Laser-Patterned and Photodeposition Ag-Functionalized TiO2 Grids on ITO Glass for Enhanced Photocatalytic Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
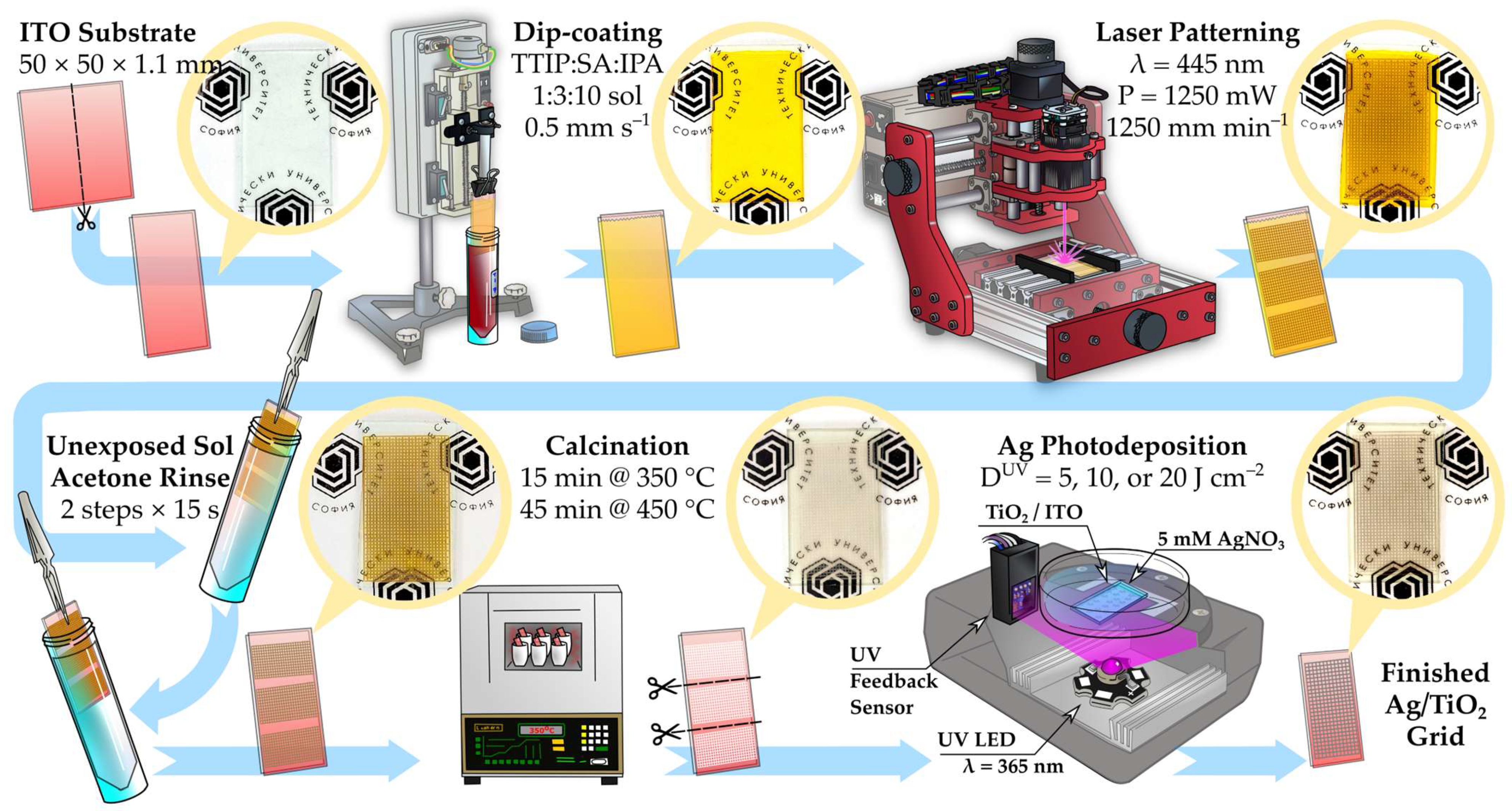
2.2. Fabrication and Ag Functionalization of Patterned TiO2 Layers
2.2.1. Sol–Gel Dip-Coating of the TiO2 Precursor Layer
2.2.2. Laser Patterning of TiO2 Sol Layers
2.2.3. Ag Photodeposition on TiO2 Grid Patterns
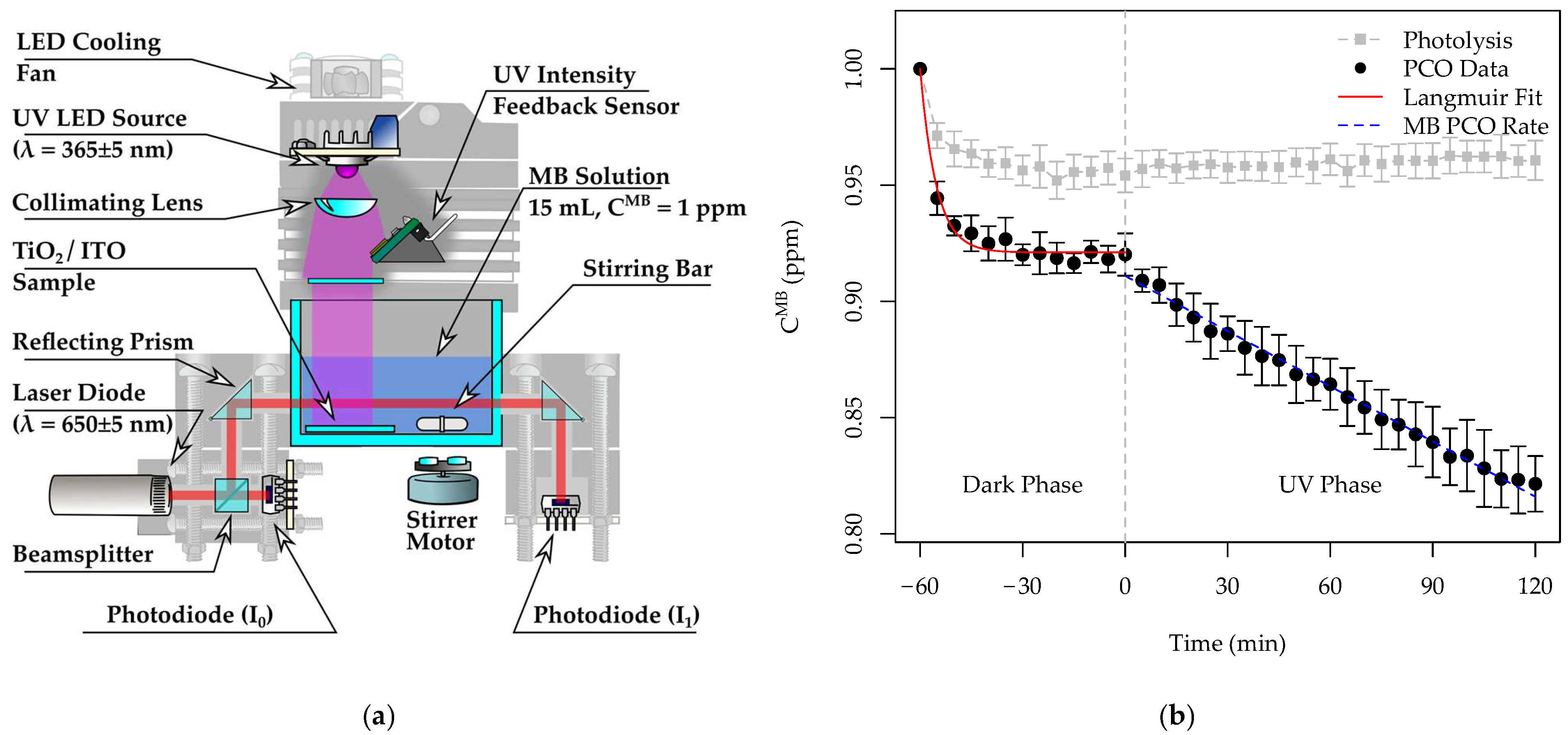
2.3. Methylene Blue Photocatalytic Oxidation Experiments
2.4. Photoelectrochemical Measurements
2.5. Characterization Techniques and Data Analysis Tools
3. Results and Discussion
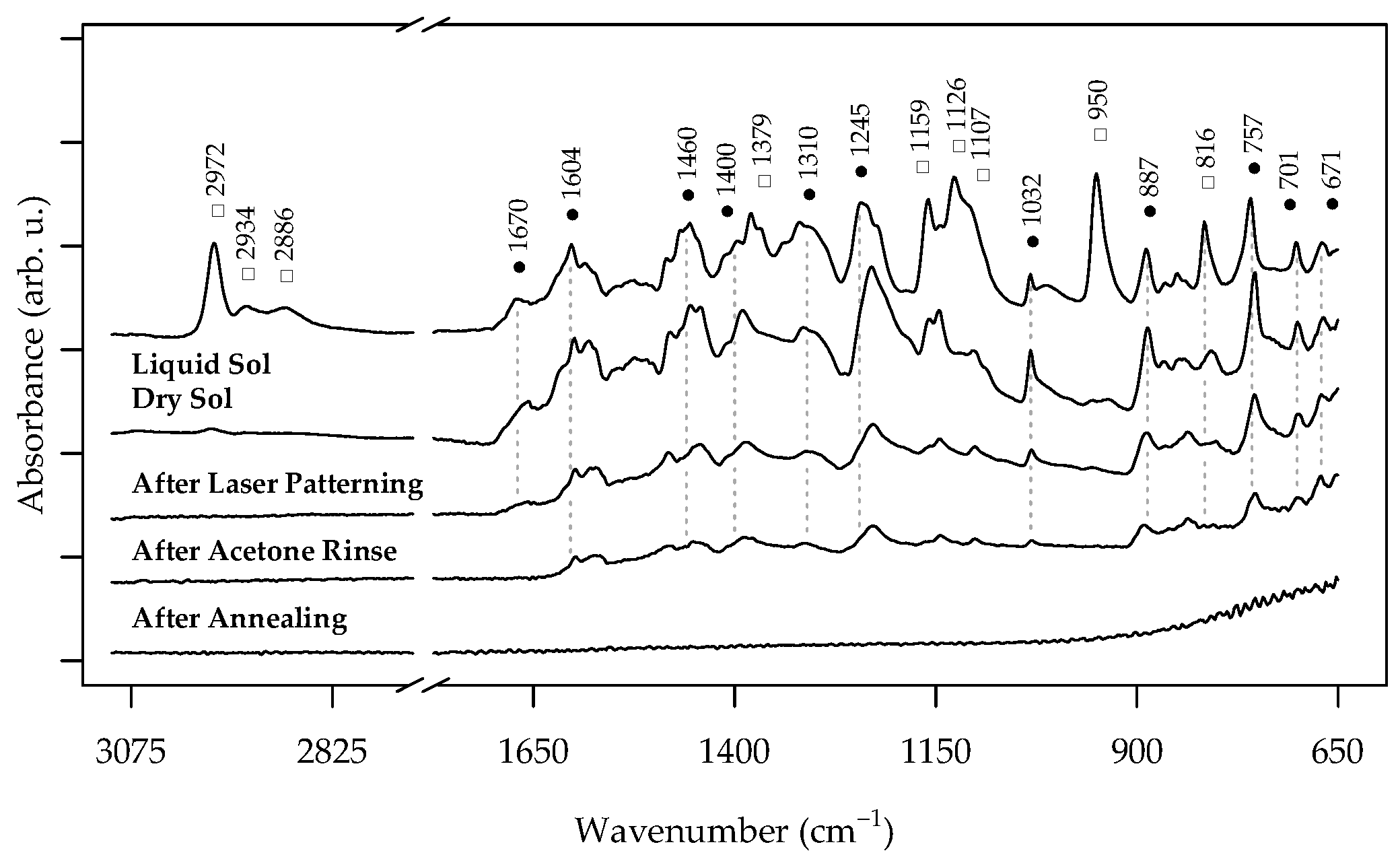
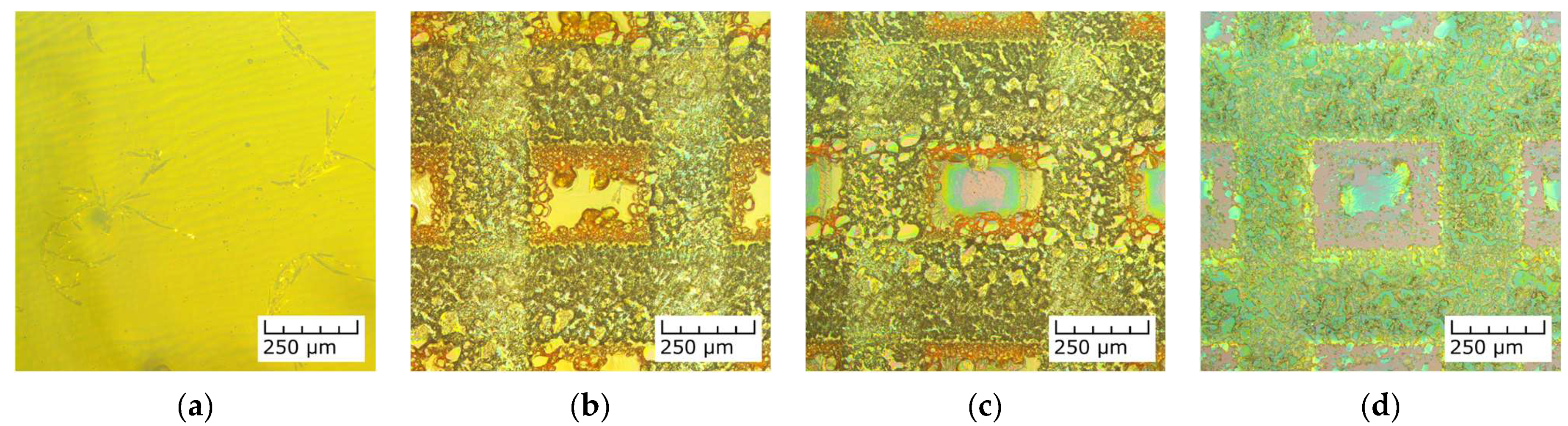
3.1. Laser Interaction with the Salycilate TiO2 Sol Dip-Coated Layers
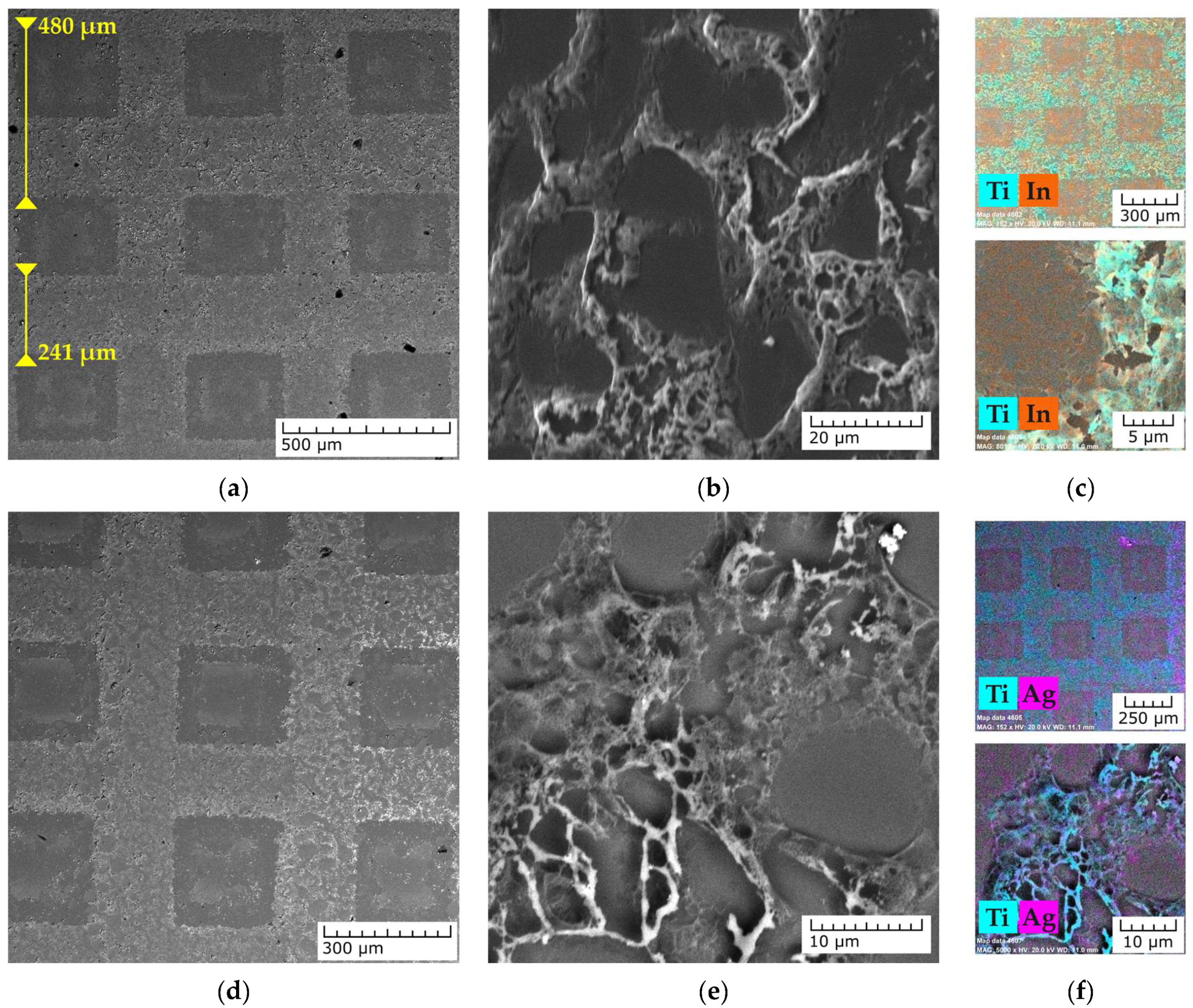
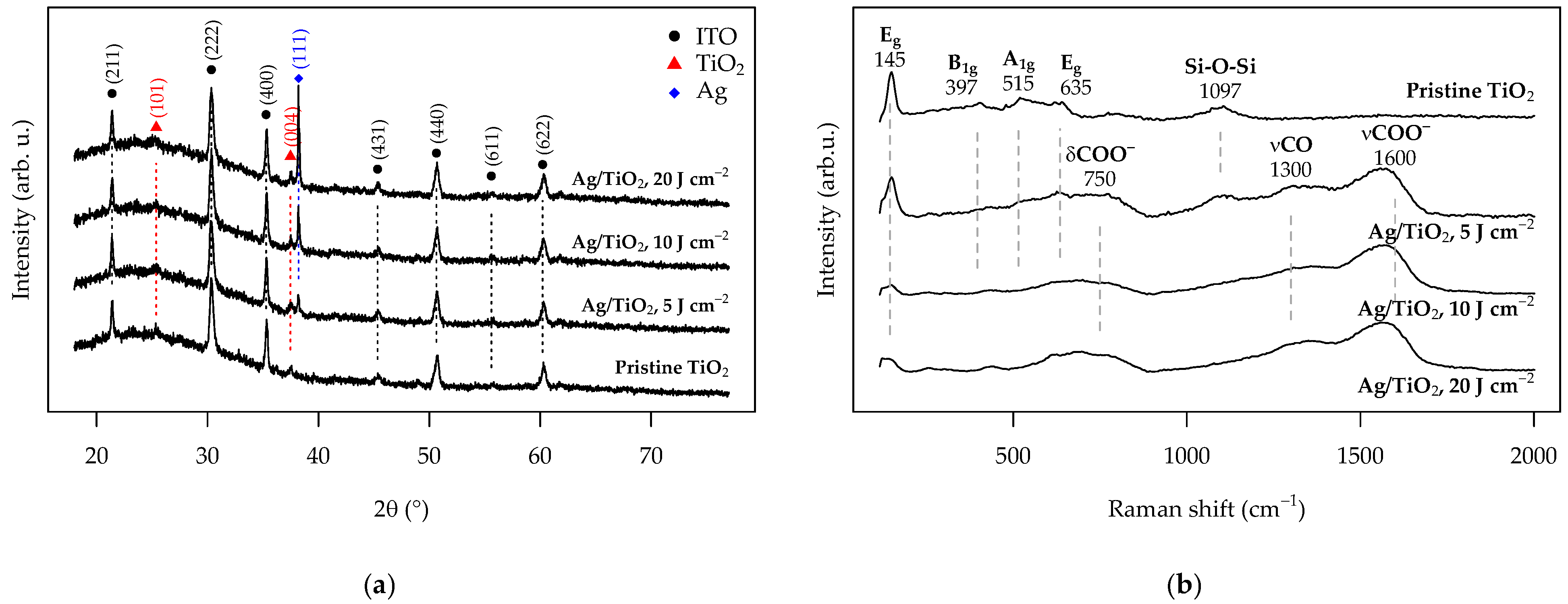
3.2. Characterization of the Pristine and Ag-Functionalized TiO2 Grid Patterns
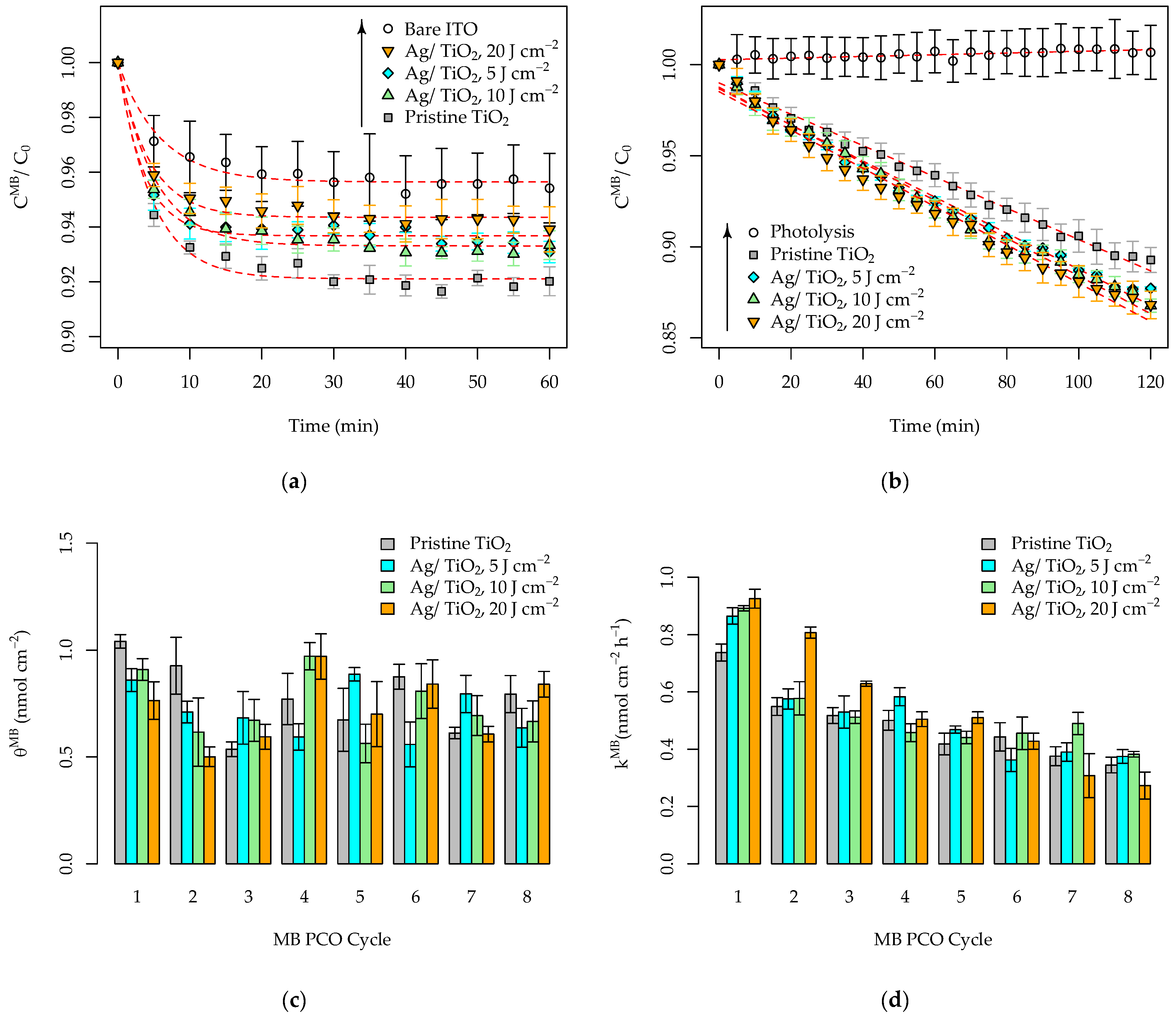
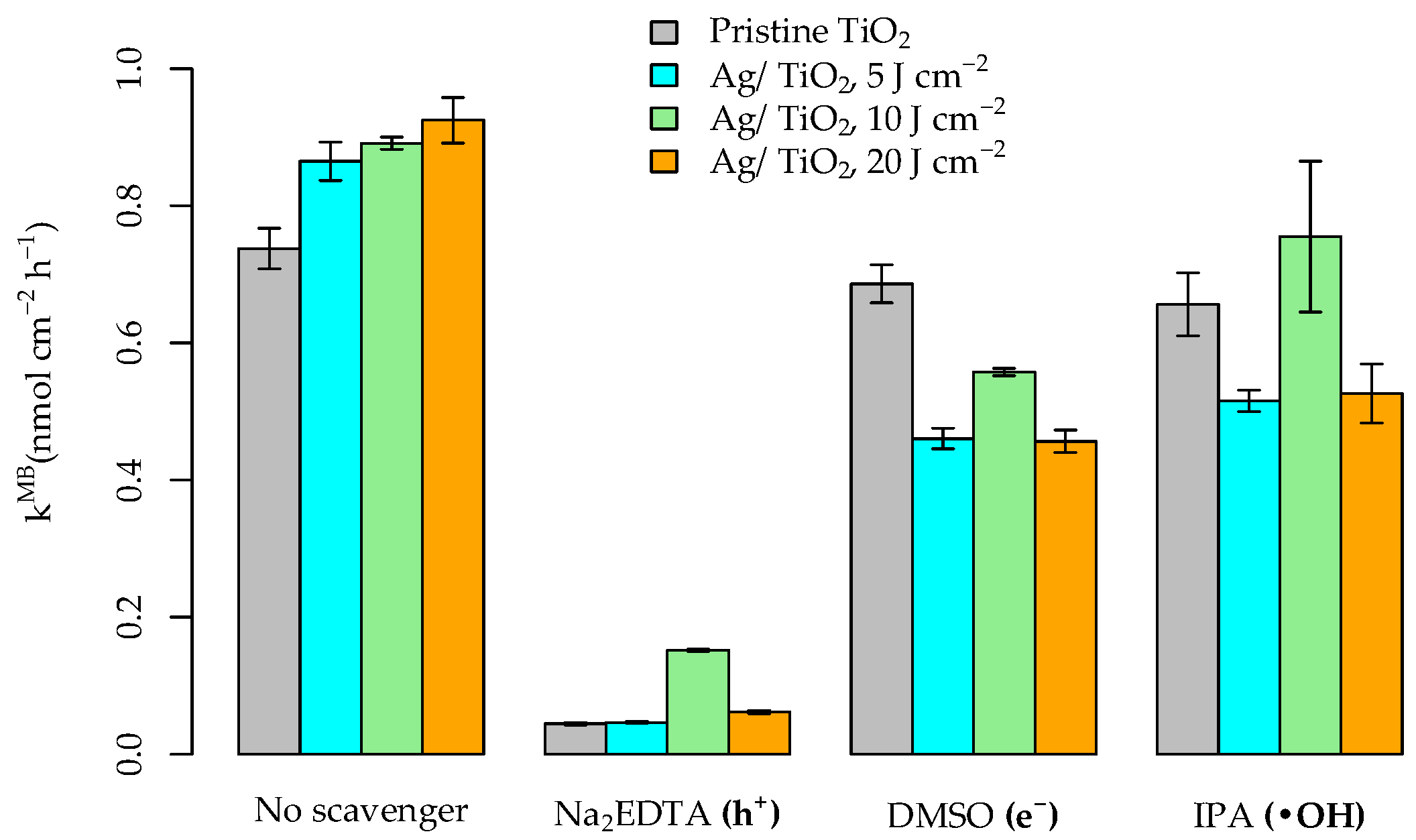
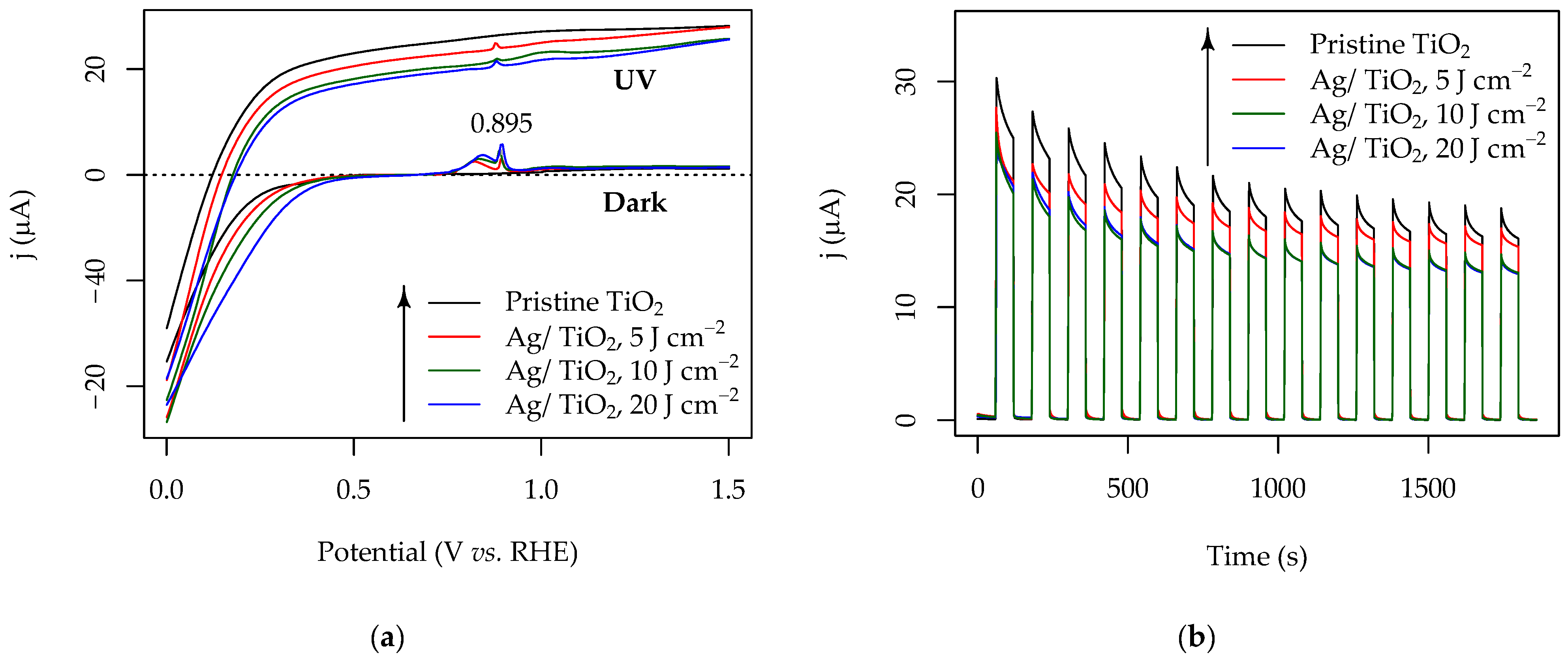
3.3. Photocatalytic Activity of the Pristine and Ag-Functionalized TiO2 Grid Patterns
| Layer Geometry | Fabrication Method | Reaction Conditions | PCO Efficiency | Ref. |
|---|---|---|---|---|
| 240 μm width, 480 μm pitch TiO2 Grid Aray | Laser treatment of TiO2 sol | 15 mL, 1 mg L−1 MB 3.75 cm2 sample area 1 W UV LED (365 nm) 0.61 mW cm−2 | 0.74 nmol cm−2 h−1 | This work |
| 240 μm width, 480 μm pitch Ag-decorated TiO2 Grid Aray | Laser treatment of TiO2 sol and Ag-photodeposition | 0.92 nmol cm−2 h−1 | ||
| 40 μm width/48 μm pitch line array | TiO2 sputter-deposition on ion-beam etching pre-patterned Si-substrate | 15 mL, 10 mg L−1 Methyl Orange 6 cm2 sample area 254 nm UV source | ~70% in 7 h * ~8 nmol cm−2 h−1 * | [69] |
| 40 μm width/48 μm pitch square array | ~70% in 7 h * ~8 nmol cm−2 h−1 * | |||
| 40 μm width/48 μm pitch hexagonal array | ~70% in 7 h ~10 nmol cm−2 h−1 * | |||
| 3 μm × 3 μm grid array | TiO2 sputter-deposition on prepatterned photoresist-coated glass substrate | 35 mL, 5 mg L−1 MB 11 cm2 sample area 18 W 254 nm UV lamp | 50% in 11 min 136 nmol cm−2 h−1 * | [70] |
| 5 μm × 5 μm grid array | 50% in 25 min 60 nmol cm−2 h−1 * | |||
| 10 mm × 10 mm infilled square | Laser treatment of Ti foil, Resulting in anatase/rutile mixture | 3 mL 10−5 MB 1 cm2 sample area 15 mW UV source | 7.22 × 10−3 min 19 nmol cm−2 h−1 * | [71] |
| 1.8 × 1.8 mm2 squares or infilled surface | Laser annealing of TiO2 nanotubes | 3 mL 40 μM MB UV LED (365 nm), 10–11 mW cm−2 | 0.013 min−1 cm−2 55 nmol cm−2 h−1 * | [72] |
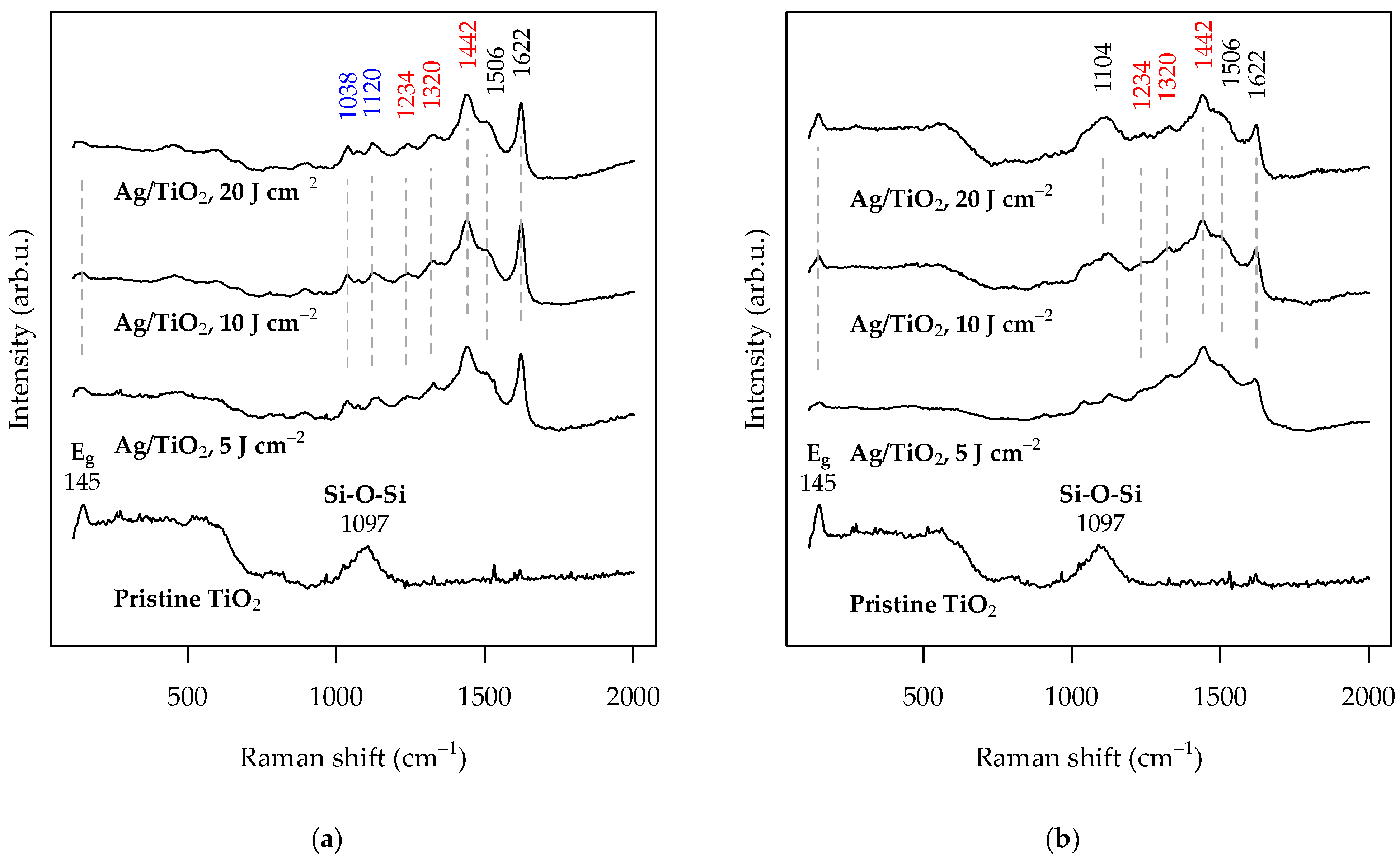
3.4. SERS Activity of the Ag-Functionalized TiO2 Grid Patterns
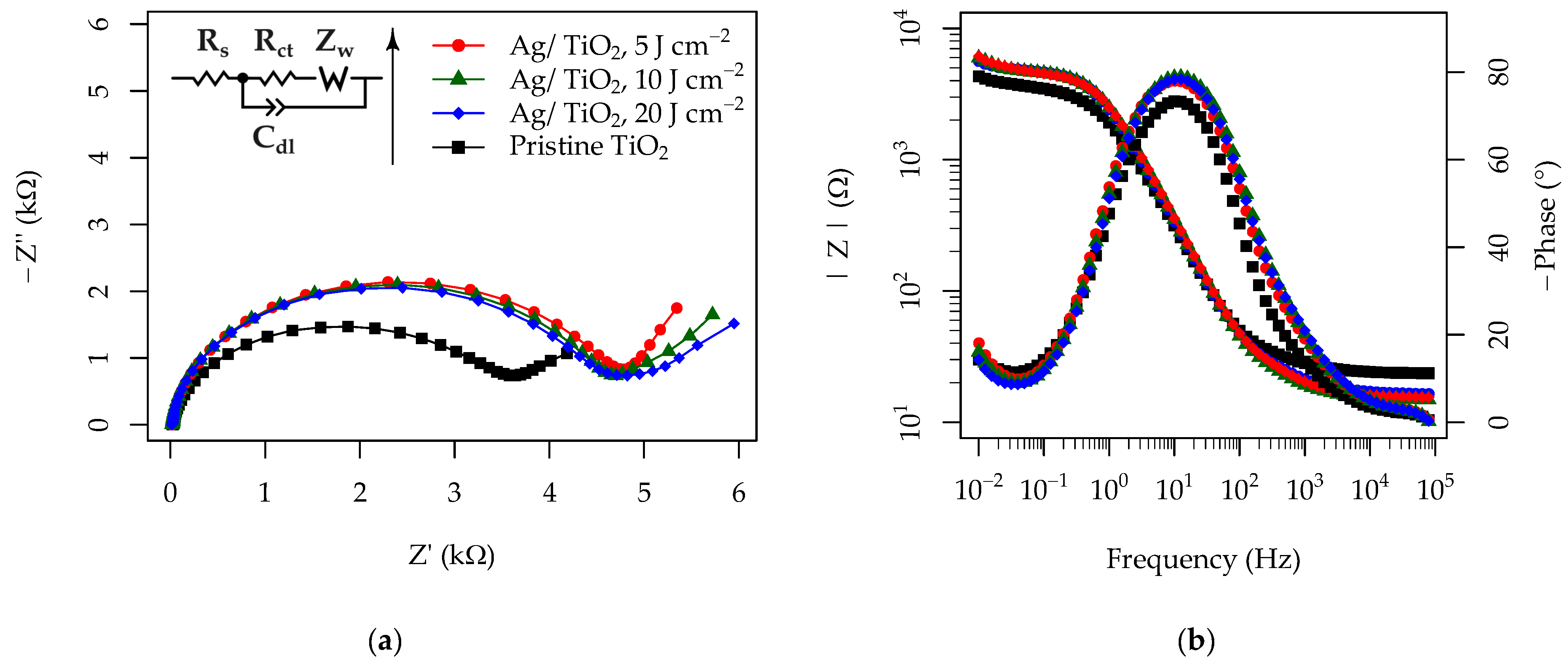
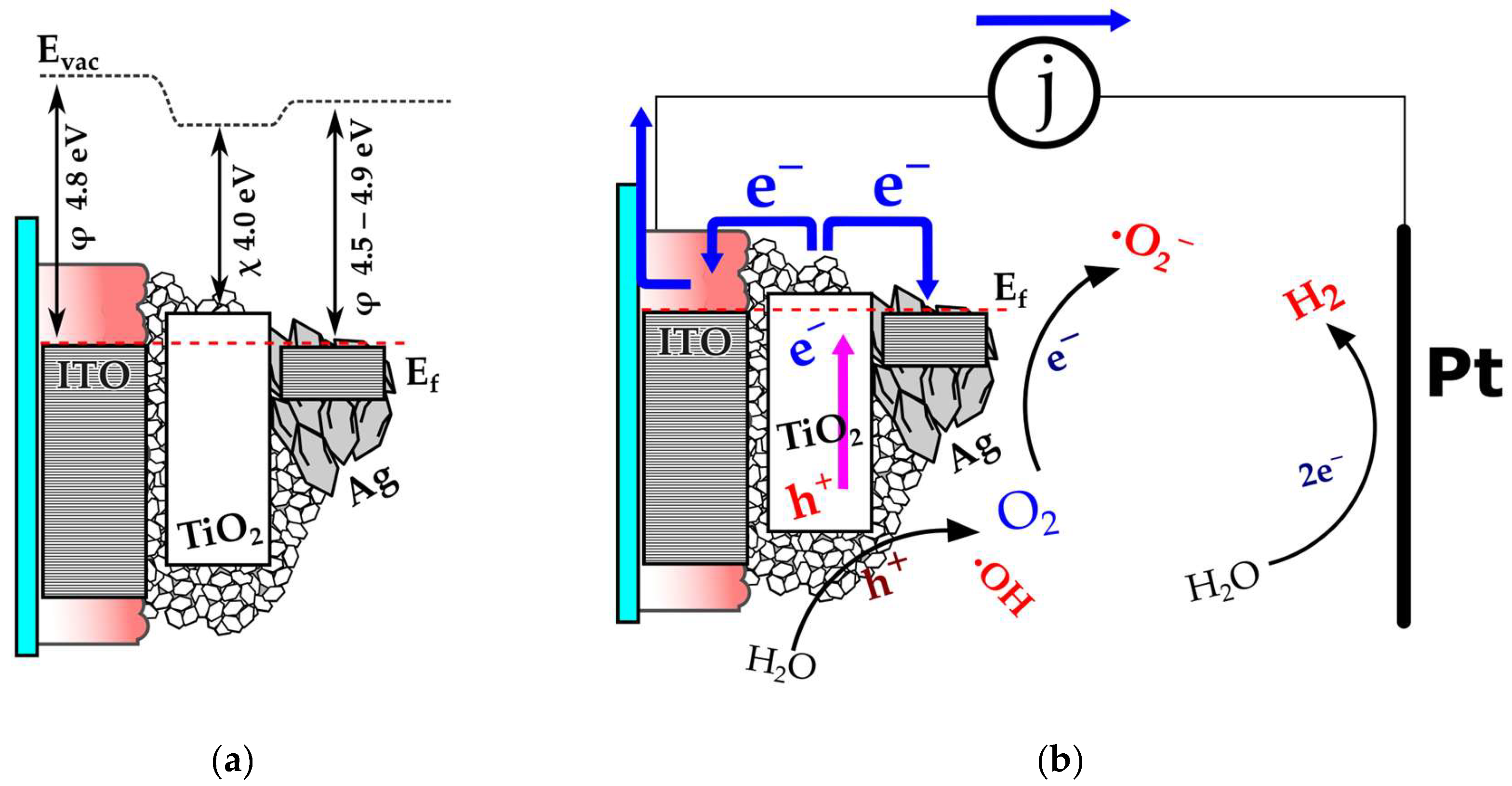
3.5. Photoelectrochemical Properties of the Pristine and Ag-Functionalized TiO2 Grid Patterns
