Modern Innovations and Applications in Plasma Electrolytic Oxidation Coatings on Aluminum, Magnesium, and Titanium
Abstract
1. Introduction
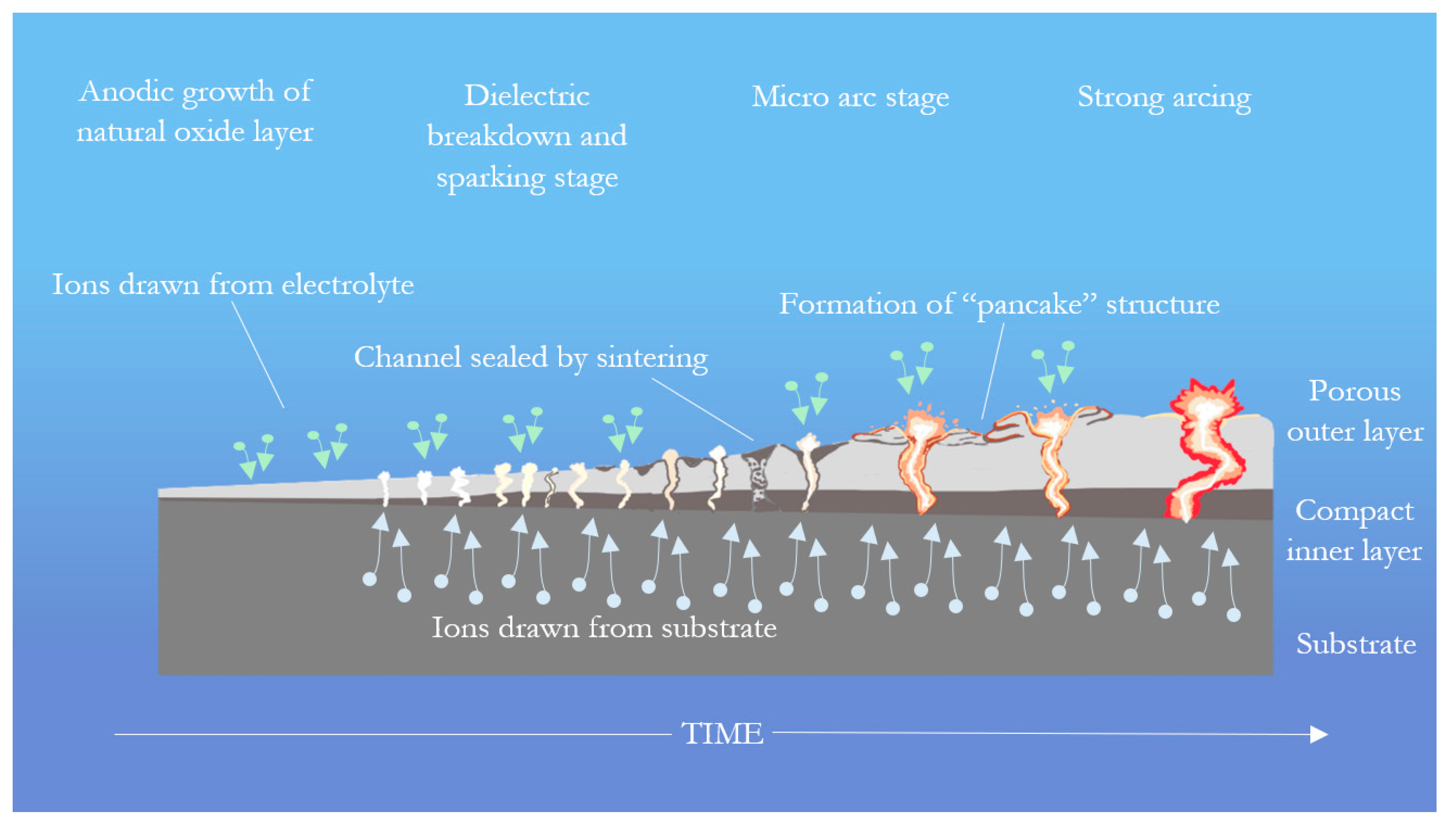
2. PEO Process
2.1. Process Overview
2.2. Process Parameters
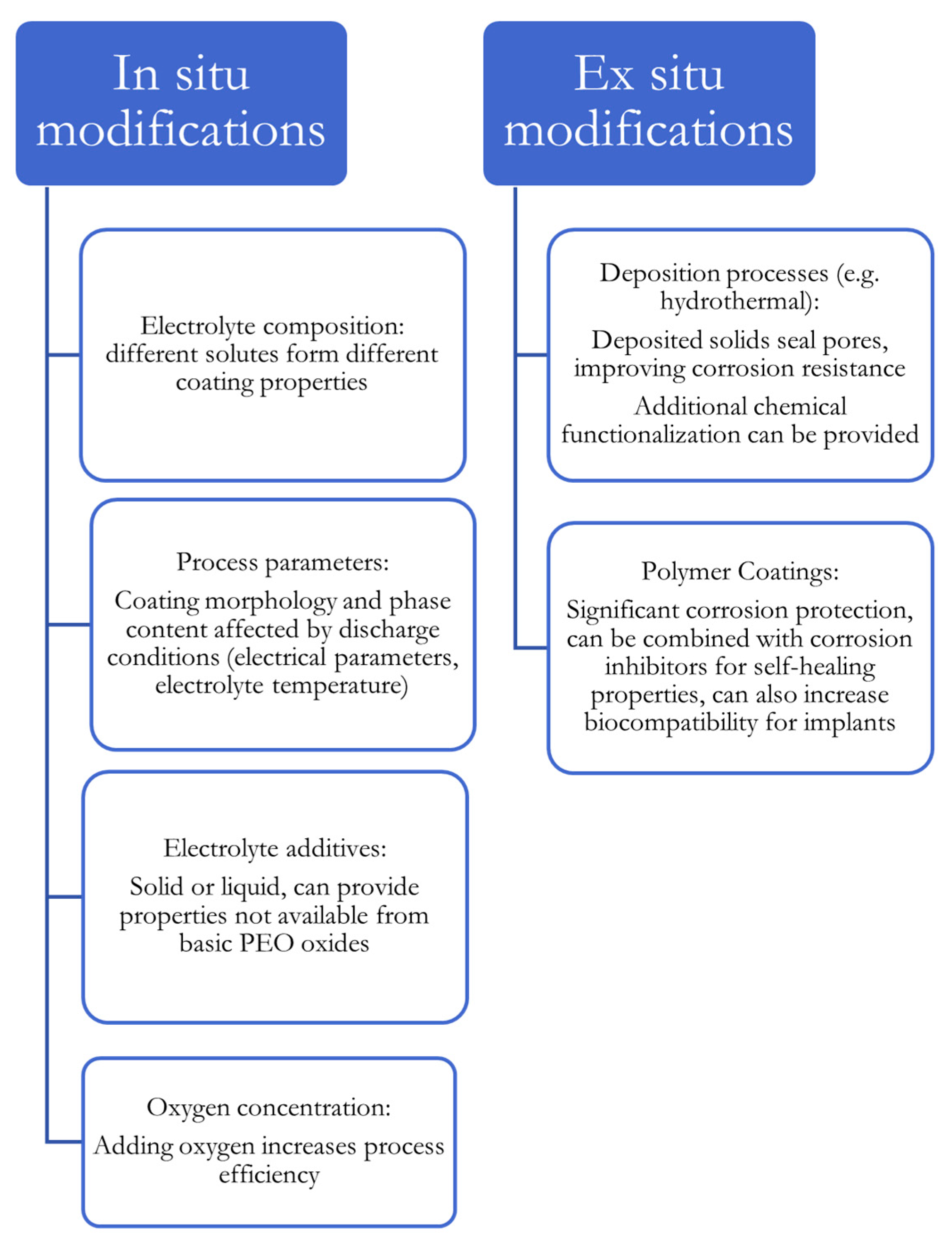
3. Modification of PEO Coatings
3.1. In Situ Modifications
3.1.1. Supplemental Oxygen
3.1.2. Molten Salt Electrolytes
3.1.3. Low-Temperature Electrolytes
3.1.4. Electrolyte Conductivity
3.1.5. Particle Additives
3.1.6. Microstructure Formation Mechanisms
3.2. Ex Situ Modifications
3.2.1. Hydrothermal Sealing
3.2.2. Polymer Sealing and Corrosion Inhibitors
4. Applications for Modified PEO Coatings
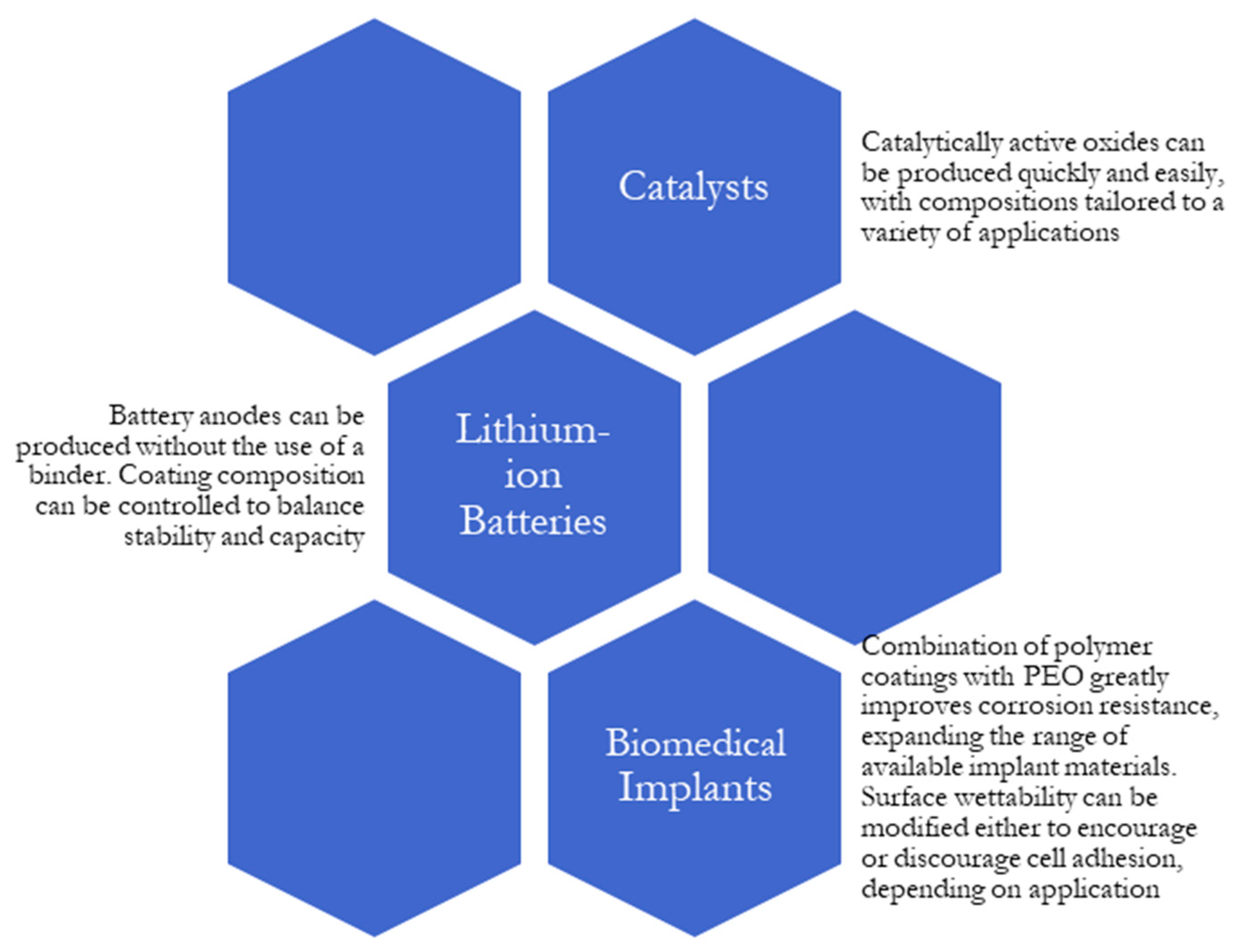
4.1. Catalysts
4.1.1. Wastewater Treatment
4.1.2. Water Splitting
4.1.3. Diesel Applications
4.1.4. Other Catalytic Applications
4.2. Lithium-Ion Batteries
4.3. Biomedical Applications
5. Future Directions and Conclusions
Funding
Conflicts of Interest
References
