Preparation and Photocatalytic Performance of Silver-Loaded Micro-Arc Oxidation TiO2 Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. TC4 Titanium Alloy Pretreatment
2.2. Configuration of Electrolyte
2.3. Micro-Arc Oxidation Treatment
2.4. Photocatalytic Experiment
2.5. Material Characterization
2.5.1. Microscopic Morphology Observation and Analysis
2.5.2. Energy Dispersive Spectroscopy (EDS) Analysis
2.5.3. XRD Phase Analysis
2.5.4. Distribution Pattern of Pore Area Analysis
2.5.5. Comparison of Photocatalytic Performance
3. Results and Discussion
3.1. XRD Phase Analysis Results
3.2. EDS Analysis
3.2.1. Composition of Undoped Silver Ion Coating Elements
3.2.2. Doping Silver Ions on the Composition of Coating Elements
3.3. Microstructure and Performance Analysis
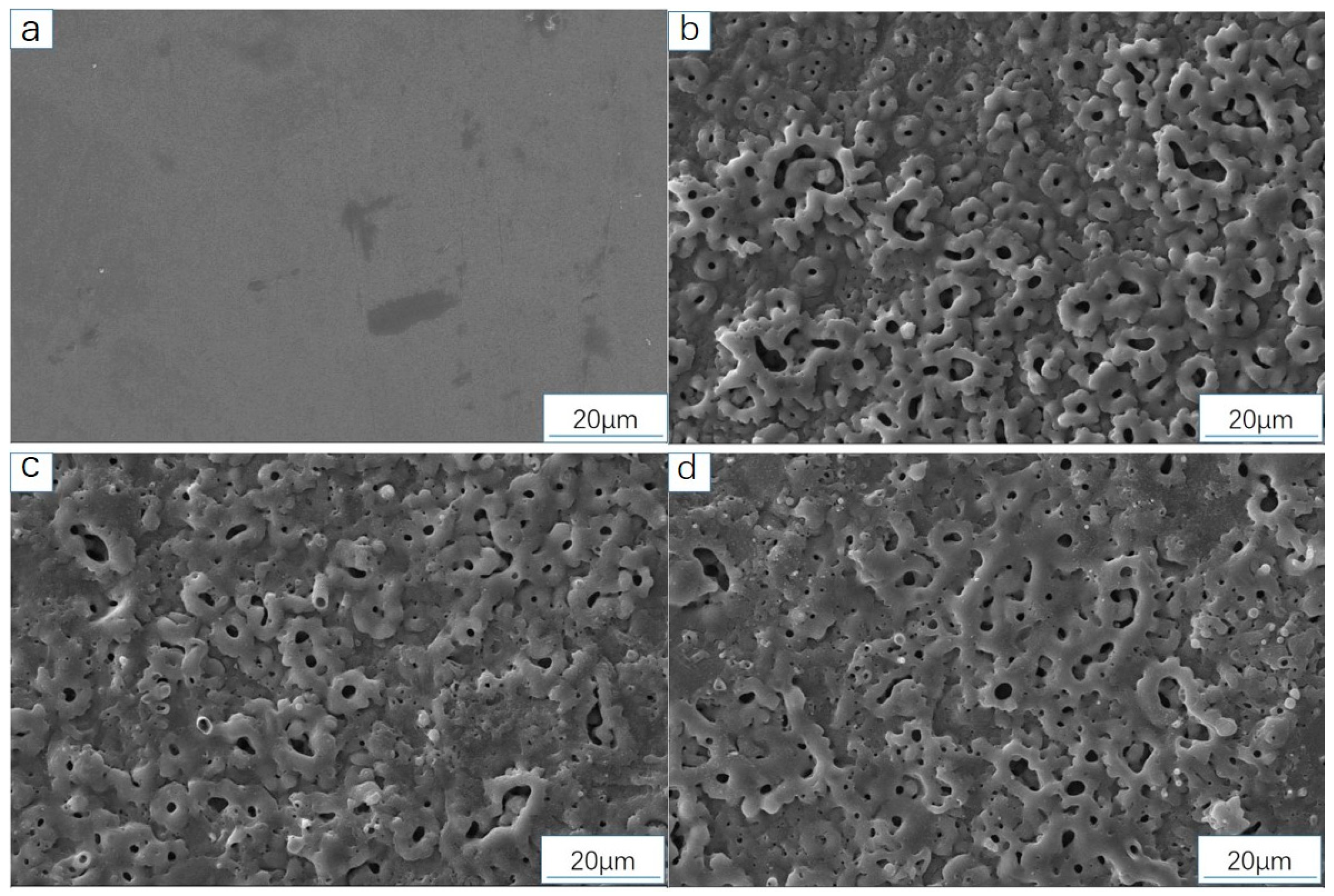
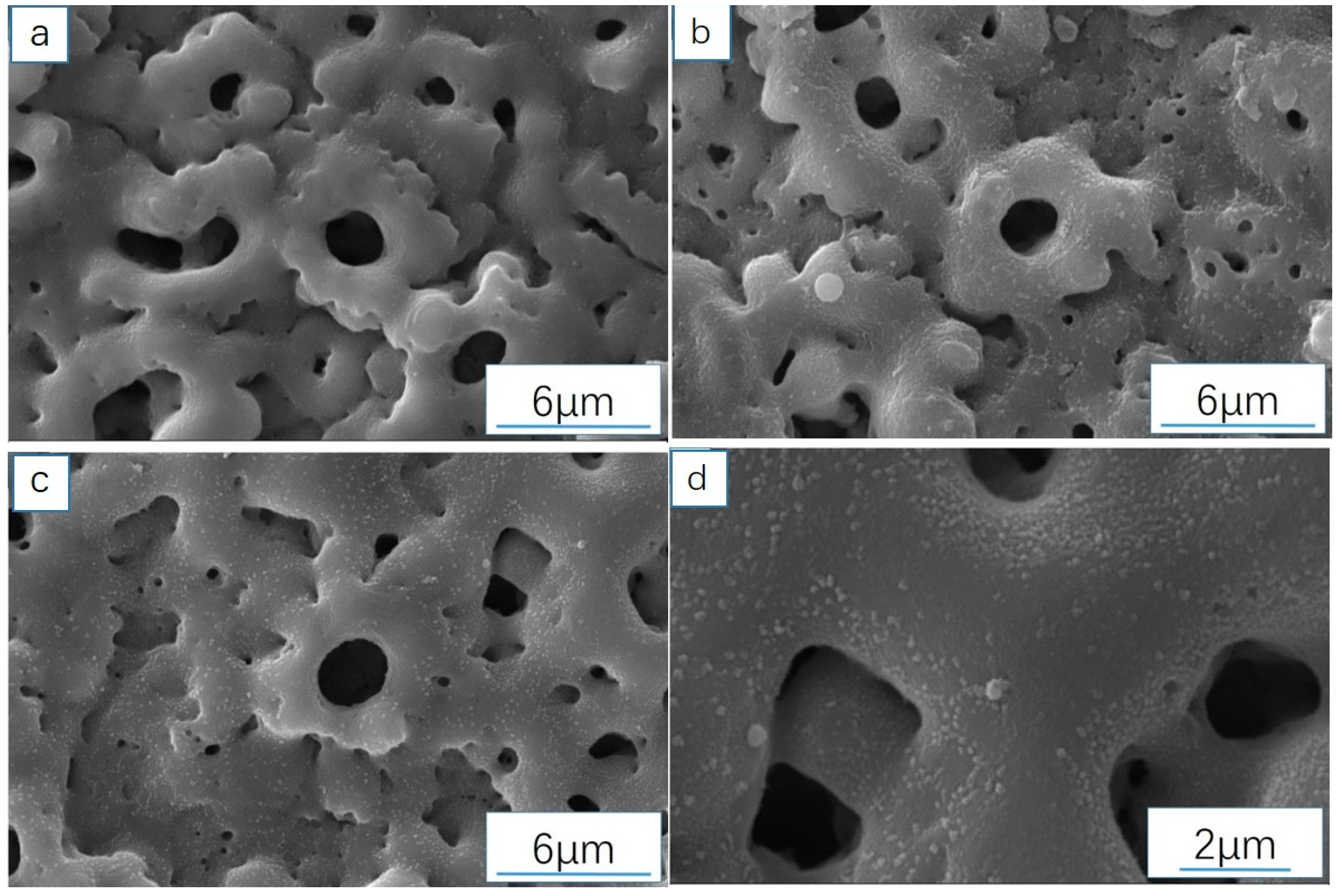
3.3.1. Analysis of Sample Surface Morphology (SEM)
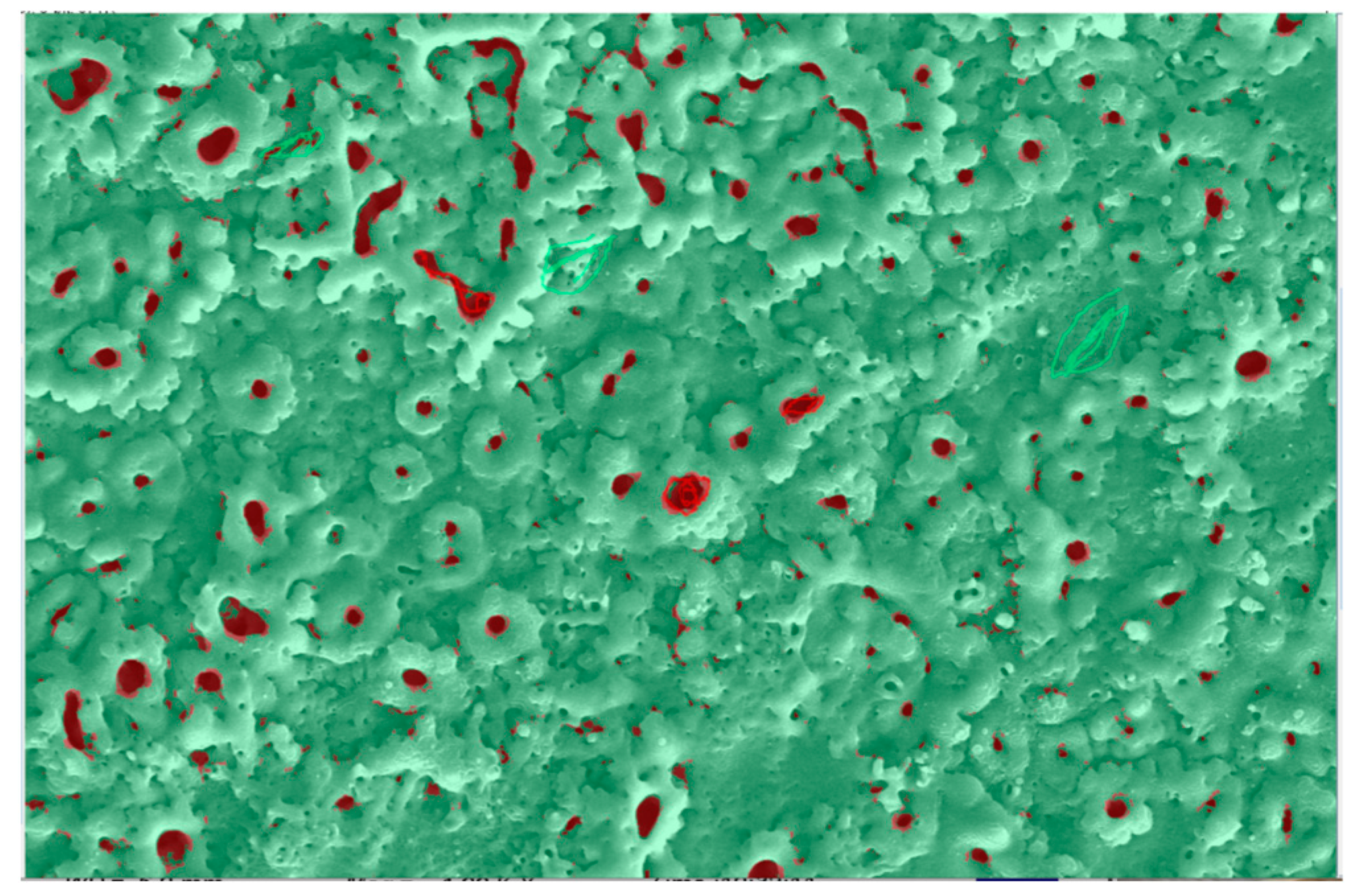
3.3.2. Distribution Pattern of Pore Area
