Integrated Microstructural and Chemical Approach for Improving CMAS Resistance in Thermal and Environmental Barrier Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
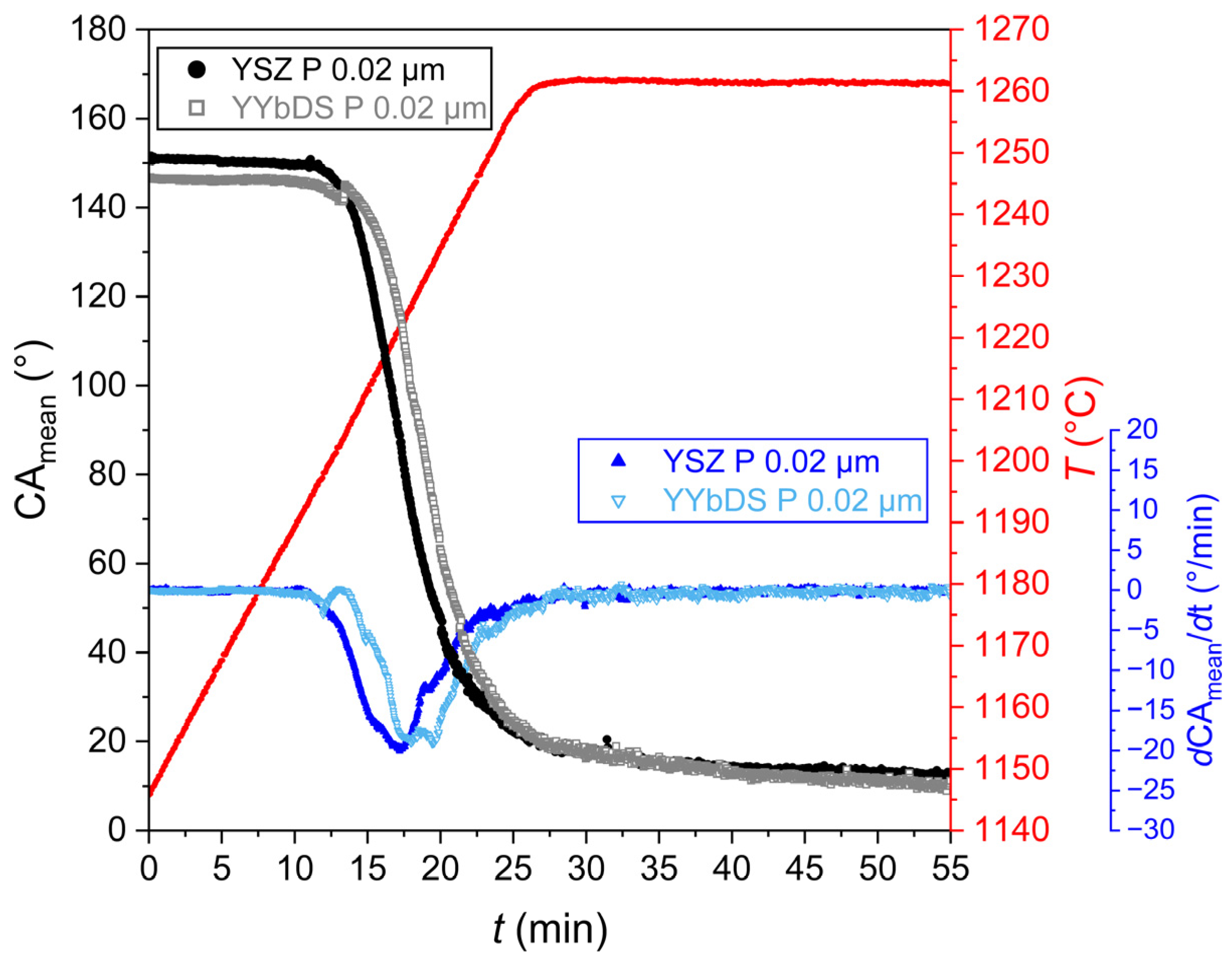
2.3. Wettability Experiments
3. Results and Discussion
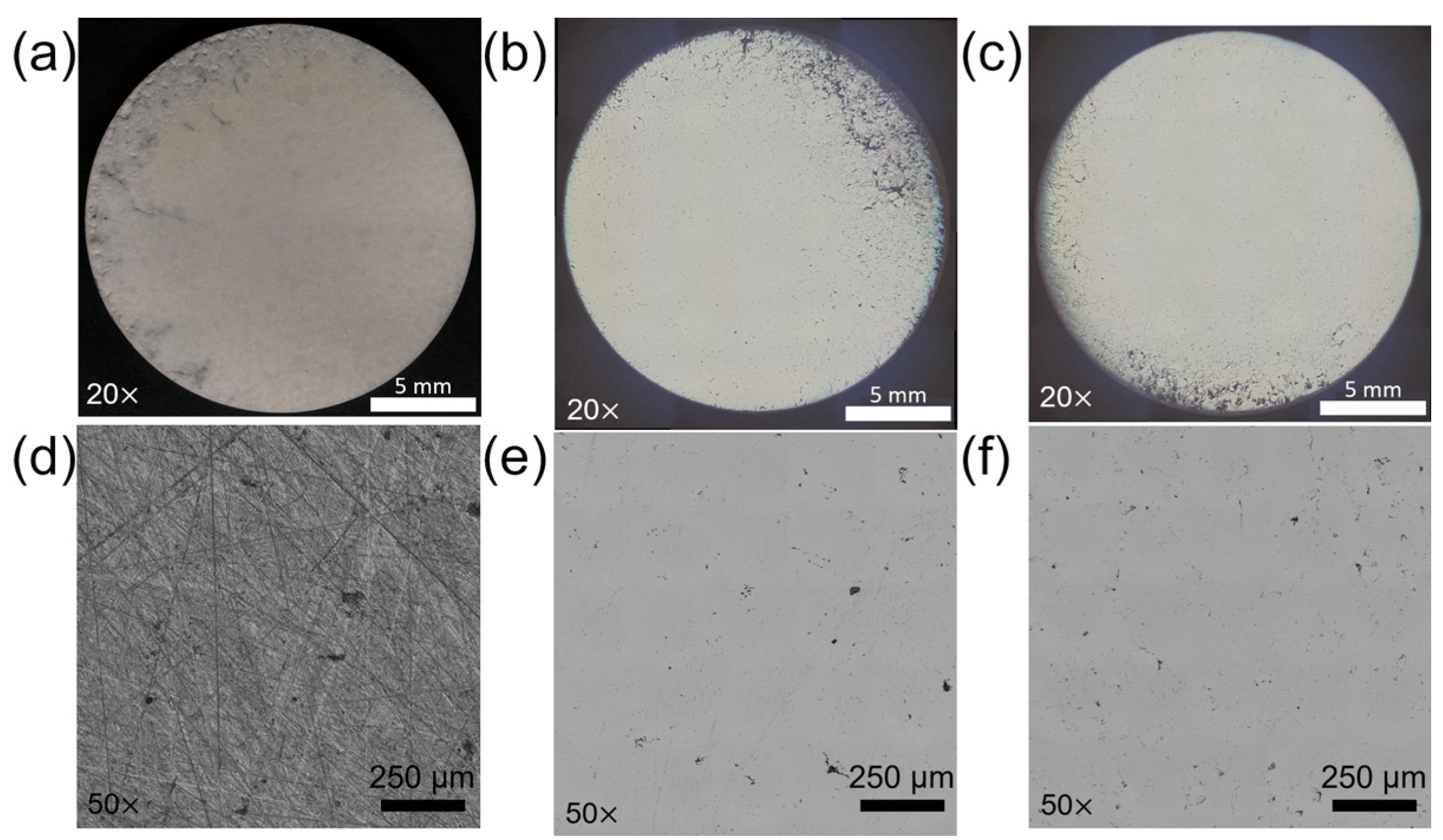
3.1. Surface Roughness Influence
3.1.1. Surface Properties and CMAS Wettability
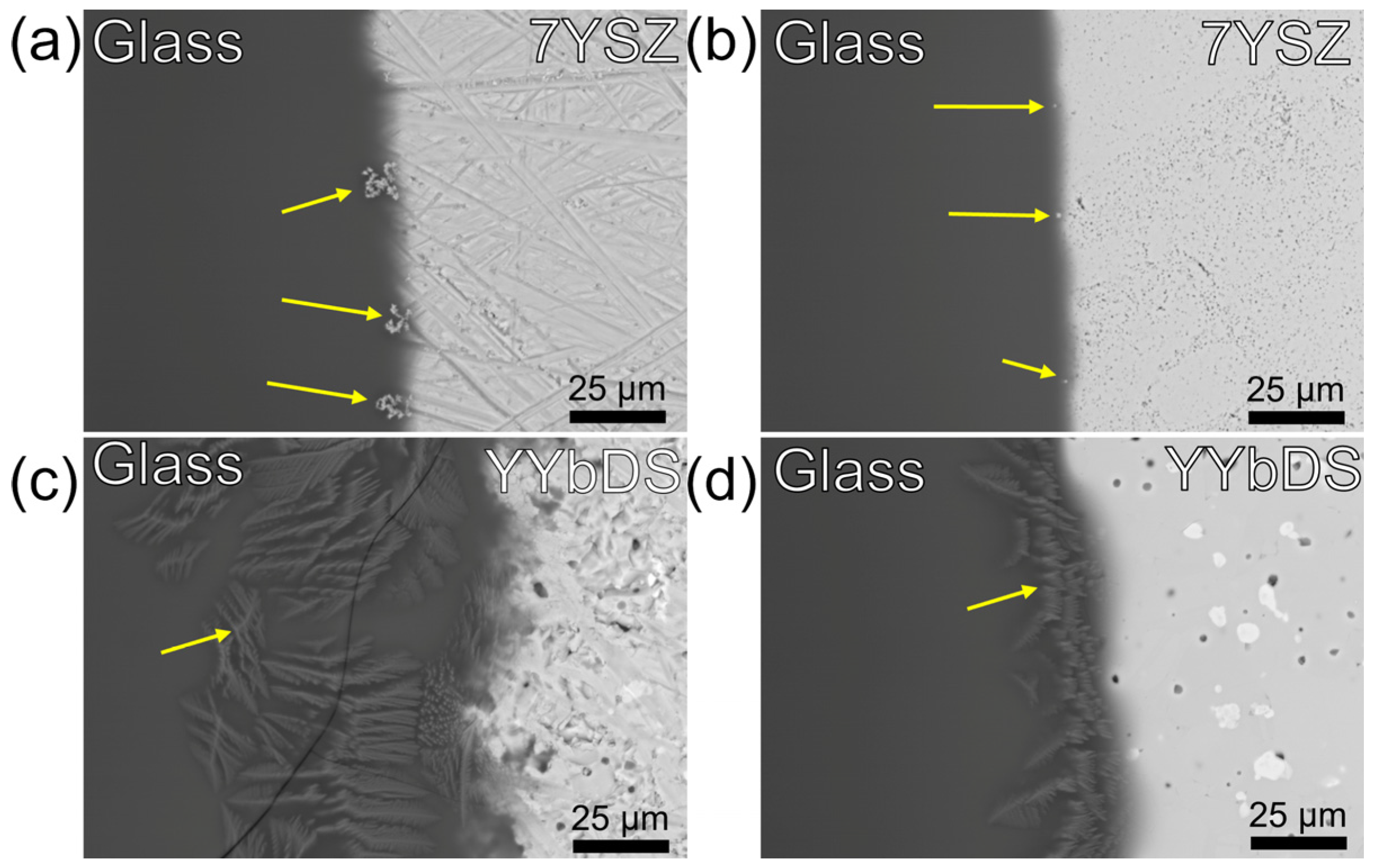
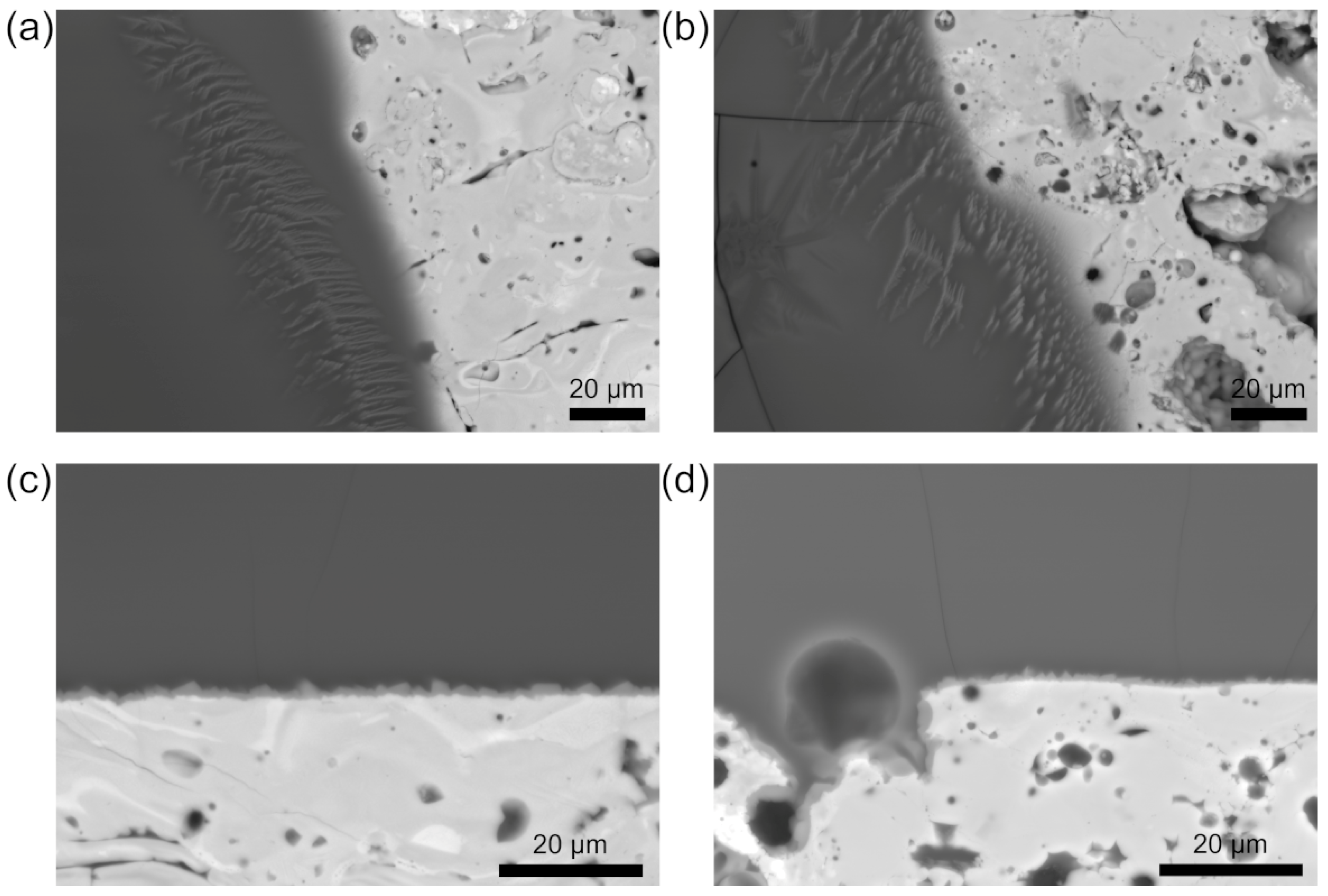
3.1.2. Microscopy of CMAS–Ceramic Interface
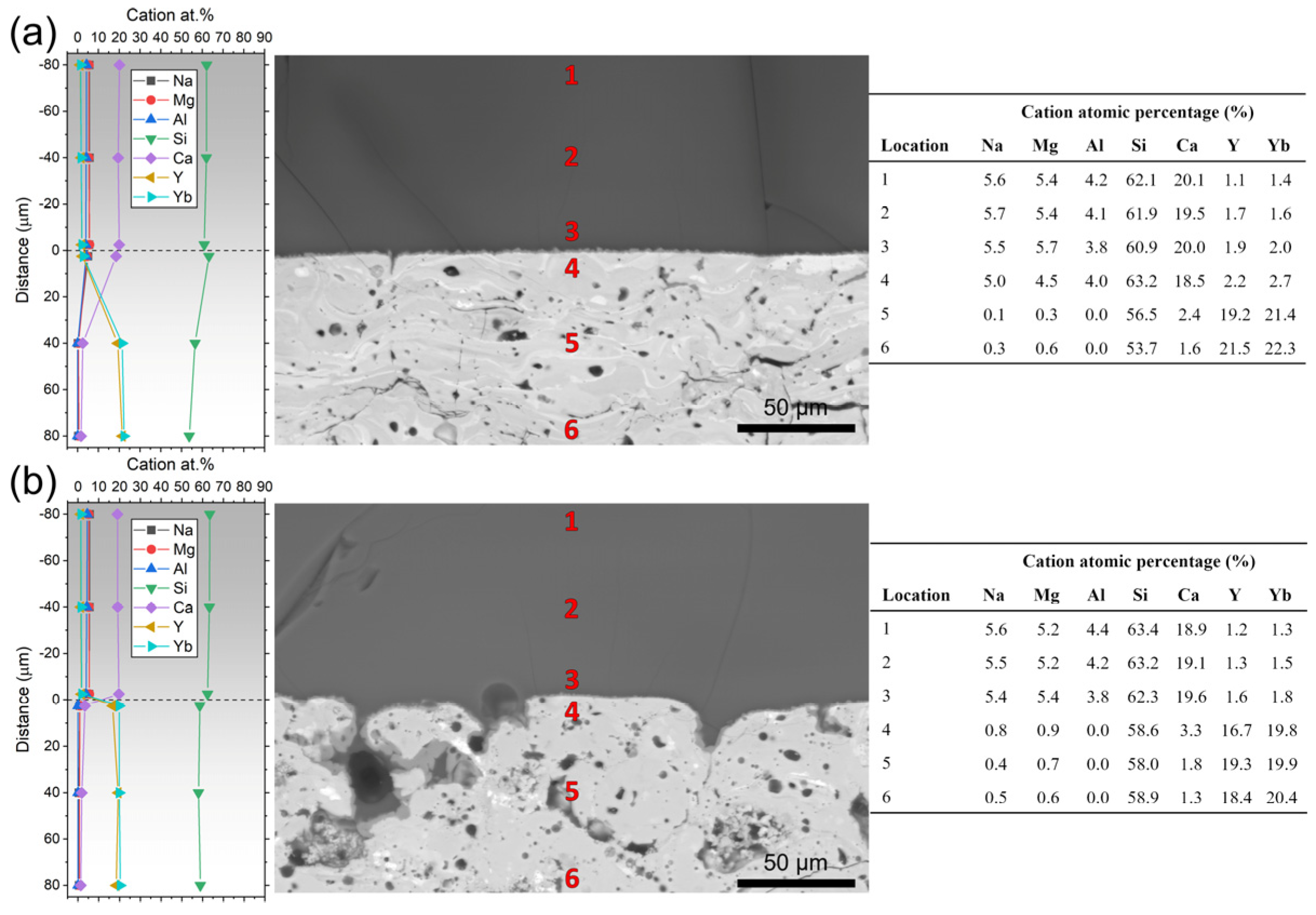
3.2. Chemistry Influence
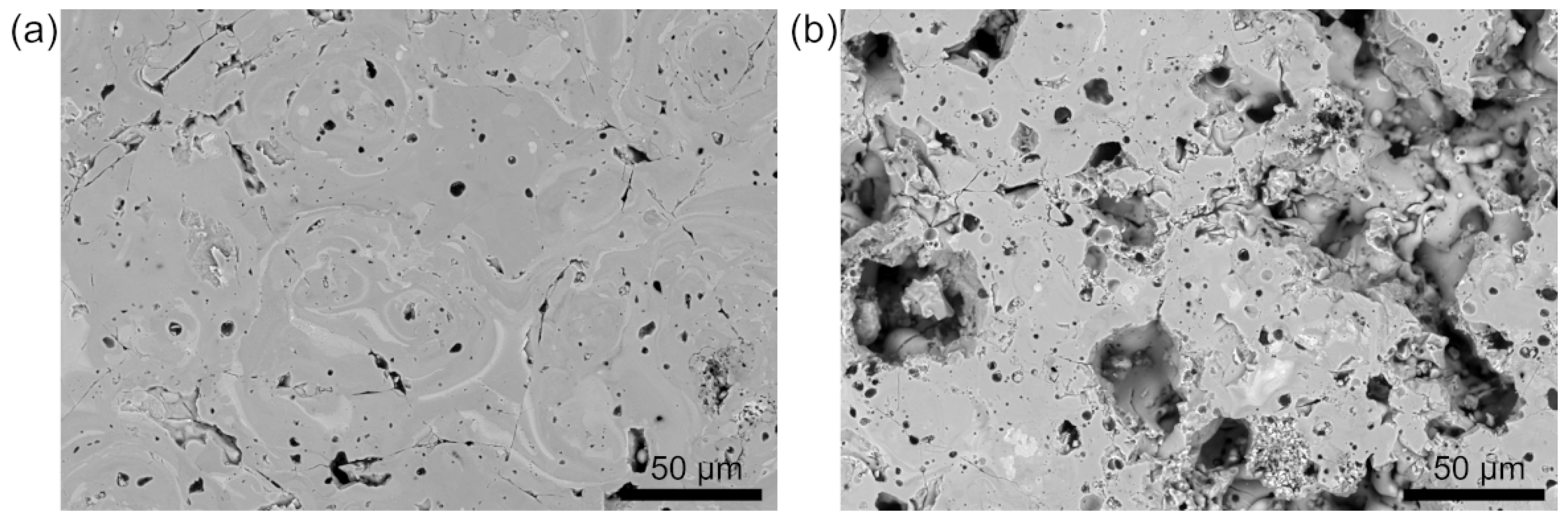
3.3. Microstructure Influence
Pore Size and Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMAS | Calcia-magnesia-alumino-silicate |
| T/EBC | Thermal/environmental barrier coating |
| 7YSZ | 7 wt.% yttria-stabilized zirconia |
| YYbDS | Yttrium ytterbium disilicate |
| SEM | Scanning electron microscopy |
| EDS | Energy dispersive spectroscopy |
| CA | Contact angle |
| Ap | Apatite |
References
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal Barrier Coatings for Gas-Turbine Engine Applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Padture, N.P. Advanced Structural Ceramics in Aerospace Propulsion. Nat. Mater. 2016, 15, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Vaßen, R.; Bakan, E.; Mack, D.E.; Guillon, O. A Perspective on Thermally Sprayed Thermal Barrier Coatings: Current Status and Trends. J. Therm. Spray Technol. 2022, 31, 685–698. [Google Scholar] [CrossRef]
- Krause, A.R.; Senturk, B.S.; Garces, H.F.; Dwivedi, G.; Ortiz, A.L.; Sampath, S.; Padture, N.P. 2ZrO2·Y2O3 Thermal Barrier Coatings Resistant to Degradation by Molten CMAS: Part I, Optical Basicity Considerations and Processing. J. Am. Ceram. Soc. 2014, 97, 3943–3949. [Google Scholar] [CrossRef]
