Identification of Potential Migrants in Food Contact Materials Labeled as Bio-Based and/or Biodegradable by GC-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Analytical Standards
2.2. Samples
2.3. Fourier-Transform Infrared Spectroscopy with Attenuated Total Reflectance (ATR-FTIR)
2.4. Sample Preparation
2.5. P&T GC-MS Method for the Determination of Volatile Compounds
2.6. GC-MS for the Determination of Semi-Volatile Compounds
2.7. Toxicity Estimation
3. Results and Discussion
3.1. Characterization of the Materials by FTIR-ATR
3.2. Screening of Volatile Compounds by P&T GC-MS
3.2.1. P&T GC-MS Method Optimization
3.2.2. Tentative Identification of Volatile Compounds
3.3. Screening of Semi-Volatile Compounds by GC-MS
3.3.1. GC-MS Method of Optimization
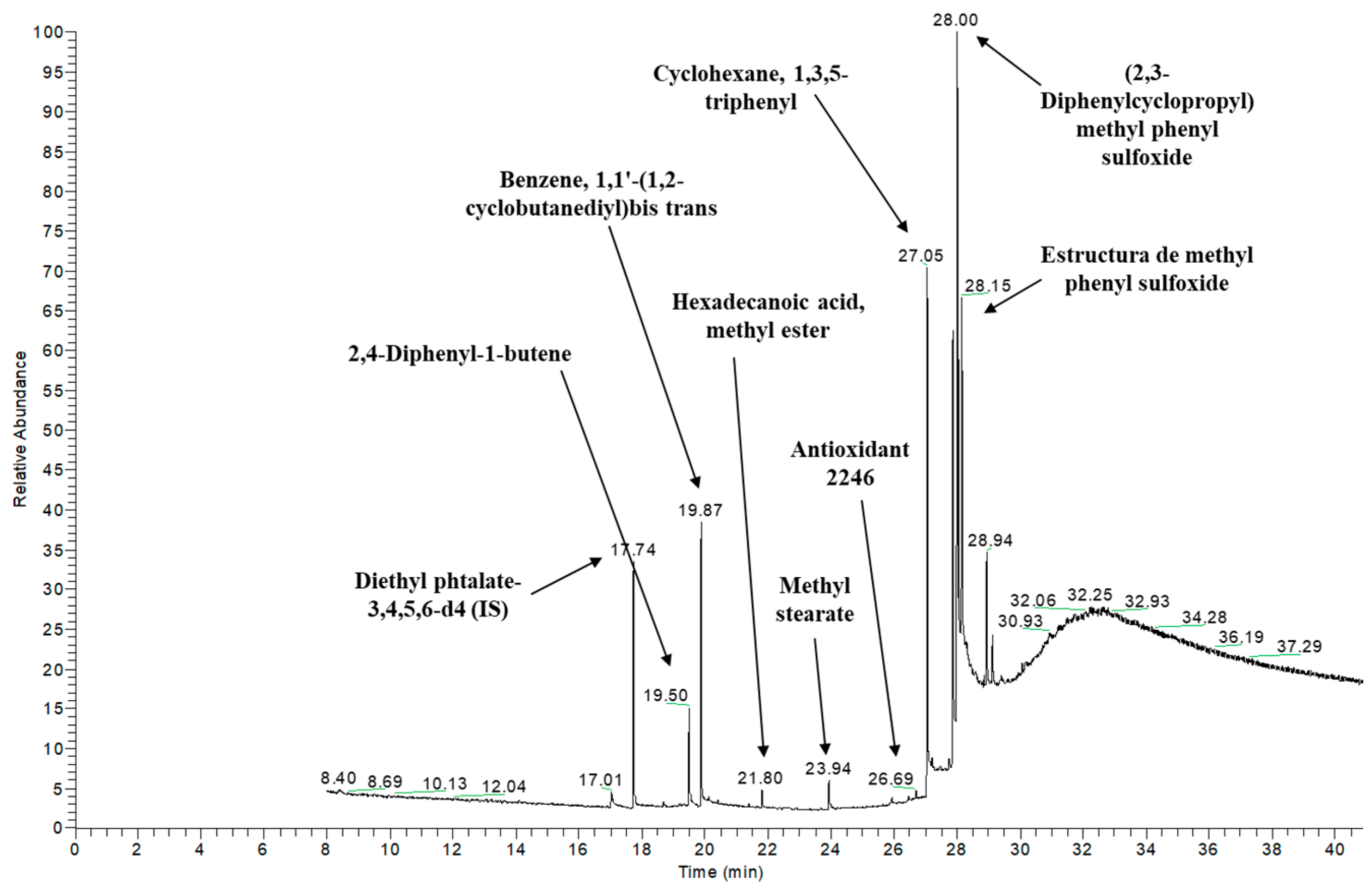
3.3.2. Tentative Identification of Semi-Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donkor, L.; Kontoh, G.; Yaya, A.; Bediako, J.K.; Apalangya, V. Bio-based and sustainable food packaging systems: Relevance, challenges, and prospects. Appl. Food Res. 2023, 3, 100356. [Google Scholar] [CrossRef]
- Bonwick, G.; Bradley, E.; Lock, I.; Romero, R. Bio-Based Materials for Use in Food Contact Applications; Fera Science 2019 (FR/001658); Report to the Food Standards Agency; Fera Science Ltd.: York, UK, 2019. Available online: https://www.food.gov.uk/sites/default/files/media/document/bio-based-materials-for-use-in-food-contact-applications_0.pdf (accessed on 20 January 2025).
- European Bioplastics. Bioplastics Facts and Figures 2020. Available online: https://docs.european-bioplastics.org/publications/EUBP_Facts_and_figures.pdf (accessed on 17 May 2024).
- Lestido-Cardama, A.; Barbosa-Pereira, L.; Sendón, R.; Bustos, J.; Losada, P.P.; De Quirós, A.R.B. Chemical safety and risk assessment of bio-based and/or biodegradable polymers for food contact: A review. Food Res. Int. 2025, 202, 115737. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(ethylene terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef] [PubMed]
- Geueke, B. Dossier—Bioplastics as Food Contact Materials; Food Packaging Forum: Zurich, Switzerland, 2014; Volume 10. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef]
- Birania, S.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Rohilla, P.; Kumar, R. Advances in development of biodegradable food packaging material from agricultural and agro-industry waste. J. Food Process Eng. 2021, 45, e13930. [Google Scholar] [CrossRef]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef]
- Regulation (EC) No. 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC. Available online: http://data.europa.eu/eli/reg/2004/1935/2021-03-27 (accessed on 13 September 2024).
- European Comission. Commission Regulation (UE) No. 10/2011, on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12, 1–89. Available online: http://data.europa.eu/eli/reg/2011/10/2023-08-31 (accessed on 9 September 2024).
- Ubeda, S.; Aznar, M.; Alfaro, P.; Nerín, C. Migration of oligomers from a food contact biopolymer based on polylactic acid (PLA) and polyester. Anal. Bioanal. Chem. 2019, 411, 3521–3532. [Google Scholar] [CrossRef]
- Tsochatzis, E.D.; Vidal, N.P.; Bai, W.; Diamantidou, D.; Theodoridis, G.; Martinez, M.M. Untargeted screening and in silico toxicity assessment of semi-and non-volatile compounds migrating from polysaccharide-based food contact materials. Food Chem. 2023, 425, 136499. [Google Scholar] [CrossRef]
- Lin, J.; Wu, W.L.; Zhong, A.H.; Xian, Y.P.; Zhong, H.N.; Dong, B.; Liang, M.; Hu, J.P.; Wu, Y.N.; Yang, X.F.; et al. Non-targeted analysis and risk assessment of intentionally and non-intentionally added substances migrating from the emerging biodegradable food contact material poly (butylene adipate-co-terephthalate)/modified starch blend film. Food Packag. Shelf Life 2023, 40, 101190. [Google Scholar] [CrossRef]
- Zimmermann, L.; Bartosova, Z.; Braun, K.; Oehlmann, J.; Völker, C.; Wagner, M. Plastic products leach chemicals that induce in vitro toxicity under realistic use conditions. Environ. Sci. Technol. 2021, 55, 11814–11823. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, S.; Aznar, M.; Nerín, C.; Kabir, A. Fabric phase sorptive extraction for specific migration analysis of oligomers from biopolymers. Talanta 2021, 233, 122603. [Google Scholar] [CrossRef] [PubMed]
- Asensio, E.; Montañés, L.; Nerín, C. Migration of volatile compounds from natural biomaterials and their safety evaluation as food contact materials. Food Chem. Toxicol. 2020, 142, 111457. [Google Scholar] [CrossRef] [PubMed]
- Riboni, N.; Bianchi, F.; Cavazza, A.; Piergiovanni, M.; Mattarozzi, M.; Careri, M. Mass spectrometry-based techniques for the detection of non-intentionally added substances in bioplastics. Separations 2023, 10, 222. [Google Scholar] [CrossRef]
- Gómez-Ramos, M.M.; Ucles, S.; Ferrer, C.; Fernández-Alba, A.R.; Hernando, M.D. Exploration of environmental contaminants in honeybees using GC-TOF-MS and GC-Orbitrap-MS. Sci. Total Environ. 2019, 647, 232–244. [Google Scholar] [CrossRef]
- Regulation (EU) 2025/40 of the European Parliament and of the Council of 19 December 2024 on Packaging and Packaging Waste, Amending Regulation (EU) 2019/1020 and Directive (EU) 2019/904, and Repealing Directive 94/62/EC. Available online: http://data.europa.eu/eli/reg/2025/40/oj (accessed on 19 June 2025).