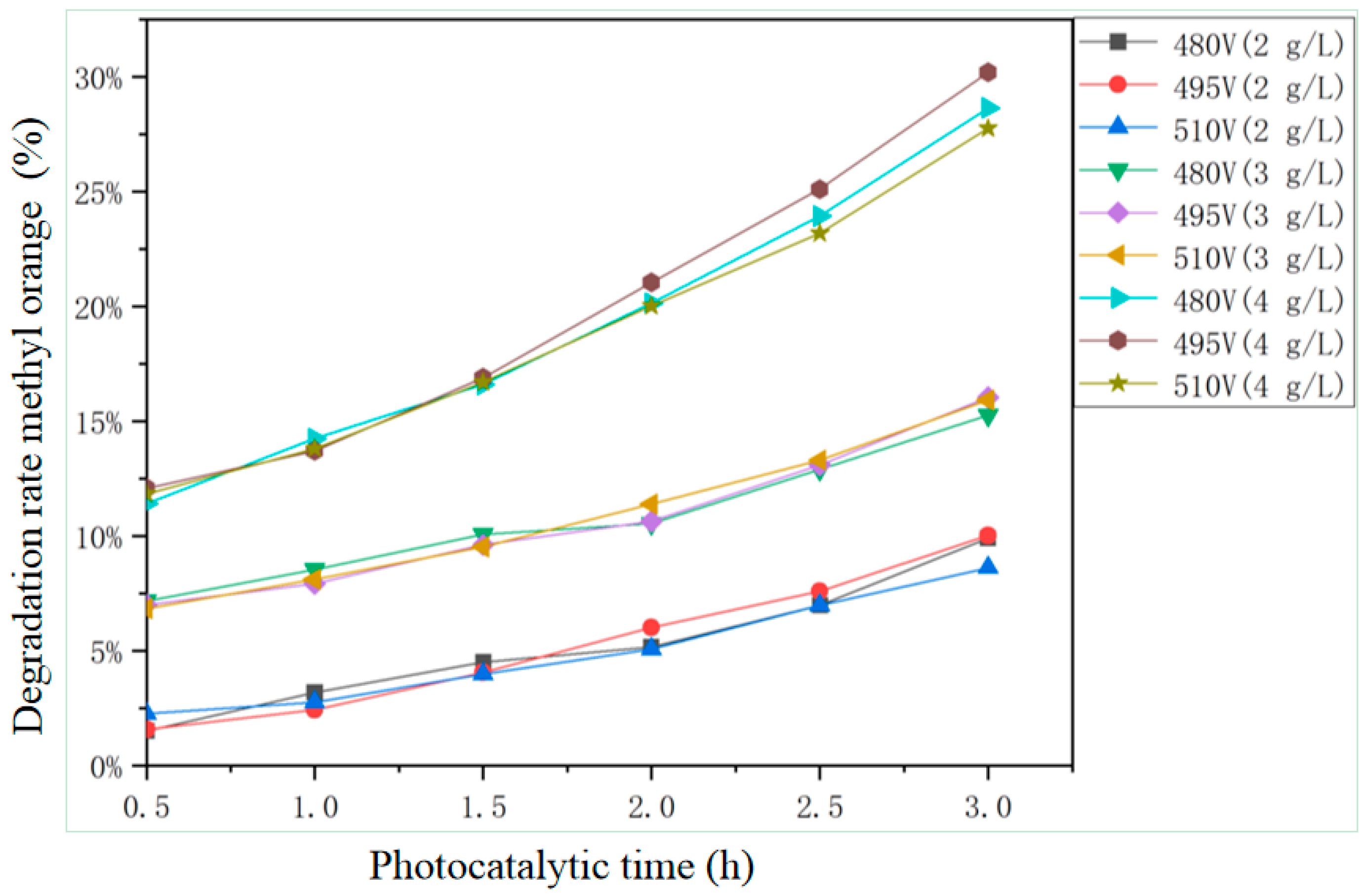
3.3.3. Photocatalytic Performance
3.4. Influence of Doping Silver Ions on Microstructure and Properties
3.4.1. Analysis of Surface Microstructure and Morphology of the Sample
3.4.2. Distribution of Pore Area After Doping with Silver
3.4.3. Influence of Doping Silver Ion Concentration on Photocatalytic Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, S.S. Research Progress in titanium dioxide doped modification and photocatalytic degradation of pollutants. Mod. Chem. Res. 2023, 16, 19–21. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.H.; Lawrence, J.; Tosine, H.M. Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous suspensions. Bull. Environ. Contam. Toxicol. 1976, 16, 697–701. [Google Scholar] [CrossRef]
- Arun, J.; Nachiappan, S.; Rangarajan, G. Synthesis and application of titanium dioxide photocatalysis for energy, decontamination and viral disinfection: A review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Li, P.L. Preparation