A Theoretical Study on Electrocatalytic Nitrogen Reduction at Boron-Doped Monolayer/Bilayer Black Phosphorene Edges
Abstract
1. Introduction
2. Methods and Computational Details
3. Results and Discussion
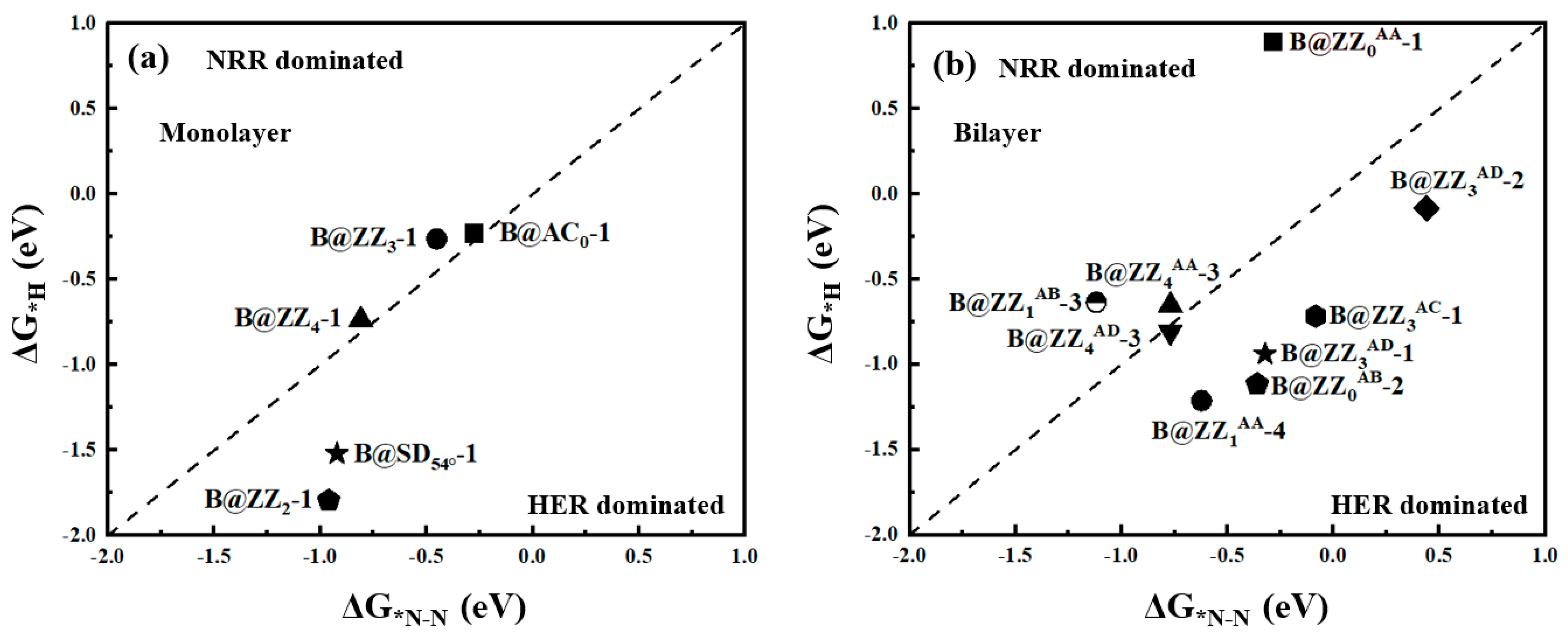
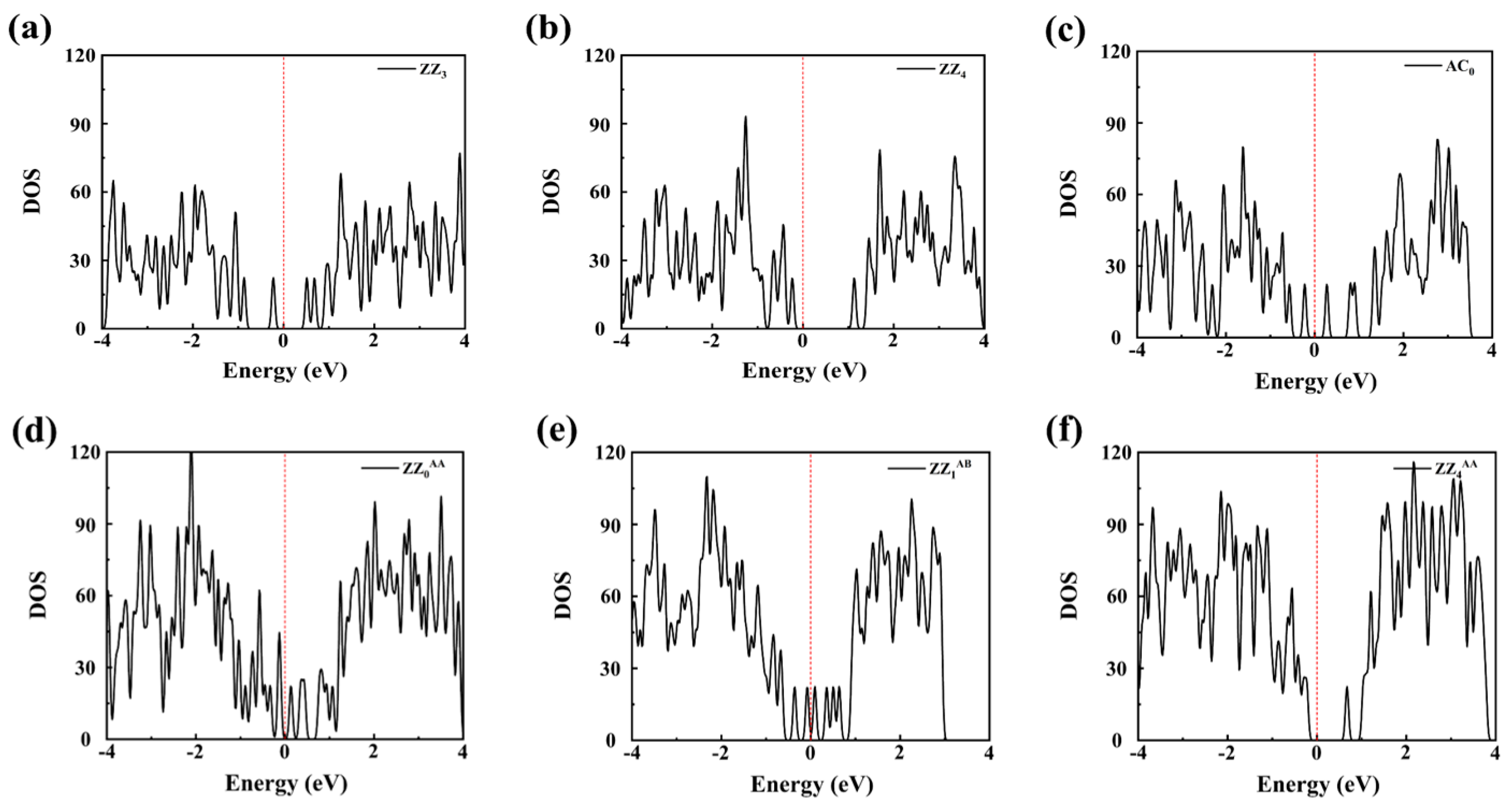
3.1. Boron-Doped BPNR Edge Site Selection and Their Investigation as NRR Catalysts
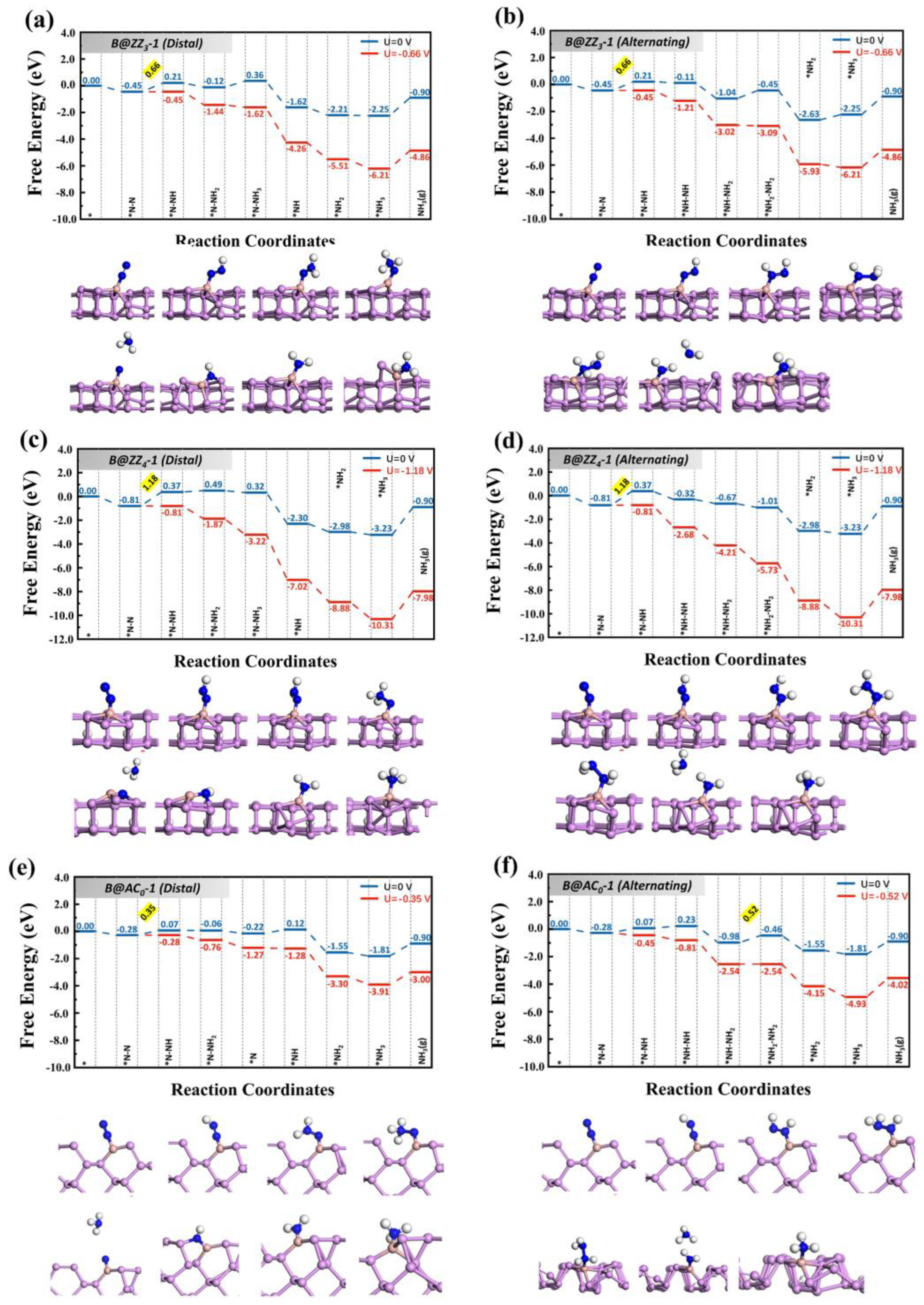
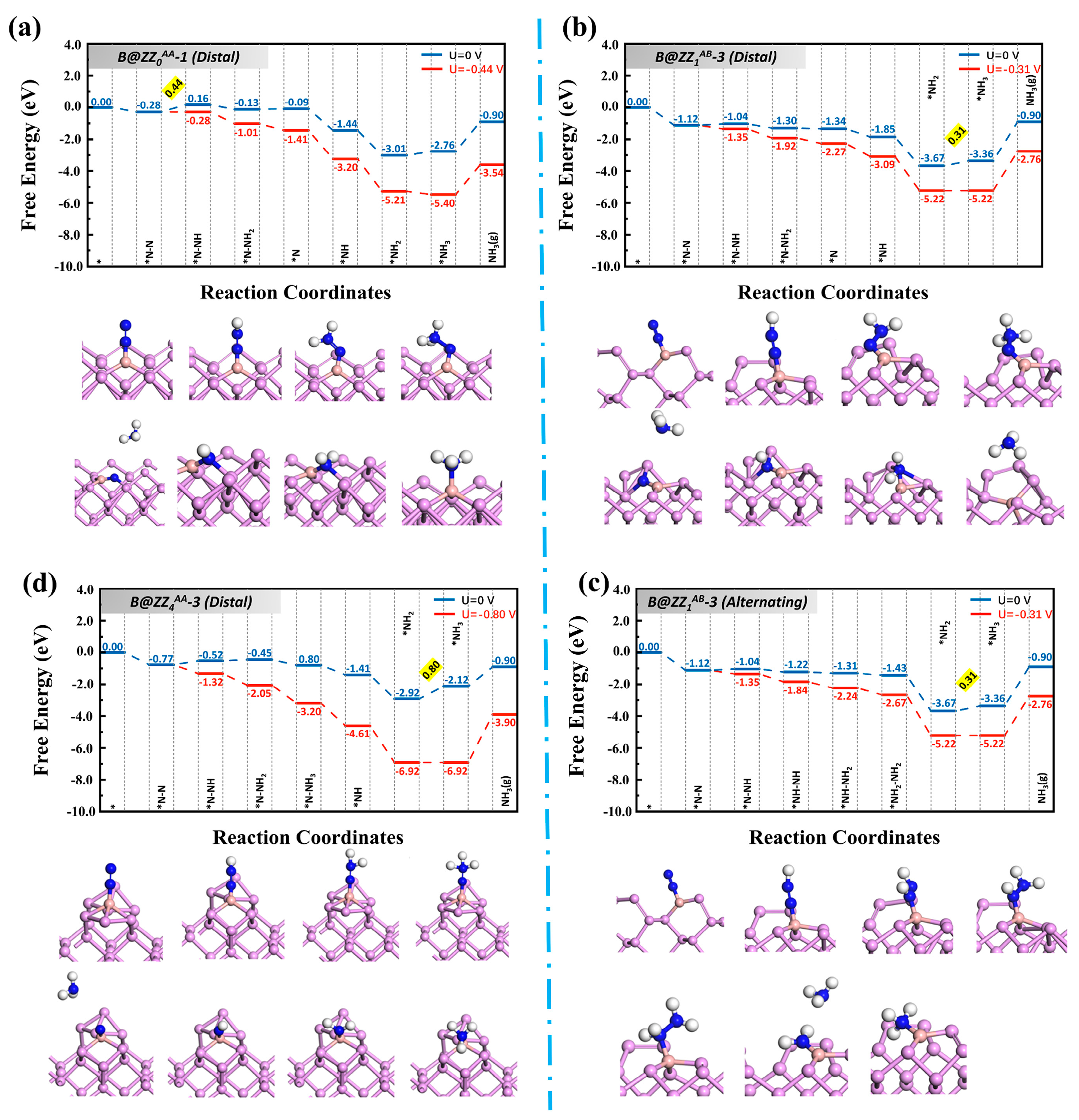
3.2. Reaction Pathways of N2 on Boron-Doped Black Phosphorene Edges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chai, W.; Bao, Y.; Jin, P.; Tang, G.; Zhou, L. A review on ammonia, ammonia-hydrogen and ammonia-methane fuels. Renew. Sustain. Energy Rev. 2021, 147, 111254. [Google Scholar] [CrossRef]
- Tian, F.; Zhou, N.; Chen, W.; Zhan, J.; Tang, L.; Wu, M. Progress in green ammonia synthesis technology: Catalytic behavior of ammonia synthesis catalysts. Adv. Sustain. Syst. 2024, 8, 2300618. [Google Scholar] [CrossRef]
- Baltrusaitis, J. Sustainable ammonia production. ACS Sustain. Chem. Eng. 2017, 5, 9527. [Google Scholar] [CrossRef]
- Oh, S.; Mun, H.; Park, J.; Lee, I. Techno-economic comparison of ammonia production processes under various carbon tax scenarios for the economic transition from grey to blue ammonia. J. Clean. Prod. 2024, 434, 139909. [Google Scholar] [CrossRef]
- Kong, J.; Choi, J.; Park, H.S. Advantages and limitations of different electrochemical NH3 production methods under ambient conditions: A review. Curr. Opin. Electrochem. 2023, 39, 101292. [Google Scholar] [CrossRef]
- Kang, L.; Pan, W.; Zhang, J.; Wang, W.; Tang, C. A review on ammonia blends combustion for industrial applications. Fuel 2023, 332, 126150. [Google Scholar] [CrossRef]