- Shackleford, J. Introduction to Materials Science for Engineers, 8th ed.; Pearson: London, UK, 2015. [Google Scholar]
- Song, G.L. Corrosion Behavior of Magnesium Alloys and Protection Techniques. In Surface Engineering of Light Alloys; Dong, H., Ed.; Woodhead Publishing: Boca Raton, FL, USA, 2010. [Google Scholar]
- Gupta, M. Utilizing Magnesium Based Materials to Reduce Green House Gas Emissions in Aerospace Sector. Aeronaut. Aerosp. Open Access J. 2017, 1, 1–6. [Google Scholar] [CrossRef][Green Version]
- Tan, J.; Ramakrishna, S. Applications of Magnesium and Its Alloys: A Review. Appl. Sci. 2021, 11, 6861. [Google Scholar] [CrossRef]
- Powell, B.R.; Krajewski, P.E.; Luo, A.A. Magnesium Alloys for Lightweight Powertrains and Automotive Structures. In Materials, Design and Manufacturing for Lightweight Vehicles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 125–186. ISBN 9780128187128. [Google Scholar]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Nadaraia, K.V.; Mashtalyar, D.V.; Piatkova, M.A.; Pleshkova, A.I.; Imshinetskiy, I.M.; Gerasimenko, M.S.; Belov, E.A.; Kumeiko, V.V.; Kozyrev, D.N.; Fomenko, K.A.; et