and Properties of MAO Coating on Titanium Fiber Surface. Master’s Thesis, Chang’an University, Xi’an, China, 2023. [Google Scholar]
- Zhang, B.W. Preparation of Modified TiO2-Based Photocatalyst and Its Photocatalytic Degradation of Organic Pollutants. Master’s Thesis, Guangxi University, Nanning, China, 2023. [Google Scholar]
- Wan, R.S. Photocatalytic Degradation of Formaldehyde and Deactivation-Regeneration of Supported TiO2. Master’s Thesis, Nanchang University, Nanchang, China, 2023. [Google Scholar]
- Li, Y.Z. Preparation and Photocatalytic Performance of Tungsten Doped TiO2 Nanotubes on the Surface of Titanium Mesh. Master’s Thesis, Liaoning Normal University, Dalian, China, 2023. [Google Scholar]
- Hung, R.Y.; Luo, X.Y.; Zhao, D.F. Preparation and photocatalytic properties of Ag doped TiO2. Nonferr. Met. Met. Sci. Eng. 2016, 7, 67–72. [Google Scholar]
- Yu, L.Z.; Li, J.W.; Lin, Y.H. Research progress on the photocatalytic property of modifiednano-TiO2 doped with transition metal ions. Energy Chem. Ind. 2019, 40, 11–17. [Google Scholar]
- Li, B.; Wang, X.M.; Wei, H.M. Effect of B-F gradient doping on photocatalytic properties of TiO2 thin films. J. Qinghai Univ. 2018, 36, 93–98. [Google Scholar]
- Lee, J.H.; Lee, Y.K.; Kim, Y.J. Surface characteristics and photocatalytic propertiy of B doped TiO2 layer synthesized by plasma electrolytic oxidation process. Korean J. Mater. Res. 2021, 31, 552–561. [Google Scholar] [CrossRef]
- Gao, X.; Feng, Q.; Fu, Y. Mechanism of microscopic adsorption of organic molecules with different functional groups on the surface of anatase phase TiO2(101). J. At. Mol. Phys. 2023, 40, 31–38. [Google Scholar]
- Yu, H.; Yao, Z.; Yu, Z.; Tian, F.Z.; Su, Y.X.; Wang, Y.B.; Yu, J. Application of black titanium dioxide and its composite materials in photocatalytic degradation of water pollutants. Fine Chem. 2025, 1, 1–18. [Google Scholar]
