Production of Self-Supporting Hollow Carbon Nanofiber Membranes with Co/Co2P Heterojunctions via Continuous Coaxial Co-Spinning for Efficient Overall Water Splitting
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Self-Supporting Co/Co2P-NCNFs-H Membranes
2.2.1. Preparation of Precursor Solutions
2.2.2. Coaxial Electrospinning
2.2.3. Post-Treatment: Pre-Oxidation, Carbonization, and Activation
2.3. Preparation of Control Samples
2.4. Catalytic Activity Measurements
3. Results and Discussion
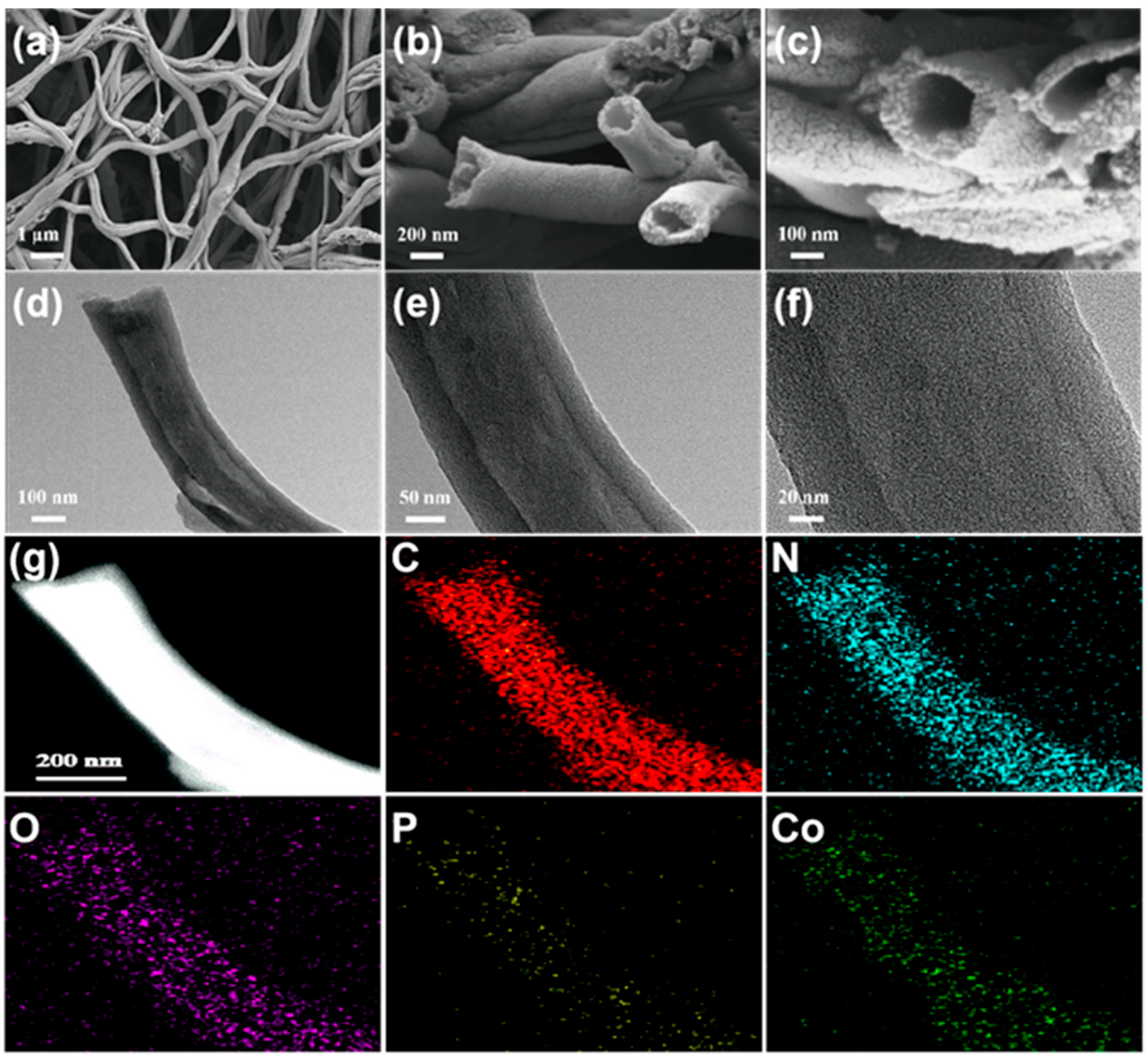
3.1. Morphology Characterization
3.2. Composition Study of the Samples
3.3. OER Activity Evaluation of Co/Co2P-NCNFs-H
3.4. HER Activity of Co/Co2P-NCNFs-H
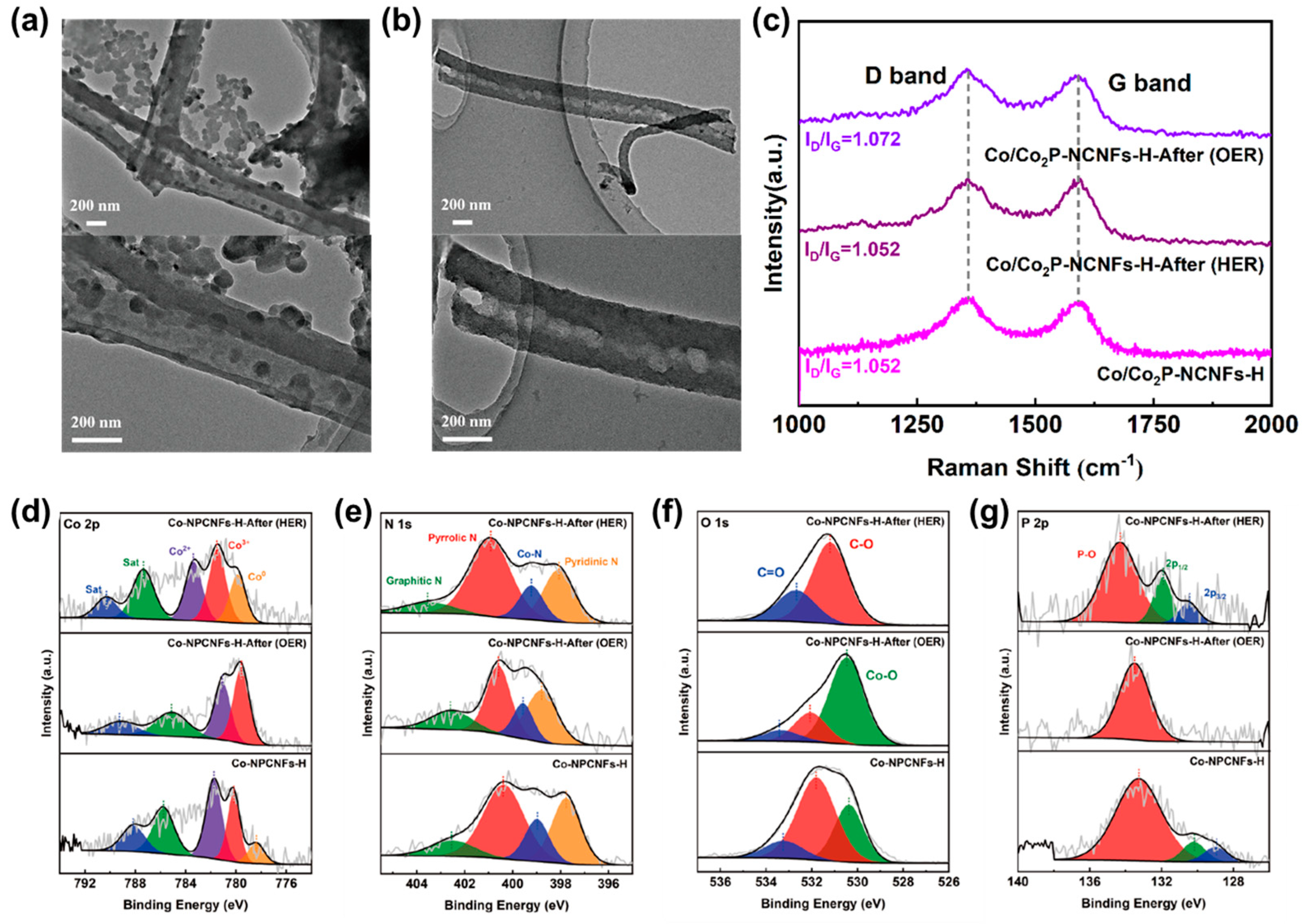
3.5. Characterization of Co/Co2P-NCNFs-H After OER and HER Stability Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Küspert, S.; Campbell, I.E.; Zeng, Z.; Balaghi, S.E.; Ortlieb, N.; Thomann, R.; Knäbbeler-Buß, M.; Allen, C.S.; Mohney, S.E.; Fischer, A. Ultrasmall and Highly Dispersed Pt Entities Deposited on Mesoporous N-doped Carbon Nanospheres by Pulsed CVD for Improved HER. Small 2024, 20, 2311260. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, M.; Gu, X.; Shi, Y.; Deng, Z.; Cai, N. Water Electrolysis toward Elevated Temperature: Advances, Challenges and Frontiers. Chem. Rev. 2023, 123, 7119–7192. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Kou, Z.; Liu, Y.; Cui, W.; Yang, B.; Li, Z.; Rodriguez, R.D.; Zhang, Q.; Dong, C.-L.; Sang, X.; Lei, L.; et al. Electronic structure optimization of metal–phthalocyanine via confining atomic Ru for all-pH hydrogen evolution. Energy Environ. Sci. 2024, 17, 1540–1548. [Google Scholar] [CrossRef]
- Zainal, B.S.; Ker, P.J.; Mohamed, H.; Ong, H.C.; Fattah, I.M.R.; Rahman, S.M.A.; Nghiem, L.D.; Mahlia, T.M.I. Recent advancement and assessment of green hydrogen production technologies. Renew. Sustain. Energy Rev. 2024, 189, 113941. [Google Scholar] [CrossRef]
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Recent progress in advanced electrocatalyst design for acidic oxygen evolution reaction. Adv. Mater. 2021, 33, 2004243. [Google Scholar] [CrossRef]
- Quan, L.; Jiang, H.; Mei, G.; Sun, Y.; You, B. Bifunctional Electrocatalysts for Overall and Hybrid Water Splitting. Chem. Rev. 2024, 124, 3694–3812. [Google Scholar] [CrossRef]
- Deng, L.; Hung, S.-F.; Lin, Z.-Y.; Zhang, Y.; Zhang, C.; Hao, Y.; Liu, S.; Kuo, C.-H.; Chen, H.-Y.; Peng, J.; et al. Valence Oscillation of Ru Active Sites for Efficient and Robust Acidic Water Oxidation. Adv. Mater. 2023, 35, 2305939. [Google Scholar] [CrossRef]
