Investigation of Superhydrophobic, Drag-Reducing and Anti-Icing Properties of Swimming Goggles
Abstract
1. Introduction
2. Experimental Section
2.1. Main Materials
2.2. Sample Preparation
2.3. Experimental Procedure
3. Results and Discussion
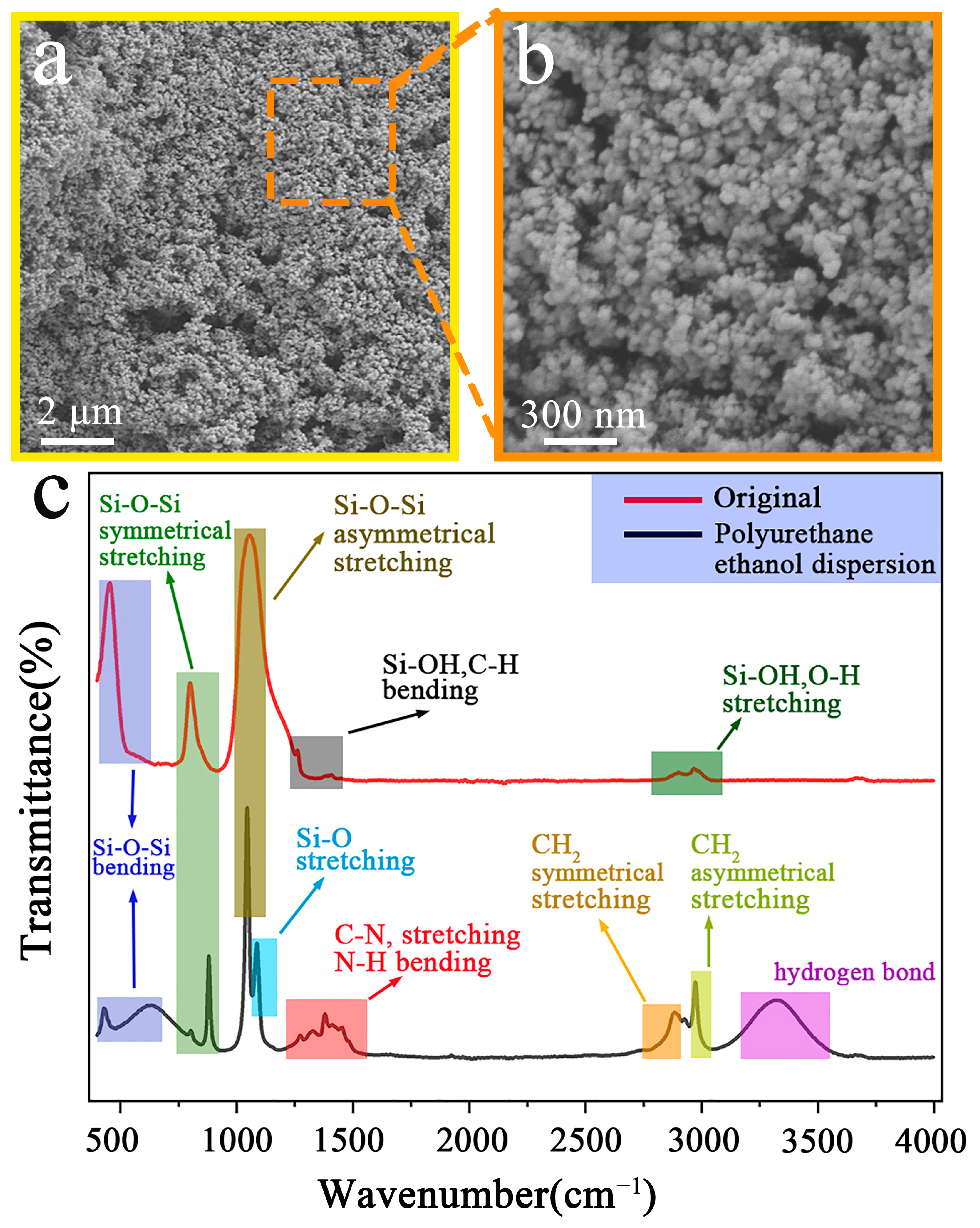
3.1. SiO2 Particle Characterization and Infrared Spectroscopy Analysis
3.2. Performance Analysis
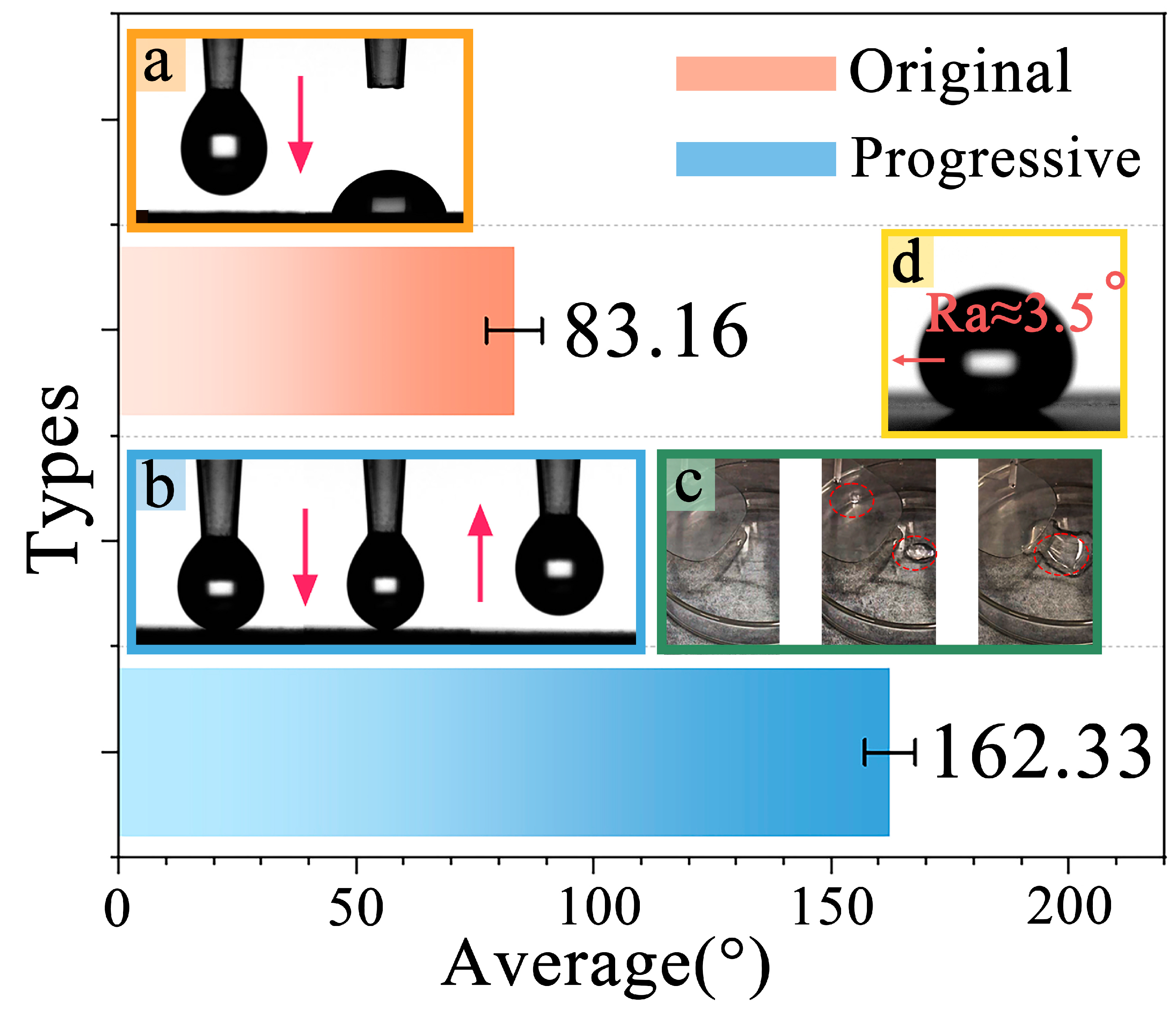
3.2.1. Hydrophobicity Analysis of Original Samples
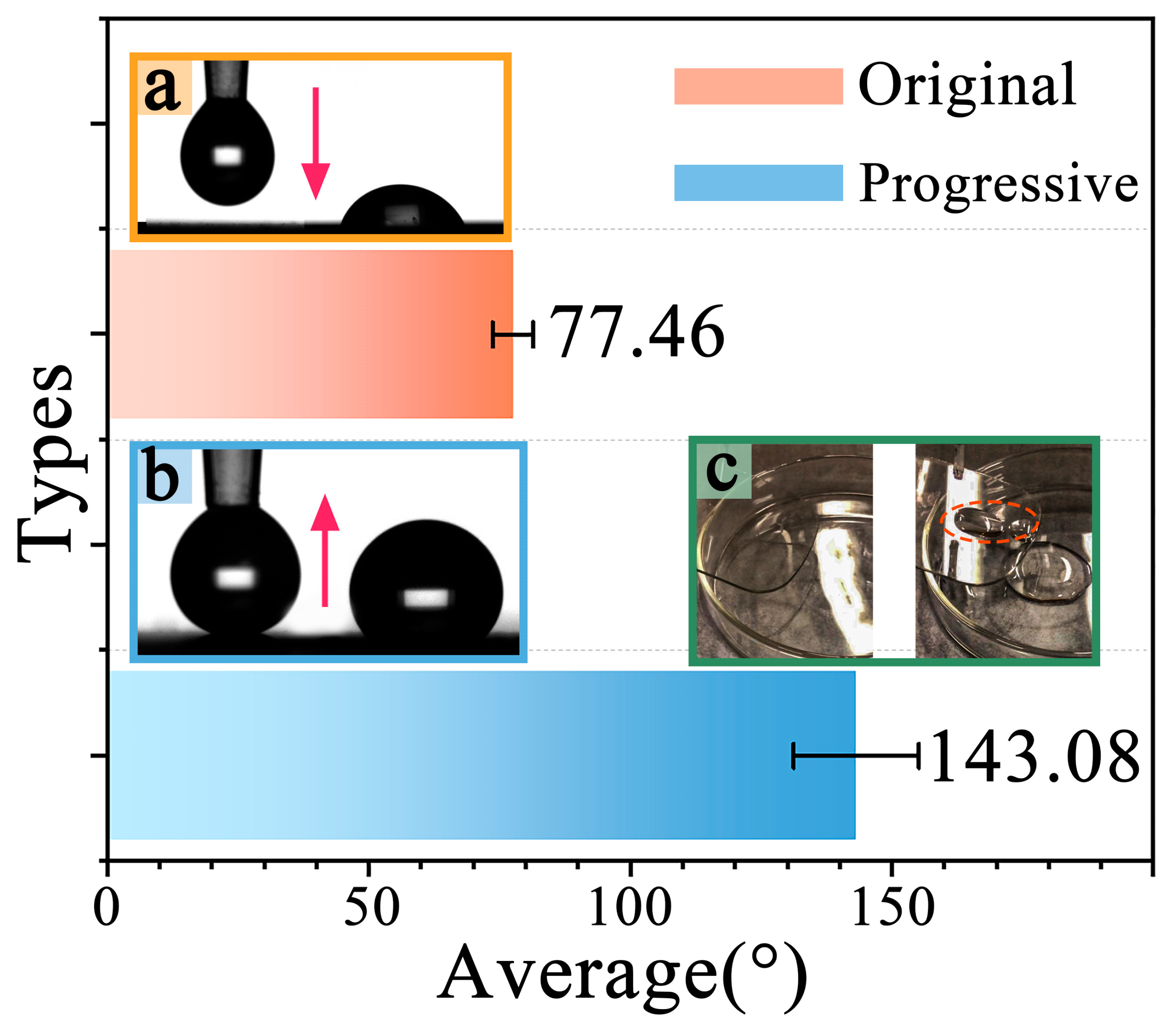
3.2.2. Hydrophobicity Analysis of Wear-Tested Samples
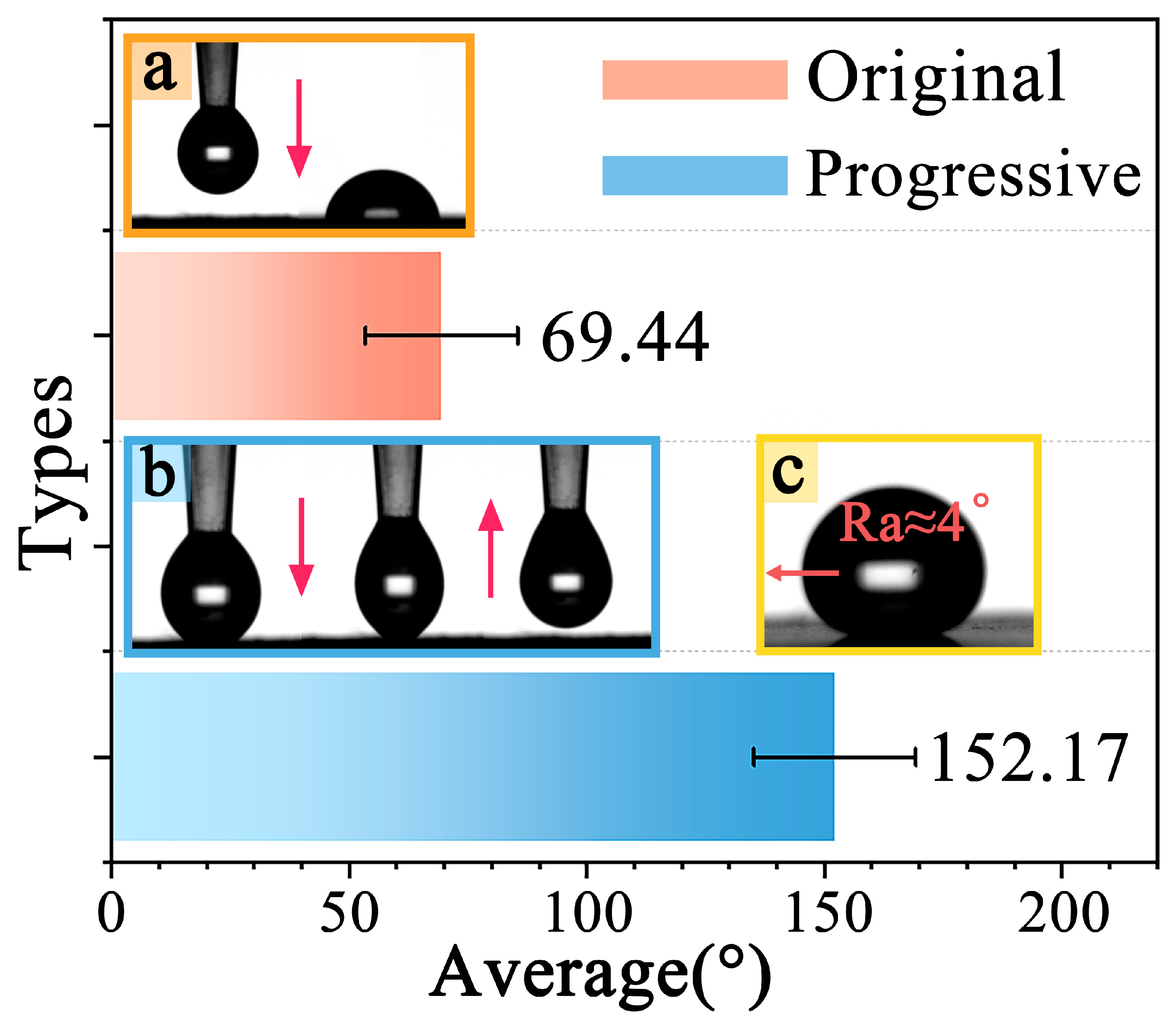
3.2.3. Hydrophobicity Analysis of Immersion-Tested Samples
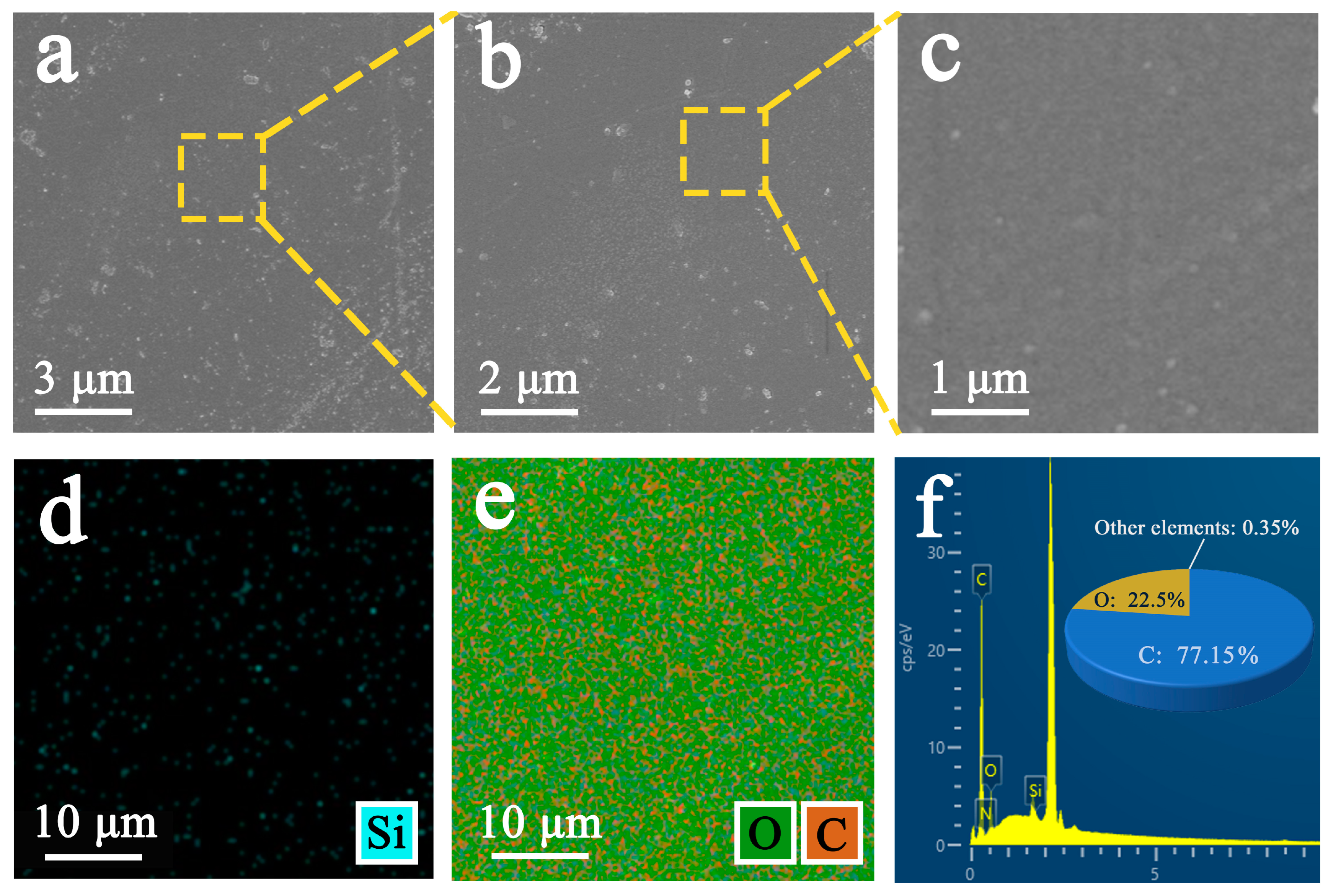
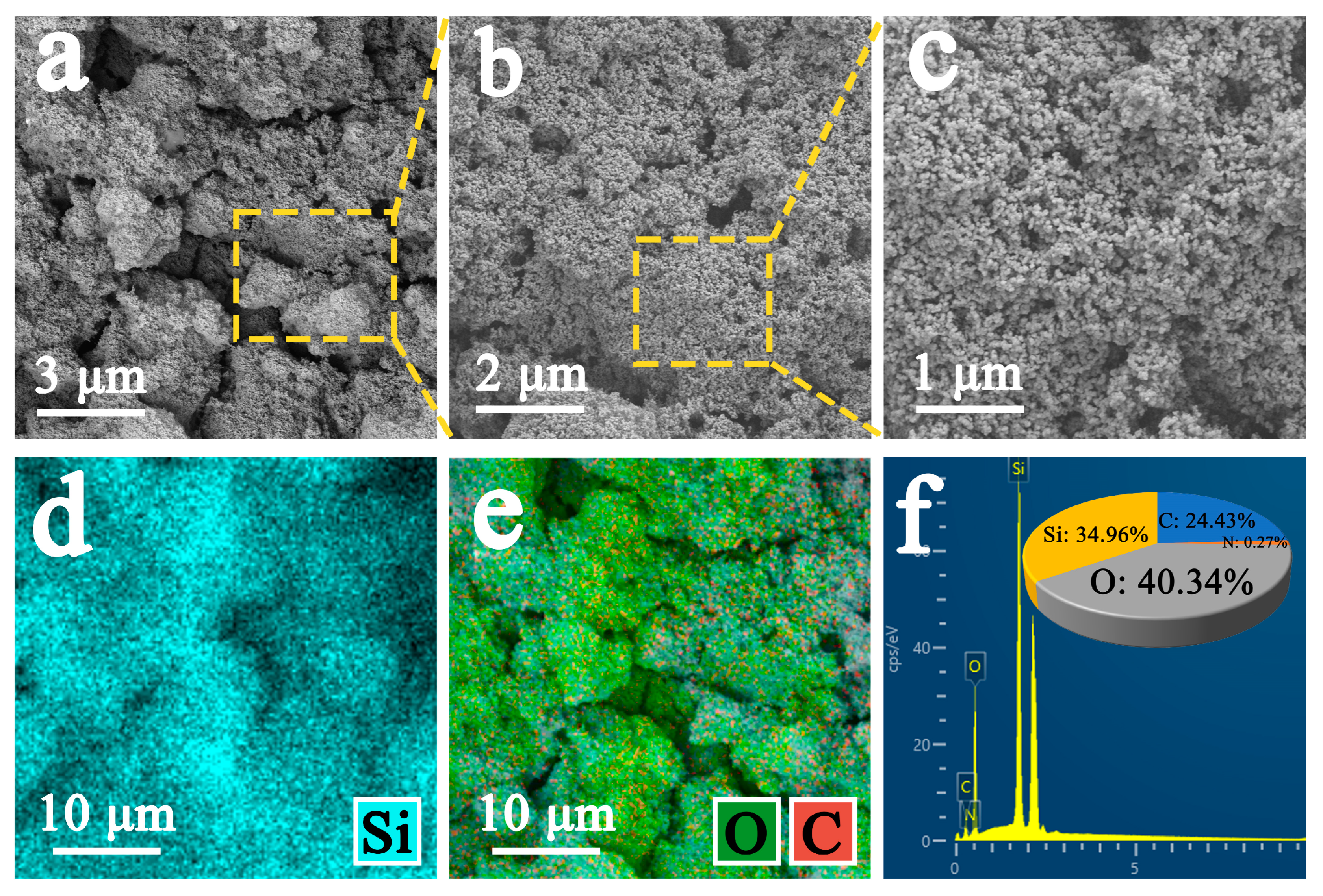
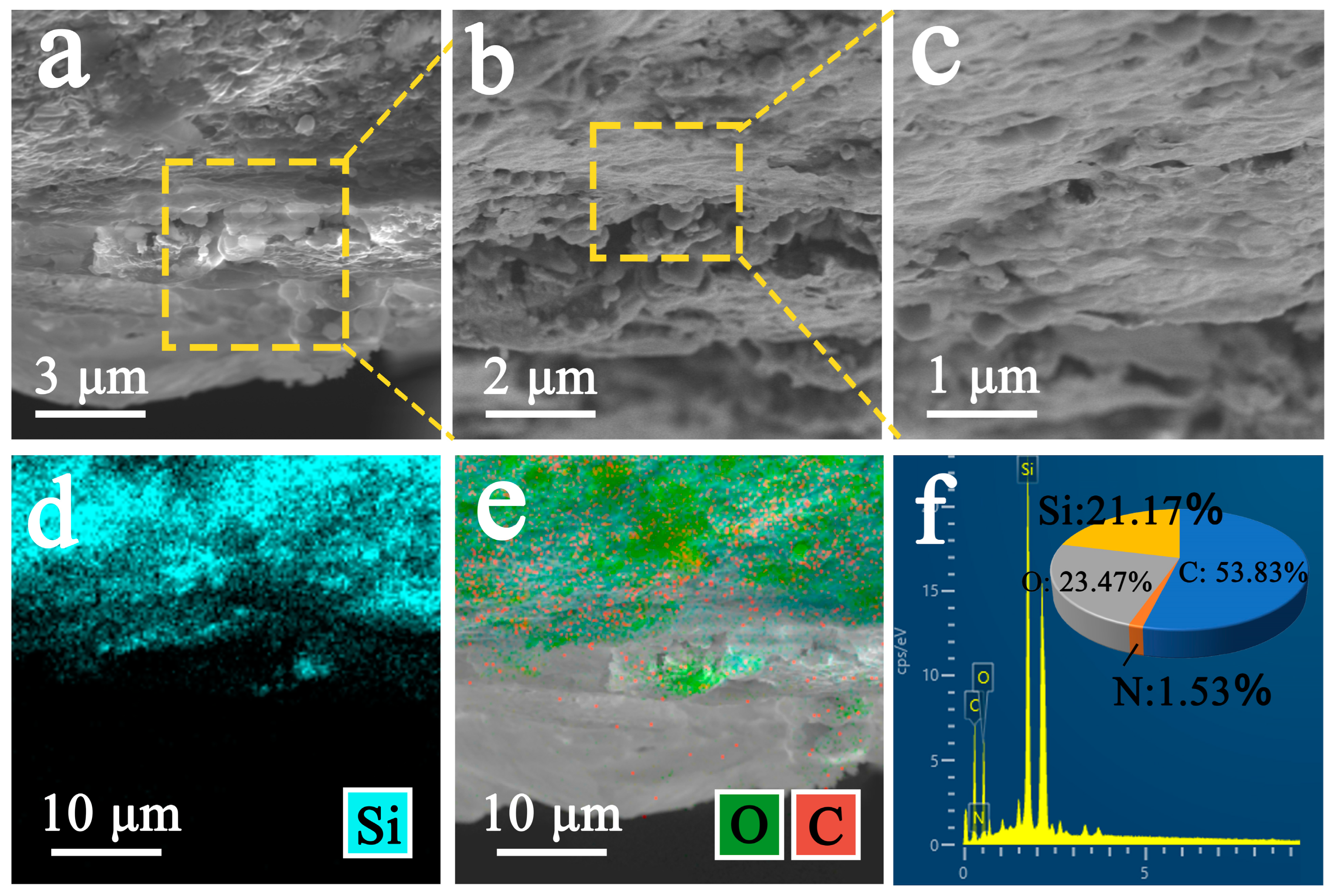
3.3. Scanning Electron Microscopy and EDS Analysis
3.3.1. Analysis of Original Samples
3.3.2. Analysis of Improved Sample
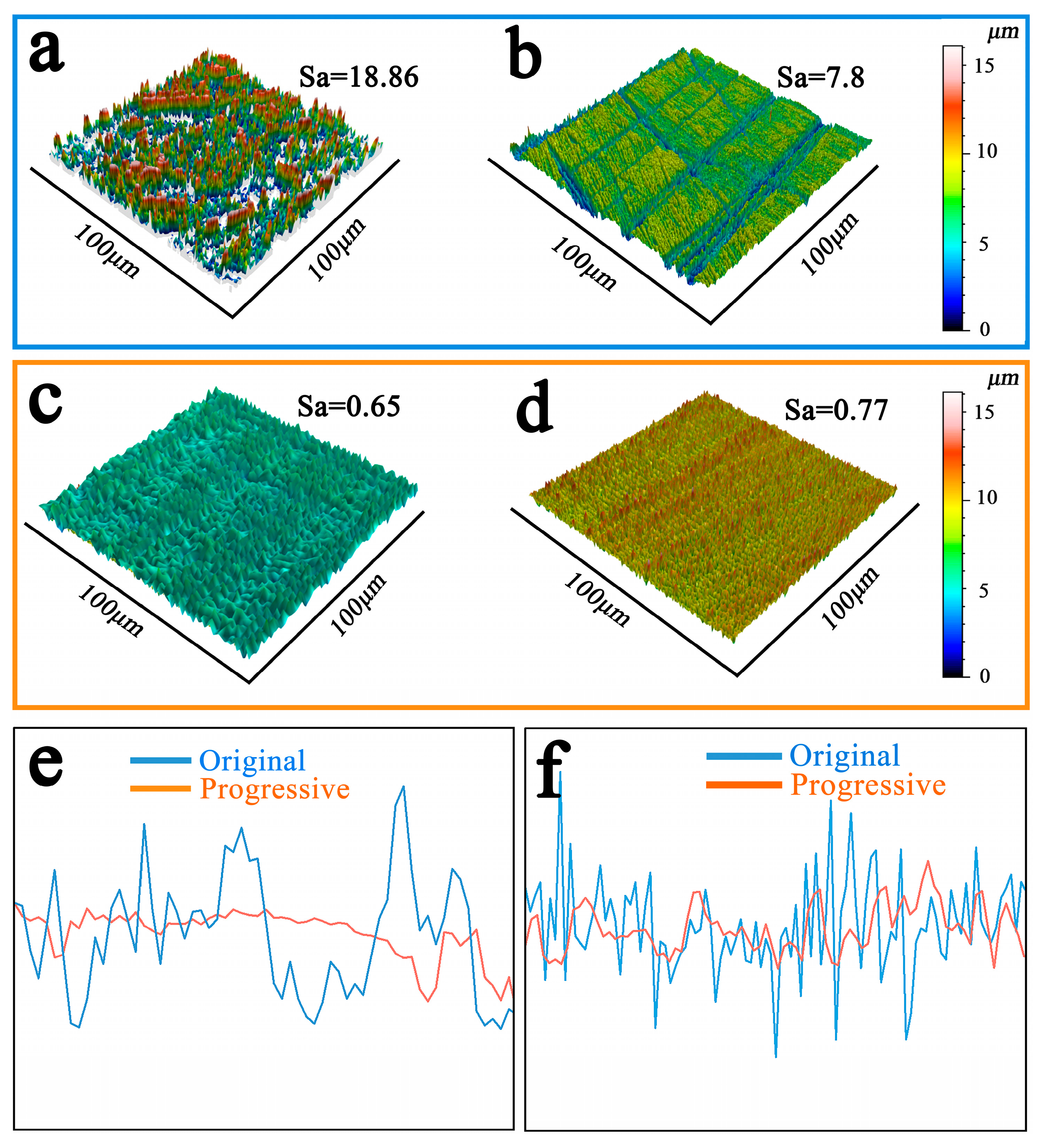
3.4. Three-Dimensional Morphological Analysis
3.5. Drag Reduction Analysis
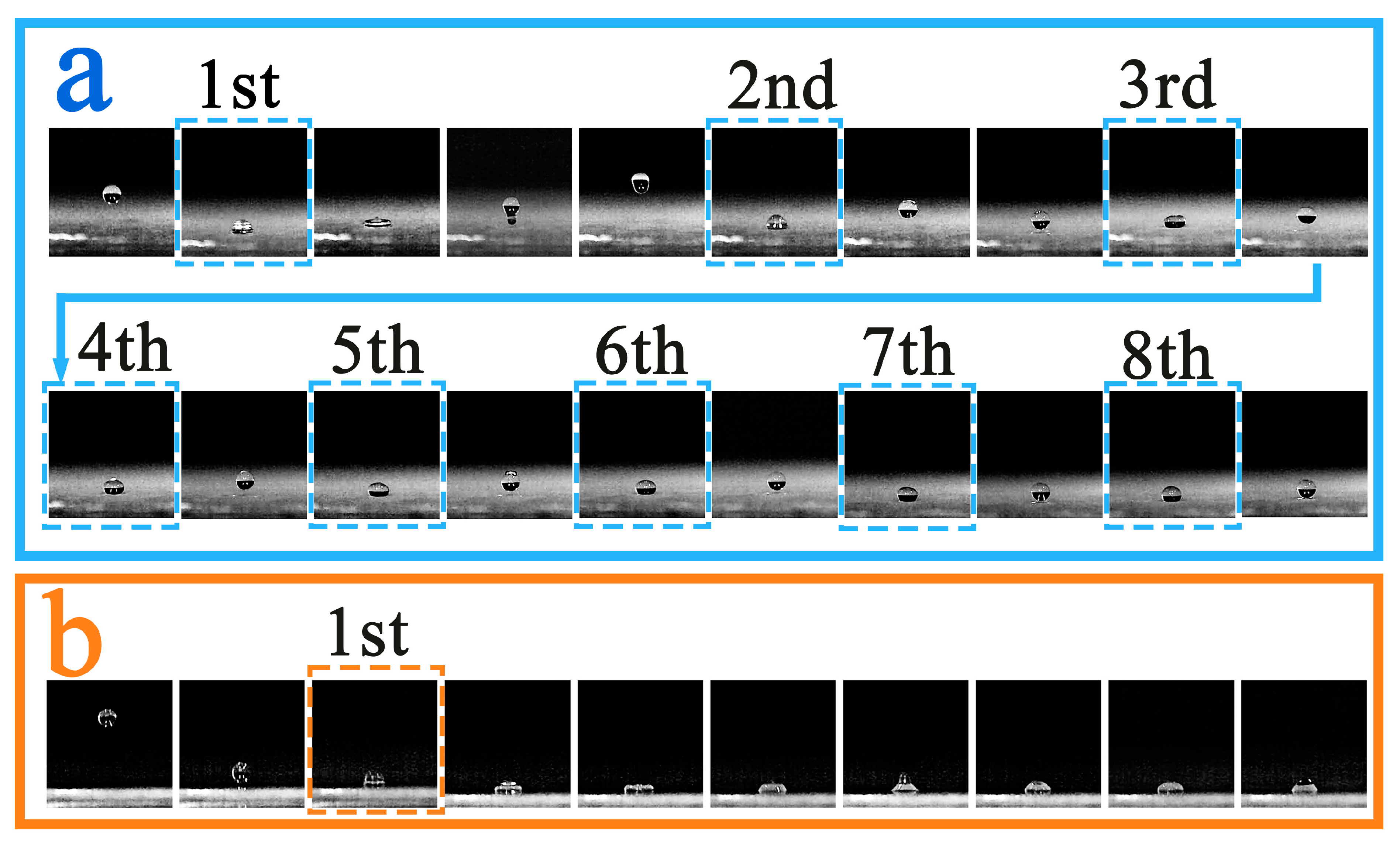
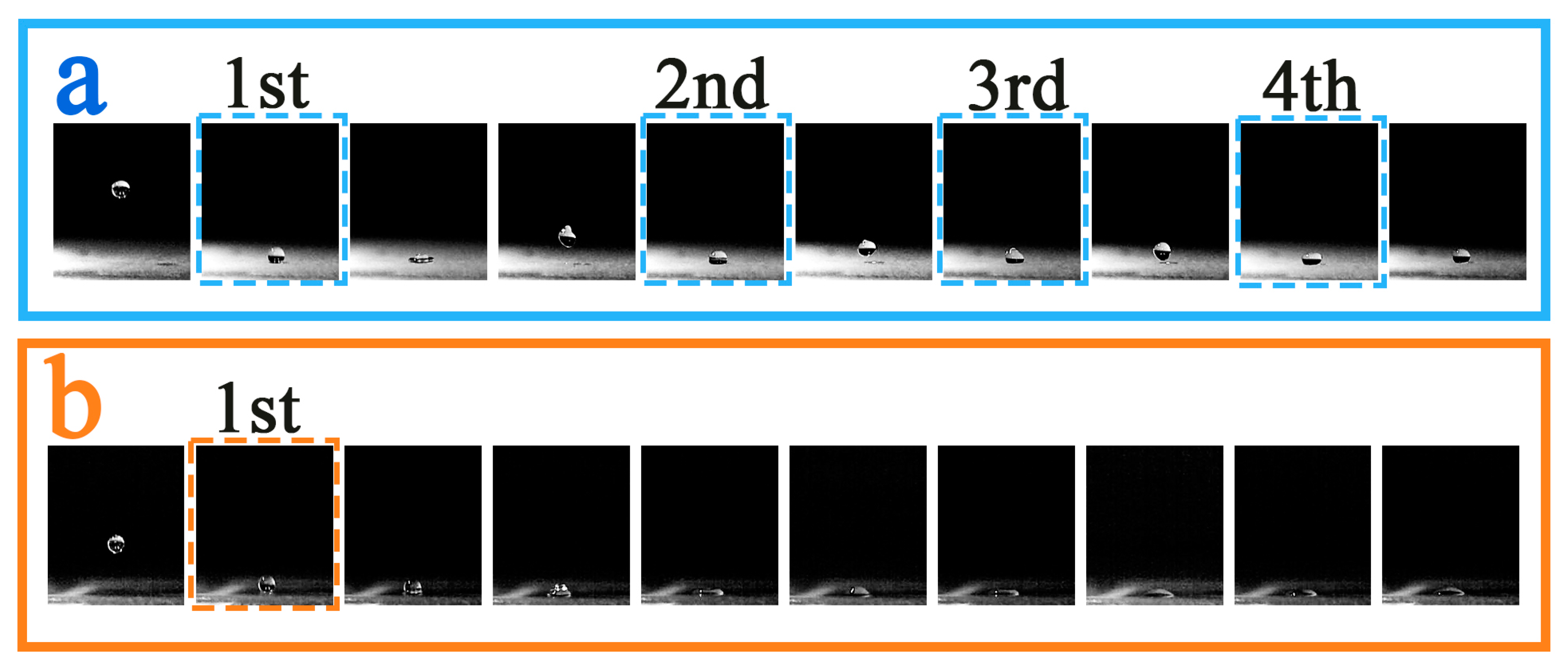
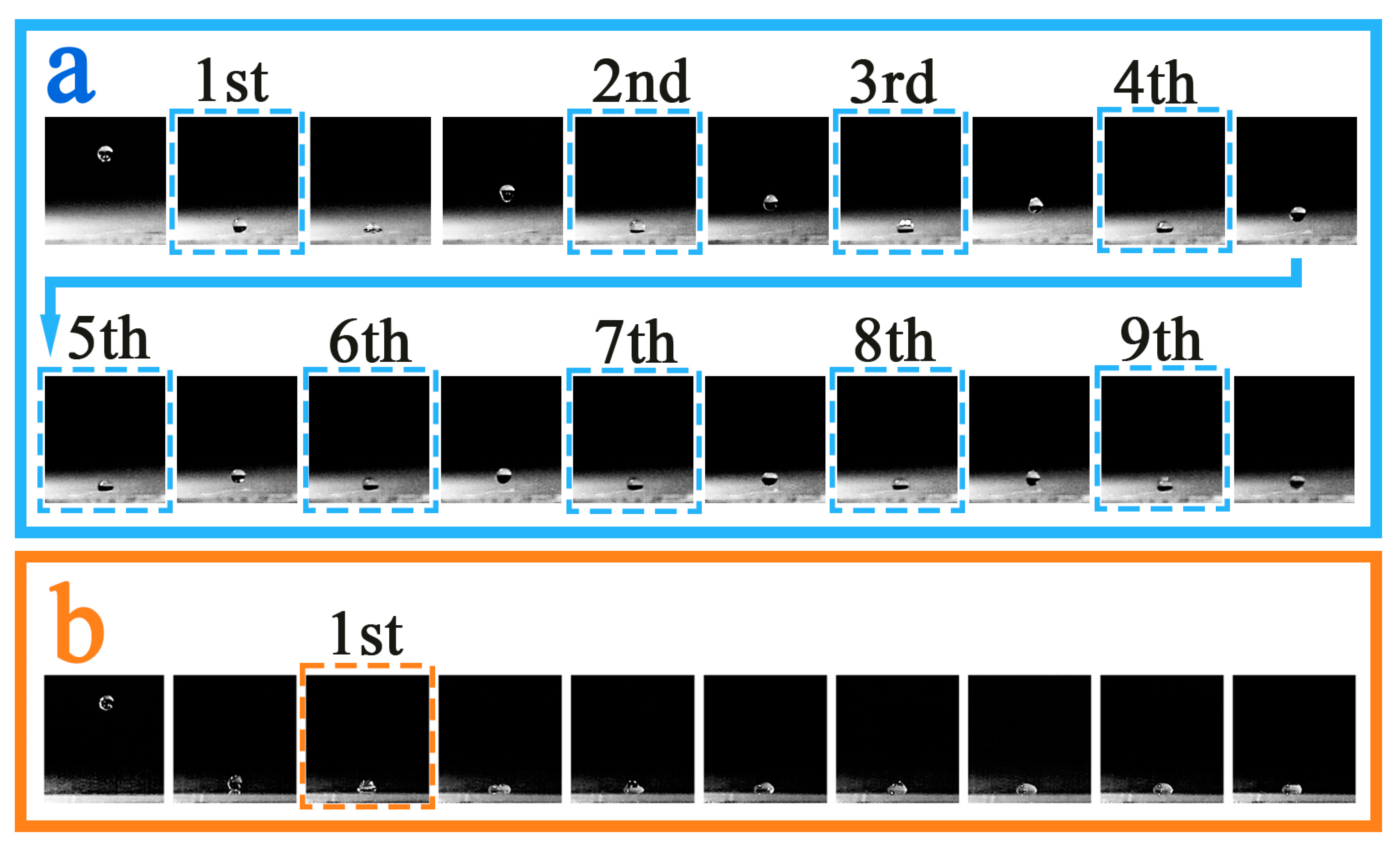
3.6. Droplet Rebound Analysis
3.6.1. Experimental Temperature 10 °C
3.6.2. Experimental Temperature −10 °C
3.6.3. Experimental Temperature: 30 °C
