Fabrication of CF–NiO Electrodes and Performance Evaluation of Microbial Fuel Cells in the Treatment of Potato Starch Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of the CF–NiO Electrode
2.3. Electrochemical Characterization of the Electrode
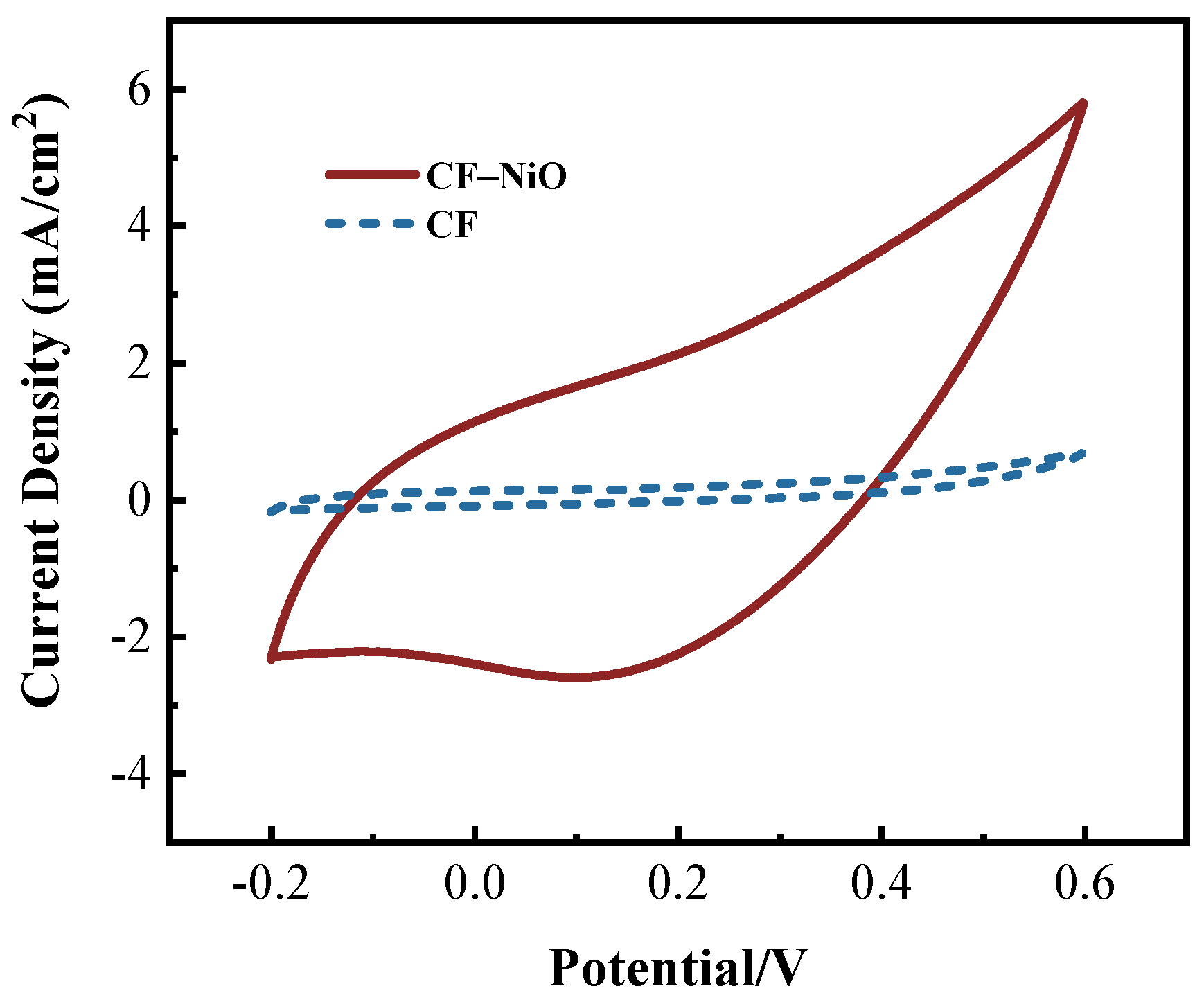
2.3.1. Cyclic Voltammetry (CV) Test
2.3.2. Electrochemical Impedance Spectroscopy (EIS) Test
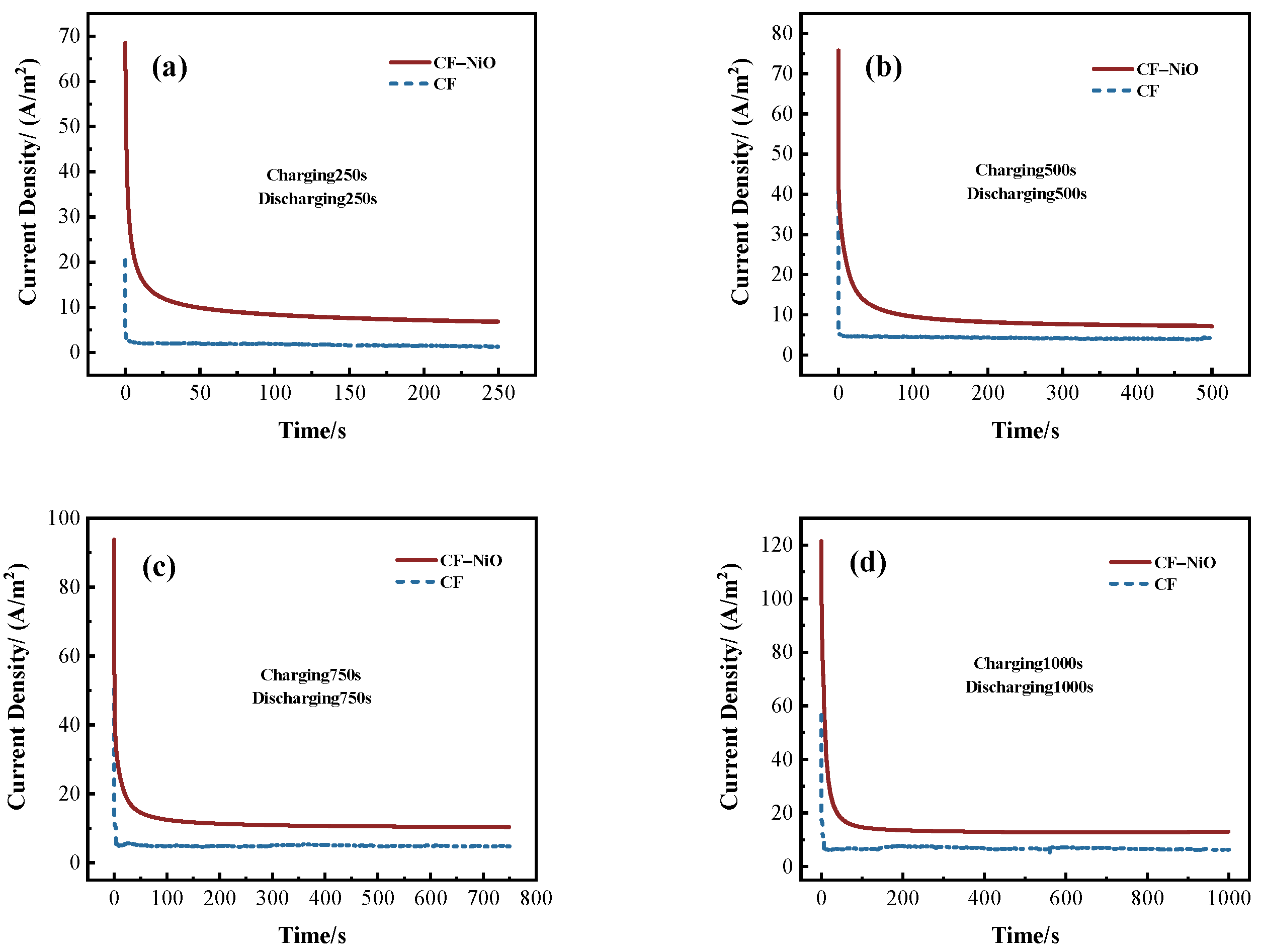
2.3.3. Chronopotentiometry (CP) Test
- Cs is the areal specific capacitance (F/cm2);
- I is the constant current (A);
- ∫Vdt is the integrated area under the voltage–time curve (V·s);
- A is the surface area of the electrode (cm2);
- Vf is the final potential (V);
- Vi is the initial potential (V).
2.4. Characterization of CF–NiO Electrode
2.5. Construction and Performance Evaluation of the MFC
2.5.1. Start-Up of the Microbial Fuel Cell
2.5.2. MFC Performance Testing
- J is the current density (A/m2);
- U is the battery potential (mV);
- R is the external resistance value (Ω);
- A is the electrode surface area (cm2).
- P is the power density (W/m2)
- U is the battery potential (mV);
- R is the external resistance value (Ω);
- A is the electrode surface area (cm2).
2.6. High-Throughput Sequencing of the MFC Microbial Community
3. Results and Discussion
3.1. Structural Characterization of CF–NiO Electrodes
3.1.1. SEM: Scanning Electron Microscopy Analysis
3.1.2. XRD: X-Ray Diffraction Analysis
3.1.3. EDS: Energy-Dispersive X-Ray Spectroscopy Analysis
3.2. Performance Evaluation of the CF–NiO Anode in Microbial Fuel Cells (MFCs)
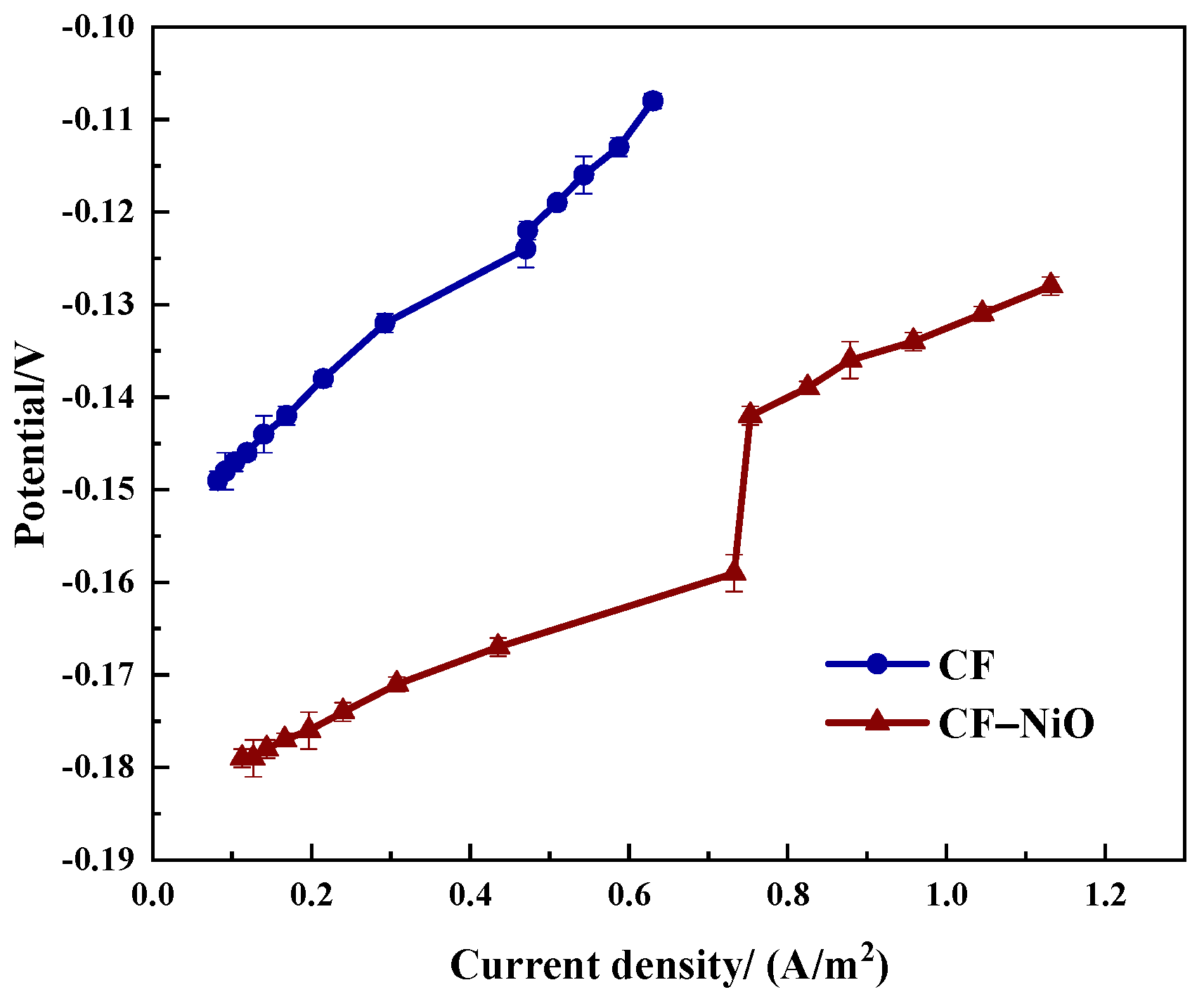
3.2.1. Polarization Curve
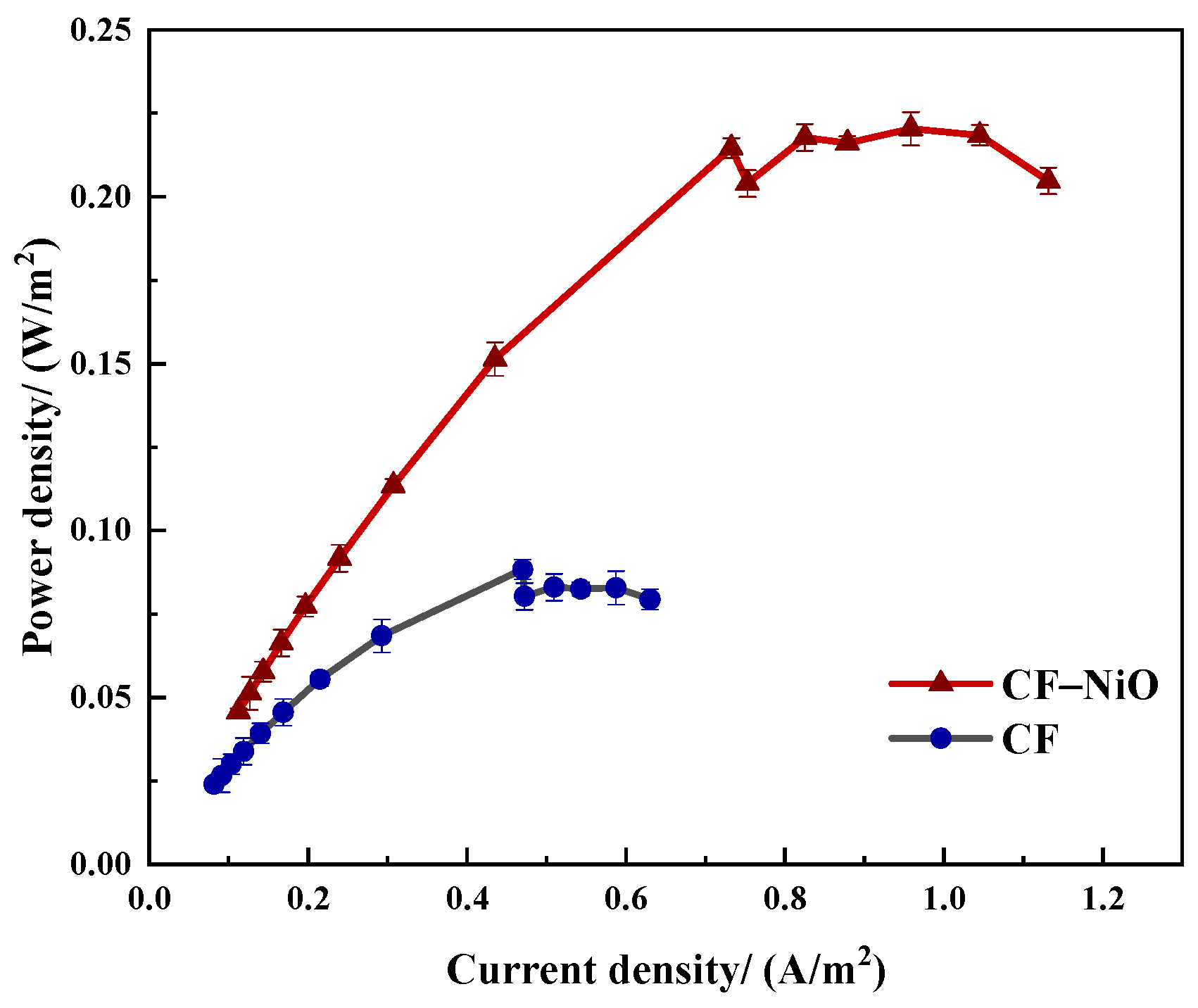
3.2.2. Power Density Curve
3.2.3. Anode Polarization Curve
3.2.4. Cyclic Voltammetry Test of the MFC
