Effect of B4C Content on Microstructure and Wear Resistance of Laser-Cladding-Enhanced 316 Stainless Steel Coatings
Abstract
1. Introduction
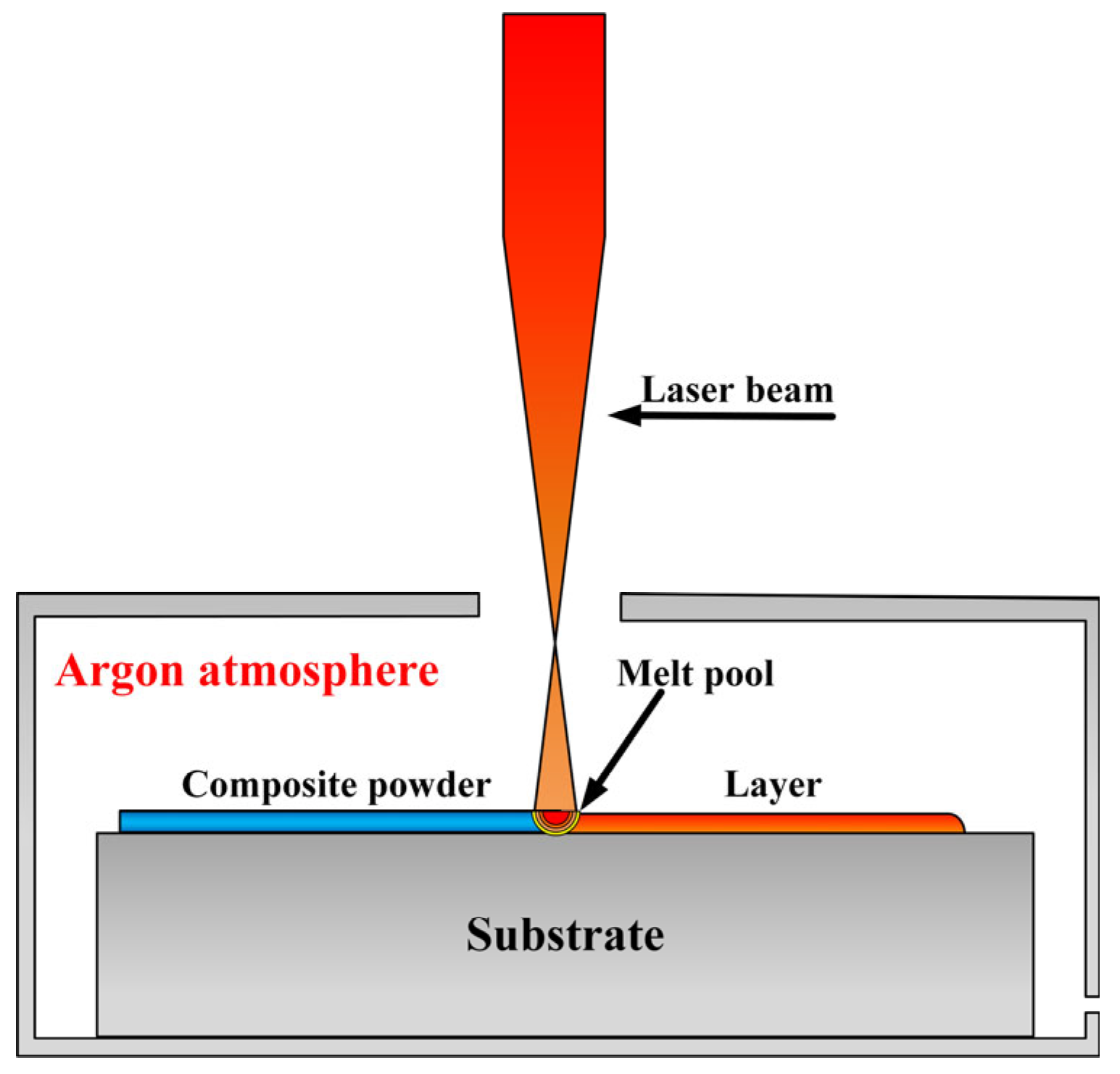
2. Materials and Methods
3. Results
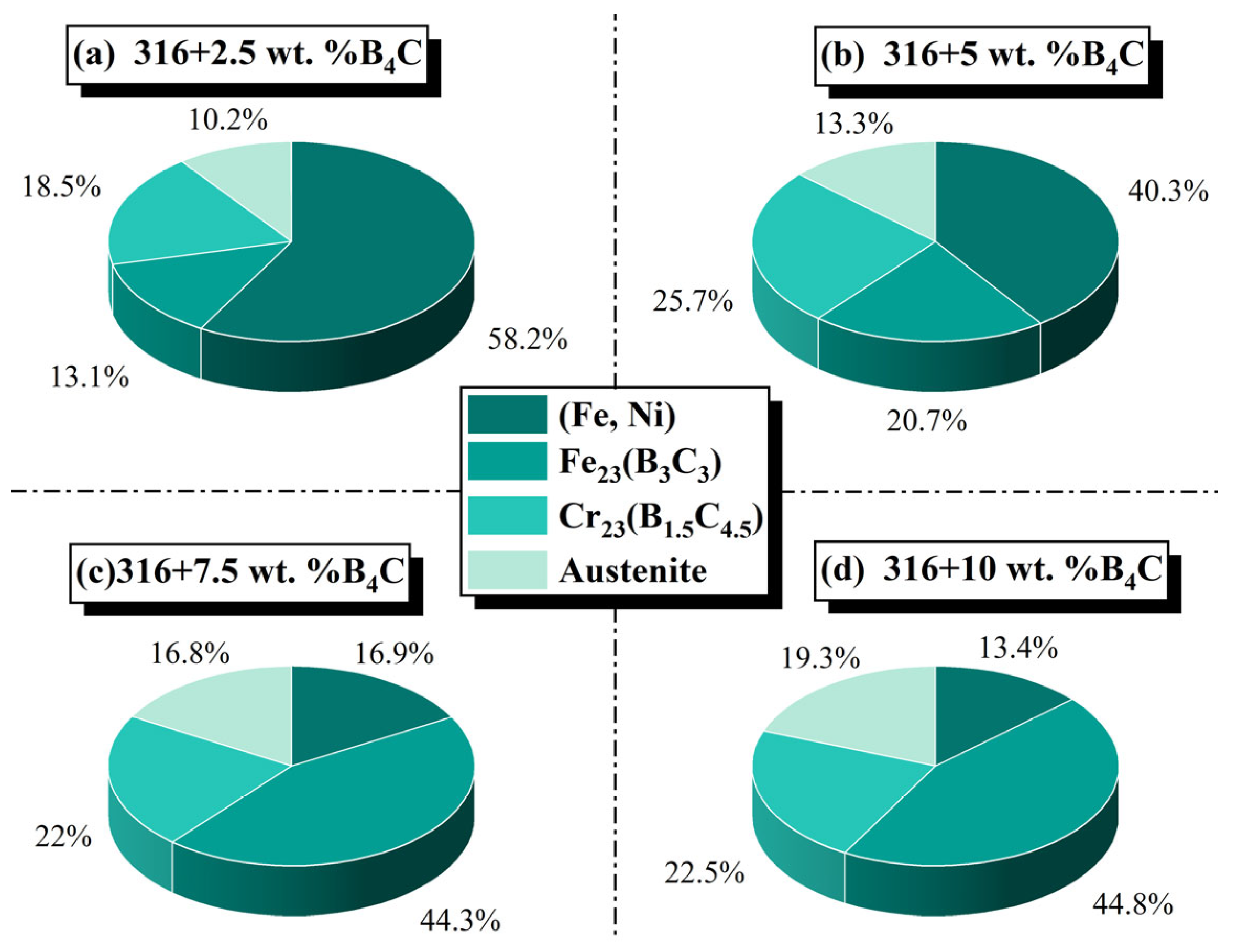
3.1. Phase Composition Analysis of the Coating
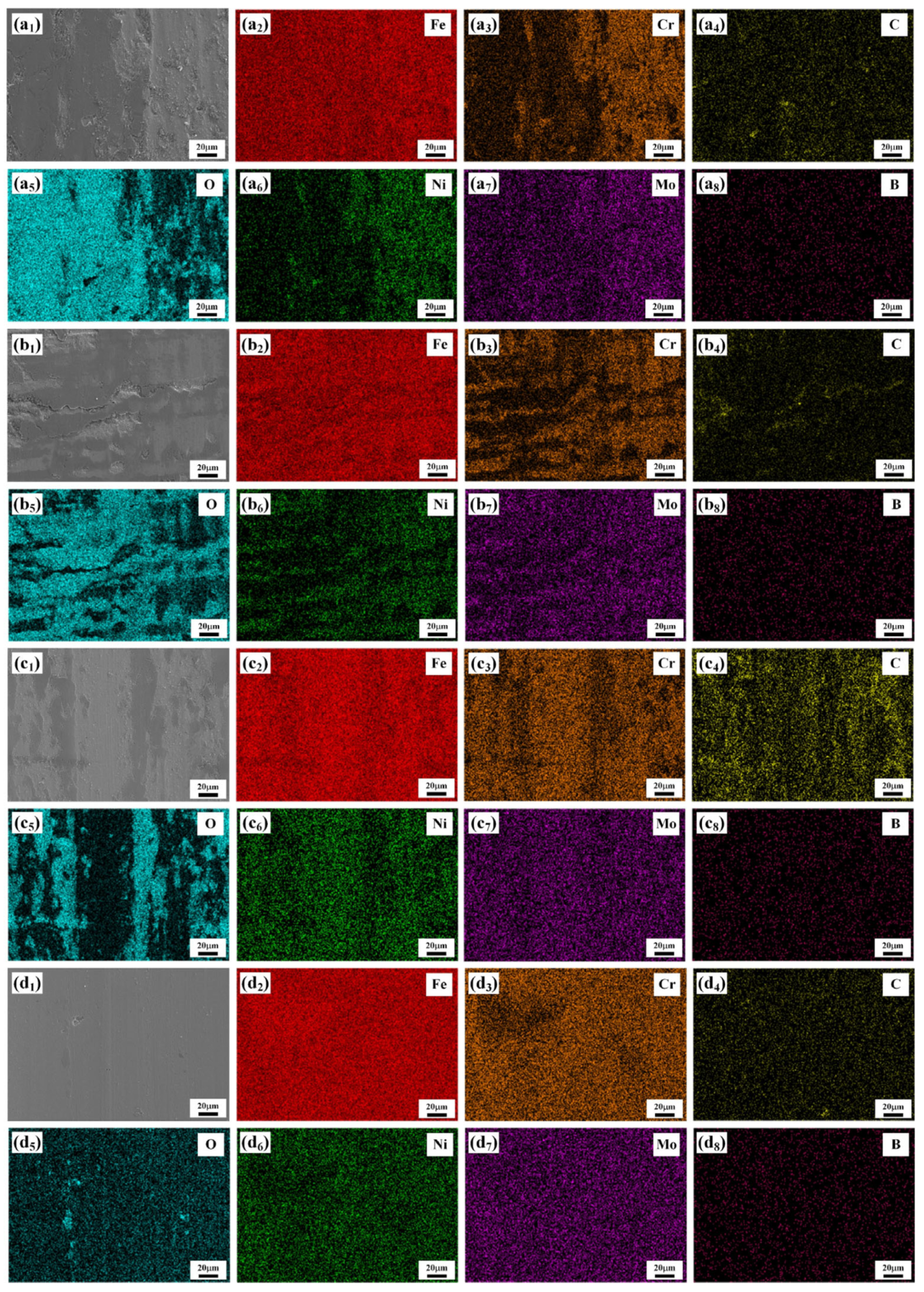
3.2. Microstructure Analysis of the Coating
3.3. Microhardness Analysis of Coatings
3.4. Tribological Analysis of Coatings

4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Z.; Song, B.; Ma, P.; Fan, W.; Gong, E.; Sun, Y.; Ding, Y.; Ju, D. Fabrication of a Novel MgO-B2O3-SiO2-Zn Coating by Thermal Spraying. Coatings 2021, 11, 907. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Z.J.; Li, Y.L.; Yuan, H.; Li, F.Y. The effect of thermal spraying on the forming performance of incremental sheet forming. J. Mater. Res. Technol. 2021, 12, 776–787. [Google Scholar] [CrossRef]
- Yuan, B.N.; Wang, Y.N.; Elnaggar, A.Y.; El Azab, I.H.; Huang, M.N.; Mahmoud, M.H.H.; El-Bahy, S.M.; Guo, M.H. Physical vapor deposition of graphitic carbon nitride (g-C3N4) films on biomass substrate: Optoelectronic performance evaluation and life cycle assessment. Adv. Compos. Hybrid Mater. 2022, 5, 813–822. [Google Scholar] [CrossRef]
- Elkins, J.; Iyengar, S.A.; Verma, O.; Shekhar, H.; Khodabandehloo, K.; Zhou, J.Y.; Pieshkov, T.; Murukeshan, J.; Nordlander, P.; Krishnamoorthy, A.; et al. Spatially Controlled Growth of Ultrathin MoO2 Polymorphs by Physical Vapor Deposition. Nano Lett. 2025, 25, 2283–2289. [Google Scholar] [CrossRef]