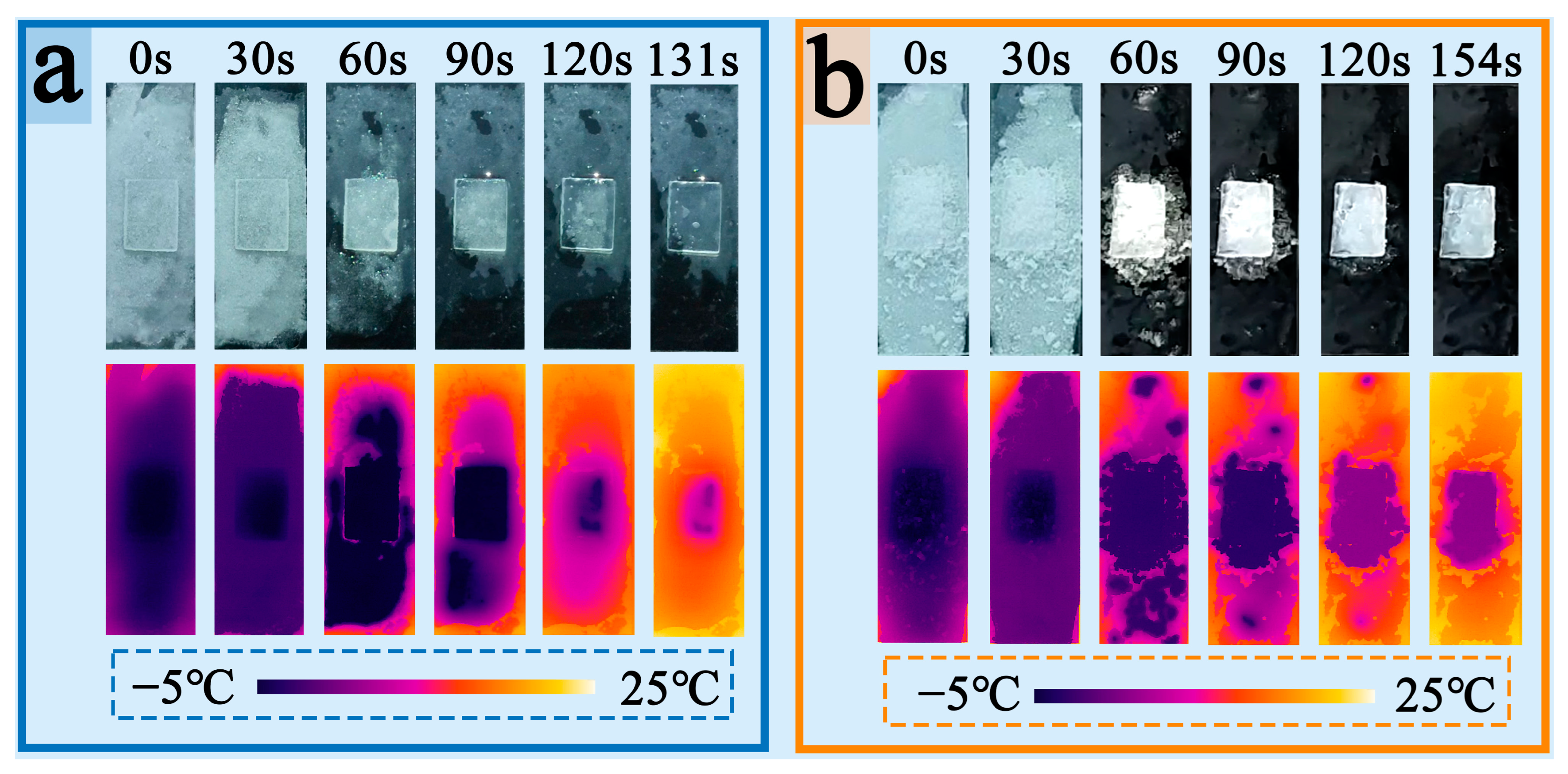
3.7. Anti-Icing Performance Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pasini, E.V.; Durand, J.; Camy, S. High pressure equilibrium data of CO2/cyclohexene oxide and CO2/limonene oxide systems in the context of polycarbonate synthesis using CO2 as a co-monomer. Fluid Phase Equilibria 2025, 595, 114406. [Google Scholar] [CrossRef]
- Sridharan, S.; Rao, J.S.; Omkar, S.N. Finite element analysis of cricket ball impact on polycarbonate-EVA sandwich. Procedia Eng. 2015, 112, 28–33. [Google Scholar] [CrossRef]
- Gohil, M.; Joshi, G. Perspective of polycarbonate composites and blends properties, applications, and future development: A review. In Green Sustainable Process for Chemical and Environmental Engineering and Science: Green Composites: Preparation, Properties, and Allied Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 393–424. [Google Scholar] [CrossRef]
- Daly, M.; Chihi, M.; Bouraoui, C.; Tarfaoui, M. Advancing composite materials: Exploring thermomechanical properties of Aerosil/polycarbonate composites via additive manufacturing. J. Manuf. Process. 2024, 131, 1770–1783. [Google Scholar] [CrossRef]
