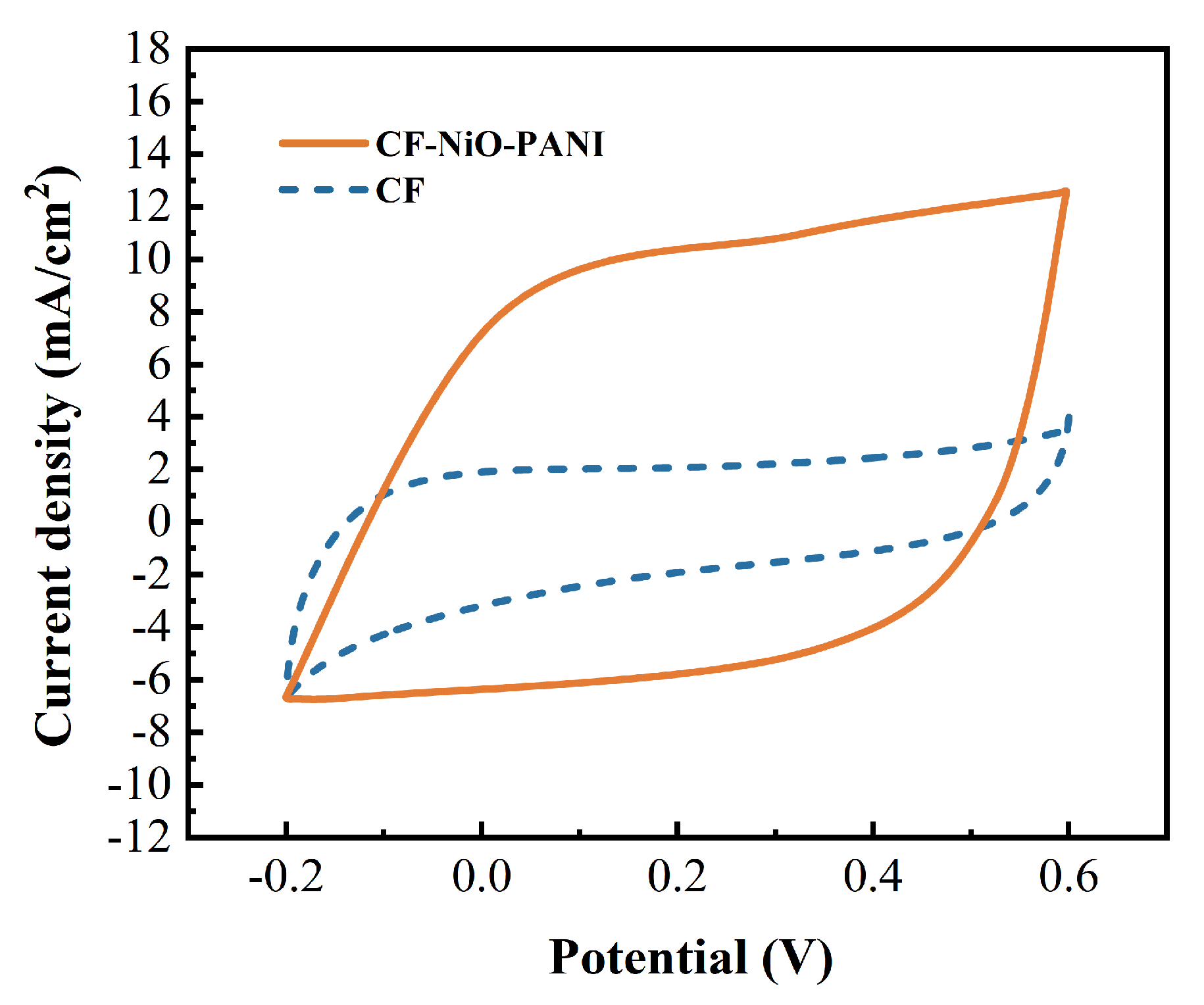
3.2.4. MFC Cyclic Voltammetry Test
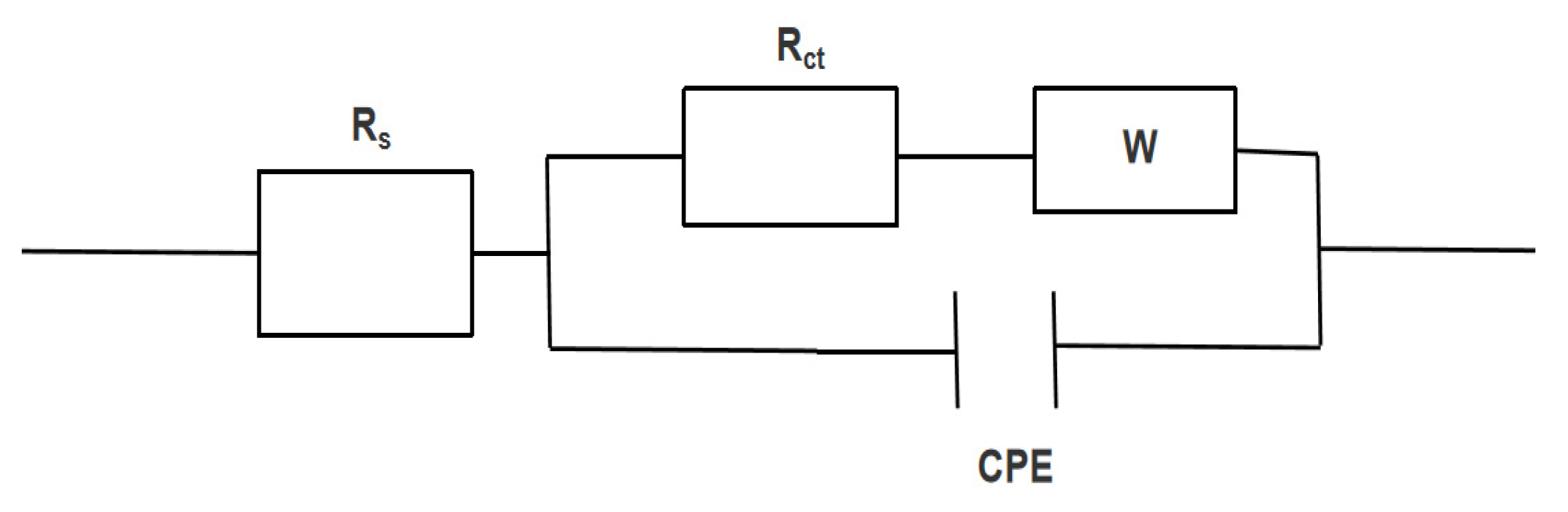
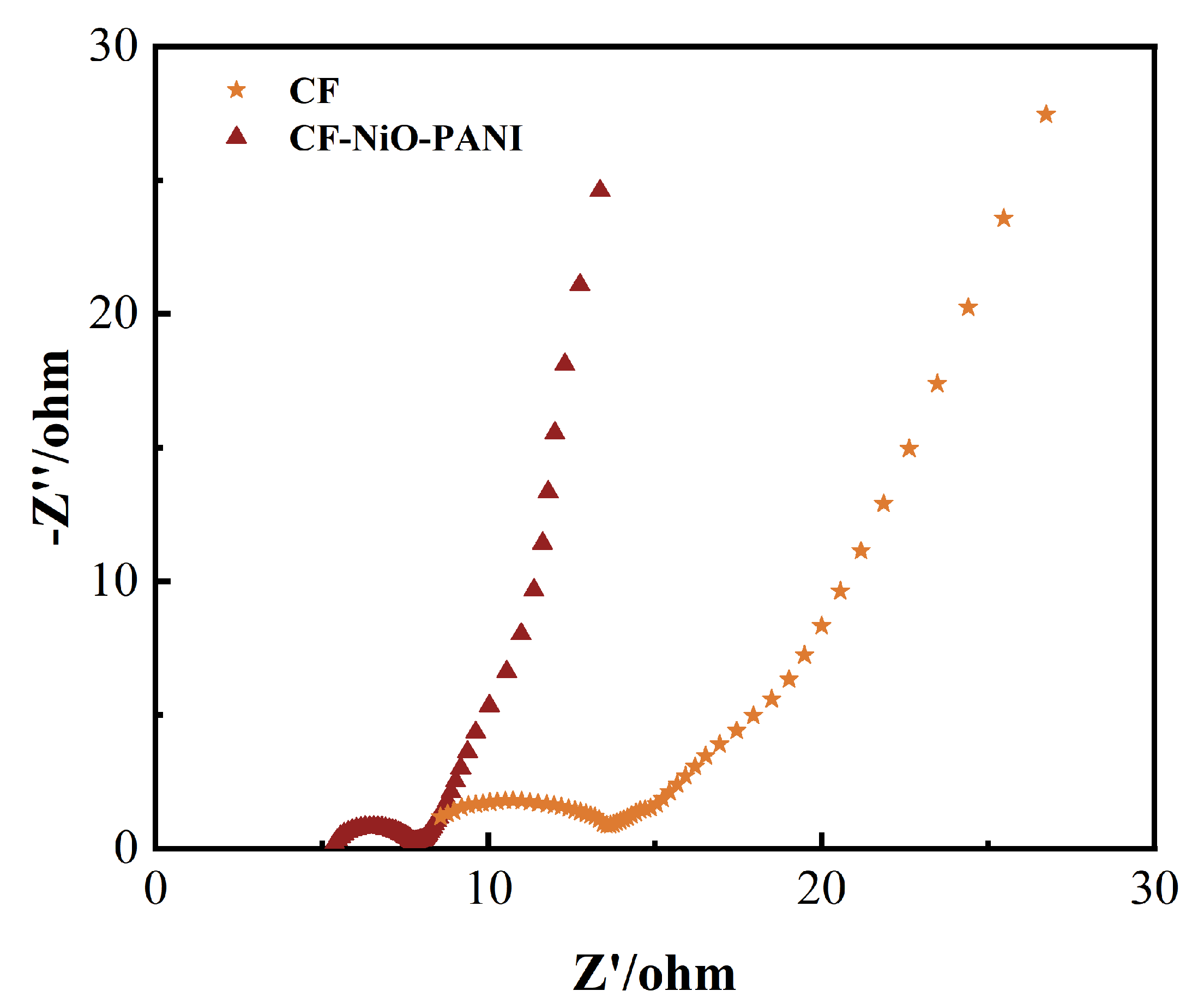
