Abstract
Preparing a bioactive surface with a hierarchical micro/nanostructure can improve the osseointegration of titanium implants. In this study, titanium was sand blasted and etched in H2SO4 solution to obtain micro-rough morphology. The samples were then hydrothermally treated in the concentrated CaHPO4 solution at 120–200 °C for 24 h to grow films consisting of anatase TiO2 and hydroxyapatite nanoparticles (size 80–240 nm). The hydrothermally calcified (200 °C) sample exhibited much better corrosion resistance in the salt solution, as well as similar cellular viability and a higher alkaline phosphatase level in the cell tests using MC3T3-E1 cells, in comparison with the polished titanium sample. The hybrid treatment is a facile and effective method to a form bioactive surface with a hierarchical micro/nanostructure on titanium.
1. Introduction
Titanium and its alloys have low density, high mechanical properties, excellent corrosion resistance, and good biocompatibility and thus are utilized to produce orthopedic and other kinds of implants [1]. The micro-scale rough titanium surface prepared generally by sand blasting technique is beneficial for the mechanical fixation of titanium implants in vivo. Moreover, different surface modification methods have been studied to form bioinert or bioactive surfaces of blasted titanium (Blast+X methods) in order to improve osseointegration, in which X can be acid etching [2,3,4], thermal oxidation [5,6], hydrothermal treatment [7,8,9], electrochemical smoothing [10], or anodization [11], and coatings by plasma polymerization, biomimetic, electrochemical, magnetron sputter, and other methods. Among the Blast+X methods, the most studied method is the sand blasting-acid etching treatment, which can generate a favorable hierarchical micro/nanostructured surface. The superimposed nanoscale topography with a significantly increased surface area generated higher adsorption of serum adhesive proteins to promote early cell attachment [12] and larger induction of osteogenesis-related genes and cell–cell signaling during the early stages of osseointegration [13].
Further treatments like alkali treatment [14,15], anodization [16], ion implantation [17], plasma treatment [18], coatings [19,20,21], molecule delivery [22], and even outfield loadings (laser, magnetism, ultrasound, etc.) have been utilized (Blast+acid etching+Y) in order to improve osseointegration and bacterial biofilm ablation of titanium implants. In comparison with surface morphology, composition, wettability, and biological properties, the corrosion resistance of titanium after the blast-based treatments has been less studied.
The use of hydrothermal treatment for the bioactivity modification of titanium has the advantages of high adhesion of the in situ grown films, simple operation, low treatment temperature, minimal impact on matrix properties, etc. [23,24]. However, according to the authors’ available references, hydrothermal treatment has rarely been studied for the surface modification of blasted and acid-etched titanium. In this study, acid etching of sand-blasted titanium was conducted, which not only cleaned the blasted surface but also exposed the fresh surface of the titanium. The following hydrothermal calcification provided a convenient method to grow a bioactive hierarchical micro/nanostructured surface of titanium. The surface morphology, microstructure, wettability, corrosion resistance, and cell compatibility of the treated titanium samples were studied.
2. Experimental
2.1. Samples Preparation
The substrates were TA2 titanium plates (10 × 10 × 1.2 mm3) and titanium foil (thickness 0.3 mm). Titanium plates were polished with SiC paper down to grits of 1200, cleaned in acetone, ethanol, deionized water sequentially with ultrasonication, and dried in air (sample Ti-PL). Titanium foil was blasted with alumina sands and cut into small pieces (sample BL, size 10 × 10 mm2). After ultrasonication cleaning, the blasted titanium foil pieces were etched in 49% H2SO4 solution at 80 °C for 1 h. The blasted and etched foils were thoroughly washed with deionized water and dried (sample BE).
The polished and the etched samples were hydrothermally treated in CaHPO4 solution with a concentration of 10 mmol/L. CaHPO4 was used because it has a slightly soluble calcium phosphate phase with a solubility of 4.3 × 10−5 g/mL at 20 °C, and it is believed to be the intermediate phase of bone mineralization. The solution had a filling ratio of about 80% in the Teflon-lined stainless-steel autoclave. The sample was placed vertically in the autoclave by using a Teflon clamp. The autoclave was sealed and heated at 120, 150, 180, and 200 °C, respectively, for 24 h (samples BEH). Polished titanium was also treated with deionized water. The samples were then taken out and rinsed with pure water several times and dried for analyses and tests.