3.2.5. Electrochemical Impedance Spectroscopy (EIS) Test of the MFC
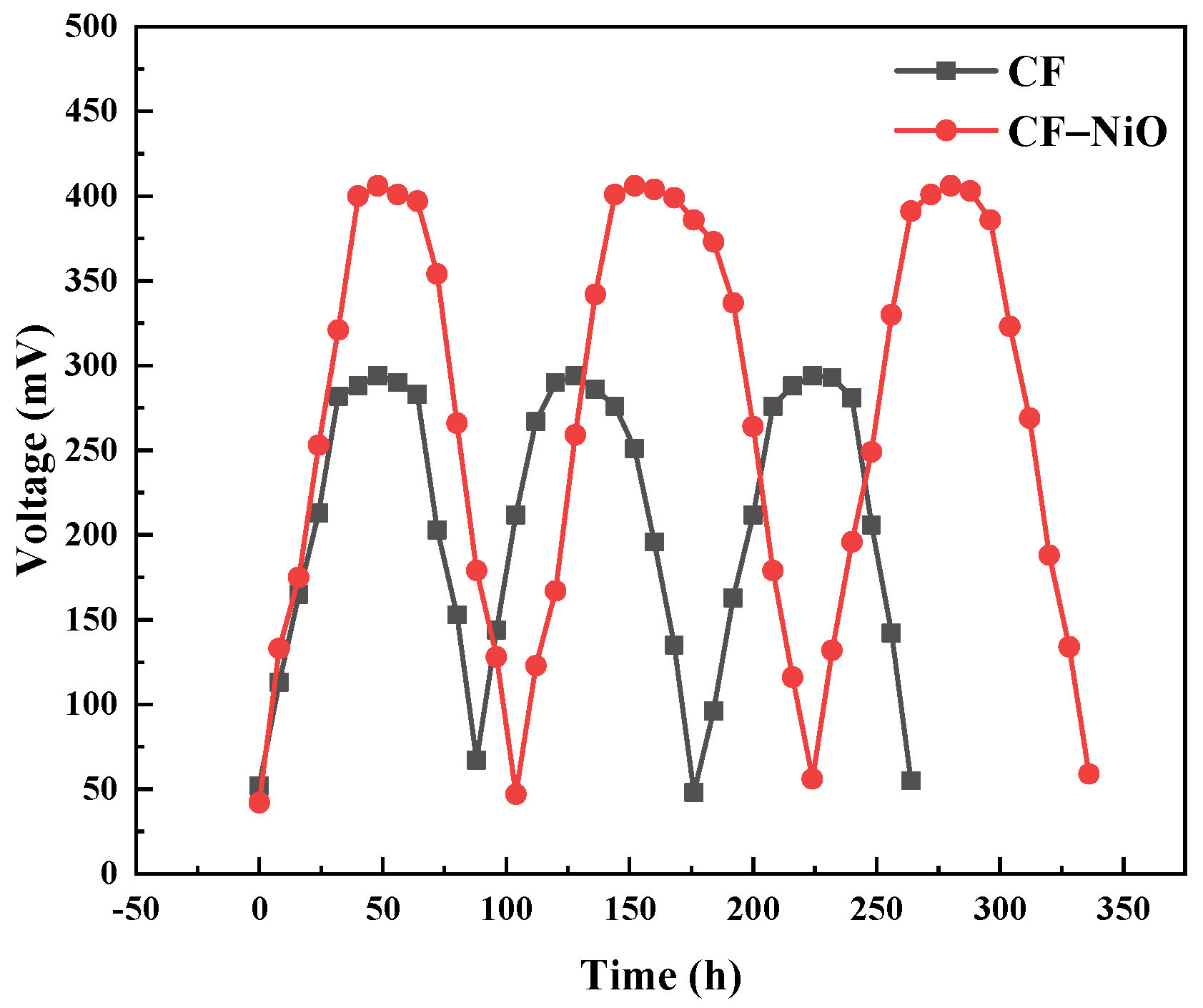
3.2.6. Energy Storage Test of the MFC
3.2.7. Cyclic Test
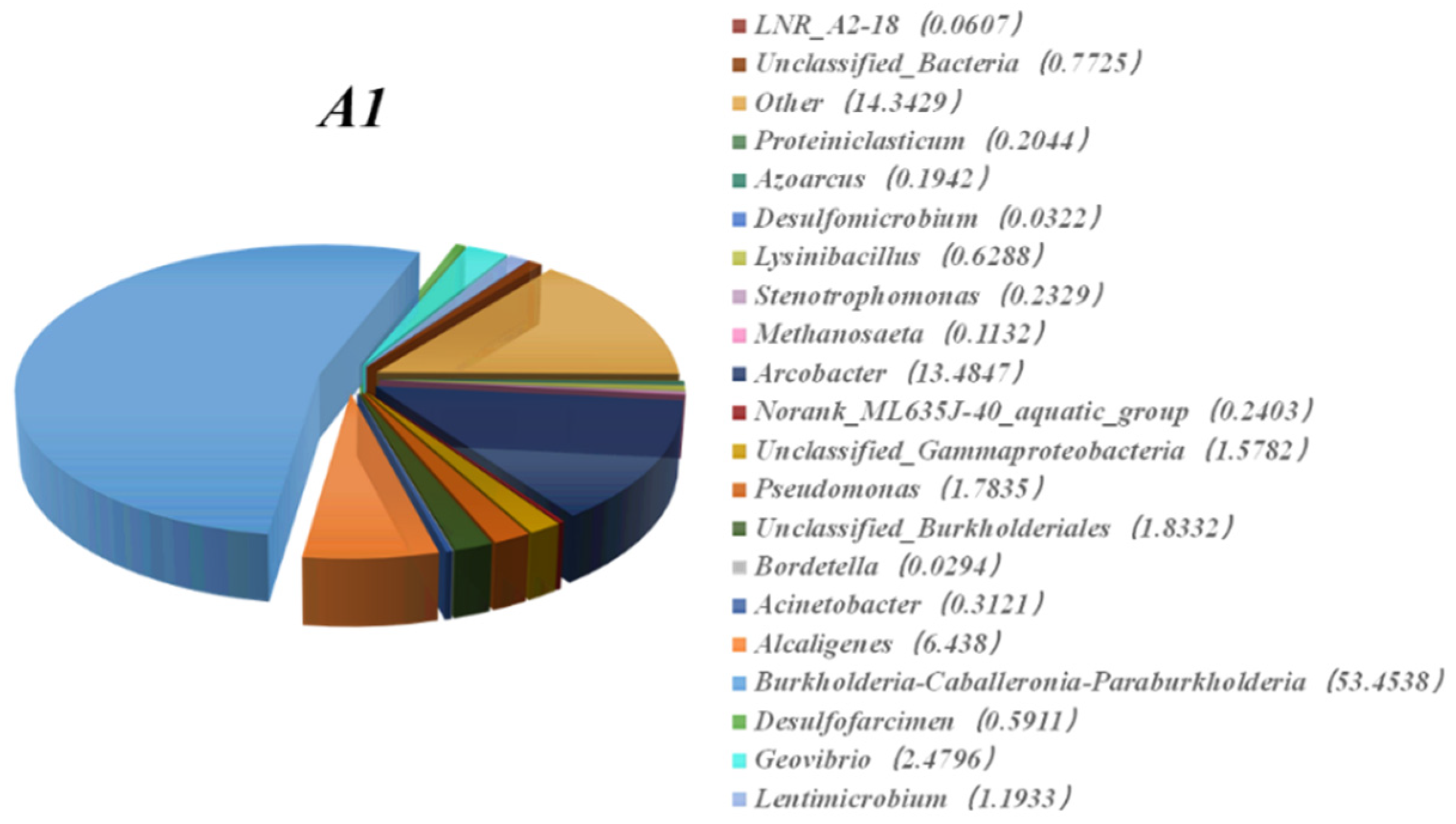
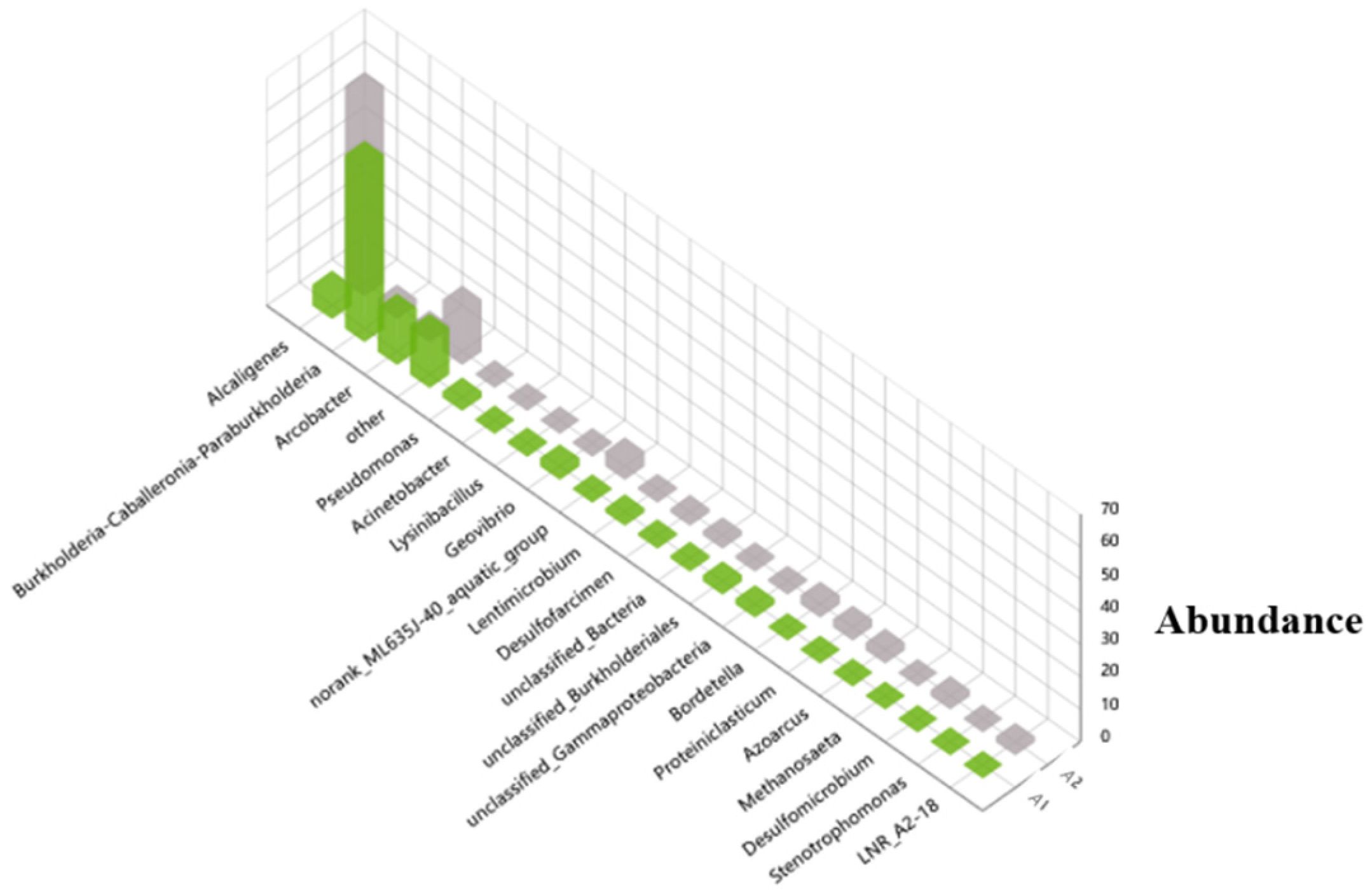
3.3. High-Throughput Sequencing of the MFC with the CF–NiO Anode



4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wijesinha-Bettoni, R.; Mouillé, B. The Contribution of Potatoes to Global Food Security, Nutrition and Healthy Diets. Am. J. Potato Res. 2019, 96, 139–149. [Google Scholar] [CrossRef]
- Ovchinnikova, A.; Krylova, E.; Gavrilenko, T.; Smekalova, T.; Zhuk, M.; Knapp, S.; Spooner, D.M. Taxonomy of cultivated potatoes (Solanum section Petota: Solanaceae). Bot. J. Linn. Soc. 2011, 165, 107–155. [Google Scholar] [CrossRef]
- De Jong, H. Impact of the potato on society. Am. J. Potato Res. 2016, 93, 415–429. [Google Scholar] [CrossRef]
- Beals, K.A. Potatoes, nutrition and health. Am. J. Potato Res. 2019, 96, 102–110. [Google Scholar] [CrossRef]
- Raigond, P.; Jayanty, S.S.; Parmar, V.; Dutt, S.; Changan, S.S.; Luthra, S.K.; Singh, B. Health-Promoting compounds in Potatoes: Tuber exhibiting great potential for human health. Food Chem. 2023, 424, 136368. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, R.; Li, S.; Guo, X.; Li, Q.; Hui, X.; Wang, Z.; Wang, H. An Evaluation of Potato Fertilization and the Potential of Farmers to Reduce the Amount of Fertilizer Used Based on Yield and Nutrient Requirements. Agronomy 2024, 14, 612. [Google Scholar] [CrossRef]