- Zhao, H.; Levi, C.G.; Wadley, H.N.G. Molten Silicate Interactions with Thermal Barrier Coatings. Surf. Coat. Technol. 2014, 251, 74–86. [Google Scholar] [CrossRef]
- Levi, C.G.; Hutchinson, J.W.; Vidal-Sétif, M.H.; Johnson, C.A. Environmental Degradation of Thermal-Barrier Coatings by Molten Deposits. MRS Bull. 2012, 37, 932–941. [Google Scholar] [CrossRef]
- Krämer, S.; Yang, J.; Levi, C.G.; Johnson, C.A. Thermochemical Interaction of Thermal Barrier Coatings with Molten CaO-MgO-Al2O3-SiO2 (CMAS) Deposits. J. Am. Ceram. Soc. 2006, 89, 3167–3175. [Google Scholar] [CrossRef]
- Peng, H.; Wang, L.; Guo, L.; Miao, W.; Guo, H.; Gong, S. Degradation of EB-PVD Thermal Barrier Coatings Caused by CMAS Deposits. Prog. Nat. Sci. Mater. Int. 2012, 22, 461–467. [Google Scholar] [CrossRef]
- Li, L.; Hitchman, N.; Knapp, J. Failure of Thermal Barrier Coatings Subjected to CMAS Attack. J. Therm. Spray Technol. 2010, 19, 148–155. [Google Scholar] [CrossRef]
- Poerschke, D.L.; Jackson, R.W.; Levi, C.G. Silicate Deposit Degradation of Engineered Coatings in Gas Turbines: Progress toward Models and Materials Solutions. Annu. Rev. Mater. Res. 2017, 47, 297–330. [Google Scholar] [CrossRef]
- Godbole, E.P.; Hewage, N.; von der Handt, A.; Poerschke, D.L. Quantifying the Efficiency of Reactions between Silicate Melts and Rare Earth Aluminate-Zirconate T/EBC Materials. J. Eur. Ceram. Soc. 2023, 43, 5626–5635. [Google Scholar] [CrossRef]
- Poerschke, D.L.; Barth, T.L.; Levi, C.G. Equilibrium Relationships between Thermal Barrier Oxides and Silicate Melts. Acta Mater. 2016, 120, 302–314. [Google Scholar] [CrossRef]