3.2.5. MFC AC Impedance Test
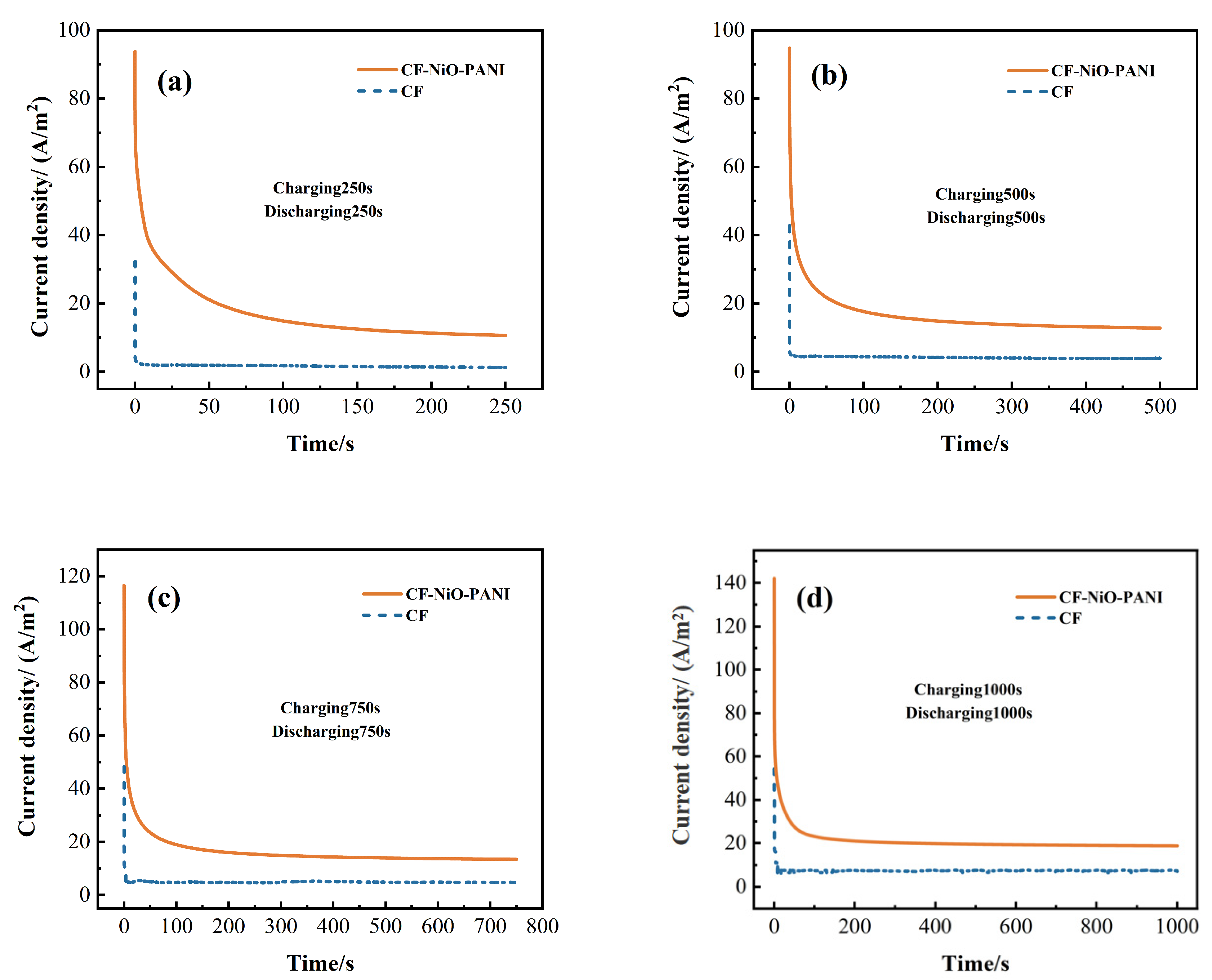
3.2.6. MFC Energy Storage Test
3.3. MFC (CF-NiO-PANI Anode) High-Throughput Sequencing