- Zhang, Q.D.; Yu, M.Y.; Zhang, B.Y.; Li, H. Effect of surface roughness of electroplating chromium coated steel on bonding strength of polymer coated steel. Polym. Polym. Compos. 2022, 30, 10. [Google Scholar] [CrossRef]
- Huang, C.A.; Shen, C.H.; Huang, W.Z.; Lo, J.S.; Lai, P.L. Grinding performance of electroplated diamond tools strengthened with Cr-C deposit using D-150 diamond particles. Int. J. Adv. Manuf. Technol. 2022, 121, 4549–4558. [Google Scholar] [CrossRef]
- Yuan, W.Y.; Li, R.F.; Chen, Z.H.; Gu, J.Y.; Tian, Y.T. A comparative study on microstructure and properties of traditional laser cladding and high-speed laser cladding of Ni45 alloy coatings. Surf. Coat. Technol. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Lou, D.Y.; Yang, S.K.; Mei, S.; Liu, Q.; Cheng, J.; Yang, Q.B.A.; Liu, D.; He, C.L. The Effect of Laser Scanning Speed on Microstructure and Performance of Cr3C2-NiCr Cermet Fabricated by in-situ Laser Cladding. Mater. Sci.-Medzg. 2021, 27, 167–174. [Google Scholar] [CrossRef]
- Vassen, R.; Bakan, E.; Mack, D.E.; Guillon, O. A Perspective on Thermally Sprayed Thermal Barrier Coatings: Current Status and Trends. J. Therm. Spray Technol. 2022, 31, 685–698. [Google Scholar] [CrossRef]
- Schalk, N.; Tkadletz, M.; Mitterer, C. Hard coatings for cutting applications: Physical vs. chemical vapor deposition and future challenges for the coatings community. Surf. Coat. Technol. 2022, 429, 127949. [Google Scholar] [CrossRef]