- Han, J.-S.; Kozukue, N.; Young, K.-S.; Lee, K.-R.; Friedman, M. Distribution of Ascorbic Acid in Potato Tubers and in Home-Processed and Commercial Potato Foods. J. Agric. Food Chem. 2004, 52, 6516–6521. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Kaur, L.; Singh, J.; Zeng, F. Functional Food Based on Potato. Foods 2023, 12, 2145. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, T.; Liu, Q.; Liu, W.; Zhao, R.; Hu, H. Effects of additives (NaCl, citric acid, and ethanol) on the cooking quality and sensory quality of vermicelli produced from freeze–thaw-dehydrated whole potato powder. Int. J. Food Sci. Technol. 2024, 59, 9459–9468. [Google Scholar] [CrossRef]
- Liu, T.; Men, Z.; Lai, C.; Lian, X. Preparation and Mechanism Analysis of Boiling Resistance of the Fresh Alum-Free Sweet Potato Vermicelli Containing Gliadin Fractions. Foods 2025, 14, 81. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Liu, H.; Zhao, L.; Wang, Y.; Wu, X.; Liao, X. Improving the production efficiency of sweet potato starch using a newly designed sedimentation tank during starch sedimentation process. J. Food Process. Preserv. 2020, 44, e14811. [Google Scholar] [CrossRef]
- Li, H.; Zeng, X.; Shi, W.; Zhang, H.; Huang, S.; Zhou, R.; Qin, X. Recovery and purification of potato proteins from potato starch wastewater by hollow fiber separation membrane integrated process. Innov. Food Sci. Emerg. Technol. 2020, 63, 102380. [Google Scholar] [CrossRef]
- Durruty, I.; Bonanni, P.S.; González, J.F.; Busalmen, J.P. Evaluation of potato-processing wastewater treatment in a microbial fuel cell. Bioresour. Technol. 2012, 105, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Cancino-Madariaga, B.; Aguirre, J. Combination treatment of corn starch wastewater by sedimentation, microfiltration and reverse osmosis. Desalination 2011, 279, 285–290. [Google Scholar] [CrossRef]