- Hoang Truong, N.; Kim, J.-S.; Lim, J.; Shin, H. Electrochemical reduction of nitrate to Ammonia: Recent progress and future directions. Chem. Eng. J. 2024, 495, 153108. [Google Scholar] [CrossRef]
- Uddin, M.A.; Honda, Y.; Kato, Y.; Takagi, K. Catalytic methanation of CO2 with NH3. Catal. Today 2017, 291, 24–28. [Google Scholar] [CrossRef]
- Chen, K.; Shen, P.; Zhang, N.; Ma, D.; Chu, K. Electrocatalytic NO Reduction to NH3 on Mo2C Nanosheets. Inorg. Chem. 2023, 62, 653–658. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, H.s.; Wang, J.; Ji, C.; Liu, Y.; Chen, D.; Zhang, H.; Wang, J.; Zhang, Y. Fertilizer N triggers native soil N-derived N2O emissions by priming gross N mineralization. Soil Biol. Biochem. 2023, 178, 108961. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, Y.; Cayuela, M.L.; Sánchez-Monedero, M.A.; Wang, Q. Compost biochemical quality mediates nitrogen leaching loss in a greenhouse soil under vegetable cultivation. Geoderma 2020, 358, 113984. [Google Scholar] [CrossRef]
- Ren, J.-T.; Wan, C.-Y.; Pei, T.-Y.; Lv, X.-W.; Yuan, Z.-Y. Promotion of electrocatalytic nitrogen reduction reaction on N-doped porous carbon with secondary heteroatoms. Appl. Catal. B 2020, 266, 118633. [Google Scholar] [CrossRef]
- Sen, S.; Bag, A.; Pal, S. Mechanistic inquisition on the reduction of C17Si(NH2)2 to NH3: A DFT study. ChemPhysChem 2024, 25, e202300723. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Guo, X.; Kong, X.; Ke, J.; Chi, M.; Li, Q.; Geng, Z.; Zeng, J. N2 electroreduction: A highly efficient metal-free electrocatalyst of F-doped porous carbon toward N2 electroreduction. Adv. Mater. 2020, 32, 2070186. [Google Scholar] [CrossRef]