- Diana, R.; Geometry, A.; Muhamad, D. Study on triphase of polymorphs TiO2 (Anatase/rutile/brookite) for boosting photocatalytic activity of metformin degradation. Chemosphere 2024, 351, 141206. [Google Scholar]
- Wang, H.; Wang, L.; Lin, D. Titanosilicate zeolite supported Pt nanoparticles with electronic metal-supportinteractions for efficient methanol steam reforming. Catal. Today 2021, 382, 42–47. [Google Scholar] [CrossRef]
- Wu, X.K.; Wang, Z.C.; Zhang, D. Solvent-free microwave synthesis of ultra-small Ru-Mo2C@CNT with strong metal-support interaction for industrial hydrogen evolution. Nat. Commun. 2021, 12, 4018–4027. [Google Scholar] [CrossRef]
- Wang, B.; Sa, G.L.; Xu, A.J. Progress of photocatalytic degradation of organic pollutants over TiO2. Ind. Catal. 2020, 28, 20–24. [Google Scholar]
- Guo, J.R.; Han, F.; Li, C.R.; Deng, H.Z.; Li, J. Study on the photocatalytic degradation of organic dye by Cu-doped TiO2. Appl. Chem. Ind. 2016, 45, 653–656. [Google Scholar]
- Zhou, J.W.; Wei, Z.B.; Sun, M.; Hao, Y.Y.; Huang, J.; Zhang, Y.; Liu, R.X. Enhancement of Photocatalytic Activity via Boron-doped Titanium Dioxide. J. Xinxiang Univ. 2019, 36, 11–16. [Google Scholar]
- Wen, Y.X.; Bao, L.H.; Ling, J.W. Photocatalytic activity of dye-sensitized titanium dioxide/graphene composite. Dye Finish 2021, 47, 7–12. [Google Scholar]
- Zhang, Q.Y.; Wu, L.M.; Tian, Y.Y.; Yu, R.F.; Zhang, Y.T. Preparation and photocatalytic properties of MOF-derived TiO2/heteropolyacids pomposites. Technol. Water Treat. 2022, 48, 71–75. [Google Scholar]
- Shi, H.Y.; Li, G.Z.; Li, J.M.; Zhang, S.L.; Luo, L.J.; Wang, H.B.; Yang, M. Study on photocatalytic degradation of methylene blue by silver doped titanium dioxide. J. Yunnan Minzu Univ. (Nat. Sci. Ed.) 2024, 33, 314–319. [Google Scholar]
- Xu, Y.K.; Luo, Y.Q.; Jiang, J.L. Influence of electrolyte Ca/P ratio on microstructure and properties of micro-arc oxidation titanium alloy coatings. Rare Metal. Mat. Eng. 2023, 52, 675–684. [Google Scholar]