- Li, M.; Zhou, M.; Tian, X.; Tan, C.; McDaniel, C.T.; Hassett, D.J.; Gu, T. Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity. Biotechnol. Adv. 2018, 36, 1316–1327. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Oliveira, V.; Simões, M.; Melo, L.; Pinto, A. Overview on the developments of microbial fuel cells. Biochem. Eng. J. 2013, 73, 53–64. [Google Scholar] [CrossRef]
- Apollon, W. An overview of microbial fuel cell technology for sustainable electricity production. Membranes 2023, 13, 884. [Google Scholar] [CrossRef]
- Aiyer, K.S. How does electron transfer occur in microbial fuel cells? World J. Microbiol. Biotechnol. 2020, 36, 19. [Google Scholar] [CrossRef]
- Ieropoulos, I.A.; Greenman, J.; Melhuish, C.; Hart, J. Comparative study of three types of microbial fuel cell. Enzym. Microb. Technol. 2005, 37, 238–245. [Google Scholar] [CrossRef]
- Oh, S.E.; Logan, B.E. Hydrogen and electricity production from a food processing wastewater using fermentation and microbial fuel cell technologies. Water Res. 2005, 39, 4673–4682. [Google Scholar] [CrossRef]
- Cecconet, D.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Agro-food industry wastewater treatment with microbial fuel cells: Energetic recovery issues. Int. J. Hydrogen Energy 2018, 43, 500–511. [Google Scholar] [CrossRef]
- Moqsud, M.A.; Omine, K.; Yasufuku, N.; Hyodo, M.; Nakata, Y. Microbial fuel cell (MFC) for bioelectricity generation from organic wastes. Waste Manag. 2013, 33, 2465–2469. [Google Scholar] [CrossRef]
- Zhou, M.; Chi, M.; Luo, J.; He, H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Logan, B.E.; Wallack, M.J.; Kim, K.-Y.; He, W.; Feng, Y.; Saikaly, P.E. Assessment of Microbial Fuel Cell Configurations and Power Densities. Environ. Sci. Technol. Lett. 2015, 2, 206–214. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G. Cellulose fibres, nanofibrils and microfibrils: The morphological sequence of MFC components from a plant physiology and fibre technology point of view. Nanoscale Res. Lett. 2011, 6, 1–7. [Google Scholar] [CrossRef]