2.2. Material Characterization and Tests
Surface profiles of the samples were collected by using a laser confocal roughness measurement system (Leica DCM8, Germany, 532 nm, confocal model). The surface morphology of the samples was analyzed with the field emission scanning electron microscope equipped with an energy dispersive analysis of X-ray (EDX) system, and the microstructure was examined by X-ray diffraction (XRD, X’Pert PRO, PANalytical B.V., The Netherlands, CuKα) and Raman scattering (Horiba HR 800, Japan, 633 nm). Contact angles of the samples were measured by injecting 3.5 μL deionized water at the sample surface with a contact angle tester under ambient conditions. The samples were enveloped in epoxy, leaving one side for testing. Corrosion resistance was evaluated in the Hank’s balanced salt solution without Ca and Mg ions (HBSS, prepared with 8.00 g/L NaCl, 0.40 g/L KCl, 0.34 g/L NaHCO3, 0.06 g/L KH2PO4, 0.12 g/L Na2HPO4·12H2O) by using an electrochemical workstation (CHI 650e, China) under ambient conditions. Platinum electrode and saturated calomel electrode (SCE) were the counter electrode and the reference electrode, respectively.
2.3. Cell Tests
The polished titanium plates and the hydrothermally treated BE foil samples (CaHPO4 solution, 200 °C, 24 h) were sterilized with Co60 at a dose of 25 kGy for the tests with MC3T3-E1 cells in vitro. The cells were cultured in Dulbecco’s modified Eagle medium (DMEM) added with 10% fetal bovine serum, 1 mM L-glutamina, penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37 °C under 5% CO2 atmosphere. The proliferative effect of the samples was tested by MTT (3-[4,5-dim-ethylthiazol-2-yl]- 2,5-diphenyltetrazolium bromide) assay [25] after 3 and 7 days of cultivation. 4-Nitrophenyldihydrogenphosphate (PNPP) was used to detect the alkaline phosphatase (ALP) enzyme activity of the cells [25] on the samples at the same time points. Statistics of the cell test results were determined using the one-way ANOVA test. The difference between two groups of samples was considered to be statistically significant with a p-value less than 0.05. The morphology of the cells on the samples was observed by SEM after treatments of fixation, dehydration, air-drying, and gold coating.
3. Results and Discussion
3.1. Microstructure and Surface Morphology of the Samples
The microstructure of the surface layer of the hydrothermally treated samples was studied by X-ray diffraction and Raman spectroscopy. As seen in the XRD patterns (Figure 1), diffraction peaks of the titanium substrate, and the weak anatase peak at 2θ = 25.2°, were present for the samples treated at 200 °C. As well, in the Raman scattering spectra (Figure 2), the characteristic peaks of anatase TiO2 at 146 (Eg), 200 (Eg), 396 (B1g), 516 (doublet of A1g and B1g), and 637 (Eg) cm−1 [26,27] were detected in the hydrothermally treated titanium sheet samples. Anatase can be formed at the surface of alkali-treated titanium by hydrothermal treatment at 100 °C or higher temperatures [28]. For the foil samples, only the peak at 146 cm−1 was obvious in the spectra, and it was strong for sample BEH. In comparison, samples BL and BE showed similar weak peaks. The oxidization in sample BL may be from the manufacture and the blasting processes. Sample BE was prepared by immersion in hot 49% H2SO4 solution. In the reaction process, the vertically placed titanium foil was etched, but at the same time an amorphous Ti(SO4)2 thin film would be formed at the sample’s surface. In addition, a slight shift of the peak at 146 cm−1 was observed for the samples treated in waterat 120 °C and 150 °C and the samples BL and BE. The shift of Raman peaks is relevant to crystal strain, oxygen vacancy, thermal stress, pressure, temperature, etc., although the causes for the peak shift of the above samples are in need of further study. As for the calcium phosphate phases, hydroxyapatite is the most thermodynamically stable phase near the neutral pH or biological pH. The characteristic Raman peak from hydroxyapatite is located at 965 cm−1, which is due to the ν1 symmetric stretching of the P-O mode [29]. This peak was not present, indicating that the hydroxyapatite layer was thin at the surface of sample BEH (CaP, 200 °C, 24 h).
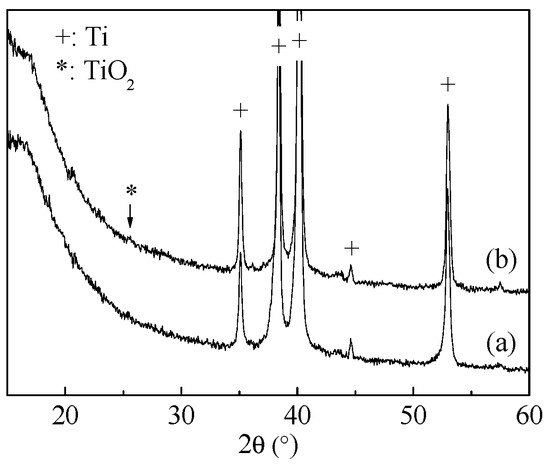
Figure 1.