- Paudel, D.R.; Pan, U.N.; Ghising, R.B.; Dhakal, P.P.; Dinh, V.A.; Wang, H.; Kim, N.H.; Lee, J.H. Interface modulation induced by the 1T Co-WS2 shell nanosheet layer at the metallic NiTe2/Ni core–nanoskeleton: Glib electrode-kinetics for HER, OER, and ORR. Nano Energy 2022, 102, 107712. [Google Scholar] [CrossRef]
- Zhao, S.; Hu, F.; Yin, L.; Li, L.; Peng, S. Manipulating electron redistribution induced by asymmetric coordination for electrocatalytic water oxidation at a high current density. Sci. Bull. 2023, 68, 1389–1398. [Google Scholar] [CrossRef]
- Song, Z.; Zhu, Y.-N.; Liu, H.; Banis, M.N.; Zhang, L.; Li, J.; Doyle-Davis, K.; Li, R.; Sham, T.-K.; Yang, L.; et al. Engineering the Low Coordinated Pt Single Atom to Achieve the Superior Electrocatalytic Performance toward Oxygen Reduction. Small 2020, 16, 2003096. [Google Scholar] [CrossRef]
- Tan, L.; Wang, H.; Qi, C.; Peng, X.; Pan, X.; Wu, X.; Wang, Z.; Ye, L.; Xiao, Q.; Luo, W.; et al. Regulating Pt electronic properties on NiFe layered double hydroxide interface for highly efficient alkaline water splitting. Appl. Catal. B Environ. 2024, 342, 123352. [Google Scholar] [CrossRef]
- Ngo, Q.P.; Nguyen, T.T.; Le, Q.T.T.; Lee, J.H.; Kim, N.H. Unveiling the Synergistic Effect of Atomic Iridium Modulated Zirconium-Doped Pure Phase Cobalt Phosphide for Robust Anion-Exchange Membrane Water Electrolyzer. Adv. Energy Mater. 2023, 13, 2301841. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Zhai, W.; Liu, H.; Sakthivel, T.; Guo, S.; Dai, Z. Metastabilizing the Ruthenium Clusters by Interfacial Oxygen Vacancies for Boosted Water Splitting Electrocatalysis. Adv. Energy Mater. 2024, 14, 2400059. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 5106. [Google Scholar] [CrossRef]
- Qian, J.; Liu, X.; Zhong, C.; Xu, G.; Li, H.; Zhou, W.; You, B.; Wang, F.; Gao, D.; Chao, D. Enhanced stability and narrowed D-band gap of Ce-doped Co3O4 for rechargeable aqueous Zn-air battery. Adv. Funct. Mater. 2023, 33, 2212021. [Google Scholar] [CrossRef]
- Yu, M.; Budiyanto, E.; Tüysüz, H. Principles of Water Electrolysis and Recent Progress in Cobalt-, Nickel-, and Iron-Based Oxides for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2022, 61, e202103824. [Google Scholar] [CrossRef]
- Browne, M.P.; Sofer, Z.; Pumera, M. Layered and two dimensional metal oxides for electrochemical energy conversion. Energy Environ. Sci. 2019, 12, 41–58. [Google Scholar] [CrossRef]
- Ren, J.-T.; Yuan, G.-G.; Weng, C.-C.; Chen, L.; Yuan, Z.-Y. Uniquely integrated Fe-doped Ni(OH)2 nanosheets for highly efficient oxygen and hydrogen evolution reactions. Nanoscale 2018, 10, 10620–10628. [Google Scholar] [CrossRef]
- Qiao, L.; Zhu, A.; Zeng, W.; Dong, R.; Tan, P.; Ding, Z.; Gao, P.; Wang, S.; Pan, J. Achieving electronic structure reconfiguration in metallic carbides for robust electrochemical water splitting. J. Mater. Chem. A 2020, 8, 2453–2462. [Google Scholar] [CrossRef]
