Preparation of Superhydrophobic Hydroxyapatite Coating on AZ31 Mg Alloy by Combining Micro-Arc Oxidation and Liquid-Phase Deposition
Abstract
1. Introduction
2. Experiments
2.1. Preparation of MAO Coating
2.2. Liquid-Phase Deposition
2.3. Morphologies and Characterizations
2.4. Bonding Strength Tests
2.5. Electrochemical Tests
2.6. In Vitro Mineralization Tests
3. Results and Discussions
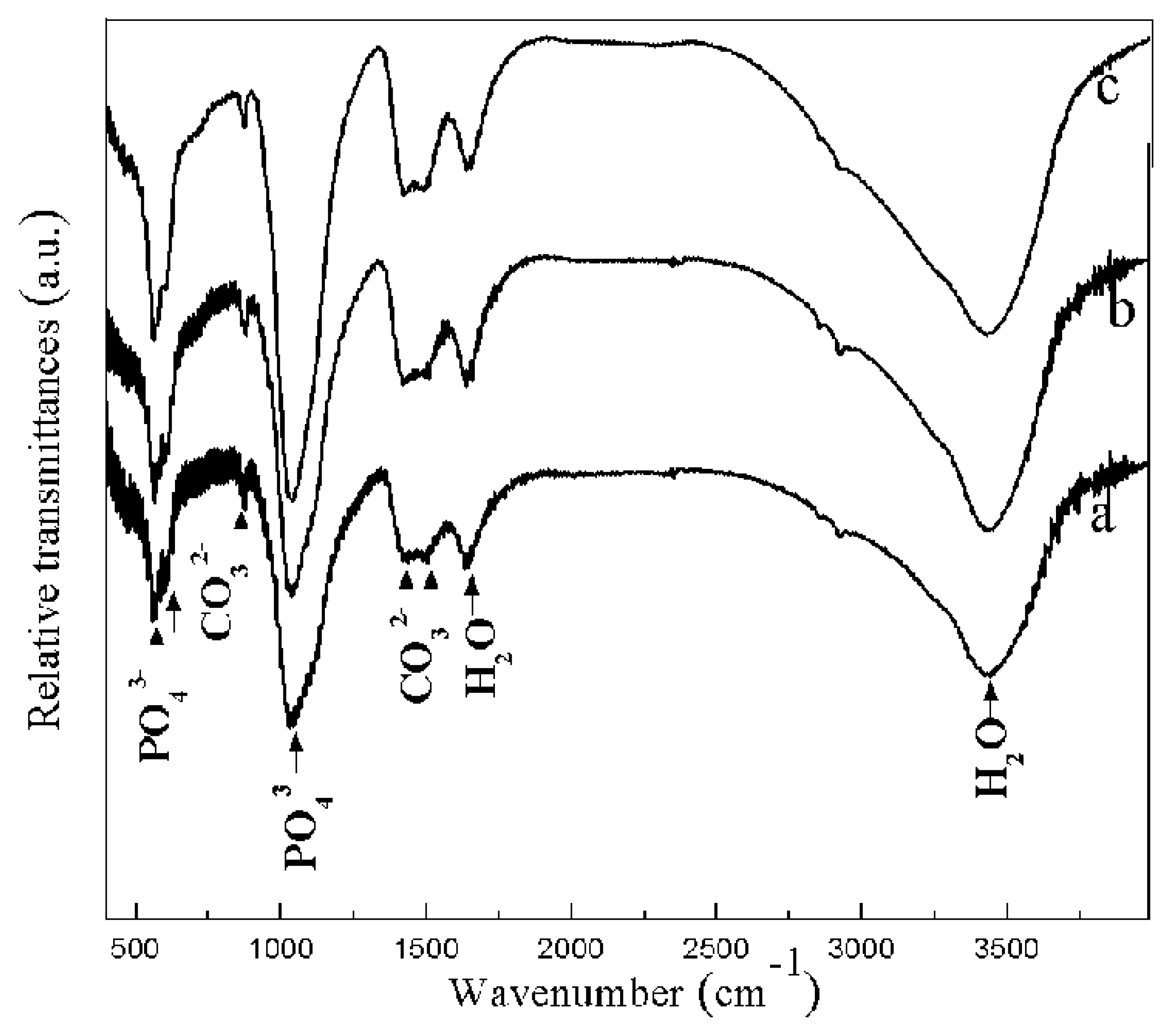
3.1. Surface Morphologies and Elemental Ratio of the Coatings
3.2. Cross-Sectional Morphology and EDS Analysis
4. Conclusions
- The composite coatings comprise an inner MAO coating and an outer HA layer. The solution penetrates the porous structure of the MAO coating, deposits within it, and subsequently fuses with the porous layer.