- Huang, G.; Guo, Y.; Lee, B.; Chen, H.; Mao, A. Research Advances and Future Perspectives of Superhydrophobic Coatings in Sports Equipment Applications. Molecules 2025, 30, 644. [Google Scholar] [CrossRef]
- Park, J.; Mehrubeoglu, M.; Kim, J.; Park, J. Comparison of design characteristics and customization protocols for swimming goggles. Electronics 2020, 9, 992. [Google Scholar] [CrossRef]
- Berg, B.K.; Irwin, C.C.; Wright, P.M.; Irwin, R.L.; Hutchinson, M. An unacceptable status quo: A sport development case study of swimming and drownings. Sport Manag. Rev. 2016, 19, 454–465. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zheng, J.; Kang, J.; Cai, G. Research on interactive sports game experience in physical training system based on digital entertainment technology and sensor devices. Entertain. Comput. 2025, 52, 100866. [Google Scholar] [CrossRef]
- Bharath, K.N.; Joshi, A.G.; Taj, A.; Gouda, P.S.; Nagamadhu, M. Evolution and recent advancements of composite materials in sports applications. In Applications of Composite Materials in Engineering; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2025; pp. 219–228. [Google Scholar]
- Carr, K.E. Shifting Currents: A World History of Swimming; Reaktion Books: London, UK, 2022. [Google Scholar]
- Wu, G.; Liu, H.B.; Ma, C.; Xu, H.M.; Ren, X.Z.; Sun, W. Developments and application of fish school swimming model in recirculating aquaculture systems. Ocean Eng. 2025, 319, 120196. [Google Scholar] [CrossRef]