- (1)
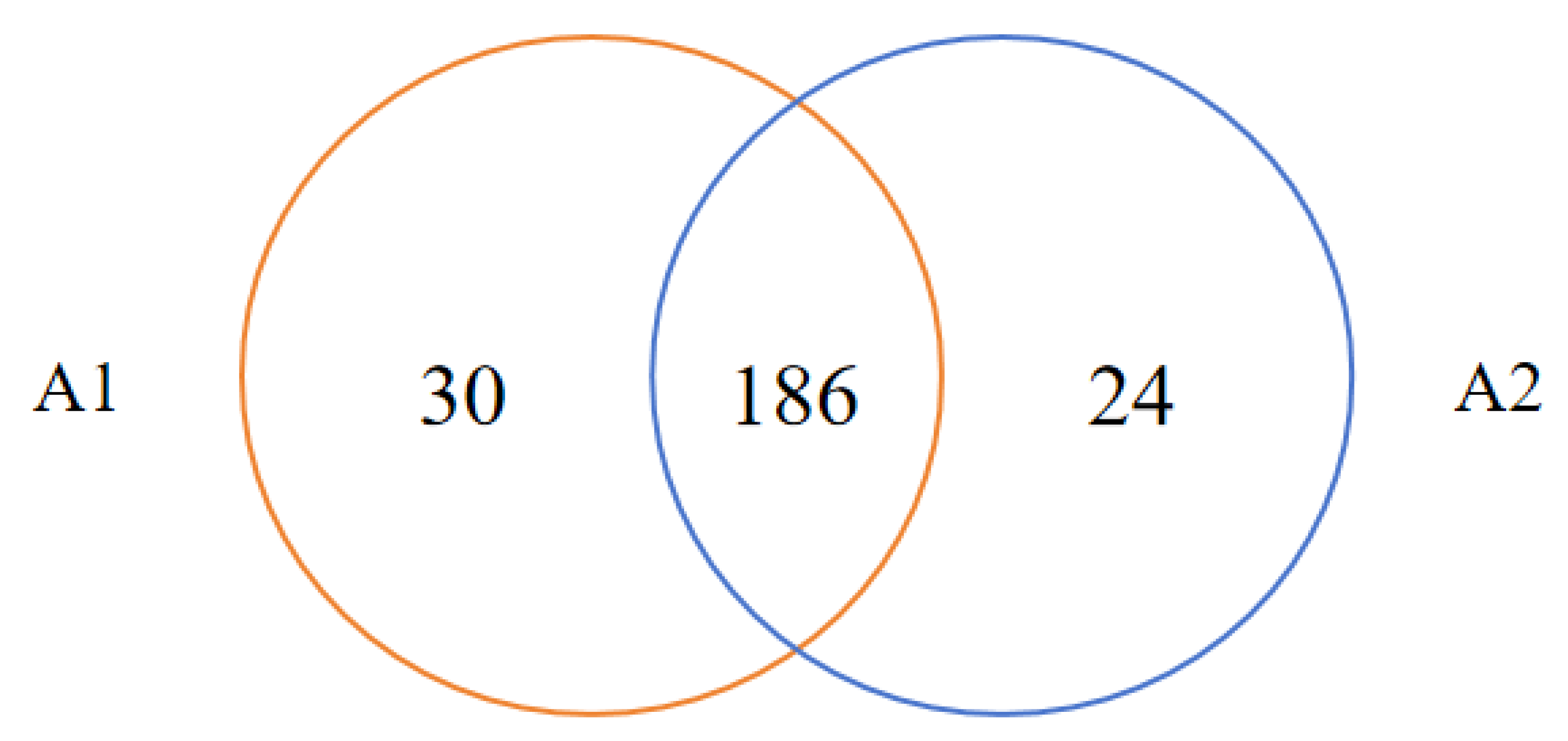
- A Venn diagram of species classification of two anodes at the family level is shown in Figure 12.