- Solution treatment enhances the hydrophobicity of the MAO coatings. A maximum contact angle of 161° is achieved at NaH2PO4 concentration of 0.1 mmol/L. This effect is attributed to the formation of dandelion-like clusters on the surface.
- The bonding strength of the composite coatings is enhanced by the incorporation of the MAO coating. All composite coatings exhibit a tensile strength exceeding 20 MPa.
- Electrochemical measurements revealed that the composite coatings exhibit significantly greater corrosion resistance than bare and MAO-coated magnesium, owing to the sealing effect and enhanced hydrophobicity.
- The composite coatings demonstrate superior apatite-forming bioactivity. The formation of new hydroxyapatite on the surface of the composite coatings was observed after 3 days of immersion in SBF.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.; Gilbert, J.L.; Lv, W.W.; Du, P.; Pan, H. Reduction reactions dominate the interactions between Mg alloys and cells: Understanding the mechanisms. Bioact. Mater. 2025, 45, 363–387. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef]
- Yang, J.; Koons, G.L.; Cheng, G.; Zhao, L.; Mikos, A.G.; Cui, F.-Z. A review on the exploitation of biodegradable magnesium-based composites for medical applications. Biomed. Mater. 2018, 13, 022001. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials. 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Xu, L.; Pan, F.; Yu, G.; Yang, L.; Zhang, E.; Yang, K. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy. Biomaterials. 2009, 30, 1512–1523. [Google Scholar] [CrossRef]
- Uppal, G.; Thakur, A.; Chauhan, A.; Bala, S. Magnesium based implants for functional bone tissue regeneration—A review. J. Magnes. Alloy. 2022, 10, 356–386. [Google Scholar] [CrossRef]
- Zhang, A.; Fan, X.; Yang, Z.; Xie, Y.; Wu, T.; Zhang, M.; Xue, Y.; Wang, Y.; Zhao, Y.; Wu, X.; et al. Optimized design and biomechanical evaluation of biodegradable magnesium alloy vascular stents. Acta Mech. Sin. 2024, 41, 1–14. [Google Scholar] [CrossRef]
- Tang, H.; Han, Y.; Wu, T.; Tao, W.; Jian, X.; Wu, Y.; Xu, F. Synthesis and properties of hydroxyapatite-containing coating on AZ31 magnesium alloy by micro-arc oxidation. Appl. Surf. Sci. 2017, 400, 391–404. [Google Scholar] [CrossRef]