- Vera, J.; Redondo, B.; Molina, R.; Jiménez, R. Effects of wearing swimming goggles on non-invasive tear break-up time in a laboratory setting. J. Optom. 2022, 15, 154–159. [Google Scholar] [CrossRef]
- Hongjiang, Y.; Xiaoqiong, H.; Yue, K.; Ping, C.; Jing, C.; Yunhua, Y. Effectiveness of 2 Pretreatment Methods in Antifogging of Goggles in a COVID-19 Isolation Ward: A Randomized Controlled Trial. J. Emerg. Nurs. 2022, 48, 571–582. [Google Scholar] [CrossRef]
- Chang, C.C.; Huang, F.H.; Chang, H.H.; Don, T.M.; Chen, C.C.; Cheng, L.P. Preparation of water-resistant antifog hard coatings on plastic substrate. Langmuir 2012, 28, 17193–17201. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; He, X. Research on super-hydrophobic surface treatment and performance of tennis hand glue. J. Polym. Res. 2022, 29, 185. [Google Scholar] [CrossRef]
- Guo, W.; Liu, C.; Li, N.; Xi, M.; Che, Y.; Jiang, C.; Zhang, S.; Wang, Z. A highly transparent and photothermal composite coating for effective anti-/de-icing of glass surfaces. Nanoscale Adv. 2022, 4, 2884–2892. [Google Scholar] [CrossRef]
- Kanzaki, M. Melting of silica up to 7 GPa. J. Am. Ceram. Soc. 1990, 73, 3706–3707. [Google Scholar] [CrossRef]