- (2)
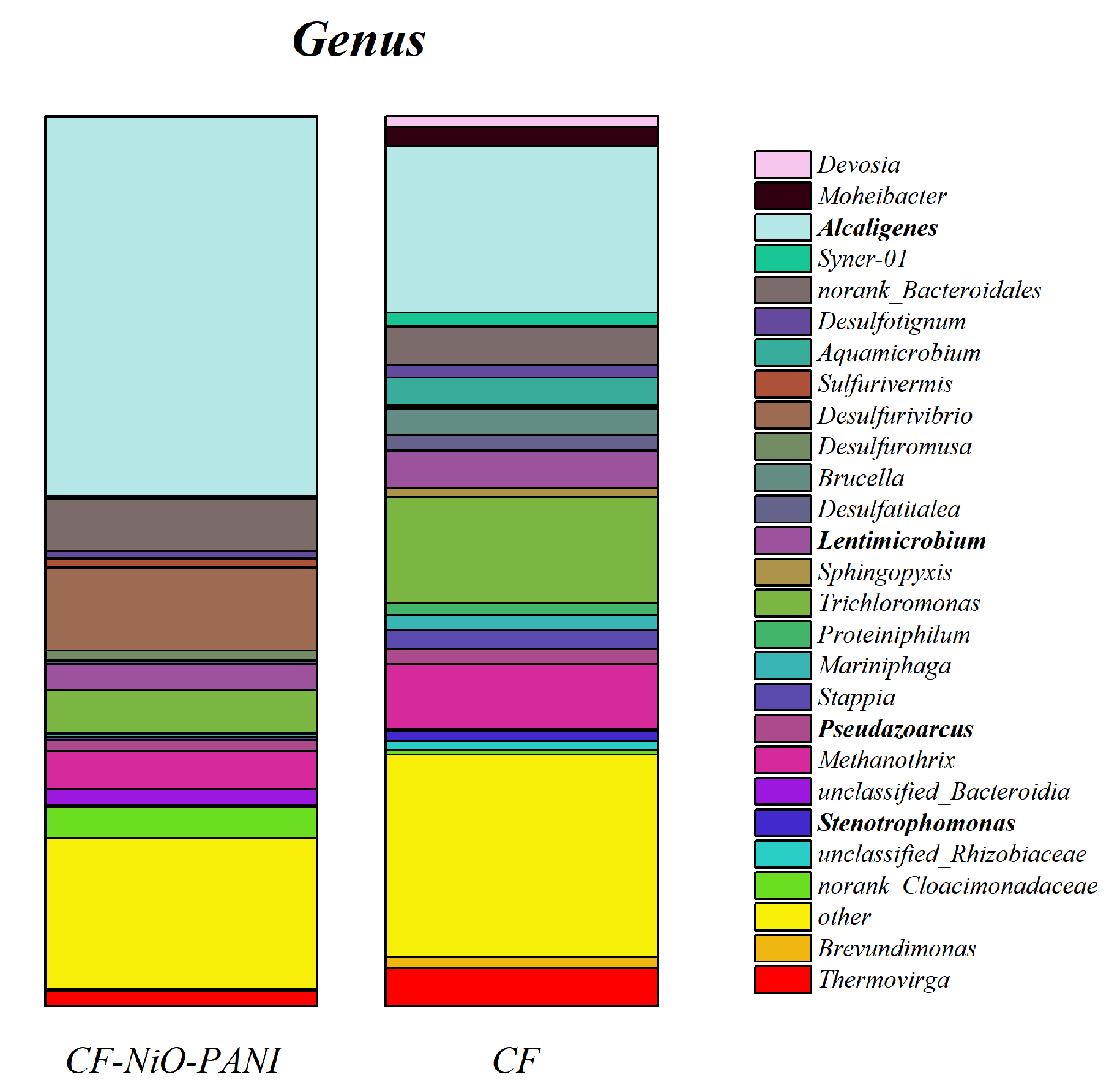
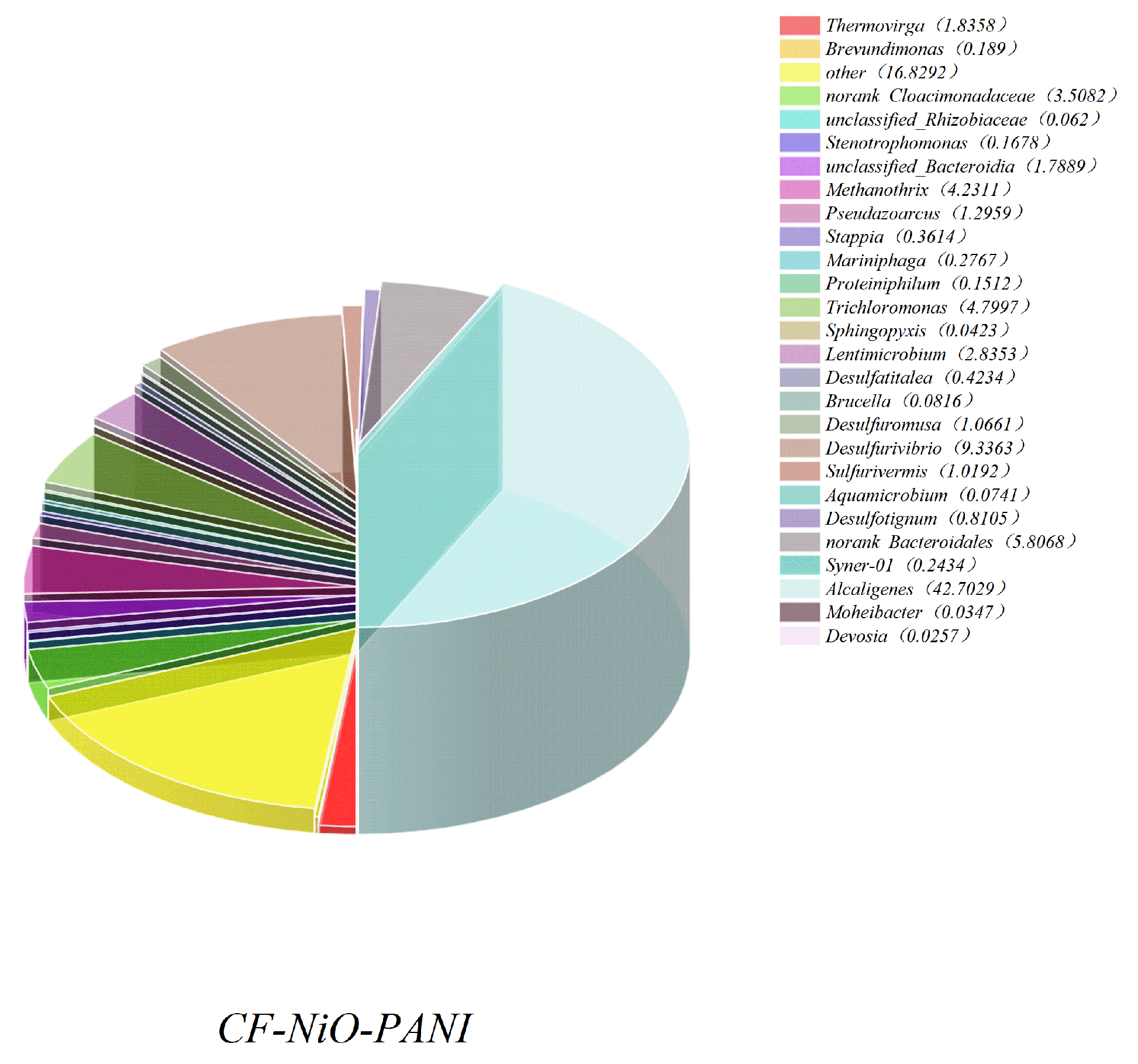
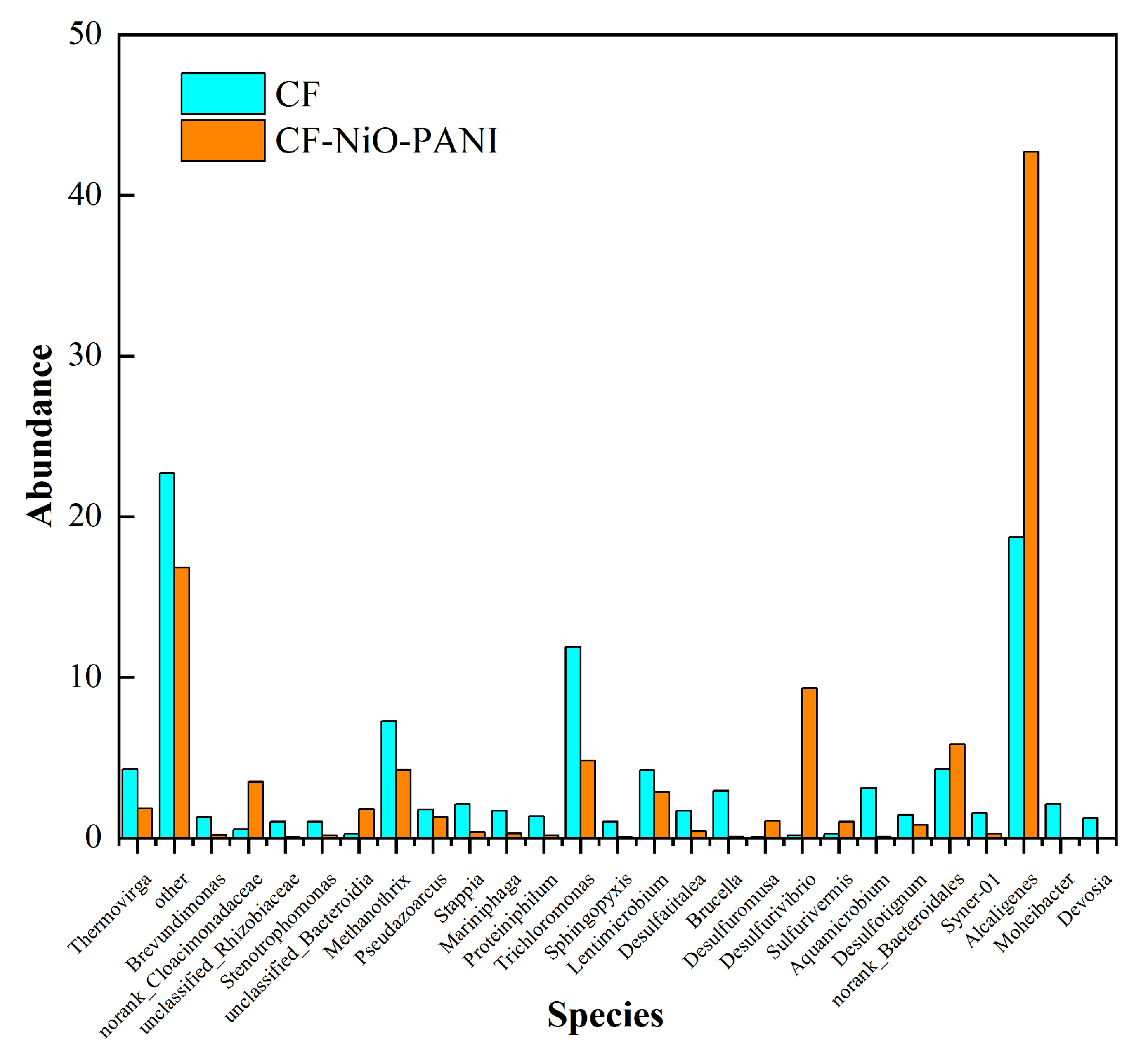
- Figure 13 shows the histogram of microbial community structure distribution of CF-NiO-PANI and CF anodes at the genus level. It can be visually seen that the microbial community of the CF-NiO-PANI anode is rich in species, among which, the electrogenic microorganisms are Stenotrophomonas, Pseudoarcus, Alcaligenes, and Lentimicrobe. Alcaligenes occupy a large proportion of the CF-NiO-PANI anode and are the main electrogenic bacteria of the CF-NiO-PANI electrode. Alcaligenes are resistant to harsh environments and have certain tolerance to high salt, extreme pH, or heavy metal pollution environments, which makes them suitable for industrial wastewater treatment. They can also improve the overall electricity generation performance through symbiosis in mixed bacteria, showing good synergy.