- Krämer, S.; Yang, J.; Levi, C.G. Infiltration-Inhibiting Reaction of Gadolinium Zirconate Thermal Barrier Coatings with CMAS Melts. J. Am. Ceram. Soc. 2008, 91, 576–583. [Google Scholar] [CrossRef]
- Poerschke, D.L.; Levi, C.G. Effects of Cation Substitution and Temperature on the Interaction between Thermal Barrier Oxides and Molten CMAS. J. Eur. Ceram. Soc. 2015, 35, 681–691. [Google Scholar] [CrossRef]
- Costa, G.C.C.; Zhu, D.; Kulis, M.J.; Acosta, W.A.; Ghoshal, A. Reactivity between Rare-Earth Oxides Based Thermal Barrier Coatings and a Silicate Melt. J. Am. Ceram. Soc. 2018, 101, 3674–3693. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Liu, Q.; Cheng, L.; Wang, Y. Calcium-Magnesium-Aluminosilicate Corrosion Behaviors of Rare-Earth Disilicates at 1400 °C. J. Eur. Ceram. Soc. 2013, 33, 3419–3428. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, J.; Zheng, L.; Hu, W.; Ren, X.; Lei, Y.; Wang, J. General Trend on the Phase Stability and Corrosion Resistance of Rare Earth Monosilicates to Molten Calcium–Magnesium–Aluminosilicate at 1300 °C. Corros. Sci. 2019, 148, 281–292. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, J.; Shan, X.; Wu, D.; Zhao, X.; Guo, F.; Xiao, P. A Promising Molten Silicate Resistant Material: Rare-Earth Oxy-Apatite RE9.33(SiO4)6O2 (RE = Gd, Nd or La). J. Eur. Ceram. Soc. 2020, 40, 4101–4110. [Google Scholar] [CrossRef]
- Cao, G.; Wang, S.Q.; Wang, Y.H.; Ding, Z.Y.; Liu, Z.G.; Ouyang, J.H.; Wang, Y.M.; Wang, Y.J. Growth Kinetics of Apatite Layer Evolved from Calcia-Magnesia-Aluminosilicate (CMAS) Hot Corrosion Reaction of (Y1-xYbx)2SiO5 Ceramics at Elevated Temperatures of 1673 K and 1773 K. J. Eur. Ceram. Soc. 2023, 43, 600–611. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, D.; Gong, X.; Liu, Y.; Li, B.; Hao, P.; Zhou, F.; Xu, B.; Zhang, X.; Wang, Y. A Systematic Analysis of the Calcium-Magnesium-Aluminosilicate Corrosion Behavior of High-Entropy (5Re0.2)2Si2O7 Materials. Corros. Sci. 2023, 219. [Google Scholar] [CrossRef]