- Chen, P.; Ye, J.; Wang, H.; Ouyang, L.; Zhu, M. Recent progress of transition metal carbides/nitrides for electrocatalytic water splitting. J. Alloys Compd. 2021, 883, 160833. [Google Scholar] [CrossRef]
- Weng, C.-C.; Ren, J.-T.; Yuan, Z.-Y. Transition Metal Phosphide-Based Materials for Efficient Electrochemical Hydrogen Evolution: A Critical Review. ChemSusChem 2020, 13, 3357–3375. [Google Scholar] [CrossRef]
- Fang, Y.; Luan, D.; Lou, X.W. Recent Advances on Mixed Metal Sulfides for Advanced Sodium-Ion Batteries. Adv. Mater. 2020, 32, 2002976. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ren, J.T.; Wang, L.; Sun, M.L.; Yang, H.M.; Lv, X.W.; Yuan, Z.Y. Synergistically enhanced activity and stability of bifunctional nickel phosphide/sulfide heterointerface electrodes for direct alkaline seawater electrolysis. J. Energy Chem. 2022, 75, 66–73. [Google Scholar] [CrossRef]
- Gao, W.; Wu, Y.J.; Wan, X.H.; Gao, J.; Wen, D. Engineering the electronic structure of FeP with rare earth elements to enhance the electrocatalytic hydrogen evolution performance. J. Mater. Chem. A 2023, 11, 18126–18134. [Google Scholar] [CrossRef]
- Li, C.; Hong, W.T.; Cai, Q.; Jian, C.Y. Directional Construction of a 1T0.63-MoSe2@MoP Multiphase-Interface Catalyst for Highly Efficient Alkaline Hydrogen Evolution. ACS Appl. Mater. Interfaces 2022, 14, 30683–30691. [Google Scholar] [CrossRef]
- Song, M.; Wang, J.B.; Zhang, X.; Guo, J.X. CoP@NC nanorod array for efficient alkaline water and alkaline seawater electrolysis. J. Alloys Compd. 2025, 1018, 179248. [Google Scholar] [CrossRef]
- Zhang, C.X.; Li, F.T.; Wu, D.; Guo, Q.M.; Liu, Z.N.; Wang, Z.K.; Kang, Z.X.; Fan, L.L.; Sun, D.F. Oxygen-coordinated MOF membrane facilitated construction of supported Co2P/CoP@C heterostructures for water electrolysis. Inorg. Chem. Front. 2025, 12, 2254–2265. [Google Scholar] [CrossRef]
- Ipadeola, A.K.; Sliem, M.H.; Mwonga, P.; Ozoemena, K.I.; Abdullah, A.M. Rational construction of N-containing carbon sheets atomically doped NiP-CoP nanohybrid electrocatalysts for enhanced green hydrogen and oxygen production. Electrochim. Acta 2024, 508, 145236. [Google Scholar] [CrossRef]
- Huo, J.J.; Ming, Y.; Huang, X.L.; Ge, R.Y.; Li, S.; Zheng, R.K.; Cairney, J.; Dou, S.X.; Fei, B.; Li, W.X. Arrayed metal phosphide heterostructure by Fe doping for robust overall water splitting. J. Colloid Interface Sci. 2025, 678, 669–681. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Guo, P.; Li, H.; Fei, B.; Guo, Y.; Pan, H.; Sun, D.; Fang, F.; Wu, R. Co/CoP Heterojunction on Hierarchically Ordered Porous Carbon as a Highly Efficient Electrocatalyst for Hydrogen and Oxygen Evolution. Adv. Energy Mater. 2021, 11, 2102134. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.Y.; He, S.Q.; Qiao, Y.X.; Yuan, A.H.; Wu, J.C.; Zhou, H. Interfacial engineering of heterostructured CoP/FeP nanoflakes as bifunctional electrocatalyts toward alkaline water splitting. J. Colloid Interface Sci. 2025, 679, 20–29. [Google Scholar] [CrossRef]