- Patlewicz, G.; Jeliazkova, N.; Safford, R.; Worth, A.; Aleksiev, B. An evaluation of the implementation of the Cramer classification scheme in the Toxtree software. SAR QSAR Environ. Res. 2008, 19, 495–524. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific opinion on exploring options for providing advice about possible human health risks based on the concept of threshold of toxicological concern (TTC). EFSA J. 2012, 10, 2750. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A Unique Polymer for Drug Delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Pinaeva, L.G.; Noskov, A.S. Biodegradable biopolymers: Real impact to environment pollution. Sci. Total Environ. 2024, 947, 174445. [Google Scholar] [CrossRef]
- Lee, H.W.; Insyani, R.; Prasetyo, D.; Prajitno, H.; Sitompul, J. Molecular Weight and Structural Properties of Biodegradable PLA Synthesized with Different Catalysts by Direct Melt Polycondensation. J. Eng. Technol. Sci. 2015, 47, 364–373. [Google Scholar] [CrossRef]
- Ibarra, V.G.; Rodríguez Bernaldo De Quirós, A.; Paseiro Losada, P.; Sendón, R. Non-target analysis of intentionally and non intentionally added substances from plastic packaging materials and their migration into food simulants. Food Packag. Shelf Life 2019, 21, 100325. [Google Scholar] [CrossRef]
- European Printing Ink Association. A sector of CEPE Aisbl. Comprising Packaging Ink Raw Materials Applied to the Non-Food Contact Surface of Food Packaging; Inventory list–Version December 2013; European Printing Ink Association: Brussels, Belgium, 2019. [Google Scholar]
- Paiva, R.; Wrona, M.; Nerín, C.; Gavril, G.L.; Cruz, S.A. Volatile Compounds and Off-odors Analysis of Recycled PLA for Packaging Applications: An Essential Factor for Ensuring Food Safety and Quality. J. Polym. Environ. 2024, 32, 6687–6697. [Google Scholar] [CrossRef]
- Salazar, R.; Domenek, S.; Plessis, C.; Ducruet, V. Quantitative determination of volatile organic compounds formed during Polylactide processing by MHS-SPME. Polym. Degrad. Stab. 2017, 136, 80–88. [Google Scholar] [CrossRef]
- Vera, P.; Uliaque, B.; Canellas, E.; Escudero, A.; Nerín, C. Identification and quantification of odorous compounds from adhesives used in food packaging materials by headspace solid phase extraction and headspace solid phase microextraction coupled to gas chromatography–olfactometry–mass spectrometry. Anal. Chim. Acta 2012, 745, 53–63. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Loureiro, P.V. Materials for Food Contact: Contribution to the Study of Chemical Safety. Ph.D. Thesis, Universidade de Santiago de Compostela, Santiago de Compostela, Spain, 2023. [Google Scholar]
- Li, D.; Zeng, Y.; Ye, Z.K.; Li, H.K.; Li, Y.Z.; Dong, B.; Su, Q.Z.; Lin, Q.B.; Xiao, J.; Zhong, H.N. Analysis of volatile organic compounds and potential odour compounds in food contact paperboard using headspace two-dimensional GC-QTOF-MS. Food Addit. Contam. Part A 2023, 40, 1482–1493. [Google Scholar] [CrossRef]
- An, H.; Lu, Z.; Wang, Z.; Abbas, M.Q.; Du, Z. Safety assessment and quality control of regenerated cellulose food packaging in different processes. Food Control. 2024, 163, 110543. [Google Scholar] [CrossRef]
- Riganakos, K.; Koller, W.; Ehlermann, D.; Bauer, B.; Kontominas, M. Effects of ionizing radiation on properties of monolayer and multilayer flexible food packaging materials. Radiat. Phys. Chem. 1999, 54, 527–540. [Google Scholar] [CrossRef]
- Panseri, S.; Chiesa, L.; Zecconi, A.; Soncini, G.; De Noni, I. Determination of Volatile Organic Compounds (VOCs) from Wrapping Films and Wrapped PDO Italian Cheeses by Using HS-SPME and GC/MS. Molecules 2014, 19, 8707–8724. [Google Scholar] [CrossRef]
- Hwang, J.B.; Lee, S.; Yeum, J.; Kim, M.; Choi, J.C.; Park, S.; Kim, J. HS-GC/MS method development and exposure assessment of volatile organic compounds from food packaging into food simulants. Food Addit. Contam. Part A 2019, 36, 1574–1583. [Google Scholar] [CrossRef]
- Song, X.; Wrona, M.; Nerin, C.; Lin, Q.; Zhong, H. Volatile non-intentionally added substances (NIAS) identified in recycled expanded polystyrene containers and their migration into food simulants. Food Packag. Shelf Life 2019, 20, 100318. [Google Scholar] [CrossRef]
- Ehret-Henry, J.; Ducruet, V.; Luciani, A.; Feigenbaum, A. Styrene and ethylbenzene migration from polystyrene into dairy products by dynamic purge-and-trap gas chromatography. J. Food Sci. 1994, 59, 990–992. [Google Scholar] [CrossRef]
- Cabanes, A.; Valdés, F.; Fullana, A. A review on VOCs from recycled plastics. Sustain. Mater. Technol. 2020, 25, e00179. [Google Scholar] [CrossRef]
- Mosquera, M.E.G.; Jiménez, G.; Tabernero, V.; Vinueza-Vaca, J.; García-Estrada, C.; Kosalková, K.; Sola-Landa, A.; Monje, B.; Acosta, C.; Alonso, R.; et al. Terpenes and Terpenoids: Building Blocks to Produce Biopolymers. Sustain. Chem. 2021, 2, 467–492. [Google Scholar] [CrossRef]
- Marin, N.; Collura, S.; Sharypov, V.I.; Beregovtsova, N.G.; Baryshnikov, S.V.; Kutnetzov, B.N.; Membrado, L.; Cebolla, V.L.; Marin, N.; Weber, A.J. Copyrolysis of wood biomass and synthetic polymers mixtures. Part II: Characterisation of the liquid phases. J. Anal. Appl. Pyrolysis 2002, 65, 41–55. [Google Scholar] [CrossRef]
- Donetzhuber, A.; Johansson, B.; Johansson, K.; Lövgren, M.; Sarin, E. Analytical characterization of the gas phases in paper and board products. Nord. Pulp Pap. Res. J. 1999, 14, 48–60. [Google Scholar] [CrossRef]
- Lago, M.A.; Ackerman, L.K. Identification of print-related contaminants in food packaging. Food Addit. Contam. Part A 2016, 33, 518–529. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Sendón, R.; Bustos, J.; Lomo, M.L.; Losada, P.P.; De Quirós, A.R.B. Dietary Exposure Estimation to Chemicals Transferred from Milk and Dairy Products Packaging Materials in Spanish Child and Adolescent Population. Foods 2020, 9, 1554. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Nerín, C. Determination of volatile compounds and their sensory impact in a biopolymer based on polylactic acid (PLA) and polyester. Food Chem. 2019, 294, 171–178. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Sendón, R.; Bustos, J.; Santillana, M.I.; Losada, P.P.; De Quirós, A.R.B. GC-MS Screening for the Identification of Potential Migrants Present in Polymeric Coatings of Food Cans. Polymers 2019, 11, 2086. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Loureiro, P.V.; Sendón, R.; Losada, P.P.; De Quirós, A.R.B. Application of chromatographic analysis for detecting components from polymeric can coatings and further determination in beverage samples. J. Chromatogr. A 2021, 1638, 461886. [Google Scholar] [CrossRef] [PubMed]
- Nerín, C.; Acosta, D.; Rubio, C. Potential migration release of volatile compounds from plastic containers destined for food use in microwave ovens. Food Addit. Contam. 2002, 19, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Bentayeb, K.; Ackerman, L.K.; Begley, T.H. Ambient Ionization–Accurate Mass Spectrometry (AMI-AMS) for the Identification of Nonvisible Set-off in Food-Contact Materials. J. Agric. Food Chem. 2012, 60, 1914–1920. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, H.; Chen, L.; Wu, X.; Wu, S.; Su, Q.; Dong, B.; Li, D.; Ma, T.; Zhong, H.; Wang, X.; et al. Characterization of volatile organic compounds in food contact paperboards and elucidation of their potential origins from the perspective of the raw materials. Food Packag. Shelf Life 2023, 37, 101062. [Google Scholar] [CrossRef]
- Domeño, C.; Aznar, M.; Nerín, C.; Isella, F.; Fedeli, M.; Bosetti, O. Safety by design of printed multilayer materials intended for food packaging. Food Addit. Contam. Part A 2017, 34, 1239–1250. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. Migration of odorous compounds from adhesives used in market samples of food packaging materials by chromatography olfactometry and mass spectrometry (GC–O–MS). Food Chem. 2014, 145, 237–244. [Google Scholar] [CrossRef]
- Canellas, E.; Vera, P.; Nerín, C. Risk assessment derived from migrants identified in several adhesives commonly used in food contact materials. Food Chem. Toxicol. 2015, 75, 79–87. [Google Scholar] [CrossRef]
- Salem, M.Z.; Zidan, Y.E.; El Hadidi, N.M.; Mansour, M.M.; Elgat, W.A.A. Evaluation of usage three natural extracts applied to three commercial wood species against five common molds. Int. Biodeterior. Biodegrad. 2016, 110, 206–226. [Google Scholar] [CrossRef]
- Ilayaraja, N.; Radhakrishnan, S.; Renganathan, N.G. Electrochemical fluorination of dimethyl glutarate and its characterization. Ionics 2010, 16, 137–144. [Google Scholar] [CrossRef]
- Román-Ramírez, L.A.; McKeown, P.; Shah, C.; Abraham, J.; Jones, M.D.; Wood, J. Chemical Degradation of End-of-Life Poly(lactic acid) into Methyl Lactate by a Zn(II) Complex. Ind. Eng. Chem. Res. 2020, 59, 11149–11156. [Google Scholar] [CrossRef]
- Silva, F.M.; Pinto, R.J.; Barros-Timmons, A.; Freire, C.S. Solventless Photopolymerizable Paper Coating Formulation for Packaging Applications. Polymers 2023, 15, 1069. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Mann, B.; Sharma, R.; Verma, A.; Panjagari, N.R.; Gandhi, K. Identification of polymer additives from multilayer milk packaging materials by liquid-solid extraction coupled with GC-MS. Food Packag. Shelf Life 2022, 34, 100975. [Google Scholar] [CrossRef]
- Asensio, E.; Peiro, T.; Nerín, C. Determination the set-off migration of ink in cardboard-cups used in coffee vending machines. Food Chem. Toxicol. 2019, 130, 61–67. [Google Scholar] [CrossRef]
- Yang, Q.H.; Lin, Q.B.; Hua, X.Y.; Liao, J.; Lu, S.Q.; Yan, L.Y.; Ma, H.S. Identification and health risk assessment of volatile and semi-volatile migrants along with chemical elements in food contact water-borne coating paper. Food Packag. Shelf Life 2024, 45, 101337. [Google Scholar] [CrossRef]
- Aurela, B. Migration of Substances from Paper and Board Food Packaging Materials. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2001. Available online: https://core.ac.uk/download/pdf/14916631.pdf (accessed on 28 November 2024).
- Knight, W.R. Recent Advances in Waterborne Acrylic Nanocomposite Paints and Coatings. Ph.D. Thesis, Lehigh University, Bethlehem, PA, USA, 2021. Available online: https://preserve.lib.lehigh.edu/ (accessed on 4 December 2024).