- Podgorbunsky, A.; Imshinetskiy, I.; Mashtalyar, D.; Sidorova, M.; Gnedenkov, A.; Sinebryukhov, S.; Gnedenkov, S. Bioresorbable composites based on magnesium and hydroxyapatite for use in bone tissue engineering: Focus on controlling and minimizing corrosion activity. Ceram. Int. 2024, 51, 423–436. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, M.; Zhao, W.; Geng, Y.; Chen, Y.; Tian, H.; Zhou, Y.; Chen, G.; Wu, R.; Zheng, Y.; et al. Insight the long-term biodegradable Mg-RE-Sr alloy for orthopaedics implant via friction stir processing. Bioact. Mater. 2024, 41, 293–311. [Google Scholar] [CrossRef]
- Hashemi, T.S.; Jaiswal, S.; Celikin, M.; McCarthy, H.O.; Levingstone, T.J.; Dunne, N.J. Strategically designed bioactive dual-layer coating of octacalcium phosphate and dicalcium phosphate dihydrate for enhancement of the corrosion resistance of pure magnesium for orthopaedic applications. Surf. Coatings Technol. 2024, 495. [Google Scholar] [CrossRef]
- Naftchali, N.K.; Aghdam, R.M.; Najjari, A.; Dehghanian, C. Investigating a nanocomposite coating of cerium ox-ide/merwinite via PEO/EPD for enhanced biocorrosion resistance, bioactivity and antibacterial activity of magnesium-based implants. Ceram. Int. 2024, 50, 42766–42779. [Google Scholar] [CrossRef]
- Tang, H.; Gao, Y. Preparation and characterization of hydroxyapatite containing coating on AZ31 magnesium alloy by micro-arc oxidation. J. Alloy Compd. 2016, 688, 699–708. [Google Scholar] [CrossRef]
- Tang, H.; Wu, T.; Wang, H.; Jian, X.; Wu, Y. Corrosion behavior of HA containing ceramic coated magnesium alloy in Hank’s solution. J. Alloy Compd. 2017, 698, 643–653. [Google Scholar] [CrossRef]
- Wang, J.; Dou, J.; Wang, Z.; Hu, C.; Liu, J.; Yu, H.; Chen, C. Corrosion resistance and biodegradability of micro-arc oxidation coatings with the variable sodium fluoride concentration on ZM21 magnesium alloys. J. Alloy Compd. 2023, 962. [Google Scholar] [CrossRef]
- Tang, H.; Li, D.; Chen, X.; Wu, C.; Wang, F. FORMATION OF HA-CONTAINING COATING ON AZ31 MAGNESIUM ALLOY BY MICRO-ARC OXIDATION. Surf. Rev. Lett. 2013, 20. [Google Scholar] [CrossRef]
- Tang, H.; Wang, F. Synthesis and properties of CaTiO3-containing coating on AZ31 magnesium alloy by micro-arc oxidation. Mater. Lett. 2013, 93, 427–430. [Google Scholar] [CrossRef]