- Li, J.W.; Hu, Y.Z.; Huang, X.; Zhu, Y.; Wang, D.L. Bimetallic Phosphide Heterostructure Coupled with Ultrathin Carbon Layer Boosting Overall Alkaline Water and Seawater Splitting. Small 2023, 19, e2206533. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Wu, Q.; Zhou, W.; Dan, J.; Zhu, H.; Lei, W.; Ma, L.-J.; Li, L. Low-loading Ir decorated cobalt encapsulated N-doped carbon nanotubes/porous carbon sheet implements efficient hydrogen/oxygen trifunctional electrocatalysis. Chem. Eng. J. 2022, 430, 132825. [Google Scholar] [CrossRef]
- Yu, B.; Liu, J.-H.; Guo, S.; Huang, G.; Zhang, S.; Chen, S.; Li, X.; Wang, Y.; Lv, L.-P. Densely populated tiny RuO2 crystallites supported by hierarchically porous carbon for full acidic water splitting. Mater. Horiz. 2023, 10, 4589–4596. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, L.; Sun, T.; Zhao, J.; Lyu, Z.; Zhuo, O.; Wang, X.; Wu, Q.; Ma, J.; Hu, Z. Significant Contribution of Intrinsic Carbon Defects to Oxygen Reduction Activity. ACS Catal. 2015, 5, 6707–6712. [Google Scholar] [CrossRef]
- Wang, H.-F.; Tang, C.; Zhang, Q. Template growth of nitrogen-doped mesoporous graphene on metal oxides and its use as a metal-free bifunctional electrocatalyst for oxygen reduction and evolution reactions. Catal. Today 2018, 301, 25–31. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, T.; Zhou, H.; Wang, Y.; Yang, X.; Liang, W.; Wu, D.; Yuan, P.; Yu, T.; He, M.; et al. Activating Fe activity and improving Ni activity via C3N4 substrate in alkaline oxygen evolution catalyzed by Ni-Fe phosphide. Appl. Catal. B Environ. 2024, 342, 123391. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, S.S.; Ning, P.; Xin, P.J.; Chen, Z.W.; Wang, Q.; Uvdal, K.; Hu, Z.J. Nested hollow architectures of nitrogen-doped carbon-decorated Fe, Co, Ni-based phosphides for boosting water and urea electrolysis. Nano Res. 2022, 15, 1916–1925. [Google Scholar] [CrossRef]
- Zhou, T.; Zhou, Y.; Ma, R.; Zhou, Z.; Liu, G.; Liu, Q.; Zhu, Y.; Wang, J. Nitrogen-doped hollow mesoporous carbon spheres as a highly active and stable metal-free electrocatalyst for oxygen reduction. Carbon 2017, 114, 177–186. [Google Scholar] [CrossRef]
- Zhu, R.L.; Yu, X.L.; Li, W.C.; Li, M.; Bo, X.J.; Gan, G.Y. Cobalt nanoparticles-embedded porous carbon nanocages uniformly dispersed hollow carbon fibers as the accelerated electrocatalysts toward water splitting. J. Alloys Compd. 2023, 947, 169488. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.T.; Li, T.; Zheng, J.L.; Yang, W.; Xiao, Y.Q. Ruthenium nanoparticles incorporated hollow carbon nanoshells for improved hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 51, 1033–1041. [Google Scholar] [CrossRef]