- (3)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jong, D.H. Impact of the Potato on Society. Am. J. Potato Res. 2016, 93, 415–429. [Google Scholar] [CrossRef]
- Ovchinnikova, A.; Krylova, E.; Gavrilenko, T.; Smekalova, T.; Zhuk, M.; Knapp, S.; Spooner, D.M. Taxonomy of cultivated potatoes (Solanum section Petota: Solanaceae). Bot. J. Linn. Soc. 2011, 165, 107–155. [Google Scholar] [CrossRef]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and human health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Zhang, J.; Zhou, Z.; Pan, Y.; Yang, Z.; Zhu, J.; Liu, Y.; Zhang, L. Crop rotations increased soil ecosystem multifunctionality by improving keystone taxa and soil properties in potatoes. Front. Microbiol. 2023, 14, 1034761. [Google Scholar] [CrossRef]
- Wierzbowska, J.; Rychcik, B.; Światły, A. The effect of different production systems on the content of micronutrients and trace elements in potato tubers. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2018, 68, 701–708. [Google Scholar] [CrossRef]
- Subramanian, N.K.; White, P.J.; Broadley, M.R.; Ramsay, G. The three-dimensional distribution of minerals in potato tubers. Ann. Bot. 2011, 107, 681–691. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Kaur, L.; Singh, J.; Zeng, F. Functional food based on Potato. Foods 2023, 12, 2145. [Google Scholar] [CrossRef]
- Pedreschi, F.; Moyano, P.; Santis, N.; Pedreschi, R. Physical properties of pre-treated potato chips. J. Food Eng. 2007, 79, 1474–1482. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, T.; Liu, Q.; Liu, W.; Zhao, R.; Hu, H. Effects of additives (NaCl, citric acid, and ethanol) on the cooking quality and sensory quality of vermicelli produced from freeze–thaw-dehydrated whole potato powder. Int. J. Food Sci. Technol. 2024, 59, 9459–9468. [Google Scholar] [CrossRef]
- Zhang, H.; Fen, X.U.; Yu, W.U.; Hu, H.H.; Dai, X.F. Progress of potato staple food research and industry development in China. J. Integr. Agric. 2017, 16, 2924–2932. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Liu, H.; Zhao, L.; Wang, Y.; Wu, X.; Liao, X. Improving the production efficiency of sweet potato starch using a newly designed sedimentation tank during starch sedimentation process. J. Food Process. Preserv. 2020, 44, e14811. [Google Scholar] [CrossRef]
- Durruty, I.; Bonanni, P.S.; González, J.F.; Busalmen, J.P. Evaluation of potato-processing wastewater treatment in a microbial fuel cell. Bioresour. Technol. 2012, 105, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, X.; Jin, M.; Chen, H.; Yi, F.; Wang, L.; Qiao, N.; Yu, D. Incorporating corn oil refining wastewater improves lipid accumulation and self-settling property of Trichosporon fermentans in corn starch wastewater. Sep. Purif. Technol. 2021, 275, 119250. [Google Scholar] [CrossRef]
- Li, M.; Zhu, X.; Yang, H.; Xie, X.; Zhu, Y.; Xu, G.; Hu, X.; Jin, Z.; Hu, Y.; Hai, Z.; et al. Treatment of potato starch wastewater by dual natural flocculants of chitosan and poly-glutamic acid. J. Clean. Prod. 2020, 264, 121641. [Google Scholar] [CrossRef]
- Moqsud, M.A.; Omine, K.; Yasufuku, N.; Hyodo, M.; Nakata, Y. Microbial fuel cell (MFC) for bioelectricity generation from organic wastes. Waste Manag. 2013, 33, 2465–2469. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Simões, M.; Melo, L.F.; Pinto, A.M.F.R. Overview on the developments of microbial fuel cells. Biochem. Eng. J. 2013, 73, 53–64. [Google Scholar] [CrossRef]
- Mohan, S.V.; Raghavulu, S.V.; Peri, D.; Sarma, P.N. Integrated function of microbial fuel cell (MFC) as bio-electrochemical treatment system associated with bioelectricity generation under higher substrate load. Biosens. Bioelectron. 2009, 24, 2021–2027. [Google Scholar] [CrossRef]
- Aiyer, K.S. How does electron transfer occur in microbial fuel cells? World J. Microbiol. Biotechnol. 2020, 36, 19. [Google Scholar] [CrossRef]