- LaRoche, J.R.; Ren, F.; Lothian, R.; Hong, J.; Pearton, S.J.; Lambers, E.; Hsu, C.H.; Wu, C.S.; Hoppe, M. Thermal stability and etching characteristics of electron beam deposited SiO and SiO2. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2000, 18, 283–287. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.Y.; Liu, Q.; Zhu, S.P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef]
- Irajizad, P.; Al-Bayati, A.; Eslami, B.; Shafquat, T.; Nazari, M.; Jafari, P.; Kashyap, V.; Masoudi, A.; Araya, D.; Ghasemi, H. Stress-localized durable icephobic surfaces. Mater. Horiz. 2019, 6, 758–766. [Google Scholar] [CrossRef]
- Li, R.; Li, M.; Wu, X.; Yu, H.; Jin, R.; Liang, J. A pine needle-like superhydrophobic Zn/ZnO coating with excellent mechanochemical robustness and corrosion resistance. Mater. Des. 2023, 225, 111583. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Lu, C.; Liu, Y.; Feng, S.; Liu, Y. Robust slippery liquid-infused porous network surfaces for enhanced anti-icing/deicing performance. ACS Appl. Mater. Interfaces 2020, 12, 25471–25477. [Google Scholar] [CrossRef]
- Liu, L.; Xie, Z.; Wen, L.; Pi, P.; Qian, Y.; Wang, S.; Wen, X. Efficient collection and directional transport of condensate on superhydrophilic-hydrophobic surfaces with bioinspired hierarchical wedge-shaped channels. Surf. Interfaces 2024, 55, 105473. [Google Scholar] [CrossRef]