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Morshedy, A.S.; El-Fawal, E.M.; Zaki, T.; El-Zahhar, A.A.; Alghamdi, M.M.; El Naggar, A.M.A. A review on heterogeneous photocatalytic materials: Mechanism, perspectives, and environmental and energy sustainability applications. Inorg. Chem. Commun. 2024, 163, 112307. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, R.; Zhan, H.; Zhao, H.; Duan, Y.; Shen, Z. Modified Titanium dioxide-based photocatalysts for water treatment: Mini review. Environ. Funct. Mater. 2024, 3, 1–12. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Kumar, L.; Ragunathan, V.; Chugh, M.; Bharadvaja, N. Nanomaterials for remediation of contaminants: A review. Environ. Chem. Lett. 2021, 19, 3139–3163. [Google Scholar] [CrossRef]
- Ismael, M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nanoparticles. Sol. Energy 2021, 19, 3139–3163. [Google Scholar] [CrossRef]
- Xu, Y.; Zangari, G. TiO2 Nanotubes Architectures for Solar Energy Conversion. Coatings 2021, 11, 931. [Google Scholar] [CrossRef]
- Zhou, C.; Xi, Z.; Stacchiola, D.J.; Liu, M. Application of ultrathin TiO2 layers in solar energy conversion devices. Energy Sci. Eng. 2022, 10, 1614–1629. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, P.; Kumar, A.; Camargo, P.H.C.; Krishnan, V. Recent Advances in Plasmonic Photocatalysis Based on TiO2 and Noble Metal Nanoparticles for Energy Conversion, Environmental Remediation, and Organic Synthesis. Small 2021, 18, 2101638. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Pandey, A.K.; Rahim, N.A. Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew. Sustain. Energy Rev. 2017, 77, 89–108. [Google Scholar] [CrossRef]
- Kaur, G.; Negi, P.; Konwar, R.J.; Kumar, H.; Devi, N.; Kaur, G.; Himani; Kaur, M.; Sharma, R.; Sati, P.C.; et al. Tailored TiO2 nanostructures for designing of highly efficient dye sensitized solar cells: A review. Nano-Struct. Nano-Objects 2023, 36, 101056. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Y.; Zhao, Y.; Ding, F.; Lei, Y.; Wang, Y.; Zhou, J.; Kang, W. Review on TiO2 nanostructured photoanode and novel dyes for dye-sensitized solar cells application. J. Mater. Sci. 2025, 60, 4975–5005. [Google Scholar] [CrossRef]
- Ge, S.; Sang, D.; Zou, L.; Yao, Y.; Zhou, C.; Fu, H.; Xi, H.; Fan, L.; Wang, C. A Review on the Progress of Optoelectronic Devices Based on TiO2 Thin Films and Nanomaterials. Nanomaterials 2023, 13, 1141–2023. [Google Scholar] [CrossRef]
- Hazra, S.; Singh, S.V.; Dahiya, S.; Aich, P.K.; Pal, B.N. Solution-Processed Ag-TiO2 Nanostructure-Based Schottky Junction Thin Films for Narrowband Hot-Electron Photodetectors. ACS Appl. Nano Mater. 2023, 6, 15119–15127. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Rahman, K.H.; Wu, C.-C.; Chen, K.-C. A Review on the Pathways of the Improved Structural Characteristics and Photocatalytic Performance of Titanium Dioxide (TiO2) Thin Films Fabricated by the Magnetron-Sputtering Technique. Catalysts 2020, 10, 598. [Google Scholar] [CrossRef]
- Vahl, A.; Veziroglu, S.; Henkel, B.; Strunskus, T.; Polonskyi, O.; Aktas, O.C.; Faupel, F. Pathways to Tailor Photocatalytic Performance of TiO2 Thin Films Deposited by Reactive Magnetron Sputtering. Materials 2019, 12, 2840. [Google Scholar] [CrossRef]