- Tao, L.; Huang, L.; Pang, K.; Li, C.; Ji, H. Fe-doped Mo2C for boosting electrocatalytic N2 reduction. Inorg. Chem. Commun. 2022, 145, 110003. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Nanocarbon for energy material applications: N2 reduction reaction. Small 2021, 17, 2007055. [Google Scholar] [CrossRef]
- Wang, F.; Mao, J. Effect of N-doping on graphene: NRR activity and N-source. Diam. Relat. Mater. 2021, 118, 108494. [Google Scholar] [CrossRef]
- Castellano-Varona, B.; Harb, M.; Araña, J.; Cavallo, L.; Azofra, L.M. In silico design of novel NRR electrocatalysts: Cobalt–molybdenum alloys. Chem. Commun. 2020, 56, 13343–13346. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Rafiq, M.; Woldu, A.R.; Tong, Q.-X.; Astruc, D.; Hu, L. Recent progress in electrocatalytic nitrogen reduction to ammonia (NRR). Coord. Chem. Rev. 2023, 478, 214981. [Google Scholar] [CrossRef]
- Liu, A.; Gao, M.; Gao, Y.; Ren, X.; Yang, Y.; Yang, Q.; Li, Y.; Gao, L.; Liang, X.; Ma, T. DFT study of Ru/graphene as high-performance electrocatalyst for NRR. Inorg. Chem. Commun. 2020, 120, 108169. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. Theoretical and experimental uncovering of Nb-TiO2 single atoms for NRR electrocatalysts. Chem. Catal. 2022, 2, 2120–2122. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Liu, Z. Bifunctional OER/NRR catalysts based on a thin-layered Co3O4–x/GO sandwich structure. ACS Appl. Mater. Interfaces 2022, 14, 43508–43516. [Google Scholar] [CrossRef]
- Liu, A.; Yang, Y.; Kong, D.; Ren, X.; Gao, M.; Liang, X.; Yang, Q.; Zhang, J.; Gao, L.; Ma, T. DFT study of the defective carbon materials with vacancy and heteroatom as catalyst for NRR. Appl. Surf. Sci. 2021, 536, 147851. [Google Scholar] [CrossRef]