- Ieropoulos, I.A.; Greenman, J.; Melhuish, C.; Hart, J. Comparative study of three types of microbial fuel cell. Enzym. Microb. Technol. 2005, 37, 238–245. [Google Scholar] [CrossRef]
- Cecconet, D.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Agro-food industry wastewater treatment with microbial fuel cells: Energetic recovery issues. Int. J. Hydrogen Energy 2018, 43, 500–511. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Hassan, S.H.A.; Oh, S.E.; Ismail, M.; Rahimnejad, M.; Jahim, J.M. Nano-structured carbon as electrode material in microbial fuel cells: A comprehensive review. J. Alloys Compd. 2013, 580, 245–255. [Google Scholar] [CrossRef]
- Zhou, M.; Chi, M.; Luo, J.; He, H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, S.; Liu, H.; Liu, S.; Zhang, J.; Yuan, J.; Fu, W.; Dang, W.; Xu, Y.; Yang, X.; et al. Heteroatom-doped porous carbon nanoparticle-decorated carbon cloth (HPCN/CC) as efficient anode electrode for microbial fuel cells (MFCs). J. Clean. Prod. 2022, 336, 130374. [Google Scholar] [CrossRef]
- Santoro, C.; Li, B.; Cristiani, P.; Squadrito, G. Power generation of microbial fuel cells (MFCs) with low cathodic platinum loading. Int. J. Hydrogen Energy 2013, 38, 692–700. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Du, Z. Polyaniline synthesis by cyclic voltammetry for anodic modification in microbial fuel cells. Int. J. Electrochem. Sci. 2014, 9, 2038–2046. [Google Scholar] [CrossRef]
- Cui, H.F.; Du, L.; Guo, P.B.; Zhu, B.; Luong, J.H. Controlled modification of carbon nanotubes and polyaniline on macroporous graphite felt for high-performance microbial fuel cell anode. J. Power Sources 2015, 283, 46–53. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S.; Hou, L.; Zuo, J.; Kong, X.; Song, Y.; Wang, Z.; Tian, Y.; Dong, J. Enhanced electricity generation and energy storage in a microbial fuel cell with a bimetallic-modified capacitive anode. Desalination 2025, 593, 118247. [Google Scholar] [CrossRef]
- Yin, T.; Lin, Z.; Su, L.; Yuan, C.; Fu, D. Preparation of vertically oriented TiO2 nanosheets modified carbon paper electrode and its enhancement to the performance of MFCs. ACS Appl. Mater. Interfaces 2015, 7, 400–408. [Google Scholar] [CrossRef]
- Gnana kumar, G.; Kirubaharan, C.J.; Udhayakumar, S.; Karthikeyan, C.; Nahm, K.S. Conductive polymer/graphene supported platinum nanoparticles as anode catalysts for the extended power generation of microbial fuel cells. Ind. Eng. Chem. Res. 2014, 53, 16883–16893. [Google Scholar] [CrossRef]
- Ma, X.; Feng, C.; Zhou, W.; Yu, H. Municipal sludge-derived carbon anode with nitrogen-and oxygen-containing functional groups for high-performance microbial fuel cells. J. Power Sources 2016, 307, 105–111. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Hu, G. Bifunctional polypyrrole/ferroferric oxide as anode material for enhanced electricity generation and energy storage in microbial fuel cell. Renew. Energy 2023, 219, 119432. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Li, S.; Lv, X.; Cheng, Y.; Wang, X. Microbial biofuel cell operating effectively through carbon nanotube blended with gold–titania nanocomposites modified electrode. Electrochim. Acta 2013, 109, 328–332. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.; Ding, L.; Ren, H.; Cui, H. Effect of conductive polymers coated anode on the performance of microbial fuel cells (MFCs) and its biodiversity analysis. Biosens. Bioelectron. 2011, 26, 4169–4176. [Google Scholar] [CrossRef] [PubMed]
- Hanafi, M.F.; Sapawe, N. A review on the current techniques and technologies of organic pollutants removal from water/wastewater. Mater. Today: Proc. 2020, 31, A158–A165. [Google Scholar] [CrossRef]
- He, Z.; Minteer, S.D.; Angenent, L.T. Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ. Sci. Technol. 2005, 39, 5262–5267. [Google Scholar] [CrossRef]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef]