al. Antibacterial HA-Coatings on Bioresorbable Mg Alloy. J. Magnes. Alloys 2024, 12, 1965–1985. [Google Scholar] [CrossRef]
- Zhang, X.P.; Zhao, Z.P.; Wu, F.M.; Wang, Y.L.; Wu, J. Corrosion and Wear Resistance of AZ91D Magnesium Alloy with and without Microarc Oxidation Coating in Hank’s Solution. J. Mater. Sci. 2007, 42, 8523–8528. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Macdonald, D.D.; Matykina, E.; Parfenov, E.V.; Egorkin, V.S.; Curran, J.A.; Troughton, S.C.; Sinebryukhov, S.L.; Gnedenkov, S.V.; Lampke, T.; et al. Review of Plasma Electrolytic Oxidation of Titanium Substrates: Mechanism, Properties, Applications, and Limitations. Appl. Surf. Sci. Adv. 2021, 5, 100121. [Google Scholar] [CrossRef]
- Xu, Y.; Qi, J.; Nutter, J.; Sharp, J.; Bai, M.; Ma, L.; Rainforth, W.M. Correlation between the Formation of Tribofilm and Repassivation in Biomedical Titanium Alloys during Tribocorrosion. Tribol. Int. 2021, 163, 107147. [Google Scholar] [CrossRef]