- Li, A.; Li, G.; Xu, Y.; Jia, Y.; Liu, Y. Superhydrophobic surface with good anti-icing properties and high durability. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134539. [Google Scholar] [CrossRef]
- He, Q.; Jia, Y.; Wang, H.; He, J.; Wang, J.; Xu, Y.; Liu, Y.; Li, A. Triple conversion strategy to build anti-de-icing sheets for the leading edge of the rotor blade. Mater. Des. 2024, 237, 112516. [Google Scholar] [CrossRef]
- He, Q.; Du, Z.; Jia, Y.; Liu, Y.; Xu, Z.; Xu, Y.; Li, A. Preparation of superhydrophobic conductive micro/nano-graphite/PDMS films on paper by simple spraying method. J. Appl. Polym. Sci. 2024, 141, e55160. [Google Scholar] [CrossRef]
- Zhang, M.; Li, H. Robust and UV-durable superhydrophobic SiO2/ZnO/micro-structures on glass substrate with high transmittance. Mater. Chem. Phys. 2025, 338, 130634. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, Z.; Yan, S.; Dong, S. Adhesion force characterization method of water-droplet sliding on superhydrophobic surface. Surf. Interfaces 2025, 60, 106073. [Google Scholar] [CrossRef]
- Li, A.; Wang, G.; Ma, Y.; Zhao, C.; Zhang, F.; He, Q.; Zhang, F. Study on preparation and properties of superhydrophobic surface of RTV silicone rubber. J. Mater. Res. Technol. 2021, 11, 135–143. [Google Scholar] [CrossRef]
- Rao, A.V.; Kulkarni, M.M.; Bhagat, S.D. Transport of liquids using superhydrophobic aerogels. J. Colloid Interface Sci. 2005, 285, 413–418. [Google Scholar]
- Xu, C.; Feng, R.; Song, F.; Wu, J.M.; Luo, Y.Q.; Wang, X.L.; Wang, Y.Z. Continuous and controlled directional water transportation on a hydrophobic/superhydrophobic patterned surface. Chem. Eng. J. 2018, 352, 722–729. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Su, F. Damage accumulation model of ice detach behavior in ultrasonic de-icing technology. Renew. Energy 2020, 153, 1396–1405. [Google Scholar] [CrossRef]
- Wen, R.; Ma, X.; Lee, Y.C.; Yang, R. Liquid-vapor phase-change heat transfer on functionalized nanowired surfaces and beyond. Joule 2018, 2, 2307–2347. [Google Scholar] [CrossRef]
- Durán, I.R.; Laroche, G. Water drop-surface interactions as the basis for the design of anti-fogging surfaces: Theory, practice, and applications trends. Adv. Colloid Interface Sci. 2019, 263, 68–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Qi, X.; Fang, X.; Teng, C.; Guo, Y.; Liu, C.; Chen, X.; Cui, H. Durable self-cleaning anti-fog and antireflective micro-nano structures fabricated by laser marker ablation of Si coated glass. Ceram. Int. 2024, 50, 31402–31417. [Google Scholar] [CrossRef]