XRD patterns of the titanium sheet samples after hydrothermal treatment (a) in water and (b) in CaHPO4 solution (200 °C, 24 h).
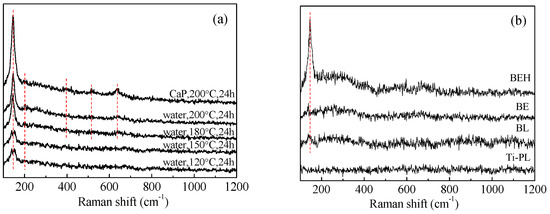
Figure 2.
Raman spectra of (a) the polished and (b) the blasted titanium samples after hydrothermal treatment in water and in CaHPO4 solution. The red dashed lines indicate the peaks positioned at 146, 200, 396, 516 and 637 cm−1, respectively.
Surface micrographs of the polished and the hydrothermally treated titanium samples are shown in Figure 3 and Figure S1. The surface of the sample treated in water at 150 °C was composed of dense nanoparticles (size 50–80 nm), and the sample treated in CaHPO4 solution had slightly bigger particles (size 80–110 nm). These nanoparticles are TiO2 nano-grains, which are hydrothermally grown on titanium surface through a dissolution–precipitation mechanism. When alkaline earth metal ions are present in the hydrothermal medium, titanate can be generated, which can provide nucleation sites for calcium phosphate. For the samples treated at 180 and 200 °C in CaHPO4 solution, there were some large white spherical particles (150–240 nm) scattered at the surface of the samples, which were identified as hydroxyapatite nanoparticles by energy dispersive X-ray spectroscopy and X-ray photoelectron spectroscopy [23].
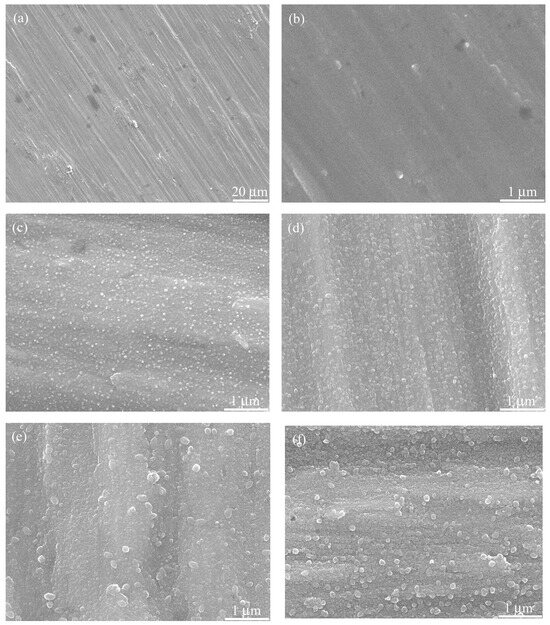
Figure 3.
SEM micrographs of the (a,b) polished titanium (Ti-PL) and the hydrothermally treated samples: (c) water at 150 °C for 24 h; (d–f) CaP solution at 150 °C, 180 °C, and 200 °C, respectively, for 24 h.
Surface micrographs of the original, the blasted, the etched, and the hydrothermally treated titanium foil samples are shown in Figure 4 and Figure S2. Acid etching can clean the surface and expose the fresh surface of titanium, which helps the subsequent hydrothermal calcification treatment. The surface of blasted/etched and hydrothermally treated titanium foil is rather rough. It has micro-scaled pits at low magnification and nano-grains at high magnification. The micro/nanostructured morphology and Ca and P composition (Figure 4f and Figure S3) of the hybrid treated titanium would be beneficial for cell growth and implant fixation.
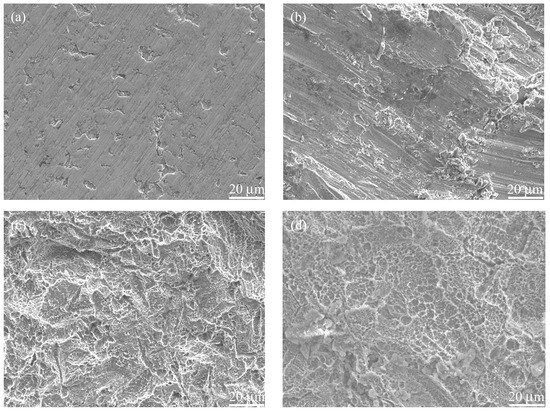
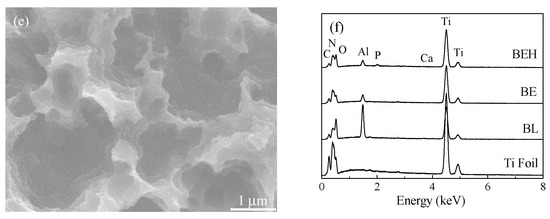
Figure 4.