- Simionescu, O.-G.; Romanițan, C.; Tutunaru, O.; Ion, V.; Buiu, O.; Avram, A. RF Magnetron Sputtering Deposition of TiO2 Thin Films in a Small Continuous Oxygen Flow Rate. Coatings 2019, 9, 442. [Google Scholar] [CrossRef]
- Dong, X.; Mamat, M.; Baikeli, Y. Effect of annealing temperature on the structural, optical, morphological, and photocatalytic properties of TiO2 thin films prepared by sol-gel spin-coating and electron beam physical vapor deposition. J. Phys. D Appl. Phys. 2024, 57, 385106. [Google Scholar] [CrossRef]
- Azpiroz, R.; Borraz, M.; González, A.; Mansilla, C.; Iglesias, M.; Pérez-Torrente, J.J. Photocatalytic Activity in the In-Flow Degradation of NO on Porous TiO2–Coated Glasses from Hybrid Inorganic–Organic Thin Films Prepared by a Combined ALD/MLD Deposition Strategy. Coatings 2022, 12, 488. [Google Scholar] [CrossRef]
- Zimbone, M.; Cantarella, M.; Sfuncia, G.; Nicotra, G.; Privitera, V.; Napolitani, E.; Impellizzeri, G. Low-temperature atomic layer deposition of TiO2 activated by laser annealing: Applications in photocatalysis. Appl. Surf. Sci. 2022, 596, 153641. [Google Scholar] [CrossRef]
- Noorasid, N.S.; Arith, F.; Aliyaselvam, O.V.; Salehuddin, F.; Mustafa, A.N.; Chelvanathan, P.; Azam, M.A.; Amin, N. Low-temperature sol-gel synthesized TiO2 with different titanium tetraisopropoxide (TTIP) molarity for flexible emerging solar cell. J. Sol-Gel Sci. Technol. 2024, 109, 826–834. [Google Scholar] [CrossRef]
- Rola, K.P.; Duda, L.; Gorantla, S.; Czyż, K.; Guzik, M.; Cybińska, J. Focused Electron Beam Micro- and Nanopatterning of Thin Films Derived from Sol–Gels Based on TiO2 Precursors for Planar Photonics. ACS Appl. Nano Mater. 2024, 7, 8692–8702. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B. Preparation and Investigation of Sol–Gel TiO2-NiO Films: Structural, Optical and Electrochromic Properties. Crystals 2024, 14, 192. [Google Scholar] [CrossRef]
- Efeoglu, H.; Güllülü, S.; Karacali, T. Resistive switching of reactive sputtered TiO2 based memristor in crossbar geometry. Appl. Surf. Sci. 2015, 350, 10–13. [Google Scholar] [CrossRef]
- Ratova, M.; Sawtell, D.; Kelly, P.J. Micro-Patterning of Magnetron Sputtered Titanium Dioxide Coatings and Their Efficiency for Photocatalytic Applications. Coatings 2020, 10, 68. [Google Scholar] [CrossRef]
- Abshari, F.; Veziroglu, S.; Adejube, B.; Vahl, A.; Gerken, M. Photocatalytic Edge Growth of Conductive Gold Lines on Microstructured TiO2–ITO Substrates. Langmuir 2024, 40, 19051–19059. [Google Scholar] [CrossRef]
- Wu, C.-T.; Soliman, A.I.A.; Tu, Y.; Utsunomiya, T.; Ichii, T.; Sugimura, H. Fabrication of TiO2 Micropatterns on Flexible Substrates by Vacuum-Ultraviolet Photochemical Treatments. Adv. Mater. Interfaces 2020, 7, 1901634. [Google Scholar] [CrossRef]
- Shavdina, O.; Berthod, L.; Kämpfe, T.; Reynaud, S.; Veillas, C.; Verrier, M.; Vocanson, F.; Fugier, P.; Jourlin, Y.; Dellea, O. Large Area Fabrication of Periodic TiO2 Nanopillars Using Microsphere Photolithography on a Photopatternable Sol–Gel Film. Langmuir 2015, 31, 7877–7884. [Google Scholar] [CrossRef]