- Fu, C.; Luo, L.; Yang, L.; Shen, S.; Wei, G.; Yin, J.; Zhang, J. Theoretical exploration of the thermodynamic process competition between NRR and HER on transition-metal-doped CoP (101) facets. J. Phys. Chem. C 2021, 125, 17051–17057. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Y.; Tian, Y.; Liu, W.; Chu, K. Activating VS2 basal planes for enhanced NRR electrocatalysis: The synergistic role of S-vacancies and B dopants. J. Mater. Chem. A 2020, 8, 16195–16202. [Google Scholar] [CrossRef]
- Li, B.; Lai, C.; Zeng, G.; Huang, D.; Qin, L.; Zhang, M.; Cheng, M.; Liu, X.; Yi, H.; Zhou, C.; et al. Black phosphorus, a rising star 2D nanomaterial in the post-graphene era: Synthesis, properties, modifications, and photocatalysis applications. Small 2019, 15, 1804565. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Degg, A.; Upadhyaya, S.; Nilges, T.; Sen Sarma, N. Synthesis, modification, and application of black phosphorus, few-layer black phosphorus (FLBP), and phosphorene: A detailed review. Mater. Adv. 2022, 3, 5557–5574. [Google Scholar] [CrossRef]
- Zanbouri, Z.; Hajati, Y.; Sabaeian, M. Investigation of anisotropic absorption in the hybrid L-shaped graphene-black phosphorene structure. Phys. E 2023, 146, 115554. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Cui, T. Edge reconstructions of black phosphorene: A global search. Nanoscale 2021, 13, 4085–4091. [Google Scholar] [CrossRef]
- Li, C.; Xie, Z.; Chen, Z.; Cheng, N.; Wang, J.; Zhu, G. Tunable bandgap and optical properties of black phosphorene nanotubes. Materials 2018, 11, 304. [Google Scholar] [CrossRef]