- Lestido-Cardama, A.; Rodríguez Bernaldo De Quirós, A.; Bustos, J.; Lomo, M.L.; Losada, P.P.; Sendón, R. Estimation of Dietary Exposure to Contaminants Transferred from the Packaging in Fatty Dry Foods Based on Cereals. Foods 2020, 9, 1038. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, Q.; Xie, C.; Liu, Y.; Zhong, H.; Gu, W.; McClements, D.J.; Ma, D. Screening and safety assessment of migrating substances released from biodegradable packaging materials into milk. Food Control 2024, 166, 110755. [Google Scholar] [CrossRef]
- Canellas, E.; Vera, P.; Nerín, C. UPLC–ESI-Q-TOF-MSE and GC–MS identification and quantification of non-intentionally added substances coming from biodegradable food packaging. Anal. Bioanal. Chem. 2015, 407, 6781–6790. [Google Scholar] [CrossRef]
- Choi, J.O.; Jitsunari, F.; Asakawa, F.; Sun Lee, D. Migration of styrene monomer, dimers and trimers from polystyrene to food simulants. Food Addit. Contam. 2005, 22, 693–699. [Google Scholar] [CrossRef]
- Vilaplana, F.; Ribes-Greus, A.; Karlsson, S. Chromatographic pattern in recycled high-impact polystyrene (HIPS)—Occurrence of low molecular weight compounds during the life cycle. Polym. Degrad. Stab. 2010, 95, 172–186. [Google Scholar] [CrossRef]
- Skjevrak, I.; Brede, C.; Steffensen, I.; Mikalsen, A.; Alexander, J.; Fjeldal, P.; Herikstad, H. Non-targeted multi-component analytical surveillance of plastic food contact materials: Identification of substances not included in EU positive lists and their risk assessment. Food Addit. Contam. 2005, 22, 1012–1022. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Beg, M.A. Structural characterization of potential endocrine disrupting activity of alternate plasticizers di-(2-ethylhexyl) adipate (DEHA), acetyl tributyl citrate (ATBC) and 2, 2, 4-trimethyl 1, 3-pentanediol diisobutyrate (TPIB) with human sex hormone-binding globulin. Reprod. Toxicol. 2019, 83, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lestido-Cardama, A.; Vázquez-Loureiro, P.; Sendón, R.; Bustos, J.; Santillana, M.I.; Losada, P.P.; De Quirós, A.R.B. Characterization of Polyester Coatings Intended for Food Contact by Different Analytical Techniques and Migration Testing by LC-MSn. Polymers 2022, 14, 487. [Google Scholar] [CrossRef] [PubMed]
- CFR. Indirect Food Additives: Adhesives and Components of Coatings- Code of Federal Regulations Title 21- Part 175-Subpart B. (s. f.). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=175.105 (accessed on 3 December 2024).
- Rahim, A.A.; Saad, B.; Osman, H.; Hashim, N.; Yahya, S.; Talib, K.M. Simultaneous determination of diethylene glycol, diethylene glycol monoethyl ether, coumarin and caffeine in food items by gas chromatography. Food Chem. 2011, 126, 1412–1416. [Google Scholar] [CrossRef]
- Binderup, M.L.; Pedersen, G.A.; Vinggaard, A.M.; Rasmussen, E.S.; Rosenquist, H.; Cederberg, T. Toxicity testing and chemical analyses of recycled fibre-based paper for food contact. Food Addit. Contam. 2002, 19, 13–28. [Google Scholar] [CrossRef]
- Bush, J.; Gilbert, J.; Goenaga, X. Spectra for the Identification of Monomers in Food Packaging; Springer Science & Business Media: Dordrcht, The Netherlands, 1993; Volume 14515. [Google Scholar]
- Aker, A.; Caron-Beaudoin, É.; Ayotte, P.; Ricard, S.; Gilbert, V.; Avard, E.; Lemire, M. Non-persistent exposures from plasticizers or plastic constituents in remote Arctic communities: A case for further research. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 400–407. [Google Scholar] [CrossRef]
- Datta, R.; Henry, M. Lactic acid: Recent advances in products, processes and technologies—a review. J. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Guart, A.; Wagner, M.; Mezquida, A.; Lacorte, S.; Oehlmann, J.; Borrell, A. Migration of plasticisers from Tritan™ and polycarbonate bottles and toxicological evaluation. Food Chem. 2013, 141, 373–380. [Google Scholar] [CrossRef]
- Dandan Doganci, M.; Doganci, E.; Balci, H.; Cetin, M. Antibacterial and cytotoxic performance of methenamine-based poly (lactic acid)/poly (ethylene glycol)(PLA/PEG) composite films. J. Appl. Polym. Sci. 2024, 141, e55412. [Google Scholar] [CrossRef]
- Astill, B.; Terhaar, C.; Fassett, D. The toxicology and fate of 2,2,4-trimethyl-1,3-pentanediol diisobutyrate. Toxicol. Appl. Pharmacol. 1972, 22, 387–399. [Google Scholar] [CrossRef]
- Bentz, K.C. Synthesis and Characterization of Linear and Branched Polylactic Acid For Use in Food Packaging Applications. Master’s Thesis, California Polytechnic State University, San Luis Obispo, CA, USA, 2011. Available online: https://www.proquest.com/dissertations-theses/synthesis-characterization-linear-branched/docview/2838330030/se-2 (accessed on 6 January 2025).
- Kleinschnitz, M.; Schreier, P. Identification and semi-quantitative determination of a migration contaminant from beverage carton packages into mineral water by on-line solid phase extraction gas chromatography-mass spectrometry (SPE-GC-MS). Chromatographia 1998, 48, 581–583. [Google Scholar] [CrossRef]
- Hu, Y.; Du, Z.; Sun, X.; Ma, X.; Song, J.; Sui, H.; Debrah, A.A. Non-targeted analysis and risk assessment of non-volatile compounds in polyamide food contact materials. Food Chem. 2020, 345, 128625. [Google Scholar] [CrossRef]
- Courgneau, C.; Domenek, S.; Guinault, A.; Avérous, L.; Ducruet, V. Analysis of the structure-properties relationships of different multiphase systems based on plasticized poly (lactic acid). J. Polym. Environ. 2011, 19, 362–371. [Google Scholar] [CrossRef]
- Singh, S.; Pereira, J.; Guerreiro, P.; Selbourne, C.; Paula, C.; Cunha, A.; Sousa, C.; Poças, F. Safety profile of ZnO active packaging PBAT based biomaterial for food packaging. First tier evaluation. Food Control 2024, 161, 110389. [Google Scholar] [CrossRef]
- Sapozhnikova, Y. Non-targeted screening of chemicals migrating from paper-based food packaging by GC-Orbitrap mass spectrometry. Talanta 2021, 226, 122120. [Google Scholar] [CrossRef]
- Cui, H.; Gao, W.; Lin, Y.; Zhang, J.; Yin, R.; Xiang, Z.; Zhang, S.; Zhou, S.; Chen, W.; Cai, K. Development of microwave-assisted extraction and dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry for the determination of organic additives in biodegradable mulch films. Microchem. J. 2021, 160, 105722. [Google Scholar] [CrossRef]
- Trumbo, D.L.; Giddings, C.L.; Wilson, L.R.A. Terpene–anhydride resins as coating materials. J. Appl. Polym. Sci. 1995, 58, 69–76. [Google Scholar] [CrossRef]
- Gavril, G.L.; Wrona, M.; Bertella, A.; Świeca, M.; Râpă, M.; Salafranca, J.; Nerín, C. Influence of medicinal and aromatic plants into risk assessment of a new bioactive packaging based on polylactic acid (PLA). Food Chem. Toxicol. 2019, 132, 110662. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. Compounds responsible for off-odors in several samples composed by polypropylene, polyethylene, paper and cardboard used as food packaging materials. Food Chem. 2020, 309, 125792. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Ghosh, T.; Purohit, S.D.; Prasannavenkadesan, V.; Rhim, J. Lignin as a sustainable and functional material for active food packaging applications: A review. J. Clean. Prod. 2024, 469, 143151. [Google Scholar] [CrossRef]
- Rai, S.; Dutta, P.K.; Mehrotra, G.K. Natural antioxidant and antimicrobial agents from agrowastes: An emergent need to food packaging. Waste Biomass Valorization 2020, 11, 1905–1916. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Su, Q.Z.; Mercado, D.; Nerín, C. Migration of volatile substances from recycled high density polyethylene to milk products. Food Packag. Shelf Life 2023, 35, 101020. [Google Scholar] [CrossRef]
- Gratia, A.; Merlet, D.; Ducruet, V.; Lyathaud, C. A comprehensive NMR methodology to assess the composition of biobased and biodegradable polymers in contact with food. Anal. Chim. Acta 2015, 853, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.; Park, S.; Volpe, S.; Torrieri, E.; Masi, P. Active packaging based on PLA and chitosan-caseinate enriched rosemary essential oil coating for fresh minced chicken breast application. Food Packag. Shelf Life 2021, 29, 100708. [Google Scholar] [CrossRef]
- Iskandar, A.F.A.; Santoso, U.; Supriyadi, S. Chemical characteristics of waru leaf (Hibiscus tiliaceus) as food packaging material. Indones. Food Nutr. Prog. 2023, 20, 72–78. [Google Scholar] [CrossRef]
- Sustaita-Rodriguez, A.; Vega-Rios, A.; Bugarin, A.; Ramos-Sanchez, V.H.; Camacho-Davila, A.A.; Rocha-Gutierrez, B.; Chavez-Flores, D. Chemoenzymatic epoxidation of highly unsaturated fatty acid methyl ester and its application as poly (lactic acid) plasticizer. ACS Sustain. Chem. Eng. 2021, 9, 17016–17024. [Google Scholar] [CrossRef]
- Song, Y.S.; Park, H.J.; Komolprasert, V. Analytical procedure for quantifying five compounds suspected as possible contaminants in recycled paper/paperboard for food packaging. J. Agric. Food Chem. 2000, 48, 5856–5859. [Google Scholar] [CrossRef]
- Tsochatzis, E.D.; Alberto Lopes, J.; Hoekstra, E.; Emons, H. Development and validation of a multi-analyte GC-MS method for the determination of 84 substances from plastic food contact materials. Anal. Bioanal. Chem. 2020, 412, 5419–5434. [Google Scholar] [CrossRef]
- Aznar, M.; Domeño, C.; Nerín, C.; Bosetti, O. Set-off of non volatile compounds from printing inks in food packaging materials and the role of lacquers to avoid migration. Dye. Pigment. 2014, 114, 85–92. [Google Scholar] [CrossRef]
- Guan, W.; He, Y.; McClements, D.J.; Chen, J.; Ma, D. Risk assessment of migrants released from multilayer packaging materials: Direct immersion-solid-phase microextraction coupled to gas chromatography-mass spectrometry. Food Packag. Shelf Life 2024, 46, 101407. [Google Scholar] [CrossRef]
- Miralles, P.; Yusà, V.; Pineda, A.; Coscollà, C. A fast and automated strategy for the identification and risk assessment of unknown substances (IAS/NIAS) in plastic food contact materials by GC-Q-Orbitrap HRMS: Recycled LDPE as a proof-of-concept. Toxics 2021, 9, 283. [Google Scholar] [CrossRef]
- Bhanot, V.; Gupta, S.; Panwar, J. Phylloplane fungus Curvularia dactyloctenicola VJP08 effectively degrades commercially available PS product. J. Environ. Manag. 2024, 351, 119920. [Google Scholar] [CrossRef]
- Martínez-Bueno, M.J.; Hernando, M.D.; Uclés, S.; Rajski, L.; Cimmino, S.; Fernández-Alba, A.R. Identification of non-intentionally added substances in food packaging nano films by gas and liquid chromatography coupled to orbitrap mass spectrometry. Talanta 2017, 172, 68–77. [Google Scholar] [CrossRef]
- Fasano, E.; Bono-Blay, F.; Cirillo, T.; Montuori, P.; Lacorte, S. Migration of phthalates, alkylphenols, bisphenol A and di (2-ethylhexyl) adipate from food packaging. Food Control 2012, 27, 132–138. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Letcher, R.J.; Zhang, Y.; Jian, K.; Zhang, J.; Su, G. A review on organophosphate Ester (OPE) flame retardants and plasticizers in foodstuffs: Levels, distribution, human dietary exposure, and future directions. Environ. Int. 2019, 127, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhou, R.; Yin, Y.; Liu, Y.; Zhao, N.; Li, H.; Zhang, A.; Li, X.; Fu, J. Occurrence of Organophosphate Esters in Food and Food Contact Materials and Related Human Exposure Risks. J. Agric. Food Chem. 2025, 73, 4455–4465. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Xia, H.; Tang, K.; Zhou, Y. Plasticizers derived from biomass resources: A short review. Polymers 2018, 10, 1303. [Google Scholar] [CrossRef]
- Sanchis, Y.; Yusà, V.; Coscollà, C. Analytical strategies for organic food packaging contaminants. J. Chromatogr. A 2017, 1490, 22–46. [Google Scholar] [CrossRef]
- Gelbke, H.; Banton, M.; Block, C.; Dawkins, G.; Eisert, R.; Leibold, E.; Pemberton, M.; Puijk, I.M.; Sakoda, A.; Yasukawa, A. Risk assessment for migration of styrene oligomers into food from polystyrene food containers. Food Chem. Toxicol. 2018, 124, 151–167. [Google Scholar] [CrossRef]
- Nieves Calvo, S. Estudios de Migración en Biomateriales Para su Uso a Alta Temperatura en Contacto con Alimentos. Bachelor’s Thesis, University of Zaragoza, Zaragoza, Spain, 2020. Available online: https://zaguan.unizar.es/record/96429?ln=es# (accessed on 3 January 2025).