- Crespo-Monteiro, N.; Hamandi, M.; Usuga Higuita, M.A.; Guillard, C.; Dappozze, F.; Jamon, D.; Vocanson, F.; Jourlin, Y. Influence of the Micro-Nanostructuring of Titanium Dioxide Films on the Photocatalytic Degradation of Formic Acid under UV Illumination. Nanomaterials 2022, 12, 1008. [Google Scholar] [CrossRef]
- Starbova, K.; Yordanova, V.; Nihtianova, D.; Hintz, W.; Tomas, J.; Starbov, J. Excimer laser processing as a tool for photocatalytic design of sol–gel TiO2 thin films. Appl. Surf. Sci. 2008, 254, 4044–4051. [Google Scholar] [CrossRef]
- Bougdid, Y.; Chenard, F.; Sugrim, J.; Kumar, R.; Kar, A. CO2 laser-assisted sintering of TiO2 nanoparticles for transparent films. J. Laser Appl. 2023, 35, 012012. [Google Scholar] [CrossRef]
- Bougdid, Y.; Kulkarni, G.; Chenard, F.; Sugrim, C.J.; Kumar, R.; Kar, A. Optical properties of transparent TiO2 films by sintering anatase nanoparticles with a CO2 laser. Opt. Mater. 2024, 156, 115969. [Google Scholar] [CrossRef]
- Noel, L.; Khitous, A.; Molinaro, C.; Zan, H.-W.; Berling, D.; Grasset, F.; Soppera, O. Laser Direct Writing of Crystallized TiO2 by Photothermal Effect Induced by Gold Nanoparticles. Adv. Mater. Technol. 2024, 9, 2300407. [Google Scholar] [CrossRef]
- Gerlein, L.F.; Benavides-Guerrero, J.A.; Cloutier, S.G. Laser-Assisted, Large-Area Selective Crystallization and Patterning of Titanium Dioxide Polymorphs. Adv. Eng. Mater. 2020, 22, 1901014. [Google Scholar] [CrossRef]
- Stefanov, B.I. Optically Transparent TiO2 and ZnO Photocatalytic Thin Films via Salicylate-Based Sol Formulations. Coatings 2023, 13, 1568. [Google Scholar] [CrossRef]
- Huang, L.; Liu, X.; Wu, H.; Wang, X.; Wu, H.; Li, R.; Shi, L.; Li, C. Surface state modulation for size-controllable photodeposition of noble metal nanoparticles on semiconductors. J. Mater. Chem. A 2020, 8, 21094–21102. [Google Scholar] [CrossRef]
- Butburee, T.; Kotchasarn, P.; Hirunsit, P.; Sun, Z.; Tang, Q.; Khemthong, P.; Sangkhun, W.; Thongsuwan, W.; Kumnorkaew, P.; Wang, H.; et al. New understanding of crystal control and facet selectivity of titanium dioxide ruling photocatalytic performance. J. Mater. Chem. A 2019, 7, 8156–8166. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y. Steering charge kinetics in photocatalysis: Intersection of materials syntheses, characterization techniques and theoretical simulations. Chem. Soc. Rev. 2015, 44, 2893–2939. [Google Scholar] [CrossRef]
- El-Zohry, A.M.; Kloo, L.; He, L. Understanding Charge Dynamics in TiO2 Using Ultrafast Mid-infrared Spectroscopy: Trapping versus Recombination. J. Phys. Chem. C 2024, 128, 4192–4199. [Google Scholar] [CrossRef]
- Okazaki, M.; Suganami, Y.; Hirayama, N.; Nakata, H.; Oshikiri, T.; Yokoi, Y.; Misawa, H.; Maeda, K. Site-Selective Deposition of a Cobalt Cocatalyst onto a Plasmonic Au/TiO2 Photoanode for Improved Water Oxidation. ACS Appl. Energy Mater. 2020, 3, 5142–5146. [Google Scholar] [CrossRef]
- Dai, W.; Wang, X.; Liu, P.; Xu, Y.; Li, G.; Fu, X. Effects of Electron Transfer between TiO2 Films and Conducting Substrates on the Photocatalytic Oxidation of Organic Pollutants. J. Phys. Chem. B 2006, 110, 13470–13476. [Google Scholar] [CrossRef] [PubMed]