- Bryce, K.; Shih, Y.T.; Huang, L.; Lian, J. Calcium-Magnesium-Aluminosilicate (CMAS) Corrosion Resistance of High Entropy Rare-Earth Phosphate (Lu0.2Yb0.2Er0.2Y0.2Gd0.2)PO4: A Novel Environmental Barrier Coating Candidate. J. Eur. Ceram. Soc. 2023, 43, 6461–6472. [Google Scholar] [CrossRef]
- Guo, L.; Feng, J.; Meng, S. Corrosion Resistance of GdPO4 Thermal Barrier Coating Candidate in the Presence of CMAS + NaVO3 and CMAS. Corros. Sci. 2022, 208. [Google Scholar] [CrossRef]
- Guo, L.; Li, G.; Gan, Z. Effects of Surface Roughness on CMAS Corrosion Behavior for Thermal Barrier Coating Applications. J. Adv. Ceram. 2021, 10, 472–481. [Google Scholar] [CrossRef]
- Chen, L.; Li, B.; Feng, J. Rare-Earth Tantalates for next-Generation Thermal Barrier Coatings. Prog. Mater. Sci. 2024, 144. [Google Scholar] [CrossRef]
- Yang, J.; Pan, W.; Han, Y.; Zhao, M.; Huang, M.; Wan, C. Mechanical Properties, Oxygen Barrier Property and Chemical Stability of RE3NbO7 for Thermal Barrier Coating. J. Am. Ceram. Soc. 2019. [Google Scholar] [CrossRef]
- Tian, L.; Huang, Y.; Song, X.; Peng, F.; Zheng, W.; Liu, Z.; Zeng, Y. Improved CMAS Corrosion Resistance of Rare Earth Niobates by High-Entropy and Composite Design. J. Mater. Res. Technol. 2024, 31, 2388–2401. [Google Scholar] [CrossRef]
- Nieto, A.; Samayoa, E.; Ansell, T.; Luo, J. Unusual Temperature-Dependent Reactivity of Ultra-High Temperature Ceramic (UHTC) Borides with Calcia-Magnesia-Alumina-Silicate (CMAS). Materialia 2021, 20, 101265. [Google Scholar] [CrossRef]