SEM micrographs of the (a) titanium foil and samples (b) BL, (c) BE, and (d,e) BEH (CaP solution, 200 °C, 24 h) and (f) their EDX spectra. The elemental atomic ratios (%) of sample BEH are Ti 61.20, O 24.85, C 5.38, N 5.84, Al 1.85, P 0.61, and Ca 0.27.
The surface profiles of the titanium samples tested by the laser confocal roughness measurement system are shown in Figure 5. At a lower resolution (50×), the scan area is larger (2640 × 3500 μm2) and the obtained roughness was also larger (Table 1). For example, Sa increased from 0.16 μm to 2.38 μm for sample Ti-PL and from 5.13 μm to 7.53 μm for sample BL when the scan range increased from 264 × 350 μm2 (500×) to 2640 × 3500 μm2 (50×). The substrate curvature may have had an effect on the roughness measurement for samples BL, BE, and BEH. Sample BE had a slightly higher roughness than sample BL, showing that etching increased roughness at the small scan area of 264 × 350 μm2. Sample BEH had a lower roughness than sample BE. The hydrothermal treatment modulated the surface morphology through the dissolution and precipitation process, leading to a reduced surface roughness.
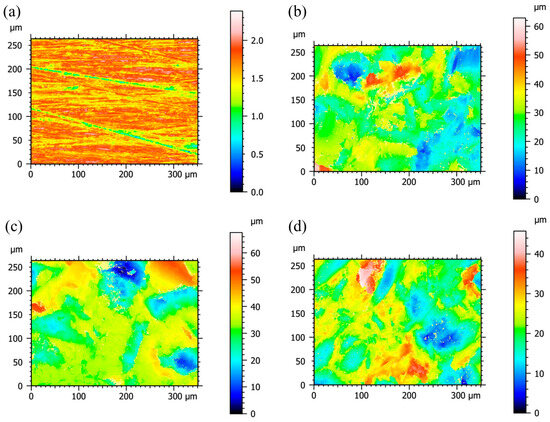
Figure 5.
Two-dimensional surface profiles of the titanium samples with a scan area of 264 × 350 μm2 (500×): (a) polished, (b) BL, (c) BE, and (d) BEH (CaP solution, 200 °C, 24 h).

Table 1.
Surface roughness values of the titanium samples.
3.2. Hydrophilicity and Corrosion Tests
The water contact angle of the polished titanium (Ti-PL) was measured to be 69.3 ± 5.2°, and the blasted titanium foil (BL) had a slightly higher contact angle (76.5 ± 4.5°, Figure 6). After the blasted foil was treated in hot H2SO4 solution, a much higher contact angle was obtained for sample BE (127.7 ± 3.8°). Seemingly, the high hydrophobicity is related to the hierarchical surface morphology (Figure 4c) and the airborne contaminations [30]. After the hydrothermal treatment in calcium phosphate solution, the contact angle obviously decreased (sample BEH, 79.5 ± 5.5°) due to the hydrophilic calcium phosphate grown at the surface. The hydrophilic surface is important for protein adsorption and cell attachment of the material [31].
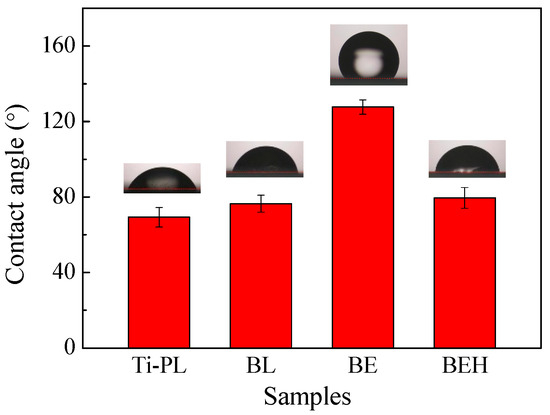
Figure 6.
Water contact angle test results of the polished and the treated titanium samples.