- Abshari, F.; Paulsen, M.; Veziroglu, S.; Vahl, A.; Gerken, M. ITO-TiO2 Heterojunctions on Glass Substrates for Photocatalytic Gold Growth Along Pattern Edges. Catalysts 2024, 14, 940. [Google Scholar] [CrossRef]
- Stefanov, B.I.; Kolev, H.G. MnOx and Pd Surface Functionalization of TiO2 Thin Films via Photodeposition UV Dose Control. Photochem 2024, 4, 474–487. [Google Scholar] [CrossRef]
- Stefanov, B. Photocatalytic reactor for in situ determination of supported catalysts activity in liquid-phase based on 3D-printed components and Arduino. In Proceedings of the 2020 XXIX International Scientific Conference Electronics (ET), Sozopol, Bulgaria, 16–18 September 2020. [Google Scholar] [CrossRef]
- Swanepoel, R. Determination of the thickness and optical constants of amorphous silicon. J. Phys. E Sci. Instrum. 1983, 16, 1214. [Google Scholar] [CrossRef]
- Kubinyi, M.; Benkö, N.; Grofcsik, A.; Jeremy Jones, W. Determination of the thickness and optical constants of thin films from transmission spectra. Thin Solid Films 1996, 286, 164–169. [Google Scholar] [CrossRef]
- Kerscher, M.; Braun, L.M.; Jander, J.H.; Rausch, M.H.; Koller, T.M.; Wasserscheid, P.; Fröba, A.P. Viscosity, Interfacial Tension, and Density of 2-Propanol and Acetone up to 423 K by Surface Light Scattering and Conventional Methods. Int. J. Thermophys. 2024, 45, 8. [Google Scholar] [CrossRef]
- Amrhein, S.; Bauer, K.C.; Galm, L.; Hubbuch, J. Non-invasive high throughput approach for protein hydrophobicity determination based on surface tension. Biotechnol. Bioeng. 2015, 112, 2485–2494. [Google Scholar] [CrossRef]
- de Gennes, P.G.; Brochard-Wyart, F.; Quéré, D. Hysteresis and Elasticity of Triple Lines. In Capillarity and Wetting Phenomena; Springer: New York, NY, USA, 2004. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, F.; Kornev, K.G. The Thickness and Structure of Dip-Coated Polymer Films in the Liquid and Solid States. Micromachines 2022, 13, 982. [Google Scholar] [CrossRef]
- Colusso, E.; Basso, M.; Carraro, C.; Cesaroni, C.; Di Russo, E.; Guglielmi, M.; Napolitani, E.; Martucci, A. Pulsed laser crystallization of sol-gel derived cerium oxide thin films. J. Sol-Gel Sci. Technol. 2025, 114, 75–86. [Google Scholar] [CrossRef]
- Matsubayashi, Y.; Nomoto, J.; Yamaguchi, I.; Tsuchiya, T. Control of the oxygen deficiency and work function of SrFeO3−δ thin films by excimer laser- assisted metal organic decomposition. CrystEngComm 2020, 22, 4685. [Google Scholar] [CrossRef]
- Txintxurreta, J.; G-Berasategui, E.; Ortiz, R.; Hernández, O.; Mendizábal, L.; Barriga, J. Indium Tin Oxide Thin Film Deposition by Magnetron Sputtering at Room Temperature for the Manufacturing of Efficient Transparent Heaters. Coatings 2021, 11, 92. [Google Scholar] [CrossRef]
- Veziroglu, S. Fabrication of Ultra-Fine Ag NPs on TiO2 Thin Films by Alcohol-Assisted Photodeposition Process for Photocatalysis-Related Applications. Materials 2024, 17, 1354. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman Spectroscopy: A New Approach to Measure the Percentage of Anatase TiO2 Exposed (001) Facets. J. Phys. Chem. C 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Liu, H.; Kaya, H.; Lin, Y.-T.; Ogrinc, A.; Kim, S.H. Vibrational spectroscopy analysis of silica and silicate glass networks. J. Am. Ceram. Soc. 2022, 105, 2355–2384. [Google Scholar] [CrossRef]