- Qu, W.; Li, S.; Chen, Z.; Li, C.; Pei, Y.; Gong, S. Hot Corrosion Behavior and Wettability of Calcium–Magnesium–Alumina–Silicate (CMAS) on LaTi2Al9O19 Ceramic. Corros. Sci. 2020, 162. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuo, X.; Fan, Z.; Mao, J.; Deng, C.; Deng, C.; Mei, X.; Cui, J.; Zhou, K.; Liu, M. Al2O3-Modified 7YSZ Thermal Barrier Coatings for Protection against Volcanic Ash Corrosion. NPJ Mater. Degrad. 2022, 6. [Google Scholar] [CrossRef]
- Yan, R.; Liang, W.; Miao, Q.; Zhao, H.; Liu, R.; Li, J.; Zang, K.; Dong, M.; He, X.; Gao, X.; et al. Mechanical, Thermal and CMAS Resistance Properties of High-Entropy (Gd0.2Y0.2Er0.2Tm0.2Yb0.2)2Zr2O7 Ceramics. Ceram. Int. 2023, 49, 20729–20741. [Google Scholar] [CrossRef]
- Qi, Y.; Ma, W.; Li, Y.; Zhang, C.; Zhang, P.; Bai, Y.; Dong, H.; Liu, L.; Yan, S. Wetting, Infiltration, and Interaction Behavior of Calcium-Magnesium-Alumino-Silicate towards Gd/Yb-Modified SrZrO3 Coatings Deposited by Solution Precursor Plasma Spray. J. Eur. Ceram. Soc. 2023. [Google Scholar] [CrossRef]
- Wright, A.J.; Kim, Y.; Mock, C.; Sharobem, T.; McGowan, R.; Bravo, L.; Murugan, M.; Dambra, C.; Keyes, B.; Ghoshal, A. Influence of Chemistry and Surface Roughness of Various Thermal Barrier Coatings on the Wettability of Molten Sand. Surf. Coat. Technol. 2024, 481, 130649. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wang, W.; Zhang, W.; Wang, J.; Li, K.; Li, H.; Liu, P.; Yang, S.; Zhang, C. Micro-Nano Dual-Scale Coatings Prepared by Suspension Precursor Plasma Spraying for Resisting Molten Silicate Deposit. Coatings 2024, 14, 1123. [Google Scholar] [CrossRef]