- Mehdinia, A.; Ziaei, E.; Jabbari, A. Facile microwave-assisted synthesized reduced graphene oxide/tin oxide nanocomposite and using as anode material of microbial fuel cell to improve power generation. Int. J. Hydrogen Energy 2014, 39, 10724–10730. [Google Scholar] [CrossRef]
- Lu, A.; Li, Y.; Jin, S.; Wang, X.; Wu, X.-L.; Zeng, C.; Ding, H.; Hao, R.; Lv, M.; Wang, C.; et al. Growth of non-phototrophic microorganisms using solar energy through mineral photocatalysis. Nat. Commun. 2012, 3, 768. [Google Scholar] [CrossRef]
- Alatraktchi, F.A.; Zhang, Y.; Angelidaki, I. Nanomodification of the electrodes in microbial fuel cell: Impact of nanoparticle density on electricity production and microbial community. Appl. Energy 2014, 116, 216–222. [Google Scholar] [CrossRef]
- Dey, N.; Samuel, G.V.; Raj, D.S.; Gajalakshmi, B. Nanomaterials as potential high performing electrode materials for microbial fuel cells. Appl. Nanosci. 2022, 13, 2625–2640. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S.; Hou, L.; Zuo, J.; Kong, X.; Song, Y.; Wang, Z.; Tian, Y.; Dong, J. Enhanced electricity generation and energy storage in a microbial fuel cell with a bimetallic-modified capacitive anode. Desalination 2024, 593, 118247. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, S.; Liu, H.; Liu, S.; Zhang, J.; Yuan, J.; Fu, W.; Dang, W.; Xu, Y.; Yang, X.; et al. Heteroatom-doped porous carbon nanoparticle-decorated carbon cloth (HPCN/CC) as efficient anode electrode for microbial fuel cells (MFCs). J. Clean. Prod. 2022, 336, 130374. [Google Scholar] [CrossRef]