- Shirpay, A.; Tavakoli, M. The behavior of the active modes of the anatase phase of TiO2 at high temperatures by Raman scattering spectroscopy. Ind. J. Phys. 2022, 96, 1673–1681. [Google Scholar] [CrossRef]
- Melvin, A.A.; Illath, K.; Das, T.; Raja, T.; Bhattacharyya, S.; Gopinath, C.S. M–Au/TiO2 (M = Ag, Pd, and Pt) nanophotocatalyst for overall solar water splitting: Role of interfaces. Nanoscale 2015, 7, 13477–13488. [Google Scholar] [CrossRef]
- Shan, W.; Liu, R.; Zhao, H.; He, Z.; Lai, Y.; Li, S.; He, G.; Liu, J. In Situ Surface-Enhanced Raman Spectroscopic Evidence on the Origin of Selectivity in CO2 Electrocatalytic Reduction. ACS Nano 2020, 14, 11363–11372. [Google Scholar] [CrossRef]
- Joshi, N.; Jain, N.; Pathak, A.; Singh, J.; Prasad, R.; Upadhyaya, C. Biosynthesis of silver nanoparticles using Carissa carandas berries and its potential antibacterial activities. J. Sol-Gel. Sci. Technol. 2018, 86, 682–689. [Google Scholar] [CrossRef]
- Pylnev, M.; Wong, M.-S. Comparative study of photocatalytic deactivation of pure and black titania thin films. J. Photochem. Photobiol. A Chem. 2019, 378, 125–130. [Google Scholar] [CrossRef]
- Tschirch, J.; Bahnemann, D.; Wark, M.; Rathouský, J. A comparative study into the photocatalytic properties of thin mesoporous layers of TiO2 with controlled mesoporosity. J. Photochem. Photobiol. A Chem. 2008, 194, 181–188. [Google Scholar] [CrossRef]
- Puga, F.; Navío, J.; Hidalgo, M. A critical view about use of scavengers for reactive species in heterogeneous photocatalysis. Appl. Catal. A Gen. 2024, 685, 119879. [Google Scholar] [CrossRef]
- Arunadevi, R.; Kavitha, B.; Rajarajan, M.; Suganthi, A. Synthesis of Ce/Mo-V4O9 nanoparticles with superior visible light photocatalytic activity for Rhodamine-B degradation. J. Environ. Chem. Eng. 2018, 6, 3349–3357. [Google Scholar] [CrossRef]
- Mills, A.; Wang, J. Photobleaching of methylene blue sensitized by TiO2: An ambiguous system? J. Photochem. Photobiol. A Chem. 1999, 127, 123–124. [Google Scholar] [CrossRef]
- Guo, M.; Ng, A.M.C.; Liu, F.; Djurišić, A.B.; Chan, W.K. Photocatalytic activity of metal oxides—The role of holes and OH radicals. Appl. Catal. B Environ. 2011, 107, 150–157. [Google Scholar] [CrossRef]
- Ishibashi, K.-I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Quantum yields of active oxidative species formed on TiO2 photocatalyst. J. Photochem. Photobiol. A 2000, 134, 139–142. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Zuo, X.; Wen, F.; Jiang, H.; Cao, H.; Pei, Y. Micro-patterned TiO2 films for photocatalysis. Matter. Lett. 2019, 254, 448–451. [Google Scholar] [CrossRef]
- Sidaraviciute, R.; Kavaliunas, V.; Puodziukynas, L.; Guobiene, A.; Martuzevicius, D.; Andrulevicius, M. Enhancement of photocatalytic pollutant decomposition efficiency of surface mounted TiO2 via lithographic surface patterning. Environ. Technol. Innov. 2020, 19, 100983. [Google Scholar] [CrossRef]