Electrochemical corrosion tests of the titanium samples were carried out in the Ca-free HBSS with the three-electrode system (Figure 7a). It can be seen from Figure 7b that the blasted sample BL had higher open circuit potential (OCP) than the other samples. In addition, sample BL showed the highest corrosion potential (Ecorr) and corrosion current density (Icorr) as well as different behaviors in the polarization and impedance plots (Figure 7c and Figure 8, Table 2), revealing the high surface reactivity of titanium after the mechanical blast treatment. The effects of blast treatment on titanium and other steels may include (1) increased roughness and surface area; (2) formation of a metastable oxide layer, e.g., Ti2O3 [32]; (3) crystal distortion, residual stress, and even grain division at the surface layer; and (4) susceptibility to pitting corrosion [33].
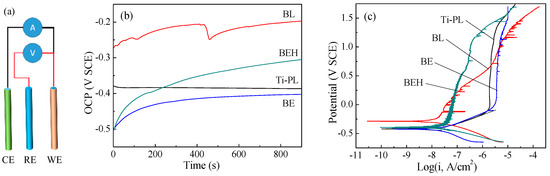
Figure 7.
(a) Schematic showing the three-electrode system for electrochemical corrosion tests: CE: counter electrode (Pt); RE: reference electrode (SCE); WE: work electrode (titanium sample). (b) Open circuit potential plots and (c) potentiodynamic polarization plots of the titanium samples.
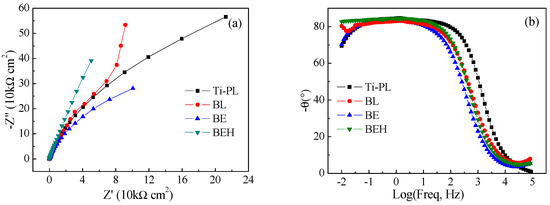
Figure 8.
(a) Nyquist plots and (b) impedance-phase angle diagrams (Bode plots) of the titanium samples.

Table 2.
Corrosion test data of the titanium samples.
In the potentiodynamic polarization test, the polished sample had the smallest Icorr value (Figure 7c), and also had the lowest surface roughness. A stable passive current density (Ipass = 2.159 μA/cm2) and a high pitting potential (Epit = 1.29 V) were obtained after a long range of anodic polarization (Table 2). The calcified sample BEH had a lower pitting potential but also had a much lower passive current density (about 1/20) than that of the polished sample, although sample BEH had a much higher surface roughness. This suggests that sample BEH has the reduced susceptibility to pitting corrosion.
In the Nyquist plots, sample BEH has a larger diameter than the other samples in the high-frequency region, indicating its higher impedance value and lower susceptibility to corrosion (Figure 8a). The hydrothermally synthesized TiO2-CaP film has obviously improved corrosion resistance of titanium, which will be beneficial for the biological properties of the hydrothermally treated titanium.
In the Bode phase plots (Figure 8b), the phase angles of the four titanium samples are within −7.9~−1.1° and −82.7~−69.4° at the high and low frequencies, respectively. The phase angle shifts to around −84° and remains almost constant at the frequency range of 0.1–10 Hz, indicating that the four samples have a response close to the capacitance. The behavior of the single capacitor reflects the single-layered surface structure of the samples.
3.3. Cell Tests
Cytocompatibility of the polished sample and the treated titanium samples was assessed using MC3T3-E1 osteoblastic cells. After 3 days of culture, sample BEH had slightly higher OD value than the polished one (p < 0.05, Figure 9a). The OD value in the MTT assay reflects the quantity of cells on the material. In the SEM micrographs, the MC3T3-E1 cells with filopodia were seen on both samples, but the cell morphology was different (Figure 10 and Figure S4). The polished sample is slightly hydrophilic (Figure 6) and cells on it exhibited more filopodia and an elongated form nearly parallel to the scratches (Figure 3a). In comparison, the cells on sample BEH were less flattened with less filopodia, indicating insufficient attachment of the osteoblastic cells. The two samples had similar OD values after 7 days of culture.
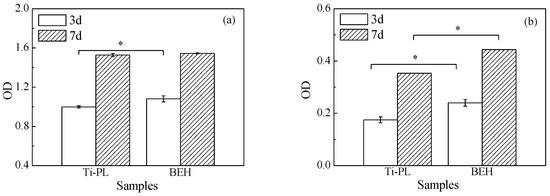
Figure 9.
(a) Viability level and (b) ALP level of MC3T3-E1 cells incubated with the polished and the BEHed titanium samples (*: p < 0.05).