- Guan, Z.; Li, J.; Li, S.; Wang, K.; Lei, L.; Wang, Y.; Zhuang, L.; Xu, Z. The transient covering of iridium species with ultrathin carbon shells via Joule-heating for robust acidic water oxidation. Mater. Chem. Front. 2024, 8, 824–835. [Google Scholar] [CrossRef]
- Poudel, M.B.; Logeshwaran, N.; Kim, A.R.; Karthikeyan, S.C.; Vijayapradeep, S.; Yoo, D.J. Integrated core-shell assembly of Ni3S2 nanowires and CoMoP nanosheets as highly efficient bifunctional electrocatalysts for overall water splitting. J. Alloys Compd. 2023, 960, 170678. [Google Scholar] [CrossRef]
- Tong, J.; Li, Y.; Bo, L.; Li, W.; Li, T.; Zhang, Q.; Kong, D.; Wang, H.; Li, C. CoP/N-doped carbon hollow spheres anchored on electrospinning core–shell N-doped carbon nanofibers as efficient electrocatalysts for water splitting. ACS Sustain. Chem. Eng. 2019, 7, 17432–17442. [Google Scholar] [CrossRef]
- Patil, S.A.; Khot, A.C.; Chavan, V.D.; Rabani, I.; Kim, D.K.; Jung, J.; Im, H.; Shrestha, N.K. Electrostatically robust CoFeOF nanosheet against chloride for green-H2 production in alkaline seawater electrolysis. Chem. Eng. J. 2024, 480, 146545. [Google Scholar] [CrossRef]
- Tran, V.A.; Do, H.H.; Le, V.T.; Vasseghian, Y.; Vo, V.; Ahn, S.H.; Kim, S.Y.; Lee, S.W. Metal-organic-framework-derived metals and metal compounds as electrocatalysts for oxygen evolution reaction: A review. Int. J. Hydrogen Energy 2022, 47, 19590–19608. [Google Scholar] [CrossRef]
- Zhao, X.L.; Yong, X.H.; Ji, Q.Z.; Yang, Z.H.; Song, Y.; Tian, T.Y.; Chen, T.; Yang, Z.G.; Xu, L.X.; Shen, X.; et al. Synthesis of all-biomass-derived carbon nanofibers for dual-functional filtration membranes and oxygen evolution reaction electrocatalysts. J. Alloys Compd. 2022, 918, 165600. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, A.A.; Lohani, P.C.; Yoo, D.J.; Kim, H.J. Assembling zinc cobalt hydroxide/ternary sulfides heterostructure and iron oxide nanorods on three-dimensional hollow porous carbon nanofiber as high energy density hybrid supercapacitor. J. Energy Storage 2023, 60, 106713. [Google Scholar] [CrossRef]
- Peng, L.C.; Wang, J.A.; Guo, S.Q.; Li, C.J. Exploratory construction of Co/Co3O4-Ni/NiO heterointerface modified macroporous interconnected hollow carbon nanofibers towards efficient and flexible electrocatalysis. Chem. Eng. J. 2022, 450, 138252. [Google Scholar] [CrossRef]
- Papkov, D.; Delpouve, N.; Delbreilh, L.; Araujo, S.; Stockdale, T.; Mamedov, S.; Maleckis, K.; Zou, Y.; Andalib, M.N.; Dargent, E. Quantifying polymer chain orientation in strong and tough nanofibers with low crystallinity: Toward next generation nanostructured superfibers. ACS Nano 2019, 13, 4893–4927. [Google Scholar] [CrossRef]