- Li, S.; Dai, M.; Wu, Y.N.; Fu, H.; Hou, X.T.; Peng, C.S.; Luo, H.H. Resource utilization of electroplating wastewater: Obstacles and solutions. Environ. Sci.-Wat. Res. Technol. 2022, 8, 484–509. [Google Scholar] [CrossRef]
- Lei, Y.W.; Sun, R.L.; Tang, Y.; Niu, W. Microstructure and phase transformations in laser clad CrxSy/Ni coating on H13 steel. Opt. Lasers Eng. 2015, 66, 181–186. [Google Scholar] [CrossRef]
- Jia, D.H.; Shi, W.Q.; Li, K.Y.; Lu, C.; An, F.J.; Lin, L.J.; Guo, F.J. Effects of rare earth oxides on wear resistance and corrosion resistance of 316L/TiC composite coating by laser cladding. Mater. Today Commun. 2024, 39, 109001. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.; Zhang, C.H.; Wu, C.L.; Chen, J.; Shahzad, M.B. Effect of Cr3C2 content on 316L stainless steel fabricated by laser melting deposition. Vacuum 2018, 147, 92–98. [Google Scholar] [CrossRef]
- Majumdar, J.D.; Kumar, A.; Li, L. Direct laser cladding of SiC dispersed AISI 316L stainless steel. Tribol. Int. 2008, 42, 750–753. [Google Scholar] [CrossRef]
- Yu, G.; Ying, L.; Lu, W.; Xiaojiao, Y.; Tao, Z.; Lungao, S.; Renquan, W. Microstructure evolution and wear resistance of laser cladded 316L stainless steel reinforced with in-situ VC-Cr7C3. Surf. Coat. Technol. 2022, 435, 128264. [Google Scholar]
- Zhang, H.; Wang, W.X.; Chang, F.; Li, C.L.; Shu, S.L.; Wang, Z.F.; Han, X.; Zou, Q.; Qiu, F.; Jiang, Q.C. Microstructure manipulation and strengthening mechanisms of 40Cr steel via trace TiC nanoparticles. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2021, 822, 141693. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, Y.Z.; Yang, H.Y.; Lin, Y.H.; Guo, R.F.; Qiu, F.; Zhang, H.; Li, C.D.; Chang, F.; Shi, F.J.; et al. Development of ceramic nanoparticles reinforced high Cr tool and die steels with high comprehensive performance. Ceram. Int. 2024, 50, 5052–5064. [Google Scholar] [CrossRef]
- Song, L.J.; Zeng, G.C.; Xiao, H.; Xiao, X.F.; Li, S.M. Repair of 304 stainless steel by laser cladding with 316L stainless steel powders followed by laser surface alloying with WC powders. J. Manuf. Process. 2016, 24, 116–124. [Google Scholar] [CrossRef]
- Yu, J.S.; Ho, H.S. Microstructure and Mechanical Properties of (Ti, Nb)C Ceramic-Reinforced 316L Stainless Steel Coating by Laser Cladding. Appl. Sci. 2022, 12, 6684. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.L.; Li, T.; Du, J.; Guo, L.Y.; Hu, K.X. Fabrication and characterization of self-lubricating anti-wear 316L stainless steel/h-BN composite coatings on Q235 substrate via laser cladding. Opt. Laser Technol. 2025, 180, 111564. [Google Scholar] [CrossRef]
- Wu, Q.L.; Long, W.M.; Zhang, L.; Zhao, H.W. A review on ceramic coatings prepared by laser cladding technology. Opt. Laser Technol. 2024, 176, 110993. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wang, Z.; Chen, L.Y.; Shu, S.L.; Qiu, F.; Zhang, L.C. Interface formation and bonding control in high-volume-fraction (TiC+TiB2)/Al composites and their roles in enhancing properties. Compos. Pt. B-Eng. 2021, 209, 108605. [Google Scholar] [CrossRef]