- Yang, J.; Li, Y.; Wang, Y.; Ruan, J.; Zhang, J.; Sun, C. Recent advances in analysis of phthalate esters in foods. TrAC Trends Anal. Chem. 2015, 72, 10–26. [Google Scholar] [CrossRef]
- Ibarra, V.G.; Sendón, R.; Bustos, J.; Losada, P.P.; De Quirós, A.R.B. Estimates of dietary exposure of Spanish population to packaging contaminants from cereal based foods contained in plastic materials. Food Chem. Toxicol. 2019, 128, 180–192. [Google Scholar] [CrossRef]
- Kirchkeszner, C.; Petrovics, N.; Nyiri, Z.; Szabó, B.S.; Eke, Z. Role of gas chromatography–single quadrupole mass spectrometry in the identification of compounds migrating from polypropylene-based food contact plastics. Microchem. J. 2022, 181, 107772. [Google Scholar] [CrossRef]
- Osorio, J.; Dreolin, N.; Aznar, M.; Nerín, C.; Hancock, P. Determination of volatile non intentionally added substances coming from a starch-based biopolymer intended for food contact by different gas chromatography-mass spectrometry approaches. J. Chromatogr. A 2019, 1599, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.L.; Honkalampi-Hämäläinen, U.; Weber, A.; Andersson, M.A.; Bertaud, F.; Castle, L.; Dahlman, O.; Hakulinen, P.; Hoornstra, D.; Lhuguenot, J.-C.; et al. The BIOSAFEPAPER project for in vitro toxicity assessments: Preparation, detailed chemical characterisation and testing of extracts from paper and board samples. Food Chem. Toxicol. 2008, 46, 2498–2509. [Google Scholar] [CrossRef] [PubMed]
- Hurd, Maycee. Thermal Oxidative Degradation of Polystyrene Plastics Used for Food Packaging During Incomplete Waste Incineration. Ph.D. Thesis, University of New Mexico, Albuquerque, NM, USA, 2024. Available online: https://digitalrepository.unm.edu/ce_etds/336 (accessed on 5 January 2025).
- Rung, C.; Welle, F.; Gruner, A.; Springer, A.; Steinmetz, Z.; Munoz, K. Identification and evaluation of (non-) intentionally added substances in post-consumer recyclates and their toxicological classification. Recycling 2023, 8, 24. [Google Scholar] [CrossRef]
- Gigli, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Finelli, L.; Munari, A.; Dalla Rosa, M. Fully aliphatic copolyesters based on poly (butylene 1, 4-cyclohexanedicarboxylate) with promising mechanical and barrier properties for food packaging applications. Ind. Eng. Chem. Res. 2013, 52, 12876–12886. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Leanza, M.; Rapisarda, M. Investigations into the characterization, degradation, and applications of biodegradable polymers by mass spectrometry. Mass Spectrom. Rev. 2023, 1–42. [Google Scholar] [CrossRef]
- Yoon, W.J.; Oh, K.S.; Koo, J.M.; Kim, J.R.; Lee, K.J.; Im, S.S. Advanced Polymerization and Properties of Biobased High Tg polyester of Isosorbide and 1,4-Cyclohexanedicarboxylic Acid through in Situ Acetylation. Macromolecules 2013, 46, 2930–2940. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Störmer, Á.; Franz, R. Dialkylketones in Paperboard Food Contact Materials—Method of Analysis in Fatty Foods and Comparative Migration into Liquid Simulants Versus Foodstuffs. Molecules 2020, 25, 915. [Google Scholar] [CrossRef]
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as Well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Available online: http://data.europa.eu/eli/reg/2006/1907/oj (accessed on 8 February 2025).


| Coding | Sample Description | Type of Material | |
|---|---|---|---|
| Internal Side | External Side | ||
| CBV | Green paper straws | Cellulose | Cellulose |
| VBT | Transparent low glass | PS | PS |
| CBX | Ice cream spoon | PLA-based | PLA-based |
| CFR | Colored plastic straws | PLGA-based | PLGA-based |
| VLT | Transparent long glass | PLA | PLA |
| BSC | Pasta packaging | Cellulose | - |
| VCT | Transparent small glass | PS | PS |
| RT (min) | m/z | Compound | IUPAC Name | Formula | CAS No | Applications | SI | RSI | TC | SML (mg/kg) | Samples | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBV | VBT | CBX | CFR | VLT | BSC | VCT | |||||||||||
| 5.14 | 41, 43, 56, 57 | Hexane | Hexane | C6H14 | 110-54-3 | Ink-related compound; degradation product [26,27] | 942 | 957 | I | NI | X | ||||||
| 5.25 | 41, 43, 56, 57 | Alkane | NIAS of chain degradation [28] | 747 | 859 | X | |||||||||||
| 5.49 | 41, 43, 57, 86 | Alkane | NIAS of chain degradation [28] | 759 | 840 | X | |||||||||||
| 7.34 | 43, 45, 60 | Acetic acid | Acetic acid | C2H4O2 | 64-19-7 | Product of PLA degradation [29] | 895 | 946 | ** | X | |||||||
| 8.60 | 43, 71, 74 | Methyl butyrate | Methyl butanoate | C5H10O2 | 623-42-7 | Resins; adhesives in FCMs [30] | 753 | 882 | I | NI | X | X | |||||
| 9.51 | 98, 100 | Toluene-d8 | C7D8 | 2037-26-5 | Internal standard (IS) | 940 | 940 | X | X | X | X | X | X | X | |||
| 9.57 | 91, 92 | Benzene, methyl | Toluene | C7H8 | 108-88-3 | Solvent [31] | 988 | 989 | I | NI | X | ||||||
| 10.33 | 41, 43, 57, 71, 85 | Heptane, 2,4-dimethyl | 2,4-Dimethylheptane | C9H20 | 2213-23-2 | Detected in PLA [32] | 853 | 921 | I | NI | X | ||||||
| 10.65 | 44, 56, 57 | Hexanal | Hexanal | C6H12O | 66-25-1 | VOCs in food contact paperboard [33] | 712 | 904 | I | NI | X | X | X | ||||
| 10.68 | 43, 45 | Propylene glycol | Propane-1,2-diol | C3H8O2 | 57-55-6 | Softener additive for cellulose regeneration [34] | 947 | 954 | ** | X | |||||||
| 11.26 | 43, 57, 71, 85 | Octane, 4-methyl | 4-Methyloctane | C9H20 | 2216-34-4 | VOC [32,35] | 731 | 952 | I | NI | X | X | X | ||||
| 11.75 | 91, 106 | Ethylbenzene | Ethylbenzene | C8H10 | 100-41-4 | Precursor of styrene and PS, VOC [36,37,38,39] | 811 | 956 | I | NI | X | X | |||||
| 11.94 | 40, 91, 106 | p-Xylene | 1,4-Xylene | C8H10 | 106-42-3 | Aromatic compound in PS [40] | 749 | 914 | I | NI | X | X | |||||
| 12.04 | 43, 57, 85 | Nonane | Nonane | C9H20 | 111-84-2 | NIAS of chain degradation [28] | 716 | 852 | I | NI | X | ||||||
| 13.10 | 105, 120 | Benzene, (1-methylethyl) | Cumene | C9H12 | 98-82-8 | Aromatic compound in PS; oxidized product of aliphatic hydrocarbon [38,40] | 813 | 915 | I | NI | X | X | |||||
| 13.10 | 39, 77, 91, 93, 105, 120 | Alpha-pinene | 2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene | C10H16 | 80-56-8 | Formation of terpene resins for adhesives, printing ink compound, coatings, and adhesion agents; monomer [41] | 765 | 835 | ** | X | |||||||
| 13.48 | 41, 55, 69, 83 | 2,2-Dimethyl-3-heptene trans | (E)-2,2-Dimethylhept-3-ene | C9H18 | 19550-75-5 | Identified in mixtures of wood biomass and polypropylene [42] | 712 | 804 | I | NI | X | ||||||
| 13.73 | 43, 57, 70 | 4-Methylhexanal | 4-Methylhexanal | C7H14O | 41065-97-8 | Aldehyde found in paper and board products [43] | 759 | 818 | I | NI | X | ||||||
| 13.75 | 91, 120 | Benzene, propyl | Propylbenzene | C9H12 | 103-65-1 | Styrene polymerization by-product [38] | 954 | 969 | I | NI | X | X | |||||
| 14.05 | 41, 55, 56, 69, 70, 83, 97 | 1-Decene | Dec-1-ene | C10H20 | 872-05-9 | Degradation of biopolymer chain [28] | 819 | 923 | 0.05 | X | |||||||
| 14.30 | 81, 82, 138 | Furan, 2-penthyl | 2-Pentylfuran | C9H14O | 3777-69-3 | Cellulose degradation product [17] | 766 | 950 | III | NI | X | X | |||||
| 14.39 | 77, 78, 115, 117, 118 | Alpha-methylstyrene | Prop-1-en-2-ylbenzene | C9H10 | 98-83-9 | Oxidized products of aliphatic hydrocarbon [38] | 940 | 961 | 0.05 | X | X | ||||||
| 14.60 | 51, 77, 105, 106 | Benzaldehyde | Benzaldehyde | C7H6O | 100-52-7 | Ink-related compound; styrene oxidation product [35,38,44,45] | 953 | 968 | ** | X | X | X | |||||
| 14.91 | 105, 134 | Benzene, 1-methylpropyl | Butan-2-ylbenzene | C10H14 | 135-98-8 | Found in PS [38] | 872 | 934 | I | NI | X | ||||||
| 15.00 | 43, 56, 84 | Octanal | Octanal | C8H16O | 124-13-0 | Odorant compound [46] | 848 | 944 | I | NI | X | X | X | ||||