- Wang, X.W.; Su, T.T.; Lu, Z.H.; Yu, L.; Sha, N.; Lv, C.M.; Xie, Y.; Ye, K. Morphological engineering of monodispersed Co2P nanocrystals for efficient alkaline water and seawater splitting. J. Colloid Interface Sci. 2025, 691, 137389. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chen, W.; Jiang, Z.J.; Jiang, Z. Co/CoO heterojunction rich in oxygen vacancies introduced by O2 plasma embedded in mesoporous walls of carbon nanoboxes covered with carbon nanotubes for rechargeable zinc–air battery. Carbon Energy 2024, 6, e457. [Google Scholar] [CrossRef]
- Kandel, M.R.; Pan, U.N.; Dhakal, P.P.; Ghising, R.B.; Nguyen, T.T.; Zhao, J.; Kim, N.H.; Lee, J.H. Unique heterointerface engineering of Ni2P− MnP nanosheets coupled Co2P nanoflowers as hierarchical dual-functional electrocatalyst for highly proficient overall water-splitting. Appl. Catal. B Environ. 2023, 331, 122680. [Google Scholar] [CrossRef]
- Peng, X.; Jin, X.; Gao, B.; Liu, Z.; Chu, P.K. Strategies to improve cobalt-based electrocatalysts for electrochemical water splitting. J. Catal. 2021, 398, 54–66. [Google Scholar] [CrossRef]
- Xia, C.; Huang, L.; Yan, D.; Douka, A.I.; Guo, W.; Qi, K.; Xia, B.Y. Electrospinning synthesis of self-standing cobalt/nanocarbon hybrid membrane for long-life rechargeable zinc–air batteries. Adv. Funct. Mater. 2021, 31, 2105021. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, K.; Wang, H.; Yao, X. The Role of Defect Sites in Nanomaterials for Electrocatalytic Energy Conversion. Chem 2019, 5, 1371–1397. [Google Scholar] [CrossRef]
- Ahn, C.H.; Yang, W.S.; Kim, J.J.; Priyanga, G.S.; Thomas, T.; Deshpande, N.G.; Lee, H.S.; Cho, H.K. Design of hydrangea-type Co/Mo bimetal MOFs and MOF-derived Co/Mo2C embedded carbon composites for highly efficient oxygen evolution reaction. Chem. Eng. J. 2022, 435, 134815. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Li, T.; Wang, Q.; Ai, Y.Y.; Hou, R.H.; Habib, A.; Shao, G.S.; Wang, F.; Zhang, P. A parallel array structured cobalt sulfide/nitrogen doped carbon nanocage/carbon fiber composite based on microfluidic spinning technology: A novel design to boost overall water splitting. J. Mater. Chem. A 2024, 12, 23872–23879. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Y.; Chen, P.; Zhang, S.; Jiang, H.; Yang, L.; Wang, Y.; Zhang, L.; Shen, J.; Zhao, X. Heterostructure Films: A Freestanding 3D Heterostructure Film Stitched by MOF-Derived Carbon Nanotube Microsphere Superstructure and Reduced Graphene Oxide Sheets: A Superior Multifunctional Electrode for Overall Water Splitting and Zn–Air Batteries (Adv. Mater. 48/2020). Adv. Mater. 2020, 32, 2070362. [Google Scholar]