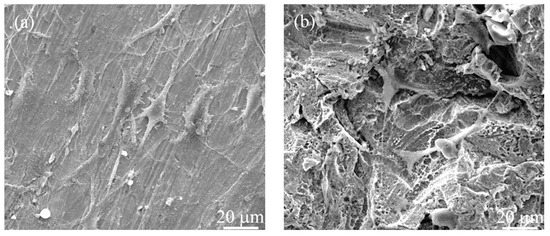
Figure 10.
SEM images of MC3T3-E1 cells on (a) the polished and (b) the BEHed titanium samples after 3 days of culture.
Sample BEH had much higher ALP activity than the polished sample at both time points (p < 0.05, Figure 9b). ALP is an important protein that is widely distributed in the bone, liver, intestine, and other organs. It is a byproduct of osteoblast cells, and it is one of the indicators of the bone formation function of osteoblasts [34]. Thus, sample BEH showed higher osteogenic differentiation of the osteoblastic cells than the polished one, possibly resulting from gradually released calcium and phosphate ions from the surface layer of sample BEH.
The hydrothermal treatment method for the surface modification of titanium and its alloys has the advantages of good film adhesion, simple operation, low treatment temperature, and minimal impact on matrix properties. Hydrothermal treatment is particularly suitable for the surface modification of rough or porous titanium, because the high-pressure environment can significantly enhance mass transfer of the solution. Studies have reported that the surface modification of titanium and its alloys has been carried out using Ca(OH)2, CaCl2, MgCl2, and other alkaline earth metal solutions, which resulted in improved biological activity of the materials and promoted growth of osteoblasts [35,36]. In this study, the blasted and acid-etched titanium was hydrothermally calcified to grow a bioactive surface layer with a hierarchical micro/nanostructure, which is a technique that has potential for the surface modification of titanium implants.
4. Conclusions
The combination of sand blasting and acid etching in H2SO4 solution was used to form a micro-rough surface layer on titanium. Hydrothermal calcification in a concentrated CaHPO4 solution at 200 °C can generate a Ca- and P-containing TiO2 surface layer on the polished and blasted/etched titanium. The hybrid-treated sample with a hierarchical micro/nanostructure has better corrosion resistance in comparison with the blasted and the blasted/etched samples. In the cell tests using MC3T3-E1 cells, the polished and hybrid-treated titanium samples showed similar cellular viability, but the latter had a higher alkaline phosphatase level, possibly due to the Ca and P elements.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings15070771/s1, Original figures.
Author Contributions
Conceptualization, K.H. and T.F.; Methodology, Y.M., Z.L., Y.Z. and H.F.; Validation, Y.M. and Z.L.; Formal analysis, Z.L.; Investigation, Y.M., Z.L. and Y.Z.; Resources, H.F. and T.F.; Data curation, Z.L. and Y.Z.; Writing—original draft, Y.M. and H.F.; Writing—review & editing, K.H. and T.F.; Supervision, K.H. and T.F.; Project administration, K.H.; Funding acquisition, K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Key Research and Development Program of Shaanxi Province (No.: 2023-YBSF-038) and the Natural Science Basic Research Program of Shaanxi Province (No.: 2022JQ-209), China. The authors would like to thank Y.F. Pan from the Instrument Analysis Center of Xi’an Jiaotong University for the SEM analysis.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ding, Y.; Yuan, Z.; Hu, J.-W.; Xu, K.; Wang, H.; Liu, P.; Cai, K.-Y. Surface modification of titanium implants with micro-nanotopography and NIR photothermal property for treating bacterial infection and promoting osseointegration. Rare Met. 2022, 41, 673–688. [Google Scholar] [CrossRef]
- Jiang, H.H.; Ma, X.; Zhou, W.J.; Dong, K.; Rausch-Fan, X.; Liu, S.T.; Li, S. The effects of hierarchical micro/nano-structured titanium surface on osteoblast proliferation and differentiation under diabetic conditions. Implant Dent. 2017, 26, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Carrasco, B.; Lemos, B.F.; Herrero-Climent, M.; Mur, F.J.G.; Ríos-Santos, J.V. Effect of the acid-etching on grit-blasted dental implants to improve osseointegration: Histomorphometric analysis of the bone-implant contact in the rabbit tibia model. Coatings 2021, 11, 1426. [Google Scholar] [CrossRef]