- Li, L.X.; Zhang, R.Z.; Liu, Z.P. Catalytic properties of different crystalline TiO2 for ozoneoxidation. New Chem. Mat. 2024, 52, 184–189. [Google Scholar]
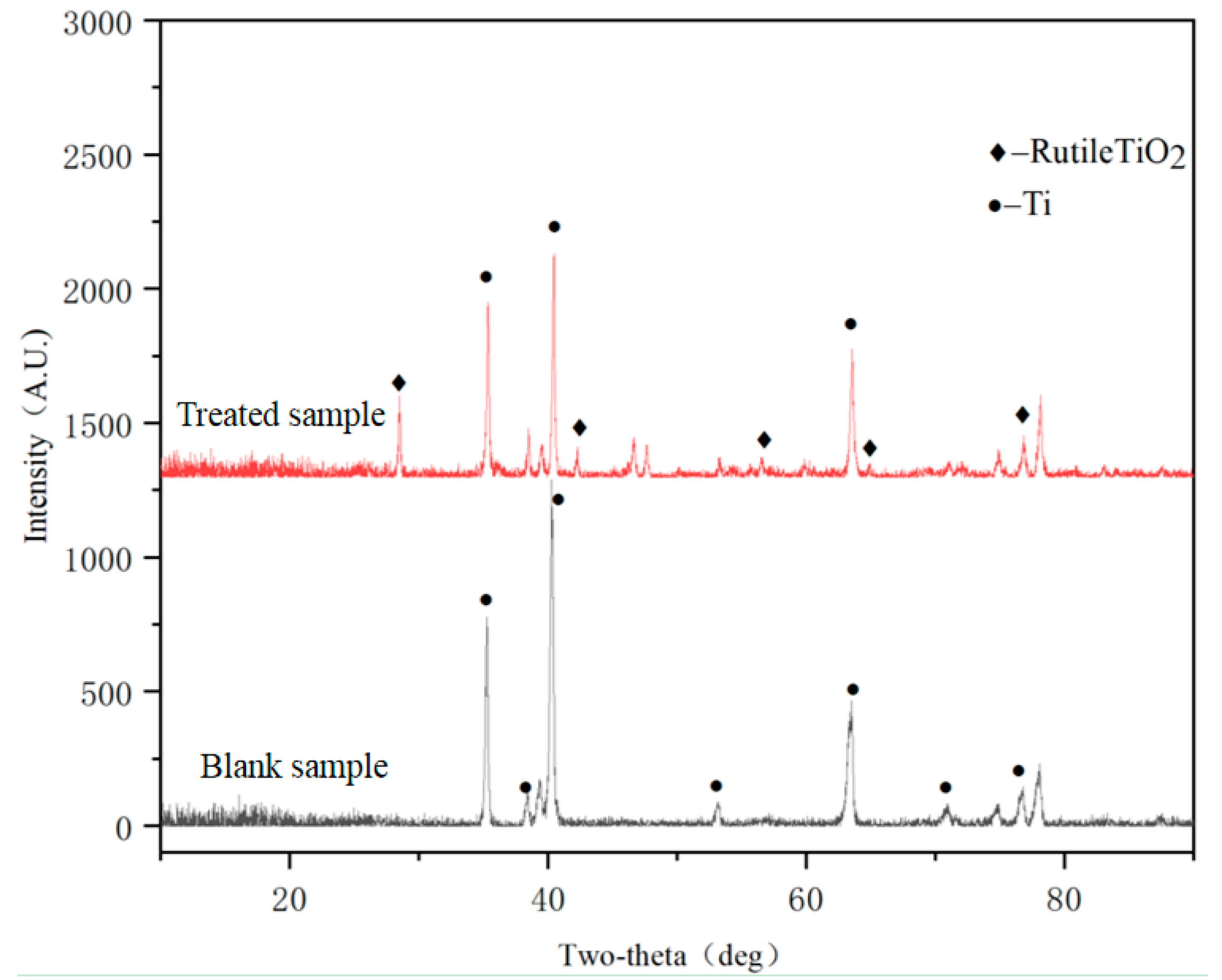
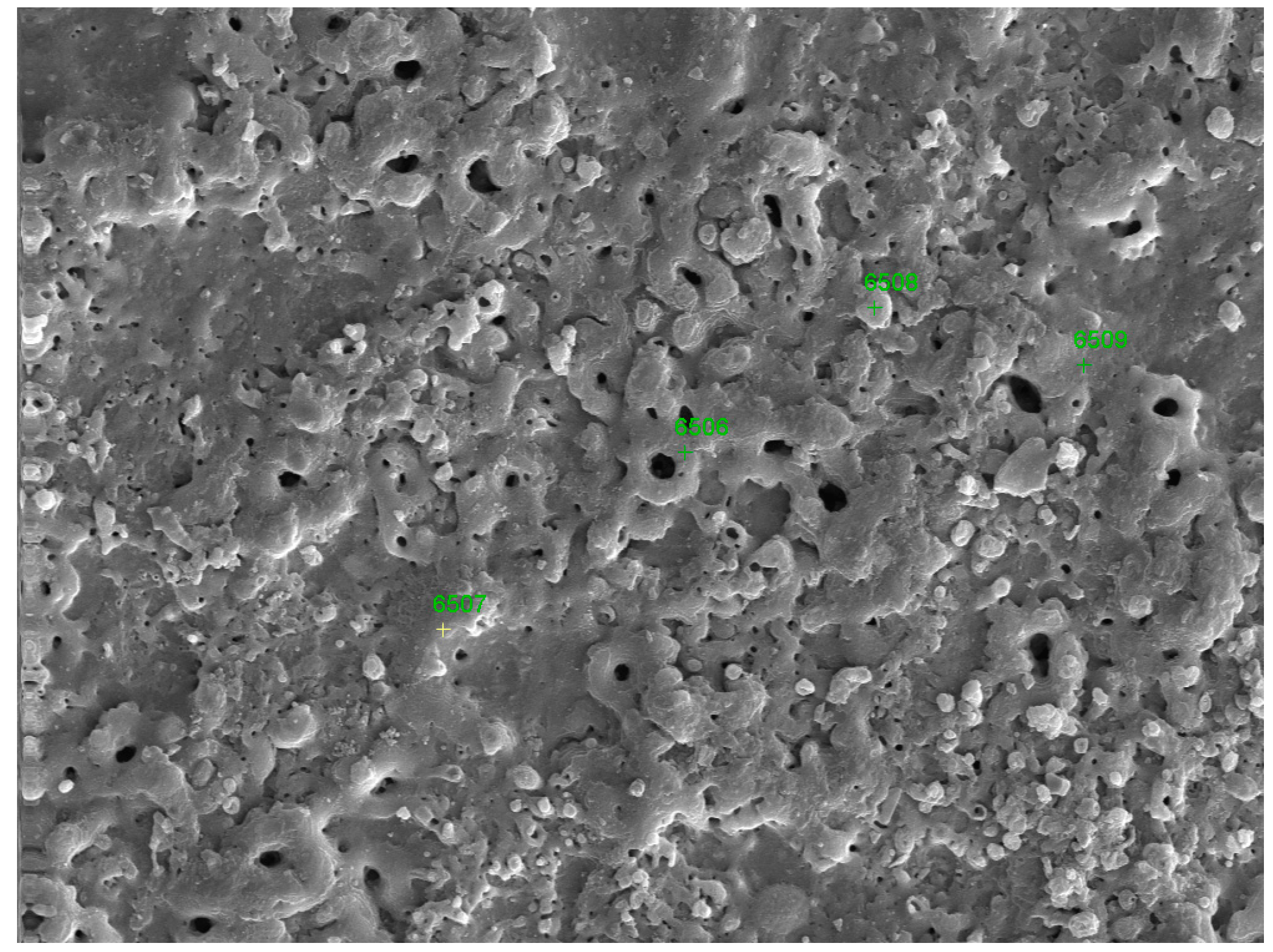
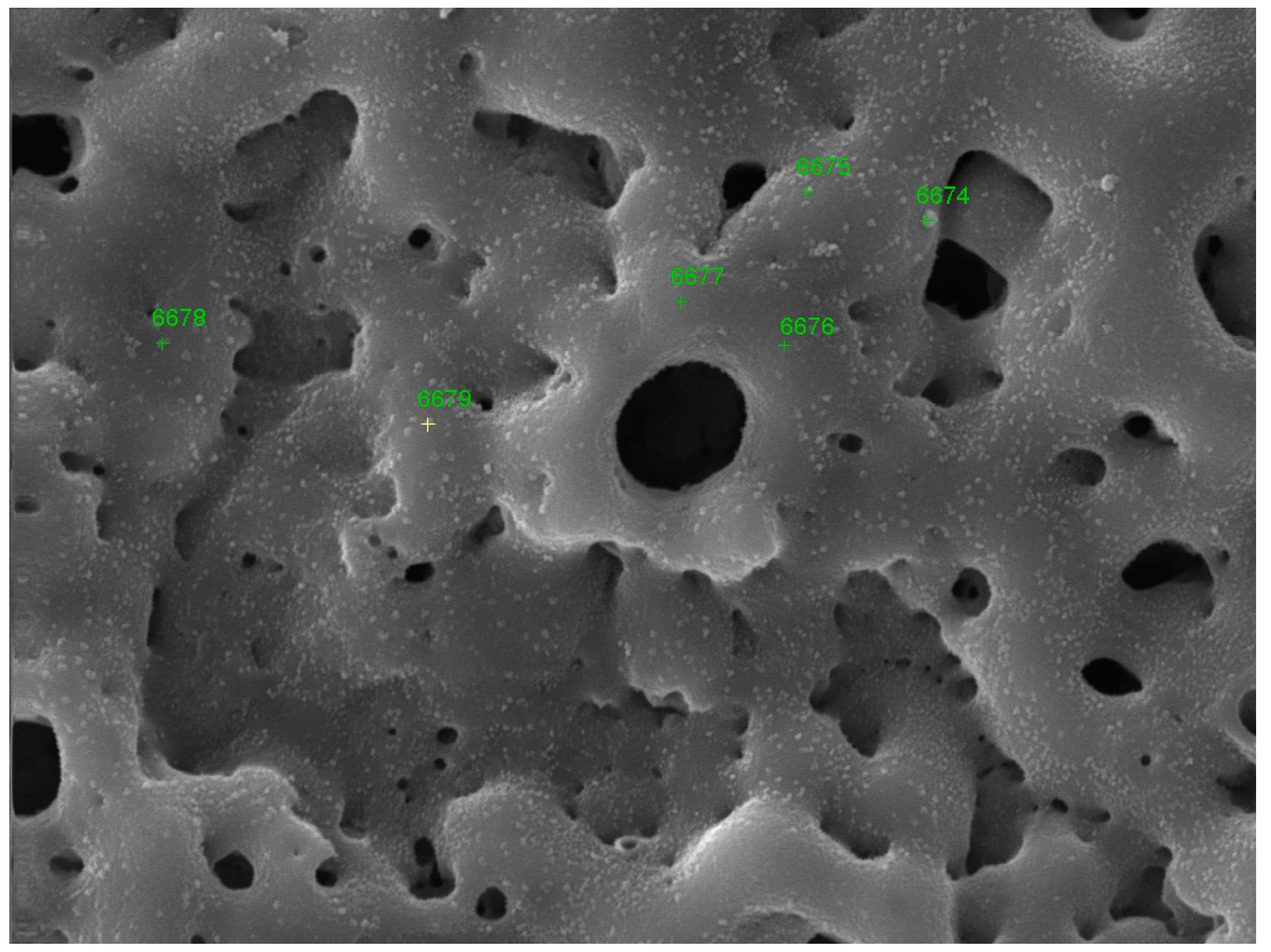
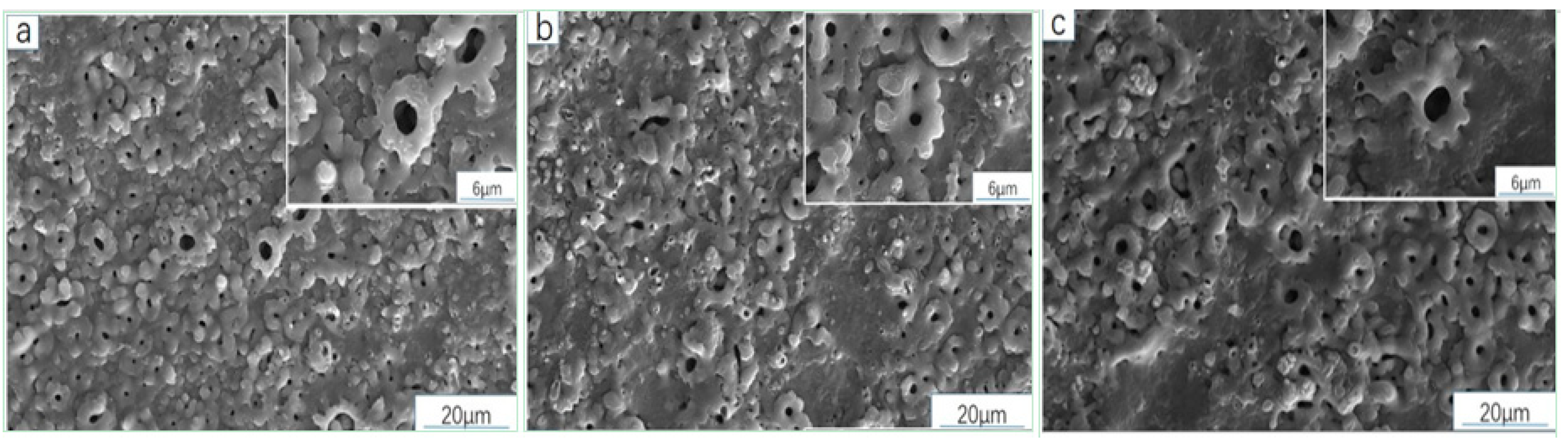



| Point | O | Al | Si | Ti | Na |
|---|---|---|---|---|---|
| 6506 | 36.81 | 8.00 | 12.09 | 42.22 | 0.88 |
| 6508 | 49.54 | 6.18 | 13.88 | 26.84 | 4.06 |
| 6507 | 33.67 | 10.22 | 8.99 | 47.12 | 0 |
| 6509 | 43.55 | 9.54 | 8.44 | 38.47 | 0 |
| Point | O | Al | Si | Ti | P | Ag |
|---|---|---|---|---|---|---|
| 6674 | 35.29 | 5.68 | 8.45 | 31.05 | 0 | 19.52 |
| 6675 | 40.66 | 7.03 | 11.52 | 40.79 | 0 | 0 |
| 6676 | 47.74 | 5.05 | 8.13 | 34.65 | 4.44 | 0 |
| 6677 | 45.15 | 4.68 | 7.88 | 37.24 | 5.05 | 0 |
| Voltage | Total Number of Holes (N) | Mean Value |
|---|---|---|
| 480 V | 223 | 1.316 |
| 495 V | 186 | 0.884 |
| 510 V | 224 | 0.939 |
| Voltage | Total Number of Holes (N) | Mean Value |
|---|---|---|
| 480 V | 350 | 1.380 |
| 495 V | 320 | 1.421 |
| 510 V | 270 | 1.793 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Xia, Y.; Fan, W.; Li, Y. Preparation and Photocatalytic Performance of Silver-Loaded Micro-Arc Oxidation TiO2 Coating. Coatings 2025, 15, 272. https://doi.org/10.3390/coatings15030272
Fan X, Xia Y, Fan W, Li Y. Preparation and Photocatalytic Performance of Silver-Loaded Micro-Arc Oxidation TiO2 Coating. Coatings. 2025; 15(3):272. https://doi.org/10.3390/coatings15030272
Chicago/Turabian StyleFan, Xingping, Ying Xia, Wei Fan, and Yulong Li. 2025. "Preparation and Photocatalytic Performance of Silver-Loaded Micro-Arc Oxidation TiO2 Coating" Coatings 15, no. 3: 272. https://doi.org/10.3390/coatings15030272
APA StyleFan, X., Xia, Y., Fan, W., & Li, Y. (2025). Preparation and Photocatalytic Performance of Silver-Loaded Micro-Arc Oxidation TiO2 Coating. Coatings, 15(3), 272. https://doi.org/10.3390/coatings15030272