| 15.42 | 115, 117, 118 | Benzene, 1-propenyl | [(E)-Prop-1-enyl]benzene | C9H10 | 873-66-5 | VOC in PS [40] | 743 | 925 | I | NI | X | ||||||
| 15.56 | 41, 43, 57, 70, 84 | 1-Hexanol, 2-ethyl | 2-Ethylhexan-1-ol | C8H18O | 104-76-7 | Adhesives; by-product of plasticizers; paper manufacturing [17,47,48] | 740 | 866 | 30 | X | |||||||
| 15.78 | 105, 119, 134 | Benzene, 1,3-diethyl | 1,3-Diethylbenzene | C10H14 | 141-93-5 | Aromatic VOC of PS [40] | 786 | 873 | I | NI | X | ||||||
| 15.79 | 111, 146, 148, 150 | 1,4-Dichlorobenzene | 1,4-Dichlorobenzene | C6H4Cl2 | 106-46-7 | Additive in FCM [49] | 726 | 834 | 12 | X | |||||||
| 15.97 | 41, 55, 69, 83, 84, 97 | 4-Undecene, 4-methyl | 4-Methylundec-4-ene | C12H24 | 61142-40-3 | NIAS of chain degradation [28] | 732 | 793 | I | NI | X | ||||||
| 16.13 | 43, 57, 71, 84, 85 | Decane, 5,6-dimethyl | 5,6-Dimethyldecane | C12H26 | 1636-43-7 | NIAS of chain degradation [28] | 746 | 805 | I | NI | X | ||||||
| 16.28 | 65, 91, 92, 120 | Benzeneacetaldehyde | 2-Phenylacetaldehyde | C8H8O | 122-78-1 | Styrene-oxidized products [38] | 801 | 922 | I | NI | X | X | |||||
| 16.29 | 41, 55, 69, 70, 83, 84 | 3-Decene, 2,2-dimethyl | (E)-2,2-Dimethyldec-3-ene | C12H24 | 55499-02-0 | NIAS of chain degradation [28] | 789 | 814 | I | NI | X | ||||||
| 16.52 | 55, 70, 83 | 2-Decene, 2,4-dimethyl | 2,4-Dimethyldec-2-ene | C12H24 | 74421-03-7 | NIAS of chain degradation [28] | 704 | 770 | I | NI | X | ||||||
| 16.73 | 77, 105, 120 | Acetophenone | 1-Phenylethanone | C8H8O | 98-86-2 | Monomer; styrene degradation product [38,44,50] | 867 | 928 | I | NI | X | X | |||||
| 16.82 | 55, 69, 83, 168 | 5-Undecene, 5-methyl | 5-Methylundec-5-ene | C12H24 | 31613-73-7 | NIAS of chain degradation [28] | 764 | 805 | I | NI | X | ||||||
| 17.05 | 119, 134 | 1,2,4,5-Tetramethylbenzene | 1,2,4,5-Tetramethylbenzene | C10H14 | 95-93-2 | VOC in food contact cardboard [51] | 830 | 932 | I | NI | X | ||||||
| 17.39 | 180, 182 | 1,2,3-Trichlorobenzene | 1,2,3-Trichlorobenzene | C6H3Cl3 | 87-61-6 | Printing inks, lacquers, resin, and pigments [52] | 858 | 951 | III | NI | X | X | X | ||||
| 17.43 | 43, 55, 70, 83 | 1-Hexanol, 2-ethyl, acetate | 2-Ethylhexyl acetate | C10H20O2 | 103-09-3 | Adhesives used in FCM [53,54] | 871 | 927 | I | NI | X | ||||||
| 17.56 | 117, 132 | 1-Allyl-2-methylbenzene | 1-Methyl-2-prop-2-enylbenzene | C10H12 | 1587-04-8 | Found in wood [55] | 717 | 818 | I | NI | X | ||||||
| 17.60 | 59, 100, 101, 129 | Pentanedioic acid, dimethyl ester (dimethyl glutarate) | Dimethyl pentanedioate | C7H12O4 | 1119-40-0 | Used in polyester paints, varnishes, lacquers, solvents, resins, and plasticizers [56] | 749 | 915 | I | NI | X | ||||||
| 17.61 | 45, 88, 89 | Propanoic acid, 2-hydroxy-methyl ester (methyl lactate) | Methyl 2-hydroxypropanoate | C4H8O3 | 547-64-8 | PLA degradation product [57] | 724 | 732 | I | NI | X | X | |||||
| 17.88 | 43, 57, 71, 85 | Dodecane | Dodecane | C12H26 | 112-40-3 | NIAS from chain degradation [28] | 895 | 935 | I | NI | X | X | |||||
| 18.40 | 51, 77, 103, 104, 132 | 2-Phenylpropenal | 2-Phenylprop-2-enal | C9H8O | 4432-63-7 | Styrene oxidation product [38] | 749 | 931 | I | NI | X | ||||||
| 18.74 | 41, 57, 70, 82 | Decanal | Decanal | C10H20O | 112-31-2 | Aliphatic hydrocarbons oxidation product in PS [38] | 832 | 911 | I | NI | X | X | |||||
| 18.83 | 127, 128, 130 | Naphthalene | Naphthalene | C10H8 | 91-20-3 | Plastic manufacturing [17] | 750 | 910 | III | NI | X | ||||||
| 18.85 | 55, 57, 70 | 2-Ethylhexyl acrylate | 2-Ethylhexyl prop-2-enoate | C11H20O2 | 103-11-7 | Acrylic monomer for solvent-free photopolymerizable paper coating [58] | 761 | 796 | 0.05 | X | |||||||
| 19.68 | 45, 56 | 1,4-Dioxane-2,5-dione, 3,6-dimethyl (DL-Lactide) | 3,6-Dimethyl-1,4-dioxane-2,5-dione | C6H8O4 | 95-96-5 | PLA oligomer [28] | 943 | 950 | I | NI | X | X | X | ||||
| 21.15 | 43, 57, 71, 85 | Tetradecane | Tetradecane | C14H30 | 629-59-4 | Alkane; possible lubricant [14,34,38,59] | 751 | 915 | I | NI | X | X | X | ||||
| 21.60 | 91, 94, 105, 119, 133, 161, 189 | Longicyclene | (2S,9S)-2,6,6,9-Tetramethyltetracyclo[5.4.0.02,9.08,10]undecane | C15H24 | 1137-12-8 | “Set-off” ink migration in carboard cups and water-borne coating paper [60,61] | 818 | 878 | I | NI | X | ||||||
| 21.77 | 42, 56, 70, 83, 89, 98 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate (TXIB) | [2,2,4-Trimethyl-3-(2-methylpropanoyloxy)pentyl] 2-methylpropanoate | C16H30O4 | 6846-50-0 | Plasticizer and ink solvent [62] | 843 | 846 | 5 | X | |||||||
| 22.00 | 42, 56, 70, 89 | Propanoic acid, 2-methyl, 3-hydroxy-2,2,4-trimethylpentyl ester | (3-Hydroxy-2,2,4-trimethylpentyl) 2-methylpropanoate | C12H24O3 | 77-68-9 | Coalescent agent [63] | 917 | 918 | II | NI | X | ||||||
| 22.11 | 43, 57, 71, 85 | Eicosane | Icosane | C20H42 | 112-95-8 | VOC found in recycled cellulose [34] | 861 | 917 | I | NI | X | ||||||
| 22.64 | 43, 57, 71, 85 | Pentadecane | Pentadecane | C15H32 | 629-62-9 | VOC found in recycled cellulose [34] | 860 | 939 | I | NI | X | X | |||||
| 23.24 | 43, 57, 71, 85 | Alkane | NIAS of chain degradation [28] | 790 | 865 | X | |||||||||||
| 23.54 | 43, 57, 71 | 2-Methylpentadecane | 2-Methylpentadecane | C16H34 | 1560-93-6 | VOC found in recycled cellulose [34] | 810 | 850 | I | NI | X | ||||||
| 23.66 | 41, 55, 57, 71, 83, 85 | 3-Methylpentadecane | 3-Methylpentadecane | C16H34 | 2882-96-4 | VOC found in recycled cellulose [34] | 754 | 807 | I | NI | X | X | |||||
| 24.04 | 41, 43, 57, 71 | Hexadecane | Hexadecane | C16H34 | 544-76-3 | NIAS of chain degradation [17,28,64] | 877 | 937 | I | NI | X | X | |||||
| 24.89 | 54, 55, 84, 100, 129 | 1,6-Dioxacyclododecane-7,12-dione | 1,6-Dioxacyclododecane-7,12-dione | C10H16O4 | 777-95-7 | VOC found in biomaterials; degradation product in resins [46,65,66] | 738 | 828 | I | NI | X | ||||||
| 25.37 | 43, 57, 71, 85 | Alkane | NIAS of chain degradation [28] | 902 | 933 | X | X | ||||||||||
| 25.80 | 91, 92, 105, 196 | Benzene, 1,1′-(1,3-propanediyl)bis | 3-Phenylpropylbenzene | C15H16 | 1081-75-0 | Isomer of the styrene dimers [67] | 820 | 893 | III | NI | X | ||||||
| 26.63 | 91, 104, 130, 208 | 2,4-Diphenyl-1-butene | 3-Phenylbut-3-enylbenzene | C16H16 | 16606-47-6 | By-product of styrene polymerization [38,68] | 736 | 938 | III | NI | X | X | |||||
| 26.90 | 55, 77, 81, 99, 105 | Methanone, (1-hydroxycyclohexyl)phenyl (Irgacure 184) | (1-Hydroxycyclohexyl)-phenylmethanone | C13H16O2 | 947-19-3 | Photoinitiators for curing UV inks [44,69] | 780 | 927 | I | NI | X | ||||||
| 27.06 | 104 | Benzene, 1, 1′-(1,2-cyclobutanediyl)bis, trans | [(1R,2R)-2-Phenylcyclobutyl]benzene | C16H16 | 20071-09-4 | By-product of styrene polymerization [38] | 906 | 920 | III | NI | X | X | |||||
| 27.36 | 130, 180, 207, 208 | 1-Phenyl-1,2,3,4-tetrahydronaphthalene | 1-Phenyl-1,2,3,4-tetrahydronaphthalene | C16H16 | 3018-20-0 | Co-monomer [44,45] | 818 | 897 | III | NI | X | ||||||
| 28.46 | 149, 150, 223 | Diisobutyl phtalate (DIBP) | Bis(2-methylpropyl) benzene-1,2-dicarboxylate | C16H22O4 | 84-69-5 | Plasticizer; printing inks; solvent to maintain color [45] | 787 | 931 | I | NI | X | ||||||
| 28.47 | 74, 87, 149 | Hexadecanoic acid, methyl ester | Methyl hexadecanoate | C17H34O2 | 112-39-0 | Intermediary for emulsifiers, stabilizers, resins, and plasticizers [17,64] | 820 | 927 | I | NI | X | ||||||
| RT (min) | m/z | Compound | IUPAC Name | Formula | CAS No | Applications | SI | RSI | TC | SML (mg/kg) | Samples | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBV | VBT | CBX | CFR | VLT | BSC | VCT | |||||||||||