- Lokachari, S.; Leng, K.; Rincon Romero, A.; Hussain, T. Effect of Microstructure on CMAS Interaction of Axial Suspension and Solution Precursor Plasma Sprayed Thermal Barrier Coatings—YSZ & GZ. In Proceedings of the Thermal Spray 2023: Proceedings from the International Thermal Spray Conference, Quebec City, QC, Canada, 22–25 May 2023; ASM International: Almere, The Netherlands, 2023; Volume 84536, pp. 653–658. [Google Scholar]
- Guo, Y.; Song, W.; Guo, L.; Li, X.; He, W.; Yan, X.; Dingwell, D.B.; Guo, H. Molten-Volcanic-Ash-Phobic Thermal Barrier Coating Based on Biomimetic Structure. Adv. Sci. 2023, 10, 2205156. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Z.; Zhuo, X.; Zhan, Y.; Liu, M.; Zhou, K.; Zhang, X. Enhancing CMAS Corrosion Resistance of PS-PVD Si/Yb2Si2O7 EBCs via Al-Modification. J. Eur. Ceram. Soc. 2023, 43, 3727–3736. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Z.; Sun, L.; Zhang, J.; Wang, J. Comprehensive Microstructural Characterization and CMAS Infiltration Resistance of Ytterbium Disilicate Coatings with Lamellar and Quasi-Columnar Structures. Corros. Sci. 2023, 221, 111316. [Google Scholar] [CrossRef]
- Wu, H.; Huo, K.; Ye, F.; Hua, Y.; Dai, F. Wetting and Spreading Behavior of Molten CMAS on the Laser Textured Thermal Barrier Coatings with the Assistance of Pt-Modification. Appl. Surf. Sci. 2023, 622, 156887. [Google Scholar] [CrossRef]
- Ushmaev, D.; Norton, A.; Kell, J. Thermally Sprayed Coatings Resistant to Environmental Degradation: Columnar-like Coatings through Laser Ablation and Surface Melting Approach. Surf. Coat. Technol. 2023, 460, 129394. [Google Scholar] [CrossRef]