- Li, B.; Han, Y.; Qi, K. Formation Mechanism, Degradation Behavior, and Cytocompatibility of a Nanorod-Shaped HA and Pore-Sealed MgO Bilayer Coating on Magnesium. ACS Appl. Mater. Interfaces. 2014, 6, 18258–18274. [Google Scholar] [CrossRef]
- Tang, H.; Tao, W.; Wang, C.; Yu, H. Fabrication of hydroxyapatite coatings on AZ31 Mg alloy by micro-arc oxidation coupled with sol–gel treatment. RSC Adv. 2018, 8, 12368–12375. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, B.; Liu, M.; Ge, Y. Corrosion characterization of micro-arc oxidization composite electrophoretic coating on AZ31B magnesium alloy. J. Alloy Compd. 2015, 621, 53–61. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Zhang, S.; Zhao, C.; Zhang, F.; Zhang, X.; Cao, L.; Fan, Q.; Tang, T. In vitro responses of human bone marrow stromal cells to a fluoridated hydroxyapatite coated biodegradable Mg–Zn alloy. Biomaterials 2010, 31, 5782–5788. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, S.-D.; Zhou, L.; Lian, J.-B.; He, J.; Li, X.-W. Preparation and characterization of calcium phosphate containing coating on plasma electrolytic oxidized magnesium and its corrosion behavior in simulated body fluids. J. Alloy Compd. 2022, 896. [Google Scholar] [CrossRef]
- Farshid, S.; Kharaziha, M.; Salehi, H.; Hakemi, M.G. Morphology-Dependent Immunomodulatory Coating of Hydroxyapatite/PEO for Magnesium-Based Bone Implants. ACS Appl. Mater. Interfaces 2023, 15, 48996–49011. [Google Scholar] [CrossRef]
- Farshid, S.; Kharaziha, M.; Atapour, M.; Di Franco, F.; Santamaria, M. Duplex plasma electrolytic oxidation/hydroxyapatite- polydopamine coating on WE43 alloy for bone implants: Long-term corrosion resistance and biological properties. Surf. Coatings Technol. 2024, 493. [Google Scholar] [CrossRef]
- Tang, H.; Xin, T.; Sun, Q.; Yi, C.; Jiang, Z.; Wang, F. Influence of FeSO4 concentration on thermal emissivity of coatings formed on titanium alloy by micro-arc oxidation. Appl. Surf. Sci. 2011, 257, 10839–10844. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wang, W.; Yu, F.; Cao, W.; Hu, S. Effect of sodium fluoride additive on microstructure and corrosion performance of micro-arc oxidation coatings on EK30 magnesium alloy. Surf. Coatings Technol. 2024, 496. [Google Scholar] [CrossRef]
- Taş, A.C. Molten Salt Synthesis of Calcium Hydroxyapatite Whiskers. J. Am. Ceram. Soc. 2001, 84, 295–300. [Google Scholar] [CrossRef]