- Yang, C.C.; Zai, S.F.; Zhou, Y.T.; Du, L.; Jiang, Q. Fe3C-Co nanoparticles encapsulated in a hierarchical structure of N-doped carbon as a multifunctional electrocatalyst for ORR, OER, and HER. Adv. Funct. Mater. 2019, 29, 1901949. [Google Scholar] [CrossRef]
- Yan, H.; Xie, Y.; Wu, A.; Cai, Z.; Wang, L.; Tian, C.; Zhang, X.; Fu, H. Anion-modulated HER and OER activities of 3D Ni–V-based interstitial compound heterojunctions for high-efficiency and stable overall water splitting. Adv. Mater. 2019, 31, 1901174. [Google Scholar] [CrossRef] [PubMed]
- Kubisztal, J.; Kubisztal, M. Pressed Ni/MFe2O4 (M = Ni, Co) powder compacts for application as bifunctional, high-performance electrodes in electrochemical water splitting. Int. J. Hydrogen Energy 2024, 56, 912–923. [Google Scholar] [CrossRef]
- Hong, Q.; Wang, Y.; Wang, R.; Chen, Z.; Yang, H.; Yu, K.; Liu, Y.; Huang, H.; Kang, Z.; Menezes, P.W. In Situ Coupling of Carbon Dots with Co-ZIF Nanoarrays Enabling Highly Efficient Oxygen Evolution Electrocatalysis. Small 2023, 19, 2206723. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Yu, X.; Li, J.; Wang, K.; Niu, J. Interfacial Interaction in NiFe LDH/NiS(2)/VS(2) for Enhanced Electrocatalytic Water Splitting. Molecules 2024, 29, 951. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, Y.; Zhang, W.; Sun, S.; Yao, S. Preparation of Co(OH)2@NiFe/NF bifunctional electrocatalyst by electrodeposition for efficient water splitting. J. Solid State Chem. 2023, 323, 124048. [Google Scholar] [CrossRef]
- Dai, X.; Tang, Z.; Yan, X.; Tao, S.; Wang, S.; Liu, Y.; Cao, J.; Deng, X.; Bo, X. In-Situ Sulfuration of Ni(OH)2 to Heterostructured Ni3S2/Ni(OH)2@Ni Catalyst for Efficient Water Splitting. Chem. Asian J. 2025, 20, e202401190. [Google Scholar] [CrossRef]
- Sang, Y.; Cao, X.; Wang, L.; Ding, G.; Wang, Y.; Yu, D.; Hao, Y.; Li, L.; Peng, S. Facile synthesis of three-dimensional spherical Ni(OH)2/NiCo2O4 heterojunctions as efficient bifunctional electrocatalysts for water splitting. Int. J. Hydrogen Energy 2020, 45, 30601–30610. [Google Scholar] [CrossRef]
- He, Y.; Yang, X.; Jiang, M.; Liu, F.; Zhang, J.; Li, H.; Cui, L.; Xu, J.; Ji, X.; Liu, J. Cr-doped NiFe sulfides nanoplate array: Highly efficient and robust bifunctional electrocatalyst for the overall water splitting and seawater electrolysis. J. Colloid Interface Sci. 2025, 680, 1079–1089. [Google Scholar] [CrossRef]
- Li, H.; Gao, X.; Li, G. Construction of Co2P-Ni3S2/NF Heterogeneous Structural Hollow Nanowires as Bifunctional Electrocatalysts for Efficient Overall Water Splitting. Small 2023, 19, 2304081. [Google Scholar] [CrossRef]