- Ponkratova, E.; Kuzmichev, A.; Rud, D.; Khubezhov, S.; Dolgintsev, D.; Ageev, E.; Veiko, V.; Sinev, D.; Zuev, D. Nanosecond Laser-Assisted Fabrication of Photocatalytically Active TiO2 Nanocoatings: Implication in Organic Dyes Degradation. ACS Appl. Nano Mater. 2024, 7, 19268–19278. [Google Scholar] [CrossRef]
- Bernhardt, A.; Lorentz, P.; Fischer, K.; Schmidt, M.; Kühnert, M.; Lotnyk, A.; Griebel, J.; Schönherr, N.; Zimmer, K.; Schulze, A. Laser-Crystallization of TiO2 Nanotubes for Photocatalysis: Influence of Laser Power and Laser Scanning Speed. Laser Photonics Rev. 2024, 18, 2300778. [Google Scholar] [CrossRef]
- Ruan, C.; Wang, W.; Gu, B. Single-molecule detection of thionine on aggregated gold nanoparticles by surface enhanced Raman scattering. J. Raman Spectrosc. 2007, 38, 568–573. [Google Scholar] [CrossRef]
- Balu, P.; Asharani, I.V.; Thirumalai, D. Catalytic degradation of hazardous textile dyes by iron oxide nanoparticles prepared from Raphanus sativus leaves’ extract: A greener approach. J. Mater. Sci. Mater. Electron. 2020, 31, 10669–10676. [Google Scholar] [CrossRef]
- Xiao, G.-N.; Man, S.-Q. Surface-enhanced Raman scattering of methylene blue adsorbed on cap-shaped silver nanoparticles. Chem. Phys. Lett. 2007, 447, 305–309. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, H.; Li, X.; Zhang, S. Enhanced photocatalytic activity of TiO2 nano-structured thin film with a silver hierarchical configuration. Appl. Surf. Sci. 2008, 254, 1630–1635. [Google Scholar] [CrossRef]
- Katsiaounis, S.; Panidi, J.; Koutselas, I.; Topoglidis, E. Fully Reversible Electrically Induced Photochromic-Like Behaviour of Ag:TiO2 Thin Films. Coatings 2020, 10, 130. [Google Scholar] [CrossRef]
- He, C.; Xiong, Y.; Chen, J.; Zha, C.; Zhu, X. Photoelectrochemical performance of Ag–TiO2/ITO film and photoelectrocatalytic activity towards the oxidation of organic pollutants. J. Photochem. Photobiol. A Chem. 2003, 157, 71–79. [Google Scholar] [CrossRef]
- Syrek, K.; Czopor, J.; Topa-Skwarczyńska, M.; Pilch, M.; Kamiński, K.; Ortyl, J.; Sulka, G. Photoelectrochemical Properties of BODIPY-Sensitized Anodic TiO2 Layers Decorated with AuNPs for Enhanced Solar Performance. J. Phys. Chem. C 2023, 127, 9471–9480. [Google Scholar] [CrossRef]
- Wierbicka, E.; Schultz, T.; Syrek, K.; Sulka, G.; Koch, N.; Pinna, N. Ultra-stable self-standing Au nanowires/TiO2 nanoporous membrane system for high-performance photoelectrochemical water splitting cells. Mater. Horiz. 2022, 9, 2797–2808. [Google Scholar] [CrossRef]
- Kavan, L. Electrochemistry and band structure of semiconductors (TiO2, SnO2, ZnO): Avoiding pitfalls and textbook errors. J. Solid State Chem. 2024, 28, 829–845. [Google Scholar] [CrossRef]
- Chen, D.; Gao, Y.; Wang, G.; Zhang, H.; Lu, W.; Li, J. Surface Tailoring for Controlled Photoelectrochemical Properties: Effect of Patterned TiO2 Microarrays. J. Phys. Chem. C 2007, 111, 13163–13169. [Google Scholar] [CrossRef]