| 8.54 | 45, 59, 72 | Diethylene glycol monoethyl ether | 2-(2-Ethoxyethoxy)ethanol | C6H14O3 | 111-90-0 | Adhesives, paints, dyes, inks, and surface coatings [72,73] | 918 | 925 | I | NI | X | X | |||||
| 8.97 | 42, 45, 59, 89 | 2-Propanol, 1,1′-oxybis | 1-(2-Hydroxypropoxy)propan-2-ol | C6H14O3 | 110-98-5 | Additive; monomer [74] | 799 | 862 | ** | X | |||||||
| 9.04 | 41, 43, 55, 57, 70, 83 | 1-Hexanol, 2-ethyl | 2-Ethylhexan-1-ol | C8H18O | 104-76-7 | Adhesives; degradation product of plasticizers; paper manufacturing [17,47,48] | 947 | 965 | 30 | X | |||||||
| 9.28 | 41, 45, 59, 103 | Polypropylene glycol | 1-(1-Butoxypropan-2-yloxy)propan-2-ol | C6H14O3 | 25322-69-4 | Adhesives; coating [54,72,75] | 735 | 797 | ** | X | |||||||
| 10.65 | 45, 88, 89 | Methyl lactate | Methyl 2-hydroxypropanoate | C4H8O3 | 547-64-8 | PLA degradation product [57] | 785 | 790 | I | NI | X | X | |||||
| 11.25 | 43, 56, 57 | 1,3-Pentanediol, 2,2,4-trimethyl | 2,2,4-Trimethylpentane-1,3-diol | C8H18O2 | 144-19-4 | Metabolite for the plasticizer TXIB [76] | 901 | 924 | II | NI | X | ||||||
| 11.66 | 43, 45, 56 | 1,4-Dioxane-2,5-dione, 3,6-dimethyl (DL-Lactide) | 3,6-Dimethyl-1,4-dioxane-2,5-dione | C6H8O4 | 95-96-5 | PLA oligomer [28] | 956 | 956 | I | NI | X | X | X | ||||
| 11.75 | 45, 74 | Lactic acid | 2-Hydroxypropanoic acid | C3H6O3 | 50-21-5 | Production of PLA [77] | 707 | 820 | ** | X | |||||||
| 11.81 | 41, 45, 57 | 2-(2-Butoxyethoxy)Ethanol | 2-(2-Butoxyethoxy)ethanol | C8H18O3 | 112-34-5 | Solvents; lacquers; inks [48,69] | 893 | 908 | I | NI | X | X | |||||
| 12.37 | 77, 94, 138 | Ethanol, 2-phenoxy | 2-Phenoxyethanol | C8H10O2 | 122-99-6 | Solvent for cellulose acetate, dyes, inks, resins, and in the synthesis of plasticizers [78] | 869 | 907 | I | NI | X | ||||||
| 12.72 | 42, 140 | Methenamine * | 1,3,5,7-Tetrazatricyclo[3.3.1.13,7]decane | C6H12N4 | 100-97-0 | Pesticides; catalytic agents; cross-linking agents [79] | 853 | 915 | 15 | X | |||||||
| 13.08 | 43, 60, 115 | Nonanoic acid | Nonanoic acid | C9H18O2 | 112-05-0 | Adhesive FCMs [17] | 748 | 861 | I | NI | X | X | |||||
| 14.19 | 45, 57, 71 | Methoxyacetic acid, 2-ethylhexyl ester | 2-Ethylhexyl 2-methoxyacetate | C11H22O3 | NA | 859 | 860 | III | NI | X | |||||||
| 14.42 | 43, 71, 83 | (1-Hydroxy-2,4,4-trimethylpentan-3-yl) 2-methylpropanoate | (1-Hydroxy-2,4,4-trimethylpentan-3-yl) 2-methylpropanoate | C12H24O3 | 74367-33-2 | Ink plasticizer [44] | 721 | 864 | I | NI | X | ||||||
| 14.43 | 43, 71, 83, 89, 98 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate (TXIB) | [2,2,4-Trimethyl-3-(2-methylpropanoyloxy)pentyl] 2-methylpropanoate | C16H30O4 | 6846-50-0 | Plasticizer; ink solvent [80] | 901 | 902 | 5 | X | |||||||
| 14.59 | 41, 60, 73 | N-decanoic acid | Decanoic acid | C10H20O2 | 334-48-5 | Additive; monomer [11] | 811 | 860 | ** | X | |||||||
| 14.77 | 41, 43, 56, 71, 89 | Propanoic acid, 2-methyl, 3-hydroxy-2,2,4-trimethylpentyl ester | (3-Hydroxy-2,2,4-trimethylpentyl) 2-methylpropanoate | C12H24O3 | 77-68-9 | PLA coating for paper and cardboard [81] | 923 | 923 | II | NI | X | X | |||||
| 15.13 | 41, 43, 57, 71, 85 | Tetradecane * | Tetradecane | C14H30 | 629-59-4 | Lubricant [14,59] | 955 | 966 | I | NI | X | ||||||
| 15.25 | 43, 109, 151 | 2,4,7,9-Tetramethyl-5-decyn-4,7-diol | 2,4,7,9-Tetramethyldec-5-yne-4,7-diol | C14H26O2 | 126-86-3 | Surfactant in water-based printing inks [82] | 866 | 882 | III | NI | X | ||||||
| 16.19 | 43, 55, 69 | 1-Dodecanol | Dodecan-1-ol | C12H26O | 112-53-8 | Paper manufacturing [17] | 874 | 941 | I | NI | X | X | |||||
| 16.63 | 45, 89 | Tetraethylene glycol | 2-[2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy]ethanol | C8H18O5 | 112-60-7 | Solvent; oligomer of the plasticizer polyethylene glycol (PEG) [44,83,84] | 942 | 942 | ** | X | X | ||||||
| 16.66 | 57, 191, 206 | 2,4-Di-tert-butylphenol | 2,4-Ditert-butylphenol | C14H22O | 96-76-4 | Antioxidant degradation product [44,64,65] | 889 | 906 | I | NI | X | ||||||
| 17.27 | 41, 54, 55 | 1,6-Dioxacyclododecane-7,12-dione | 1,6-Dioxacyclododecane-7,12-dione | C10H16O4 | 777-95-7 | PBAT (polybutylene adipate-co-terephthalate) cyclic oligomer [85] | 913 | 913 | I | NI | X | ||||||
| 17.37 | 41, 60, 73 | Dodecanoic acid | Dodecanoic acid | C12H24O2 | 143-07-7 | Additive; monomer; antimicrobial agent; adhesive [11,17,44] | 822 | 860 | ** | X | |||||||
| 17.74 | 153, 181 | Diethyl phthalate-3,4,5,6-d4 | C12H10D4O4 | 93952-12-6 | Internal standard (IS) | 878 | 933 | X | X | X | X | X | X | X | |||
| 17.75 | 149, 177 | Diethyl phtalate (DEP) * | Diethyl benzene-1,2-dicarboxylate | C12H14O4 | 84-66-2 | Plasticizer; printing ink; solvent to maintain color [45,64,86] | 857 | 950 | I | NI | X | X | X | X | X | ||
| 17.90 | 41, 43, 57, 71, 85 | Hexadecane | Hexadecane | C16H34 | 544-76-3 | NIAS from chain degradation [28] | 935 | 935 | I | NI | X | ||||||
| 17.91 | 91, 131, 176 | 3-Pentenoic acid, 4-phenyl | (E)-4-Phenylpent-3-enoic acid | C11H12O2 | 53774-19-9 | Antioxidant degradation product [44] | 756 | 888 | I | NI | X | ||||||
| 18.00 | 41, 43, 45, 57 | Methoxyacetic acid, decyl ester | Decyl 2-methoxyacetate | C13H26O3 | 259141-02-1 | 807 | 821 | III | NI | X | |||||||
| 18.16 | 146, 160, 235 | 2,6-Diisopropylphenylcarbamic acid methyl ester | Methyl N-[2,6-di(propan-2-yl)phenyl]carbamate | C14H21NO2 | 39076-23-8 | Additive [87] | 834 | 836 | I | NI | X | ||||||
| 18.69 | 91, 92, 105 | Benzene, 1,1′-(1,3-propanediyl)bis | 3-Phenylpropylbenzene | C15H16 | 1081-75-0 | Isomer of the styrene dimers [67] | 831 | 934 | III | NI | X | X | |||||
| 18.74 | 43, 95, 121 | Alpha-cadinol | (1R,4S,4aR,8aR)-1,6-Dimethyl-4-propan-2-yl-3,4,4a,7,8,8a-hexahydro-2H-naphthalen-1-ol | C15H26O | 481-34-5 | Adhesives; printing inks; coatings; adhering agents [88] | 851 | 885 | III | NI | X | ||||||
| 18.83 | 65, 91, 155, 171 | p-Toluenesulfonamide | 4-Methylbenzenesulfonamide | C7H9NO2S | 70-55-3 | Adhesives [72] | 906 | 927 | III | NI | X | ||||||
| 18.88 | 43, 55, 69, 83 | 1-Tetradecanol | Tetradecan-1-ol | C14H30O | 112-72-1 | Resin monomers; precursors; raw materials [26,89] | 898 | 935 | I | NI | X | ||||||
| 18.89 | 43, 55, 57, 69 | 1-Tridecanol | Tridecan-1-ol | C13H28O | 26248-42-0 | Lubricant; precursor of surfactants; plasticizers [90] | 860 | 935 | I | NI | X | ||||||
| 18.93 | 45, 57, 71, 74, 89 | Methoxyacetic acid family | 772 | 790 | X | ||||||||||||
| 19.19 | 77, 81, 99 | 1-Hydroxycyclohexyl phenyl ketone | (1-Hydroxycyclohexyl)-phenylmethanone | C13H16O2 | 947-19-3 | Photoinitiator; paints; printing inks [44,69] | 926 | 949 | I | NI | X | ||||||
| 19.2 | 78, 104 | Benzene, 1,1′-(1,2-cyclobutanediyl)bis, cis | [(1S,2R)-2-Phenylcyclobutyl]benzene | C16H16 | 7694-30-6 | Antioxidant degradation product; by-products of styrene polymerization [38,44] | 733 | 855 | III | NI | X | ||||||
| 19.46 | 74, 87, 143, 199 | Methyl tetradecanoate | Methyl tetradecanoate | C15H30O2 | 124-10-7 | Slip agent [64] | 921 | 953 | I | NI | X | ||||||
| 19.50 | 91, 104, 130 | 2,4-Diphenyl-1-butene | 3-Phenylbut-3-enylbenzene | C16H16 | 16606-47-6 | By-product of styrene polymerization [38,68] | 738 | 951 | III | NI | X | X | |||||
| 19.65 | 107, 135, 147, 178 | Coniferyl aldehyde | (E)-3-(4-Hydroxy-3-methoxyphenyl)prop-2-enal | C10H10O3 | 458-36-6 | Lignin precursor [91,92] | 754 | 793 | I | NI | X | ||||||
| 19.69 | 91, 124, 137, 180 | Coniferyl alcohol | 4-[(E)-3-Hydroxyprop-1-enyl]-2-methoxyphenol | C10H12O3 | 458-35-5 | Lignin precursor [91] | 850 | 945 | I | NI | X | ||||||
| 19.87 | 78, 103, 104 | Benzene, 1,1′-(1,2-cyclobutanediyl)bis trans | [(1R,2R)-2-Phenylcyclobutyl]benzene | C16H16 | 20071-09-4 | Monomer by-product [44] | 919 | 923 | III | NI | X | X | |||||