- Ju, S.; Liu, Y.; Pei, M.; Shuai, Y.; Zhai, Z.; Yan, W.; Wang, Y.-J.; Zhang, J. Amorphization-induced abundant coordinatively unsaturated Ni active sites in NiCo(OH)2 for boosting catalytic OER and HER activities at high current densities for water-electrolysis. J. Colloid Interface Sci. 2024, 653, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Zhu, Y.-R.; Chen, Y.; Dou, S.-Y.; Chen, X.-Y.; Dong, B.; Guo, B.-Y.; Liu, D.-P.; Liu, C.-G.; Chai, Y.-M. Hydrogen evolution under large-current-density based on fluorine-doped cobalt-iron phosphides. Chem. Eng. J. 2020, 399, 125831. [Google Scholar] [CrossRef]
- Huang, C.; Zhou, J.; Duan, D.; Zhou, Q.; Wang, J.; Peng, B.; Yu, L.; Yu, Y. Roles of heteroatoms in electrocatalysts for alkaline water splitting: A review focusing on the reaction mechanism. Chin. J. Catal. 2022, 43, 2091–2110. [Google Scholar] [CrossRef]





| Catalysts | Overpotential at 100 mA cm−2 (mV) | Tafel Slope (mV dec−1) | Ref. |
|---|---|---|---|
| Co/Co2P-NCNFs-H | 405.6 | 76.2 | This work |
| p-Co9S8/NC/CF | 398 | 75 | [59] |
| Ni@N-HCGHF | 470 | 63 | [60] |
| Fe3C-Co/NC | 450 | - | [61] |
| Ni2P-VP2/NF | 398 | 56 | [62] |
| Ni/NiFe2O4 | 365 | 86 | [63] |
| Co-ZIF/CDs/CC | 401 | 147 | [64] |
| NiFe LDH/NiS2/VS2 | 384 | 99 | [65] |
| Catalyst | Overpotential at 100 mA cm−2 (mV) | Tafel Slope (mV dec−1) | Ref. |
|---|---|---|---|
| Co/Co2P-NCNFs-H | 247.9 | 67.7 | This work |
| Co(OH)2@NiFe/NF | 311 | 74 | [66] |
| Ni3S2/Ni(OH)2-5h | 360 | 80.8 | [67] |
| Ni(OH)2/NiCo2O4 | 189 (η10) | 41 | [68] |
| NiFeCrSx/NF | 236 | 67.4 | [69] |
| Co2P-Ni3S2/NF | 110 | 114.2 | [70] |
| ac-NiCo(OH)2/NF | 320 | 90 | [71] |
| F-Co2P/Fe2P/IF | 151.8 | 115.01 | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, R.; Ding, J.; Fan, J.; Zhuang, L. Production of Self-Supporting Hollow Carbon Nanofiber Membranes with Co/Co2P Heterojunctions via Continuous Coaxial Co-Spinning for Efficient Overall Water Splitting. Coatings 2025, 15, 772. https://doi.org/10.3390/coatings15070772
Duan R, Ding J, Fan J, Zhuang L. Production of Self-Supporting Hollow Carbon Nanofiber Membranes with Co/Co2P Heterojunctions via Continuous Coaxial Co-Spinning for Efficient Overall Water Splitting. Coatings. 2025; 15(7):772. https://doi.org/10.3390/coatings15070772
Chicago/Turabian StyleDuan, Ruidan, Jianhang Ding, Jiawei Fan, and Linzhou Zhuang. 2025. "Production of Self-Supporting Hollow Carbon Nanofiber Membranes with Co/Co2P Heterojunctions via Continuous Coaxial Co-Spinning for Efficient Overall Water Splitting" Coatings 15, no. 7: 772. https://doi.org/10.3390/coatings15070772
APA StyleDuan, R., Ding, J., Fan, J., & Zhuang, L. (2025). Production of Self-Supporting Hollow Carbon Nanofiber Membranes with Co/Co2P Heterojunctions via Continuous Coaxial Co-Spinning for Efficient Overall Water Splitting. Coatings, 15(7), 772. https://doi.org/10.3390/coatings15070772