- Kang, Y.X.; Bai, Y.; Du, G.Q.; Yu, F.L.; Bao, C.G.; Wang, Y.T.; Ding, F. High Temperature Wettability between CMAS and YSZ Coating with Tailored Surface Microstructures. Mater. Lett. 2018, 229, 40–43. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Z. Recent Advances of Bioinspired Functional Materials with Specific Wettability: From Nature and beyond Nature. Nanoscale Horiz. 2019, 4, 52–76. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, C.; Blackman, B.R.K.; Eduardo, S. Mimicking Nature to Control Bio-Material Surface Wetting and Adhesion. Int. Mater. Rev. 2022, 67, 658–681. [Google Scholar] [CrossRef]
- Su, B.; Tian, Y.; Jiang, L. Bioinspired Interfaces with Superwettability: From Materials to Chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Lu, X.; Hu, M.; Chen, M.; Zhang, H.; Tian, Y.; Liu, B.; Jing, Q. Comparison of Corrosion Behaviors and Wettability of CMAS on Ta2O5-Y2O3 Co-Stabilized ZrO2 and YSZ Thermal Barrier Coatings. J. Eur. Ceram. Soc. 2023, 43, 5636–5651. [Google Scholar] [CrossRef]
- Wu, J.; Guo, H.; Gao, Y.; Gong, S. Microstructure and Thermo-Physical Properties of Yttria Stabilized Zirconia Coatings with CMAS Deposits. J. Eur. Ceram. Soc. 2011, 31, 1881–1888. [Google Scholar] [CrossRef]
- Pujol, G.; Ansart, F.; Bonino, J.P.; Malié, A.; Hamadi, S. Step-by-Step Investigation of Degradation Mechanisms Induced by CMAS Attack on YSZ Materials for TBC Applications. Surf. Coat. Technol. 2013, 237, 71–78. [Google Scholar] [CrossRef]
- Stokes, J.L.; Harder, B.J.; Wiesner, V.L.; Wolfe, D.E. High-Temperature Thermochemical Interactions of Molten Silicates with Yb2Si2O7 and Y2Si2O7 Environmental Barrier Coating Materials. J. Eur. Ceram. Soc. 2019, 39, 5059–5067. [Google Scholar] [CrossRef]






| Composition | ID | Porosity (%) |
|---|---|---|
| Y0.077Zr0.923O2-δ | 7YSZ P | 3.1 |
| (Y1/2Yb1/2)2Si2O7 | YYbDS P | 2.9 |
| YYbDS C | 6.3 | |
| YYbDS C 5P | 22.7 |
| ID | Finish | Sa (µm) | Ssk | Sdq |
|---|---|---|---|---|