- Santos, J.S.; Tarek, M.; Sikora, M.S.; Praserthdam, S.; Praserthdam, P. Anodized TiO2 nanotubes arrays as microbial fuel cell (MFC) electrodes for wastewater treatment: An overview. J. Power Sources 2023, 564, 232872. [Google Scholar] [CrossRef]
- Santoro, C.; Li, B.; Cristiani, P.; Squadrito, G. Power generation of microbial fuel cells (MFCs) with low cathodic platinum loading. Int. J. Hydrogen Energy 2013, 38, 692–700. [Google Scholar] [CrossRef]
- Gude, V.G. Wastewater treatment in microbial fuel cells—An overview. J. Clean. Prod. 2016, 122, 287–307. [Google Scholar] [CrossRef]
- He, Z.; Minteer, S.D.; Angenent, L.T. Electricity Generation from Artificial Wastewater Using an Upflow Microbial Fuel Cell. Environ. Sci. Technol. 2005, 39, 5262–5267. [Google Scholar] [CrossRef]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of Electricity during Wastewater Treatment Using a Single Chamber Microbial Fuel Cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef]
- Arun, J.; SundarRajan, P.; Pavithra, K.G.; Priyadharsini, P.; Shyam, S.; Goutham, R.; Le, Q.H.; Pugazhendhi, A. New insights into microbial electrolysis cells (MEC) and microbial fuel cells (MFC) for simultaneous wastewater treatment and green fuel (hydrogen) generation. Fuel 2024, 355, 129530. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Verma, P. Microbial fuel cell: A green approach for the utilization of waste for the generation of bioelectricity. Bioresour. Bioprocess. 2016, 3, 1–14. [Google Scholar] [CrossRef]
- Hamedani, E.A.; Abasalt, A.; Talebi, S. Application of microbial fuel cells in wastewater treatment and green energy production: A comprehensive review of technology fundamentals and challenges. Fuel 2024, 370, 131855. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, V.; Mustafa, S.; Aftab, U.; Laghari, Z.A.; Jatoi, A.S.; Mashooque, S.; Ahmed, M. Graphene-loaded nickel oxide nanocomposite as anode material for microbial fuel cell. Biomass Convers. Biorefinery 2022, 13, 13245–13252. [Google Scholar] [CrossRef]