- Liu, N.A.; Jing, C.N.; Lin, T.; Tu, Y.M.; Fu, T.L.; Li, Z.W. Effect of B4C content on microstructure and properties of AlCoCrFeNi coatings by laser cladding. J. Mater. Sci. 2025, 60, 4846–4863. [Google Scholar] [CrossRef]
- Guo, C.H.; Xu, S.C.; Chen, Z.B.; Gao, H.B.; Jiang, G.R.; Sun, W.Y.; Wang, X.H.; Jiang, F.C. Effect of B4C content and particle sizes on the laser cladded B4C/Inconel 625 composite coatings: Process, microstructure and corrosion property. J. Mater. Res. Technol. 2024, 30, 6278–6290. [Google Scholar] [CrossRef]
- Liu, D.; He, D.Y.; Li, H.L.; Li, N.L.; Ma, L.X.; Li, H.C.; Xu, Y.; Yu, J.M. Multi-ceramic phases synergistically reinforced titanium matrix composite coating deposited by laser cladding: Effect of B4C content on the microstructure and wear properties. Surf. Coat. Technol. 2025, 496, 131648. [Google Scholar] [CrossRef]
- Guan, C.; Yu, T.B.; Zhao, Y.; Chen, L.Y.; Chen, Y. Repair of Gear by Laser Cladding Ni60 Alloy Powder: Process, Microstructure and Mechanical Performance. Appl. Sci. 2023, 13, 319. [Google Scholar] [CrossRef]
- Chen, W.J.; Chen, H.; Li, C.C.; Wang, X.L.; Cai, Q. Microstructure and fatigue crack growth of EA4T steel in laser cladding remanufacturing. Eng. Fail. Anal. 2017, 79, 120–129. [Google Scholar] [CrossRef]
- Nie, M.H.; Zhang, S.; Wang, Z.Y.; Zhang, C.H.; Chen, H.T.; Chen, J. Effect of laser power on microstructure and interfacial bonding strength of laser cladding 17-4PH stainless steel coatings. Mater. Chem. Phys. 2022, 275, 125236. [Google Scholar] [CrossRef]








| Element | C | Si | Mn | Cr | Ni | Mo | V | Fe |