- Li, L.J.; Kim, S.N.; Cho, S.A. Comparison of alkaline phosphatase activity of MC3T3-E1 cells cultured on different Ti surfaces: Modified sandblasted with large grit and acid-etched (MSLA), laser-treated, and laser and acid-treated Ti surfaces. J. Adv. Prosthodont. 2016, 8, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Barranco, V.; Escudero, M.L.; García-Alonso, M.C. Influence of the microstructure and topography on the barrier properties of oxide scales generated on blasted Ti6Al4V surfaces. Acta Biomater. 2011, 7, 2716–2725. [Google Scholar] [CrossRef]
- Saldaña, L.; Barranco, V.; González-Carrasco, J.L.; Rodríguez, M.; Munuera, L.; Vilaboa, N. Thermal oxidation enhances early interactions between human osteoblasts and alumina blasted Ti6Al4V alloy. J. Biomed. Mater. Res. Part A 2007, 81, 334–346. [Google Scholar] [CrossRef]
- Park, J.W. Osseointegration of two different phosphate ion-containing titanium oxide surfaces in rabbit cancellous bone. Clin. Oral Implant Res. 2013, 24, 145–151. [Google Scholar] [CrossRef]
- Park, J.W.; An, C.H.; Jeong, S.H.; Suh, J.Y. Osseointegration of commercial microstructured titanium implants incorporating magnesium: A histomorphometric study in rabbit cancellous bone. Clin. Oral Implant Res. 2012, 23, 294–300. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.J.; Jang, J.H.; Suh, J.Y. Surface characteristics and primary bone marrow stromal cell response of a nanostructured strontium-containing oxide layer produced on a microrough titanium surface. J. Biomed. Mater. Res. Part A 2012, 100, 1477–1487. [Google Scholar] [CrossRef]
- Kityk, A.; Hnatko, M.; Pavlik, V.; Balog, M.; Soltys, J.; Labudova, M. Advancing biomedical substrate engineering: An eco-friendly route for synthesizing micro- and nanotextures on 3D printed Ti-6Al-4V. J. Mater. Res. Technol. 2024, 28, 2098–2115. [Google Scholar] [CrossRef]
- Iwata, N.; Nozaki, K.; Horiuchi, N.; Yamashita, K.; Tsutsumi, Y.; Miura, H.; Nagai, A. Effects of controlled micro-/nanosurfaces on osteoblast proliferation. J. Biomed. Mater. Res. Part A 2017, 105, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Kohavi, D.; Badihi, L.; Rosen, G.; Steinberg, D.; Sela, M.N. An in vivo method for measuring the adsorption of plasma proteins to titanium in humans. Biofouling 2013, 29, 1215–1224. [Google Scholar] [CrossRef]
- Bryington, M.; Mendonça, G.; Nares, S.; Cooper, L.F. Osteoblastic and cytokine gene expression of implant-adherent cells in humans. Clin. Oral Implants Res. 2014, 25, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Ge, X.Y.; Jia, S.N.; Jiang, X.; Zhang, Y.; Lin, Y. The influence of titanium surfaces treated by alkalis on macrophage and osteoblast-like cell adhesion and gene expression in vitro. RSC Adv. 2015, 5, 81378–81387. [Google Scholar] [CrossRef]
- Jia, S.N.; Zhang, Y.; Ma, T.; Chen, H.F.; Lin, Y. Enhanced hydrophilicity and protein adsorption of titanium surface by sodium bicarbonate solution. J. Nanomater. 2015, 2015, 536801. [Google Scholar] [CrossRef]
- Huang, J.Y.; Zhang, X.C.; Yan, W.X.; Chen, Z.P.; Shuai, X.T.; Wang, A.X.; Wang, Y. Nanotubular topography enhances the bioactivity of titanium implants. Nanomed.-Nanotechnol. Biol. Med. 2017, 13, 1913–1923. [Google Scholar] [CrossRef]
- Kim, B.S.; Kim, J.S.; Park, Y.M.; Choi, B.Y.; Lee, J. Mg ion implantation on SLA-treated titanium surface and its effects on the behavior of mesenchymal stem cell. Mater. Sci. Eng. C 2013, 33, 1554–1560. [Google Scholar] [CrossRef]
- Chou, W.C.; Wang, R.C.C.; Liu, C.; Yang, C.Y.; Lee, T.M. Surface modification of direct-current and radio-frequency oxygen plasma treatments enhance cell biocompatibility. Materials 2017, 10, 1223. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, Y.X.; Qiao, S.C.; Zhou, L.Y.; Shi, J.Y.; Lai, H.C. Bacterial and mammalian cells adhesion to tantalum-decorated micro-/nano-structured titanium. J. Biomed. Mater. Res. Part A 2017, 105, 871–878. [Google Scholar] [CrossRef]