- Khabthani, J.J.; Chika, K.; Jemaï, G.; Mayou, D.; Trambly de Laissardière, G. Electronic structure and conductivity in functionalized multilayer black phosphorene. Phys. Rev. B 2024, 110, 045150. [Google Scholar] [CrossRef]
- Luo, Y.; Ren, C.; Wang, S.; Li, S.; Zhang, P.; Yu, J.; Sun, M.; Sun, Z.; Tang, W. Adsorption of transition metals on black phosphorene: A first-principles study. Nanoscale Res. Lett. 2018, 13, 282. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Zhang, M.; Zhao, W.; Liu, Y.; Qin, H. The electronic and magnetic properties of multi-atom doped black phosphorene. Nanomaterials 2019, 9, 311. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Padmashree, R.; Prasad, A. Recent advancement of surface modification techniques of 2-D nanomaterials. Mater. Sci. Eng. B 2023, 297, 116817. [Google Scholar] [CrossRef]
- Liu, H.; Cao, X.; Ding, L.-X.; Wang, H. Sn-doped black phosphorene for enhancing the selectivity of nitrogen electroreduction to ammonia. Adv. Funct. Mater. 2022, 32, 2111161. [Google Scholar] [CrossRef]
- Wang, C.; Gao, J.; Zhao, J.-G.; Yan, D.-J.; Zhu, X.-D. Synergistically coupling black phosphorus quantum dots with MnO2 nanosheets for efficient electrochemical nitrogen reduction under ambient conditions. Small 2020, 16, 1907091. [Google Scholar] [CrossRef]
- Lai, J.; Liu, H.; Ding, L.-X.; Wang, J.; Chen, G.-F.; Wang, H. Black phosphorene with removable aluminum ion protection for enhanced electrochemical nitrogen fixation. Adv. Energy Mater. 2024, 14, 2303963. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; He, C.; Ren, X.; Zhang, P.; Mi, H. Recent progress in 2D catalysts for photocatalytic and electrocatalytic artificial nitrogen reduction to ammonia. Adv. Energy Mater. 2021, 11, 2003294. [Google Scholar] [CrossRef]