| 19.89 | 43, 55, 73, 129, 185 | Myristic acid | Tetradecanoic acid | C14H28O2 | 544-63-8 | Lubricant; adhesive; paper manufacturing [17,32,64] | 879 | 907 | ** | X | X | X | X | X | |||
| 20.04 | 43, 45, 57, 71, 85 | 3-Methylheptadecane | 3-Methylheptadecane | C18H38 | 6418-44-6 | Lubricant [44] | 824 | 884 | I | NI | X | ||||||
| 20.12 | 130, 179, 208 | Naphthalene, 1,2,3,4-tetrahydro-1-phenyl | 1-Phenyl-1,2,3,4-tetrahydronaphthalene | C16H16 | 3018-20-0 | Co-monomer [44,45] | 841 | 913 | III | NI | X | X | X | ||||
| 20.15 | 45, 89 | Pentaethylene glycol | 2-[2-[2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy]ethoxy]ethanol | C10H22O6 | 4792-15-8 | Polyester resin; PEG oligomer; plasticizer [32,83,84] | 957 | 957 | I | NI | X | ||||||
| 20.38 | 43, 57, 71, 85 | Alkane | NIAS of chain degradation [28] | 787 | 903 | I | NI | X | X | X | |||||||
| 20.41 | 91, 128, 206 | Naphthalene, 1,2-dihydro-4-phenyl | 4-Phenyl-1,2-dihydronaphthalene | C16H14 | 7469-40-1 | Residue of polymerization in recycled PS [68] | 799 | 897 | III | NI | X | X | |||||
| 20.49 | 120, 121, 138 | 2-Ethylhexyl salicylate | 2-Ethylhexyl 2-hydroxybenzoate | C15H22O3 | 118-60-5 | Photoinitiator; UV filter [64,93] | 793 | 916 | I | NI | X | ||||||
| 20.65 | 43, 55, 57, 102 | Isopropyl myristate | Propan-2-yl tetradecanoate | C17H34O2 | 110-27-0 | Cellulose plasticizer, pigment dispersant, and binding agent [60,64] | 759 | 814 | I | NI | X | ||||||
| 21.05 | 41, 43, 55, 60, 73 | Pentadecanoic acid | Pentadecanoic acid | C15H30O2 | 1002-84-2 | Adhesives; paper/board packaging manufacturing [34] | 812 | 830 | I | NI | X | ||||||
| 21.09 | 41, 57, 149 | Diisobutyl phthalate (DIBP) * | Bis(2-methylpropyl) benzene-1,2-dicarboxylate | C16H22O4 | 84-69-5 | Plasticizer; printing inks; solvent to maintain color [17,45] | 858 | 913 | I | NI | X | X | |||||
| 21.31 | 43, 55, 57, 83 | 1-Hexadecanol | Hexadecan-1-ol | C16H34O | 36653-82-4 | Adhesives; plasticizer; ionic surfactant; foam stabilizer [26,61,94] | 862 | 928 | ** | X | X | X | X | ||||
| 21.53 | 43, 57, 71 | Alkane | NIAS of chain degradation [28] | 701 | 855 | X | |||||||||||
| 21.62 | 57, 175, 205, 217 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | 7,9-Ditert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione | C17H24O3 | 82304-66-3 | Antioxidant Irganox 1010 degradation product [47] | 842 | 879 | III | NI | X | X | |||||
| 21.79 | 43, 74, 87 | Hexadecanoic acid, methyl ester | Methyl hexadecanoate | C17H34O2 | 112-39-0 | Intermediary for emulsifiers, stabilizers, resins, and plasticizers [64] | 934 | 944 | I | NI | X | X | X | ||||
| 22.21 | 43, 55, 60, 73, 129 | Palmitic acid | Hexadecanoic acid | C16H32O2 | 57-10-3 | Varnishes; slip agent; adhesives; lubricant in paper manufacturing [17,46,47,64] | 925 | 930 | ** | X | X | X | X | X | |||
| 22.55 | 43, 45, 57, 71 | Diethylene glycol monododecyl ether | 2-(2-Dodecoxyethoxy)ethanol | C16H34O3 | 3055-93-4 | Adhesives [72] | 794 | 844 | I | NI | X | ||||||
| 22.64 | 43, 57, 71, 85 | Alkane | NIAS of chain degradation [28] | 921 | 927 | X | |||||||||||
| 22.86 | 57, 79, 107, 134 | Juvabione | Methyl (4R)-4-[(2R)-6-methyl-4-oxoheptan-2-yl]cyclohexene-1-carboxylate | C16H26O3 | 17904-27-7 | Natural compound in some kinds of wood [84] | 836 | 844 | I | NI | X | ||||||
| 23.26 | 41, 43, 57, 73, 129 | Heptadecanoic acid | Heptadecanoic acid | C17H34O2 | 506-12-7 | Adhesives; paper manufacturing [17] | 737 | 894 | I | NI | X | ||||||
| 23.28 | 45, 89 | Ethylene glycol family | Plasticizer for PLA [84] | 936 | 936 | X | |||||||||||
| 23.37 | 69, 81, 95, 137 | 13-Epimanool | (3S)-5-[(1S,4aS,8aS)-5,5,8a-Trimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl]-3-methylpent-1-en-3-ol | C20H34O | 1438-62-6 | Natural component in wood and bark [86] | 902 | 910 | III | NI | X | ||||||
| 23.52 | 41, 43, 55, 69, 83, 97 | Heptadecanol | Heptadecan-1-ol | C17H36O | 1454-85-9 | Plasticizer [26,95] | 923 | 957 | I | NI | X | X | X | X | |||
| 23.63 | 67, 81, 95 | Methyl linolelaidate | Methyl (9E,12E)-octadeca-9,12-dienoate | C19H34O2 | 2566-97-4 | Fatty acid methyl esters (FAMEs) [96] | 789 | 808 | I | NI | X | ||||||
| 23.75 | 41, 55, 69, 83, 97, 264 | 11-Octadecenoic acid, methyl ester | Methyl (E)-octadec-11-enoate | C19H36O2 | 52380-33-3 | FAMEs plasticizers [97,98] | 935 | 938 | I | NI | X | ||||||
| 23.94 | 43, 74, 87 | Methyl stearate | Methyl octadecanoate | C19H38O2 | 112-61-8 | Solvent; defoamers; slip agent [44,50,99] | 914 | 917 | I | NI | X | X | X | ||||
| 24.05 | 41; 55; 69 | Oleic acid | (Z)-Octadec-9-enoic acid | C18H34O2 | 112-80-1 | Lubricant; paper manufacturing; adhesives [17,47] | 800 | 811 | ** | X | X | ||||||
| 24.29 | 112, 156, 158 | Tributyl aconitate | Tributyl (Z)-prop-1-ene-1,2,3-tricarboxylate | C18H30O6 | 7568-58-3 | Plasticizer by-product [100] | 920 | 939 | I | NI | X | ||||||
| 24.30 | 41, 43, 57, 60, 73 | Stearic acid | Octadecanoic acid | C18H36O2 | 57-11-4 | Lubricant; slip agent in adhesives [32] | 916 | 925 | ** | X | X | ||||||
| 24.50 | 41, 57, 129, 185 | Tributyl citrate | Tributyl 2-hydroxypropane-1,2,3-tricarboxylate | C18H32O7 | 77-94-1 | Plasticizer [64] | 906 | 920 | III | NI | X | ||||||
| 24.70 | 43, 57, 71, 85 | N-docosane | Docosane | C22H46 | 629-97-0 | Adhesives [54] | 867 | 924 | I | NI | X | ||||||
| 24.95 | 55, 57, 70, 112 | Bis(2-ethylhexyl) fumarate (DEHF) | Bis(2-ethylhexyl) (E)-but-2-enedioate | C20H36O4 | 141-02-6 | Plasticizer [86] | 906 | 906 | I | NI | X | ||||||
| 25.13 | 129, 185, 259 | Tributyl acetylcitrate (ATBC) * | Tributyl 2-acetyloxypropane-1,2,3-tricarboxylate | C20H34O8 | 77-90-7 | Plasticizer [64,84] | 920 | 933 | 60 expressed as the sum of group substances | X | X | X | |||||
| 25.20 | 45, 72, 87, 100 | Dimethylpalmitamide | N,N-Dimethylhexadecanamide | C18H37NO | 3886-91-7 | Polyamide blend films [31] | 897 | 900 | III | NI | X | ||||||
| 25.33 | 43, 45, 57, 71, 89 | Triethylene glycol monododecyl ether | 2-[2-(2-Dodecoxyethoxy)ethoxy]ethanol | C18H38O4 | 3055-94-5 | Ink [101] | 776 | 815 | I | NI | X | ||||||
| 25.51 | 159, 173, 185, 269, 284 | Dehydroabietal | (1R,4aS,10aR)-1,4a-Dimethyl-7-propan-2-yl-2,3,4,9,10,10a-hexahydrophenanthrene-1-carbaldehyde | C20H28O | 13601-88-2 | Degradation product of adhesives [86] | 892 | 897 | III | NI | X | ||||||
| 25.56 | 43, 55, 57, 69, 83 | 1-Octadecanol | Octadecan-1-ol | C18H38O | 112-92-5 | Adhesives; paper manufacturing; lubricant; ink solvent [17,26,102] | 899 | 916 | I | NI | X | ||||||
| 25.64 | 43, 57, 71, 85 | Alkane | NIAS of chain degradation [28] | 771 | 915 | X | |||||||||||
| 25.85 | 161, 178 | 2-Propenoic acid, 3-(4-methoxyphenyl), 2-ethylhexyl ester | 2-Ethylhexyl (E)-3-(4-methoxyphenyl)prop-2-enoate | C18H26O3 | 5466-77-3 | UV filter [64] | 914 | 927 | I | NI | X | ||||||
| 25.91 | 43, 55, 74, 87 | Eicosanoic acid, methyl ester | Methyl icosanoate | C21H42O2 | 1120-28-1 | FAME [103] | 812 | 855 | I | NI | X | ||||||
| 25.93 | 91, 105, 129, 207 | R-methyl phenyl sulfoxide | Possible degradation product of PS [104] | 702 | 753 | X | X | ||||||||||
| 26.04 | 45, 89 | Ethylene glycol family | Possible plasticizer for PLA [84] | 951 | 951 | X | |||||||||||
| 26.13 | 239, 240, 241 | Methyl dehydroabietate | Methyl (1R,4aS,10aR)-1,4a-dimethyl-7-propan-2-yl-2,3,4,9,10,10a-hexahydrophenanthrene-1-carboxylate | C21H30O2 | 1235-74-1 | Cellulose derivative, varnishes, printing inks, and adhesives [64,69,86] | 886 | 893 | I | NI | X | ||||||
| 26.15 | 58, 100, 115 | Dodecanamide, N,N-diethyl | N,N-Diethyldodecanamide | C16H33NO | 3352-87-2 | Slipping and anti-blocking agent [105] | 841 | 909 | III | NI | X | ||||||
| 26.22 | 41, 55, 59, 72 | 9-Octadecenamide, (Z) | (Z)-Octadec-9-enamide | C18H35NO | 301-02-0 | Slip agent [46,50] | 770 | 818 | ** | X | |||||||