- Mujahid, K.; Iqbal, F.; Ali, A.; Butt, M.; Bukhari, N.; Nosheen, S.; Sharif, F.; Abbas, Z. Zinc-doped phosphate coatings for en-hanced corrosion resistance, antibacterial properties, and biocompatibility of AZ91D Mg alloy. J. Alloys Com-Pounds 2024, 1005. [Google Scholar]
- Guan, Q.; Hu, T.; Zhang, L.; Yu, M.; Niu, J.; Ding, Z.; Yu, P.; Yuan, G.; An, Z.; Pei, J. Concerting magnesium implant degradation facilitates local chemotherapy in tumor-associated bone defect. Bioact. Mater. 2024, 40, 445–459. [Google Scholar] [CrossRef]
- Kong, J.; Kolooshani, A.; Kolahdouz, A.; Nejad, M.G.; Toghraie, D. Fabrication and characterization of magnesium implants coated with magnetic nanoparticles-wollastonite-hydroxyapatite for medical and sports injury applications: Finite element analysis. Ceram. Int. 2023, 50, 5755–5765. [Google Scholar] [CrossRef]
- Drotarova, L.; Slamecka, K.; Balint, T.; Remesova, M.; Hudak, R.; Zivcak, J.; Schnitzer, M.; Celko, L.; Montufar, E.B. Biodegradable WE43 Mg alloy/hydroxyapatite interpenetrating phase composites with reduced hydrogen evolution. Bioact. Mater. 2024, 42, 519–530. [Google Scholar]
- Ahmadkhaniha, D.; Fedel, M.; Sohi, M.H.; Hanzaki, A.Z.; Deflorian, F. Corrosion behavior of magnesium and magnesium–hydroxyapatite composite fabricated by friction stir processing in Dulbecco’s phosphate buffered saline. Corros. Sci. 2016, 104, 319–329. [Google Scholar] [CrossRef]
- Chen, Z.; Ji, H.; Geng, X.; Chen, X.; Yong, X.; Zhang, S. 3-D distribution characteristics of the micro-defects in the PEO coating on ZM6 mg-alloy during corrosion. Corros. Sci. 2020, 174. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Gao, S.-D.; Li, P.-P.; Zeng, R.-C.; Zhang, F.; Li, S.-Q.; Han, E.-H. Corrosion resistance of a self-healing micro-arc oxidation/polymethyltrimethoxysilane composite coating on magnesium alloy AZ31. Corros. Sci. 2017, 118, 84–95. [Google Scholar] [CrossRef]
- Cui, X.-J.; Lin, X.-Z.; Liu, C.-H.; Yang, R.-S.; Zheng, X.-W.; Gong, M. Fabrication and corrosion resistance of a hydrophobic micro-arc oxidation coating on AZ31 Mg alloy. Corros. Sci. 2015, 90, 402–412. [Google Scholar] [CrossRef]