- Pei, W.; Zhou, S.; Zhao, J.; Du, Y.; Dou, S. Optimization of photocarrier dynamics and activity in phosphorene with intrinsic defects for nitrogen fixation. J. Mater. Chem. A 2020, 8, 20570–20580. [Google Scholar] [CrossRef]
- Xu, F.; Wu, F.; Zhu, K.; Fang, Z.; Jia, D.; Wang, Y.; Jia, G.; Low, J.; Ye, W.; Sun, Z.; et al. Boron doping and high curvature in Bi nanorolls for promoting photoelectrochemical nitrogen fixation. Appl. Catal. B Environ. 2021, 284, 119689. [Google Scholar] [CrossRef]
- Kang, B.; Yuan, Y.; Lv, Y.; Ai, H.; Yong Lee, J. Synergistic ultra-high activity of double B doped graphyne for electrocatalytic nitrogen reduction. Chem. Eng. J. 2022, 428, 131318. [Google Scholar] [CrossRef]
- Zhang, J.A.-O.; Fu, C.; Song, S.; Du, H.A.-O.; Zhao, D.; Huang, H.; Zhang, L.; Guan, J.A.-O.; Zhang, Y.; Zhao, X.A.-O.; et al. Changing the phosphorus allotrope from a square columnar structure to a planar zigzag nanoribbon by increasing the diameter of carbon nanotube nanoreactors. Nano. Lett. 2020, 20, 1280–1285. [Google Scholar] [CrossRef]
- Xiong, J.; Gong, Q.; Feng, T.; Wang, M.; Zhang, X.; Liu, G.; Qiao, G.; Xu, Z. Enhance hydrogen evolution reaction performance via double-stacked edges of black phosphorene. Inorg. Chem. 2023, 62, 21115–21127. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Z. Single Mo atom supported on defective boron nitride monolayer as an efficient electrocatalyst for nitrogen fixation: A computational study. J. Am. Chem. Soc. 2017, 139, 12480–12487. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Niu, X.; Li, Q.; Du, A.; Wang, J. Metal-free single atom catalyst for N2 fixation driven by visible light. J. Am. Chem. Soc. 2018, 140, 14161–14168. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-D.; Wei, Z.-X.; Dou, Y.-H.; Feng, Y.-Z.; Ma, J.-M. Ru-doped phosphorene for electrochemical ammonia synthesis. Rare Met. 2020, 39, 874–880. [Google Scholar] [CrossRef]
- Liu, K.; Fu, J.; Zhu, L.; Zhang, X.; Li, H.; Liu, H.; Hu, J.; Liu, M. Single-atom transition metals supported on black phosphorene for electrochemical nitrogen reduction. Nanoscale 2020, 12, 4903–4908. [Google Scholar] [CrossRef]
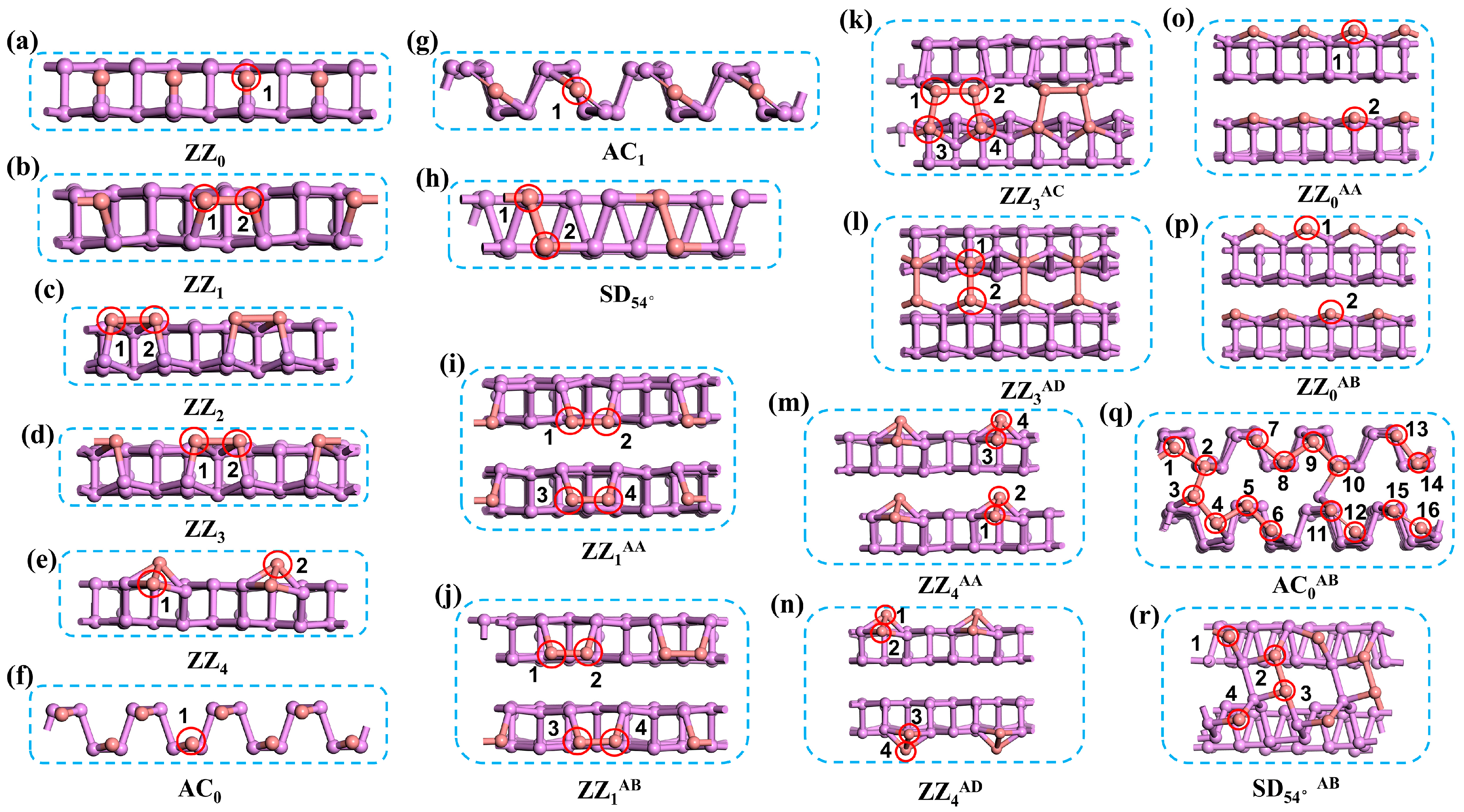
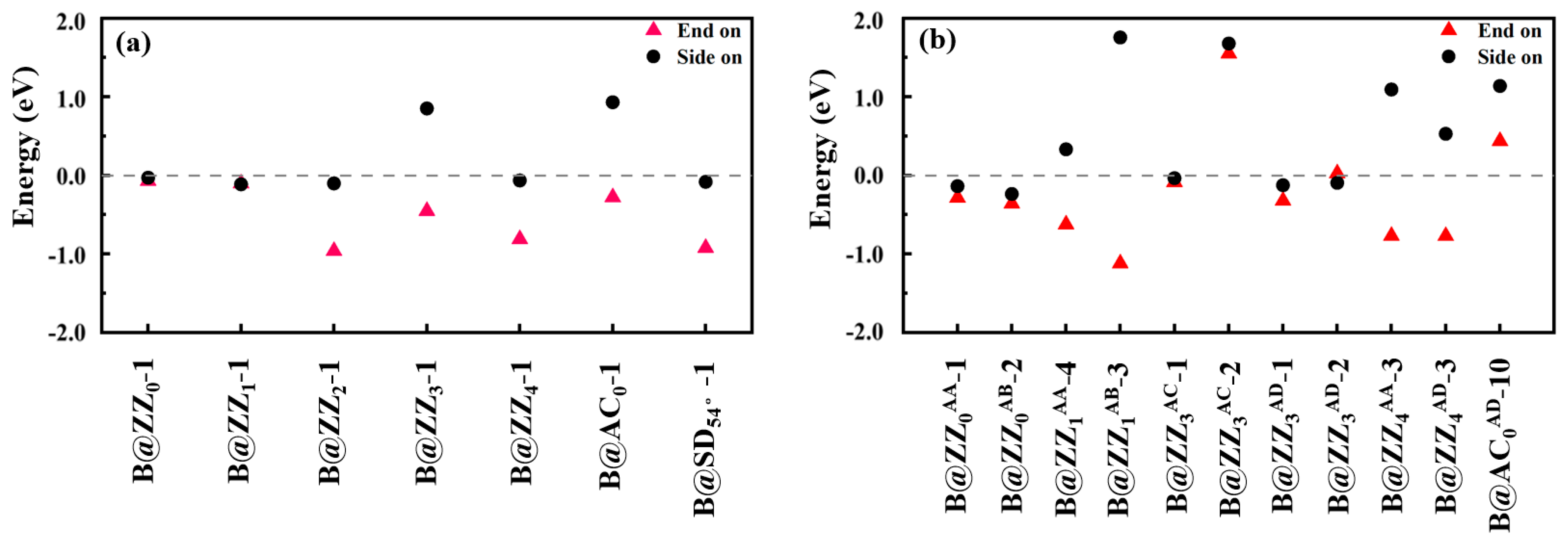
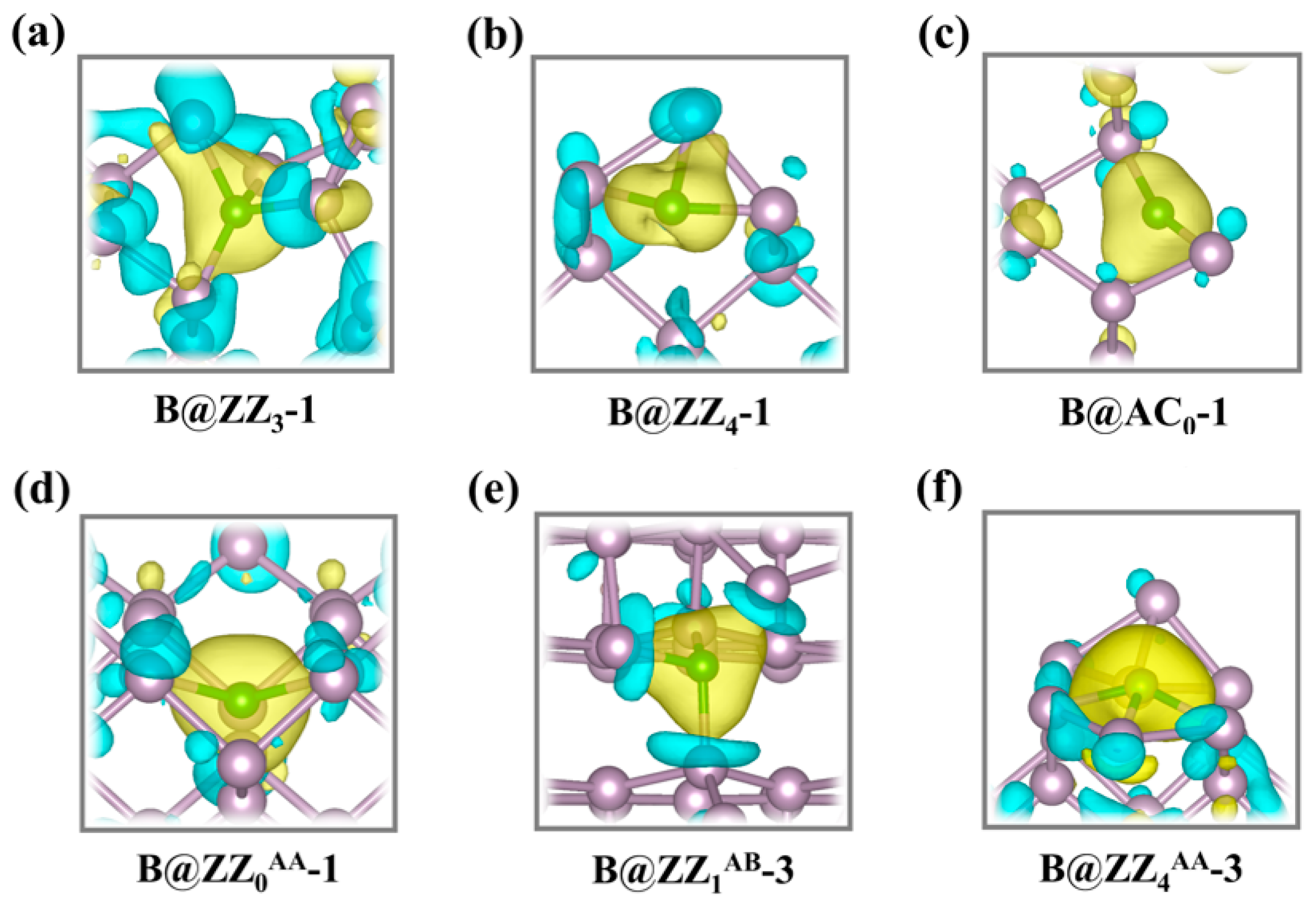
| Catalyst | EBPNR (eV) | Edoped (eV) | EB (eV) | EBinding (eV) |
|---|---|---|---|---|
| B@ZZ0-1 | −277.57 | −277.16 | −6.19 | −0.17 |
| B@ZZ1-1 | −300.47 | −300.49 | −6.19 | −0.60 |
| B@ZZ2-1 | −299.62 | −300.03 | −6.19 | −0.18 |
| B@ZZ3-1 | −298.46 | −300.53 | −6.19 | −2.66 |
| B@ZZ4-1 | −322.82 | −322.68 | −6.19 | −0.44 |
| B@AC0-1 | −333.91 | −334.62 | −6.19 | −1.29 |
| B@AC1-1 | −333.91 | - | −6.19 | - |
| B@SD54°-1 | −158.13 | −158.01 | −6.19 | −0.46 |
| Catalyst | EBPNR (eV) | Edoped (eV) | EB (eV) | EBinding (eV) |
|---|---|---|---|---|
| B@ZZ0AA-1 | −560.75 | −561.25 | −6.19 | −1.08 |
| B@ZZ0AB-2 | −561.88 | −561.97 | −6.19 | −0.67 |
| B@ZZ1AA-4 | −606.26 | −606.27 | −6.19 | −0.60 |
| B@ZZ1AB-3 | −605.69 | −605.71 | −6.19 | −0.60 |
| B@ZZ3AC-1 | −608.25 | −609.51 | −6.19 | −1.84 |
| B@ZZ3AC-2 | −608.25 | −610.53 | −6.19 | −2.86 |
| B@ZZ3AD-1 | −609.73 | −610.36 | −6.19 | −1.22 |
| B@ZZ3AD-2 | −609.73 | −610.53 | −6.19 | −1.39 |
| B@ZZ4AA-3 | −650.93 | −650.91 | −6.19 | −0.56 |