|---|---|---|---|---|---|---|---|---|
| Component | 0.16–0.18 | ≤1.00 | ≤1.00 | 11.50–14.00 | ≤0.60 | 0.80–1.20 | ≤0.30 | Bal. |
| Element | C | Si | Mn | Mo | Ni | Cr | Fe |
|---|---|---|---|---|---|---|---|
| Component | 0.06 | 0.92 | 1.51 | 2.55 | 13.23 | 17.24 | Bal. |
| B4C Content | Diffracted Intensity | Half High Width | Grain Size |
|---|---|---|---|
| 2.5 wt.%B4C | 373 | 0.656 | 132 |
| 5 wt.%B4C | 285 | 0.721 | 120 |
| 7.5 wt.%B4C | 252 | 0.974 | 88 |
| 10 wt.%B4C | 177 | 0.921 | 94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Li, H.; Liu, Y.; Jiang, J.; Zhang, Y. Effect of B4C Content on Microstructure and Wear Resistance of Laser-Cladding-Enhanced 316 Stainless Steel Coatings. Coatings 2025, 15, 681. https://doi.org/10.3390/coatings15060681
Zhang D, Li H, Liu Y, Jiang J, Zhang Y. Effect of B4C Content on Microstructure and Wear Resistance of Laser-Cladding-Enhanced 316 Stainless Steel Coatings. Coatings. 2025; 15(6):681. https://doi.org/10.3390/coatings15060681
Chicago/Turabian StyleZhang, Dongdong, Haozhe Li, Yu Liu, Jingyu Jiang, and Yufeng Zhang. 2025. "Effect of B4C Content on Microstructure and Wear Resistance of Laser-Cladding-Enhanced 316 Stainless Steel Coatings" Coatings 15, no. 6: 681. https://doi.org/10.3390/coatings15060681
APA StyleZhang, D., Li, H., Liu, Y., Jiang, J., & Zhang, Y. (2025). Effect of B4C Content on Microstructure and Wear Resistance of Laser-Cladding-Enhanced 316 Stainless Steel Coatings. Coatings, 15(6), 681. https://doi.org/10.3390/coatings15060681