| 7YSZ P | 30 µm | 0.33 | −2.61 | 1.01 |
| 3 µm | 0.05 | 1.16 | 0.13 | |
| 0.02 µm | 0.05 | 0.00 | 0.15 | |
| YYbDS P | 30 µm | 0.61 | −5.32 | 1.46 |
| 3 µm | 0.08 | 9.11 | 0.36 | |
| 0.02 µm | 0.05 | 6.22 | 0.29 | |
| YYbDS C | 0.02 µm | 0.74 | −7.22 | 0.78 |
| YYbDS C 5P | 0.02 µm | 8.21 | −2.03 | 3.39 |
| Chemistry | Optimal Sa (µm) | Optimal Porosity (%) | Interfacial Product | Infiltration Depth (µm) |
|---|---|---|---|---|
| 7YSZ | <3 | N/A | None | 6–8 |
| YYbDS | <3 | ~22.7 | Ca2RE8(SiO4)6O2 | ≤2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wright, A.J.; Mock, C.; Sharobem, T.; Sotiropoulos, N.; Dambra, C.; Keyes, B.; Ghoshal, A. Integrated Microstructural and Chemical Approach for Improving CMAS Resistance in Thermal and Environmental Barrier Coatings. Coatings 2025, 15, 680. https://doi.org/10.3390/coatings15060680
Wright AJ, Mock C, Sharobem T, Sotiropoulos N, Dambra C, Keyes B, Ghoshal A. Integrated Microstructural and Chemical Approach for Improving CMAS Resistance in Thermal and Environmental Barrier Coatings. Coatings. 2025; 15(6):680. https://doi.org/10.3390/coatings15060680
Chicago/Turabian StyleWright, Andrew J., Clara Mock, Timothy Sharobem, Nickolas Sotiropoulos, Chris Dambra, Brian Keyes, and Anindya Ghoshal. 2025. "Integrated Microstructural and Chemical Approach for Improving CMAS Resistance in Thermal and Environmental Barrier Coatings" Coatings 15, no. 6: 680. https://doi.org/10.3390/coatings15060680
APA StyleWright, A. J., Mock, C., Sharobem, T., Sotiropoulos, N., Dambra, C., Keyes, B., & Ghoshal, A. (2025). Integrated Microstructural and Chemical Approach for Improving CMAS Resistance in Thermal and Environmental Barrier Coatings. Coatings, 15(6), 680. https://doi.org/10.3390/coatings15060680