- Mohammadnejad, L.; Theurer, A.; Alber, J.; Illing, B.; Kimmerle-Mueller, E.; Schultheiss, J.; Krajewski, S.; Rupp, F. Surface-mediated modulation of different biological responses on anatase-coated titanium. J. Funct. Biomater. 2024, 15, 29. [Google Scholar] [CrossRef]
- Zhang, J.M.; Wang, T.; Tang, C.B.; Wang, Q.N.; Qian, H.M.; Miao, R.J. Preparation and property of PDA/CPP bilayer on SLA surfaces. J. Inorg. Mater. 2017, 32, 1264–1268. [Google Scholar]
- Deng, J.Y.; Cohen, D.J.; Sabalewski, E.L.; Van Duyn, C.; Wilson, D.S.; Schwartz, Z.; Boyan, B.D. Semaphorin 3A delivered by a rapidly polymerizing click hydrogel overcomes impaired implant osseointegration in a rat type 2 diabetes model. Acta Biomater. 2023, 157, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Li, H.-W.; Sun, J.-m.; Li, G.; Li, W.; Zhang, H.-m. Facile hydrothermal synthesis of TiO2-CaP nano-films on Ti6Al4V alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 1122–1127. [Google Scholar] [CrossRef]
- Li, H.; Fu, T.; Li, W.; Alajmi, Z.; Sun, J. Hydrothermal growth of TiO2-CaP nano-films on a Ti-Nb-based alloy in concentrated calcium phosphate solutions. J. Nanopart. Res. 2016, 18, 4. [Google Scholar] [CrossRef]
- Fu, T.; Sun, J.M.; Zhao, Y.T.; Wang, L.J.; Zhou, Y.C.; Ma, X. Hydrothermally crystallized Sr-containing bioactive glass film and its cytocompatibility. Ceram. Int. 2017, 43, 13689–13695. [Google Scholar] [CrossRef]
- Cho, H.W.; Liao, K.L.; Yang, J.S.; Wu, J.J. Revelation of rutile phase by Raman scattering for enhanced photoelectrochemical performance of hydrothermally-grown anatase TiO2 film. Appl. Surf. Sci. 2018, 440, 125–132. [Google Scholar] [CrossRef]
- Zanatta, A.R. A fast-reliable methodology to estimate the concentration of rutile or anatase phases of TiO2. AIP Adv. 2017, 7, 075201. [Google Scholar] [CrossRef]
- Fu, T.; Fan, J.T.; Shen, Y.G.; Sun, J.M. Hydrothermal calcification of alkali treated titanium in CaHPO4 solution. Mater. Chem. Phys. 2017, 189, 105–110. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Alamara, K.; Saber-Samandari, S. Calcium phosphate coatings: Morphology, micro-structure and mechanical properties. Ceram. Int. 2014, 40, 563–572. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Tian, Y. Insights into the wettability transition of nanosecond laser ablated surface under ambient air exposure. J. Colloid Interface Sci. 2019, 533, 268–277. [Google Scholar] [CrossRef]
- Tan, J.; Li, L.; Li, B.; Tian, X.; Song, P.; Wang, X. Titanium surfaces modified with graphene oxide/gelatin composite coatings for enhanced antibacterial properties and biological activities. ACS Omega 2022, 7, 27359–27368. [Google Scholar] [CrossRef] [PubMed]
- Jeyalakshmi, P.; Ramkumar, P. The synergetic effect of micro-blasting and thermal oxidation on the corrosion performance of Ti6Al4V. Surf. Coat. Technol. 2023, 467, 129727. [Google Scholar] [CrossRef]
- Barranco, V.; Onofre, E.; Escudero, M.L.; Garcia-Alonso, M.C. Characterization of roughness and pitting corrosion of surfaces modified by blasting and thermal oxidation. Surf. Coat. Technol. 2010, 204, 3783–3793. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, W.; Qiu, K.; Chen, L.; Wang, W.; Nie, W.; Mo, X.; He, C. BMP-2 derived peptide and dexamethasone incorporated mesoporous silica nanoparticles for enhanced osteogenic differentiation of bone mesenchymal stem cells. ACS Appl. Mater. Interfaces 2015, 7, 15777–15789. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Li, S.J.; Tao, X.J.; Hao, Y.L.; Yang, R. Surface modification of Ti-Nb-Zr-Sn alloy by thermal and hydrothermal treatments. Mater. Sci. Eng. C 2009, 29, 1245–1251. [Google Scholar] [CrossRef]
- Chen, X.-B.; Li, Y.-C.; Du Plessis, J.; Hodgson, P.D.; Wen, C.E. Influence of calcium ion deposition on apatite-inducing ability of porous titanium for biomedical applications. Acta Biomater. 2009, 5, 1808–1820. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).