- Makhtar, M.M.Z.; Don, M.M.; Tajarudin, H.A. Microbial fuel cell (MFC) development from anaerobic digestion system. In Anaerobic Digestion Processes: Applications and Effluent Treatment; Springer: Singapore, 2018; pp. 9–31. [Google Scholar]
- Hindatu, Y.; Annuar, M.S.M.; Gumel, A.M. Mini-review: Anode modification for improved performance of microbial fuel cell. Renew. Sustain. Energy Rev. 2017, 73, 236–248. [Google Scholar] [CrossRef]
- Arun, J.; SundarRajan, P.S.; Pavithra, K.G.; Priyadharsini, P.; Shyam, S.; Goutham, R.; Le, Q.; Pugazhendhi, A. New insights into microbial electrolysis cells (MEC) and microbial fuel cells (MFC) for simultaneous wastewater treatment and green fuel (hydrogen) generation. Fuel 2024, 355, 129530. [Google Scholar] [CrossRef]
- Hamedani, E.A.; Abasalt, A.; Talebi, S. Application of microbial fuel cells in wastewater treatment and green energy production: A comprehensive review of technology fundamentals and challenges. Fuel 2024, 370, 131855. [Google Scholar] [CrossRef]
- Wu, Q.; Jiao, S.; Ma, M.; Peng, S. Microbial fuel cell system: A promising technology for pollutant removal and environmental remediation. Environ. Sci. Pollut. Res. 2020, 27, 6749–6764. [Google Scholar] [CrossRef]
- Wei, G.; Chun, X.; Sheng, W.; Jiang, S. Capacitive performance of polyaniline prepared by soft template method. Procedia Eng. 2012, 27, 1378–1385. [Google Scholar] [CrossRef]
- Mahanta, D.; Manna, U.; Madras, G.; Patil, S. Multilayer self-assembly of TiO2 nanoparticles and polyaniline-grafted-chitosan copolymer (CPANI) for photocatalysis. ACS Appl. Mater. Interfaces 2011, 3, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Gong, S. Electrochemical Synthesis of Chitosan-co-polyaniline/WO3⋅nH2O Composite Electrode for Amperometric Detection of NO2 Gas. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2008, 20, 1775–1781. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhang, D.; Kong, X.; Song, Y.; Ma, S.; Duan, Y.; Vyshnikin, A.; Palchykov, V. Carbon Felt/Nickel Oxide/Polyaniline Nanocomposite as a Bifunctional Anode for Simultaneous Power Generation and Energy Storage in a Dual-Chamber MFC. Coatings 2025, 15, 356. [Google Scholar] [CrossRef]
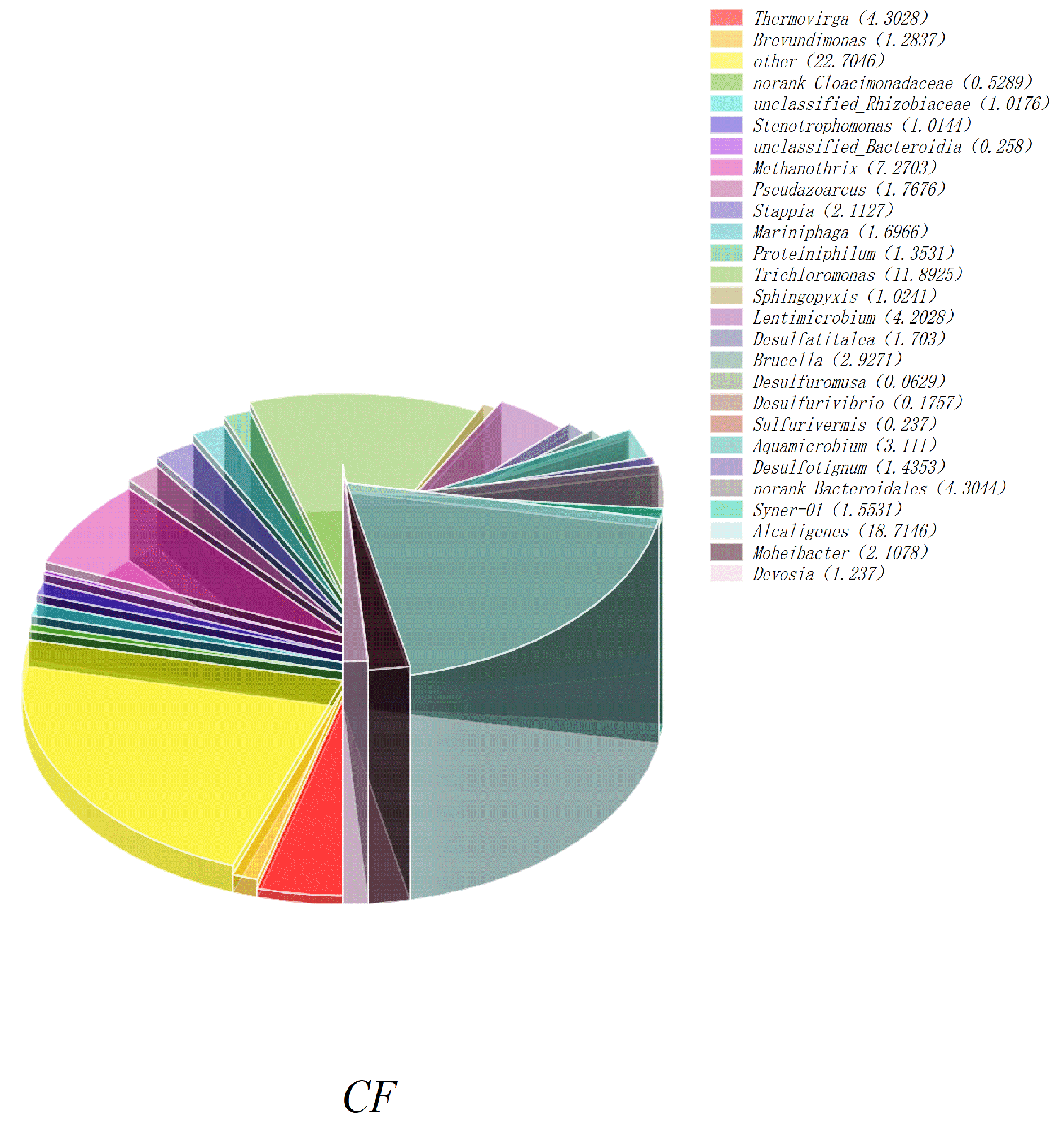
| Electrode | CF | CF-NiO-PANI |
|---|---|---|
| RΩ (Ω) | 7.23 | 1.57 |
| Rct (Ω) | 10.18 | 4.48 |
| Electrode | Current and Capacity | C250/D250 | C500/D500 | C750/D750 | C1000/D1000 |
|---|---|---|---|---|---|
| CF | ih (A/m2) | 37.37 | 42.76 | 48.37 | 54.37 |
| is (A/m2) | 1.28 | 3.93 | 4.73 | 6.95 | |
| Qs (C/m2) | 109.96 | 125.60 | 177.89 | 348.77 | |
| Qt (C/m2) | 419.96 | 2091.85 | 3655.64 | 7303.77 | |
| CF-NiO-PANI | ih (A/m2) | 93.80 | 94.76 | 116.52 | 142.13 |
| is (A/m2) | 10.64 | 12.80 | 13.41 | 18.78 | |
| Qs (C/m2) | 1625.34 | 1757.85 | 2205.90 | 2607.06 | |
| Qt (C/m2) | 4287.30 | 8157.85 | 12,263.40 | 20,886.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Wang, J.; Jiang, L.; Lei, J.; Li, W.; Yang, T.; Wang, Z.; Zuo, J.; Wang, Y. Preparation of CF-NiO-PANI Electrodes and Study on the Efficiency of MFC in Recovering Potato Starch Wastewater. Coatings 2025, 15, 776. https://doi.org/10.3390/coatings15070776
Han Y, Wang J, Jiang L, Lei J, Li W, Yang T, Wang Z, Zuo J, Wang Y. Preparation of CF-NiO-PANI Electrodes and Study on the Efficiency of MFC in Recovering Potato Starch Wastewater. Coatings. 2025; 15(7):776. https://doi.org/10.3390/coatings15070776
Chicago/Turabian StyleHan, Yiwei, Jingyuan Wang, Liming Jiang, Jiuming Lei, Wenjing Li, Tianyi Yang, Zhijie Wang, Jinlong Zuo, and Yuyang Wang. 2025. "Preparation of CF-NiO-PANI Electrodes and Study on the Efficiency of MFC in Recovering Potato Starch Wastewater" Coatings 15, no. 7: 776. https://doi.org/10.3390/coatings15070776
APA StyleHan, Y., Wang, J., Jiang, L., Lei, J., Li, W., Yang, T., Wang, Z., Zuo, J., & Wang, Y. (2025). Preparation of CF-NiO-PANI Electrodes and Study on the Efficiency of MFC in Recovering Potato Starch Wastewater. Coatings, 15(7), 776. https://doi.org/10.3390/coatings15070776