| 26.46 | 57, 70, 71, 129 | Hexanedioic acid, dioctyl ester (dioctyl adipate) | Dioctyl hexanedioate | C22H42O4 | 123-79-5 | Plasticizer [69] | 721 | 925 | I | NI | X | ||||||
| 26.47 | 55, 57, 70, 129 | Hexanedioic acid, bis(2-ethylhexyl) ester (DEHA) | Bis(2-ethylhexyl) hexanedioate | C22H42O4 | 103-23-1 | Plasticizer [106] | 708 | 924 | 18 (60 expressed as the sum of group substances) | X | |||||||
| 26.58 | 43, 57, 71, 85 | Tetracosane | Tetracosane | C24H50 | 646-31-1 | Lubricant [87] | 859 | 872 | I | NI | X | ||||||
| 26.62 | 45, 57, 85, 125 | Ethanol, 2-butoxy, phosphate | Tris(2-butoxyethyl) phosphate | C18H39O7P | 78-51-3 | Flame retardant, lacquer, paint, and plasticizer [107,108,109] | 877 | 885 | III | NI | X | ||||||
| 26.69 | 149, 161, 164, 177 | Phenol, 2,2′-methylenebis[6-(1,1-dimethylethyl)-4-methyl (Antioxidant 2246) | 2-Tert-butyl-6-[(3-tert-butyl-2-hydroxy-5-methylphenyl)methyl]-4-methylphenol | C23H32O2 | 119-47-1 | Antioxidant [110] | 907 | 918 | 1.5 expressed as the sum of group substances | X | X | X | |||||
| 26.74 | 45, 89 | Ethylene glycol family | Plasticizer for PLA [84] | 821 | 887 | X | |||||||||||
| 26.90 | 45, 57, 90 | Methoxyacetic acid, octadecyl ester family | 784 | 824 | X | ||||||||||||
| 27.04 | 91, 117 | Cyclohexane, 1,3,5-triphenyl | (3,5-Diphenylcyclohexyl)benzene | C24H24 | 28336-57-4 | PS by-product [111] | 711 | 823 | III | NI | X | X | |||||
| 27.20 | 57, 145 | Hexanoic acid, 2-ethyl, hexadecyl ester | Hexadecyl 2-ethylhexanoate | C24H48O2 | 59130-69-7 | Emollient agent [112] | 823 | 877 | I | NI | X | ||||||
| 27.28 | 43, 57, 71, 155 | Bis(2-ethylhexyl)hexahydro phthalate | Bis(2-ethylhexyl) cyclohexane-1,2-dicarboxylate | C24H44O4 | 84-71-9 | Plasticizer [113] | 809 | 826 | I | NI | X | ||||||
| 27.29 | 77, 105, 149 | Diethylene glycol dibenzoate | 2-(2-Benzoyloxyethoxy)ethyl benzoate | C18H18O5 | 120-55-8 | Plasticizer [64,86] | 966 | 968 | I | NI | X | ||||||
| 27.46 | 43, 57, 71, 85 | Pentacosane | Pentacosane | C25H52 | 629-99-2 | Adhesives; lubricant [87,114] | 751 | 917 | I | NI | X | X | X | ||||
| 27.61 | 43, 57, 98, 134, 239 | 2-Palmitoylglycerol | 1,3-Dihydroxypropan-2-yl hexadecanoate | C19H38O4 | 23470-00-0 | Slip agent [96,115] | 835 | 862 | I | NI | X | ||||||
| 27.74 | 149, 167 | Di(2-ethylhexyl) phthalate (DEHP) | Bis(2-ethylhexyl) benzene-1,2-dicarboxylate | C24H38O4 | 117-81-7 | Plasticizer; printing inks; solvent [45,86] | 802 | 913 | 0.6 (60 expressed as the sum of group substances) | X | X | X | |||||
| 27.84 | 43, 59, 255, 315 | 15-Hydroxydehydroabietic acid, methyl ester | Methyl (1R,4aS,10aR)-7-(2-hydroxypropan-2-yl)-1,4a-dimethyl-2,3,4,9,10,10a-hexahydrophenanthrene-1-carboxylate | C21H30O3 | 29461-23-2 | Oxidation product of Pinaceae resin [116] | 805 | 822 | I | NI | X | ||||||
| 27.86 | 91, 129, 207 | R-methyl phenyl sulfoxide | Degradation product of PS [104,117] | 812 | 822 | X | X | ||||||||||
| 27.99 | 91, 129, 207 | (2,3-Diphenylcyclopropyl)methyl phenyl sulfoxide | [2-(Benzenesulfinylmethyl)-3-phenylcyclopropyl]benzene | C22H20OS | 131758-71-9 | Degradation product of PS [117,118] | 809 | 813 | III | NI | X | X | |||||
| 28.01 | 43, 45, 55, 57, 91 | Methoxyacetic acid, hexadecyl ester | Hexadecyl 2-methoxyacetate | C19H38O3 | NA | 732 | 835 | III | NI | X | |||||||
| 28.05 | 91, 129, 207 | R-methyl phenyl sulfoxide | Degradation product of PS [104,117] | 803 | 809 | X | X | ||||||||||
| 28.14 | 91, 129, 207 | R-methyl phenyl sulfoxide | Degradation product of PS [104,117] | 813 | 819 | X | X | ||||||||||
| 28.35 | 43, 57, 71, 85 | Alkane | NIAS of chain degradation [28] | 917 | 943 | X | |||||||||||
| 28.51 | 45, 89, 134 | Ethyleneglycol family | Plasticizer for PLA [84] | 894 | 896 | X | |||||||||||
| 28.74 | 57, 71, 127, 155 | Cyclohexanedicarboxylic acid family | Found in PLGA [119,120] | 715 | 718 | X | |||||||||||
| 28.93 | 91, 129, 207 | R-methyl phenyl sulfoxide | Degradation product of PS [104,117] | 724 | 794 | X | X | ||||||||||
| 28.95 | 43, 57, 71, 85, 127, 155 | Cyclohexanedicarboxylic acid family | Monomer [121] | 743 | 759 | X | |||||||||||
| 29.11 | 45, 89 | Ethylene glycol family | Plasticizer for PLA [84] | 814 | 851 | X | |||||||||||
| 29.12 | 91, 129, 207 | R-methyl phenyl sulfoxide | Degradation product of PS [104,117] | 701 | 784 | X | |||||||||||
| 29.13 | 55, 57, 69, 83, 97 | 1-Eicosanol | Icosan-1-ol | C20H42O | 629-96-9 | Lubricant [74,102] | 857 | 908 | I | NI | X | ||||||
| 29.14 | 43, 57, 71, 85 | Heptacosane | Heptacosane | C27H56 | 593-49-7 | Alkane in paper-based food packaging [86] | 751 | 808 | I | NI | X | ||||||
| 29.16-29.63 | 85, 127, 156 | Cyclohexanedicarboxylic acid family | Monomer [121] | 807 | 833 | X | |||||||||||
| 29.65 | 43, 45, 47 | Methoxyacetic acid, octadecyl ester | Octadecyl 2-methoxyacetate | C21H42O3 | NA | 756 | 801 | III | NI | X | |||||||
| 29.78-30.06 | 85, 127, 157 | Cyclohexanedicarboxylic acid family | Monomer [121] | 809 | 835 | X | |||||||||||
| 30.07 | 69, 81 | Squalene | (6E,10E,14E,18E)-2,6,10,15,19,23-Hexamethyltetracosa-2,6,10,14,18,22-hexaene | C30H50 | 111-02-4 | Plasticizer; oxygen scavenger [45,59,64] | 836 | 866 | I | NI | X | X | |||||
| 30.18-30.39 | 81, 126, 155 | Cyclohexanedicarboxylic acid family | Monomer [121] | 809 | 853 | X | |||||||||||
| 31.22 | 43, 57, 229 | Myristyl myristate | Tetradecyl tetradecanoate | C28H56O2 | 3234-85-3 | Ester formed by the reaction between fatty acids used as lubricants and alcohols [96] | 850 | 856 | I | NI | X | ||||||
| 31.27 | 45, 89 | Ethyleneglycol family | Plasticizer for PLA [84] | 806 | 813 | X | |||||||||||
| 33.00 | 43, 57, 229 | Tetradecanoic acid, hexadecyl ester | Hexadecyl tetradecanoate | C30H60O2 | 2599-01-1 | Ester formed by the reaction between fatty acids used as lubricants and alcohols [96] | 822 | 847 | I | NI | X | ||||||
| 34.34 | 57, 71, 239 | Palmitone | Hentriacontan-16-one | C31H62O | 502-73-8 | NIAS of sizing agents in paper and cardboard [86,122] | 818 | 845 | II | NI | X | ||||||
| 35.40 | 43, 55, 57, 257 | Hexadecanoic acid, octadecyl ester | Octadecyl hexadecanoate | C34H68O2 | 2598-99-4 | Ester formed by the reaction between fatty acids used as lubricants and alcohols [96] | 802 | 912 | I | NI | X | ||||||
| 37.37 | 43, 57, 71, 239 | Dialkyl ketone family | NIAS of sizing agents in paper and cardboard [122] | 719 | 782 | X | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Sanvicente, E.; Barbosa-Pereira, L.; Sendón, R.; Rodríguez Bernaldo de Quirós, A.; Lestido-Cardama, A. Identification of Potential Migrants in Food Contact Materials Labeled as Bio-Based and/or Biodegradable by GC-MS. Coatings 2025, 15, 751. https://doi.org/10.3390/coatings15070751
López Sanvicente E, Barbosa-Pereira L, Sendón R, Rodríguez Bernaldo de Quirós A, Lestido-Cardama A. Identification of Potential Migrants in Food Contact Materials Labeled as Bio-Based and/or Biodegradable by GC-MS. Coatings. 2025; 15(7):751. https://doi.org/10.3390/coatings15070751
Chicago/Turabian StyleLópez Sanvicente, Emma, Letricia Barbosa-Pereira, Raquel Sendón, Ana Rodríguez Bernaldo de Quirós, and Antía Lestido-Cardama. 2025. "Identification of Potential Migrants in Food Contact Materials Labeled as Bio-Based and/or Biodegradable by GC-MS" Coatings 15, no. 7: 751. https://doi.org/10.3390/coatings15070751
APA StyleLópez Sanvicente, E., Barbosa-Pereira, L., Sendón, R., Rodríguez Bernaldo de Quirós, A., & Lestido-Cardama, A. (2025). Identification of Potential Migrants in Food Contact Materials Labeled as Bio-Based and/or Biodegradable by GC-MS. Coatings, 15(7), 751. https://doi.org/10.3390/coatings15070751