- Sethi, S.K.; Manik, G. Recent progress in super hydrophobic/hydrophilic self-cleaning surfaces for various industrial applications: A review. Polym.-Plast. Technol. Eng. 2018, 57, 1932–1952. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Lin, H.; Guo, X.; Wang, G.; Jia, Y.; Tang, L. Investigation of Superhydrophobic, Drag-Reducing and Anti-Icing Properties of Swimming Goggles. Coatings 2025, 15, 664. https://doi.org/10.3390/coatings15060664
Ding J, Lin H, Guo X, Wang G, Jia Y, Tang L. Investigation of Superhydrophobic, Drag-Reducing and Anti-Icing Properties of Swimming Goggles. Coatings. 2025; 15(6):664. https://doi.org/10.3390/coatings15060664
Chicago/Turabian StyleDing, Junyi, Haiqi Lin, Xubin Guo, Guangfei Wang, Yangyang Jia, and Lu Tang. 2025. "Investigation of Superhydrophobic, Drag-Reducing and Anti-Icing Properties of Swimming Goggles" Coatings 15, no. 6: 664. https://doi.org/10.3390/coatings15060664
APA StyleDing, J., Lin, H., Guo, X., Wang, G., Jia, Y., & Tang, L. (2025). Investigation of Superhydrophobic, Drag-Reducing and Anti-Icing Properties of Swimming Goggles. Coatings, 15(6), 664. https://doi.org/10.3390/coatings15060664