- Zhong, D.; Liu, Y.; Liao, X.; Zhong, N.; Xu, Y. Facile preparation of binder-free NiO/MnO2-carbon felt anode to enhance electricity generation and dye wastewater degradation performances of microbial fuel cell. Int. J. Hydrogen Energy 2018, 43, 23014–23026. [Google Scholar] [CrossRef]
- Li, X.; Hu, B.; Suib, S.; Lei, Y.; Li, B. Electricity generation in continuous flow microbial fuel cells (MFCs) with manganese dioxide (MnO2) cathodes. Biochem. Eng. J. 2011, 54, 10–15. [Google Scholar] [CrossRef]
- Wu, X.; Sun, D.; Li, M.; Zhang, X.; Al-Dhabi, N.A.; Fang, Q.; Tang, W.; Tang, Q.; Kou, J.; Wang, Z.; et al. Nano-Fe3O4/polymerize aniline/carbon cathode based microbial fuel cell for efficient power generation and uranium separation. Chem. Eng. J. 2024, 487, 150634. [Google Scholar] [CrossRef]
- Dehghanian, M.; Mahdavi, M.A.; Gheshlaghi, R.; Darband, G.B.; Absalan, Y. Synthesis of TiC@C–TiO2 as an emerging efficient nanostructure for anode modification in high-power microbial fuel cells. Int. J. Hydrogen Energy 2025, 116, 591–600. [Google Scholar] [CrossRef]
- Zeng, F.; Wu, Y.; Bo, L.; Zhang, L.; Liu, W.; Zhu, Y. Coupling of electricity generation and denitrification in three-phase single-chamber MFCs in high-salt conditions. Bioelectrochemistry 2020, 133, 107481. [Google Scholar] [CrossRef]






| Materials | Power Density W/m2 | References |
|---|---|---|
| CF-MnO2 | 0.161 | [43] |
| nFe3O4/PA/CB-MFC | 0.01517 | [44] |
| TiO2@CC | 0.188 | [45] |
| CF–NiO | 0.22 | This work |
| Electrode | CF | CF–NiO |
|---|---|---|
| RΩ (Ω) | 14.83 ± 0.03 | 11.55 ± 0.05 |