| B@ZZ4AD-3 | −650.45 | −650.45 | −6.19 | −0.58 |
| B@AC0AB-10 | −674.50 | −675.69 | −6.19 | −1.78 |
| B@SD54°AB-1 | −323.57 | - | −6.19 | - |
| System | Pathway | PDS | η (V) |
|---|---|---|---|
| B@ZZ3-1 | Distal | *N-N→*N-NH | 0.50 |
| Alternating | *N-N→*N-NH | 0.50 | |
| B@ZZ4-1 | Distal | *N-N→*N-NH | 1.02 |
| Alternating | *N-N→*N-NH | 1.02 | |
| B@AC0-1 | Distal | *N-N→*N-NH | 0.19 |
| Alternating | *NH-NH2→*NH2-NH2 | 0.36 | |
| B@ZZ0AA-1 | Distal | *N-N→*N-NH | 0.28 |
| B@ZZ1AB-3 | Distal | *NH2→*NH3 | 0.15 |
| Alternating | *NH2→*NH3 | 0.15 | |
| B@ZZ4AA-3 | Distal | *NH2→*NH3 | 0.64 |
| Ru@P [46] | Enzymatic | *N-NH→*NH-NH | 0.70 |
| DV-(5|8|5)@BP [39] | Alternating | *NH-NH2→*NH2-NH2 | 0.51 |
| W@BP [47] | Alternating | *NH-NH2→*NH2-NH2 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, W.; Xiong, J.; Xu, Z. A Theoretical Study on Electrocatalytic Nitrogen Reduction at Boron-Doped Monolayer/Bilayer Black Phosphorene Edges. Coatings 2025, 15, 755. https://doi.org/10.3390/coatings15070755
Bao W, Xiong J, Xu Z. A Theoretical Study on Electrocatalytic Nitrogen Reduction at Boron-Doped Monolayer/Bilayer Black Phosphorene Edges. Coatings. 2025; 15(7):755. https://doi.org/10.3390/coatings15070755
Chicago/Turabian StyleBao, Wenkai, Jianling Xiong, and Ziwei Xu. 2025. "A Theoretical Study on Electrocatalytic Nitrogen Reduction at Boron-Doped Monolayer/Bilayer Black Phosphorene Edges" Coatings 15, no. 7: 755. https://doi.org/10.3390/coatings15070755
APA StyleBao, W., Xiong, J., & Xu, Z. (2025). A Theoretical Study on Electrocatalytic Nitrogen Reduction at Boron-Doped Monolayer/Bilayer Black Phosphorene Edges. Coatings, 15(7), 755. https://doi.org/10.3390/coatings15070755