- Di Valentin, C.; Selloni, A. Bulk and Surface Polarons in Photoexcited Anatase TiO2. J. Phys. Chem. Lett. 2011, 2, 2223–2228. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Chelvayohan, M.; Mee, C. Work function measurements on (110), (100) and (111) surfaces of silver. J. Phys. C Solid State Phys. 1982, 15, 2305. [Google Scholar] [CrossRef]
- Seong, M.; Kim, H.; Lee, S.; Kim, D.; Oh, S. Engineering the work function of solution-processed electrodes of silver nanocrystal thin film through surface chemistry modification. APL Mater. 2018, 6, 121105. [Google Scholar] [CrossRef]
- Li, W.-X.; Stampfl, C.; Scheffler, M. Subsurface oxygen and surface oxide formation at Ag(111): A density-functional theory investigation. Phys. Rev. B 2003, 67, 045408. [Google Scholar] [CrossRef]
- Zhao, W.; Wen, L.; Parkin, I.; Zhao, X.; Liu, B. Fermi-level shift, electron separation, and plasmon resonance change in Ag nanoparticle-decorated TiO2 under UV light illumination. Phys. Chem. Chem. Phys. 2023, 25, 20134–20144. [Google Scholar] [CrossRef]



| Material | MB PCO Rate, (nmol cm−2 h−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | Cycle 6 | Cycle 7 | Cycle 8 | |
| Pristine TiO2 | 0.74 ± 0.03 (0.992) | 0.55 ± 0.03 (0.994) | 0.52 ± 0.03 (0.996) | 0.50 ± 0.03 (0.992) | 0.42 ± 0.04 (0.976) | 0.44 ± 0.05 (0.992) | 0.38 ± 0.03 (0.971) | 0.34 ± 0.03 (0.976) |
| Ag/TiO2 5 J cm−2 | 0.86 ± 0.03 (0.989) | 0.58 ± 0.04 (0.995) | 0.53 ± 0.06 (0.995) | 0.58 ± 0.03 (0.996) | 0.47 ± 0.01 (0.993) | 0.36 ± 0.04 (0.945) | 0.39 ± 0.03 (0.984) | 0.37 ± 0.02 (0.981) |
| Ag/TiO2 10 J cm−2 | 0.89 ± 0.01 (0.990) | 0.58 ± 0.06 (0.991) | 0.51 ± 0.02 (0.997) | 0.46 ± 0.03 (0.994) | 0.44 ± 0.02 (0.994) | 0.46 ± 0.06 (0.991) | 0.49 ± 0.04 (0.990) | 0.38 ± 0.01 (0.988) |
| Ag/TiO2 20 J cm−2 | 0.92 ± 0.03 (0.987) | 0.81 ± 0.02 (0.990) | 0.63 ± 0.01 (0.979) | 0.50 ± 0.03 (0.989) | 0.51 ± 0.02 (0.995) | 0.43 ± 0.03 (0.990) | 0.31 ± 0.08 (0.973) | 0.27 ± 0.05 (0.941) |
| Material | EIS Fitting Results | (μA) | ||||
|---|---|---|---|---|---|---|
| (Ω) | (Ω) | (Ω) | ||||
| (μF) | (α) | |||||
| Pristine TiO2 | 25 | 3270 | 73 | 0.91 | 335 | 17.2 |
| Ag/TiO2, 5 J cm−2 | 29 | 4218 | 53 | 0.99 | 451 | 15.9 |
| Ag/TiO2, 10 J cm−2 | 25 | 4236 | 51 | 0.97 | 444 | 13.7 |
| Ag/TiO2, 20 J cm−2 | 22 | 4358 | 52 | 0.95 | 423 | 13.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanov, B.I. Laser-Patterned and Photodeposition Ag-Functionalized TiO2 Grids on ITO Glass for Enhanced Photocatalytic Degradation. Coatings 2025, 15, 709. https://doi.org/10.3390/coatings15060709
Stefanov BI. Laser-Patterned and Photodeposition Ag-Functionalized TiO2 Grids on ITO Glass for Enhanced Photocatalytic Degradation. Coatings. 2025; 15(6):709. https://doi.org/10.3390/coatings15060709
Chicago/Turabian StyleStefanov, Bozhidar I. 2025. "Laser-Patterned and Photodeposition Ag-Functionalized TiO2 Grids on ITO Glass for Enhanced Photocatalytic Degradation" Coatings 15, no. 6: 709. https://doi.org/10.3390/coatings15060709
APA StyleStefanov, B. I. (2025). Laser-Patterned and Photodeposition Ag-Functionalized TiO2 Grids on ITO Glass for Enhanced Photocatalytic Degradation. Coatings, 15(6), 709. https://doi.org/10.3390/coatings15060709