- Dai, J.; Yang, J.; Zhang, X.; Zhang, L.; Sun, B.; Li, X.; Bai, J.; Xue, F.; Chu, C. Synergistic effects of BSA adsorption and shear stress on corrosion behaviors of WE43 alloy under simulated physiological flow field. Corros. Sci. 2024, 237. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, C.; Wei, J.; Wen, Z.; Zhang, S.; Lin, L. Calcium phosphate/chitosan composite coating: Effect of different concentrations of Mg2+ in the m-SBF on its bioactivity. Appl. Surf. Sci. 2013, 280, 256–262. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef]










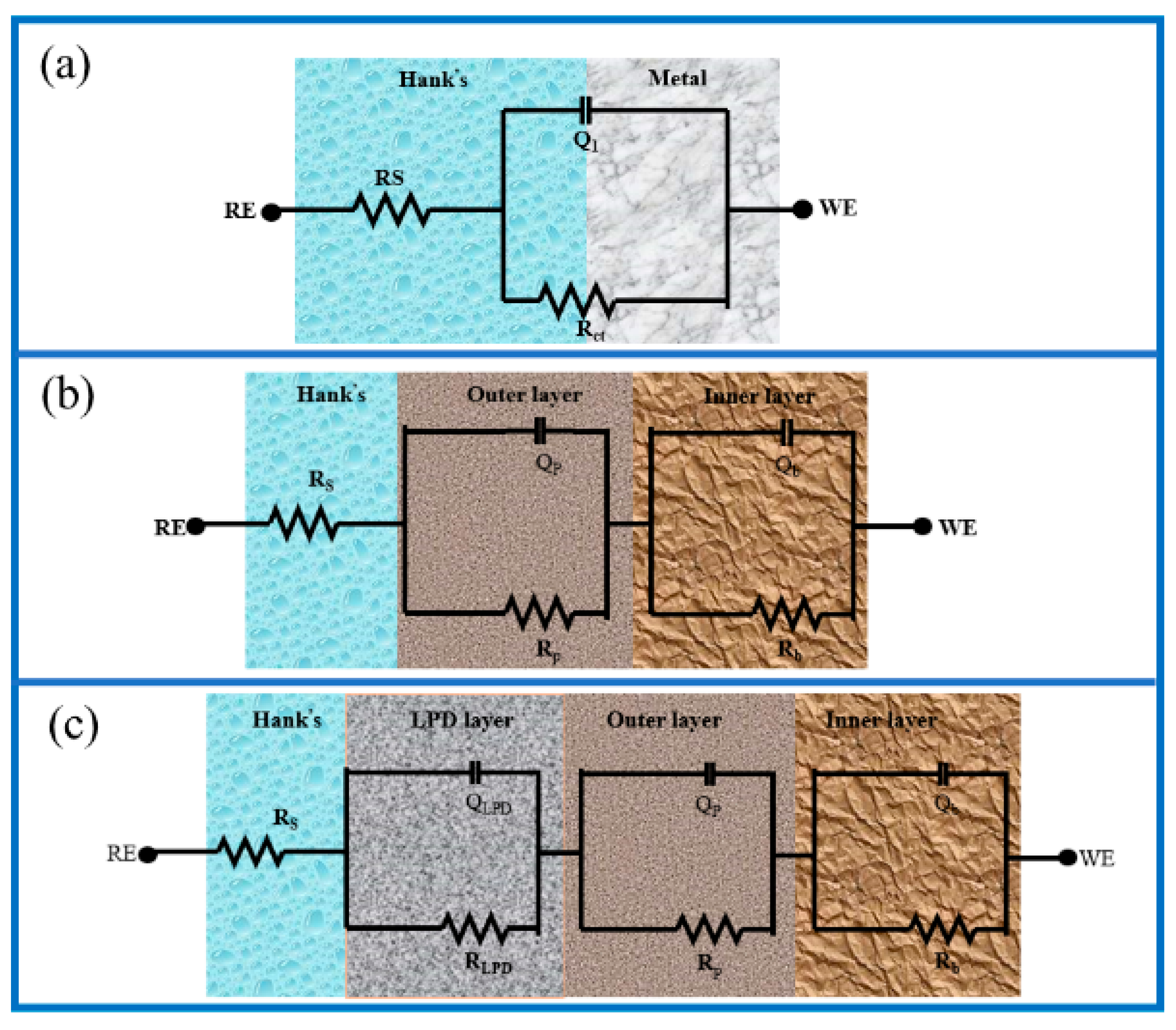

| Samples | Ecorr (mV) | Icorr (A/cm2) |
|---|---|---|
| AZ31 alloy | −137 | 86.7 |
| MAO coating | −95 | 5.23 |
| MAO-LPD-1 | −83 | 0.933 |
| MAO-LPD-2 | −77 | 0.548 |
| MAO-LPD-3 | −65 | 0.281 |
| Samples | Rs(Ω·cm2) | Q1 − Y0 | Q1−n | R1(Ω·cm2) | Q2 − Y0 | Q2−n | R2(Ω·cm2) | Q3 − Y0 | Q3−n | R3(Ω·cm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| (Ω−1 cm−2 s−n) | (Ω−1 cm−2 s−n) | (Ω−1 cm−2 s−n) | ||||||||
| Sub | 7.3 × 10−2 | 1.65 × 10−5 | 0.87 | 5015 | ||||||
| MAO | 9.4 × 10−4 | 7.88 × 10−7 | 0.83 | 5712 | 3.76 × 10−4 | 0.81 | 7132 | |||
| MAO-LPD-1 | 1.7 × 10−6 | 7.53 × 10−5 | 0.81 | 972 | 1.65 × 10−6 | 0.76 | 1.1 × 104 | 4.72 × 10−4 | 0.79 | 8163 |
| MAO-LPD-2 | 7.2 × 10−6 | 1.87 × 10−7 | 0.77 | 2873 | 9.55 × 10−6 | 0.71 | 1.6 × 104 | 8.12 × 10−4 | 0.74 | 9447 |
| MAO-LPD-3 | 9.5 ×10−6 | 2.61 × 10−7 | 0.79 | 3223 | 9.78 × 10−6 | 0.67 | 1.8 × 104 | 7.75 × 10−4 | 0.71 | 9852 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Liang, X.; Yuan, Y.; Jian, F.; Tang, H. Preparation of Superhydrophobic Hydroxyapatite Coating on AZ31 Mg Alloy by Combining Micro-Arc Oxidation and Liquid-Phase Deposition. Coatings 2025, 15, 675. https://doi.org/10.3390/coatings15060675
Hu Y, Liang X, Yuan Y, Jian F, Tang H. Preparation of Superhydrophobic Hydroxyapatite Coating on AZ31 Mg Alloy by Combining Micro-Arc Oxidation and Liquid-Phase Deposition. Coatings. 2025; 15(6):675. https://doi.org/10.3390/coatings15060675
Chicago/Turabian StyleHu, Yanqing, Xin Liang, Yujie Yuan, Feiyu Jian, and Hui Tang. 2025. "Preparation of Superhydrophobic Hydroxyapatite Coating on AZ31 Mg Alloy by Combining Micro-Arc Oxidation and Liquid-Phase Deposition" Coatings 15, no. 6: 675. https://doi.org/10.3390/coatings15060675
APA StyleHu, Y., Liang, X., Yuan, Y., Jian, F., & Tang, H. (2025). Preparation of Superhydrophobic Hydroxyapatite Coating on AZ31 Mg Alloy by Combining Micro-Arc Oxidation and Liquid-Phase Deposition. Coatings, 15(6), 675. https://doi.org/10.3390/coatings15060675