| Rct (Ω) | 24.8 ± 0.02 | 11.72 ± 0.02 |
| Electrode | Current and Charge | C250/D250 | C500/D500 | C750/D750 | C1000/D1000 |
|---|---|---|---|---|---|
| CF | ih (A/m2) | 20.43 | 45.45 | 50.37 | 56.55 |
| is (A/m2) | 1.27 | 4.06 | 4.75 | 6.29 | |
| Qs (C/m2) | 61.26 | 78.57 | 93.67 | 452.9 | |
| Q (C/m2) | 839.51 | 2093.57 | 3655.97 | 6742.67 | |
| CF/NiO | ih (A/m2) | 68.43 | 75.75 | 93.83 | 121.45 |
| is (A/m2) | 6.71 | 7.13 | 10.26 | 12.86 | |
| Qs (C/m2) | 742.82 | 986.53 | 989.69 | 1290.03 | |
| Q (C/m2) | 2285.29 | 4549.47 | 8674.49 | 14,150.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Xue, S.; Jiang, L.; Lei, J.; Li, W.; Han, Y.; Wang, Z.; Zuo, J.; Wang, Y. Fabrication of CF–NiO Electrodes and Performance Evaluation of Microbial Fuel Cells in the Treatment of Potato Starch Wastewater. Coatings 2025, 15, 760. https://doi.org/10.3390/coatings15070760
Yang T, Xue S, Jiang L, Lei J, Li W, Han Y, Wang Z, Zuo J, Wang Y. Fabrication of CF–NiO Electrodes and Performance Evaluation of Microbial Fuel Cells in the Treatment of Potato Starch Wastewater. Coatings. 2025; 15(7):760. https://doi.org/10.3390/coatings15070760
Chicago/Turabian StyleYang, Tianyi, Song Xue, Liming Jiang, Jiuming Lei, Wenjing Li, Yiwei Han, Zhijie Wang, Jinlong Zuo, and Yuyang Wang. 2025. "Fabrication of CF–NiO Electrodes and Performance Evaluation of Microbial Fuel Cells in the Treatment of Potato Starch Wastewater" Coatings 15, no. 7: 760. https://doi.org/10.3390/coatings15070760
APA StyleYang, T., Xue, S., Jiang, L., Lei, J., Li, W., Han, Y., Wang, Z., Zuo, J., & Wang, Y. (2025). Fabrication of CF–NiO Electrodes and Performance Evaluation of Microbial Fuel Cells in the Treatment of Potato Starch Wastewater. Coatings, 15(7), 760. https://doi.org/10.3390/coatings15070760