- Revathi, A.; Borrás, A.D.; Muñoz, A.I.; Richard, C.; Manivasagam, G. Degradation Mechanisms and Future Challenges of Titanium and Its Alloys for Dental Implant Applications in Oral Environment. Mater. Sci. Eng. C 2017, 76, 1354–1368. [Google Scholar] [CrossRef]
- Manoj, A.; Kasar, A.K.; Menezes, P.L. Tribocorrosion of Porous Titanium Used in Biomedical Applications. J. Bio Tribo Corros. 2019, 5, 3. [Google Scholar] [CrossRef]
- Garcia-Cabezón, C.; Rodríguez-Méndez, M.L.; Borrás, V.A.; Bayón, R.; Salvo-Comino, C.; Garcia-Hernandez, C.; Martin-Pedrosa, F. Improvements in Tribological and Anticorrosion Performance of Porous Ti-6Al-4V via PEO Coating. Friction 2021, 9, 1303–1318. [Google Scholar] [CrossRef]
- Dong, H. Tribological Properties of Titaniuim-Based Alloys. In Surface Engineering of Light Alloys; Dong, H., Ed.; Woodhead Publishing: Cambridge, UK, 2010. [Google Scholar]
- Dohda, K.; Yamamoto, M.; Hu, C.; Dubar, L.; Ehmann, K.F. Galling Phenomena in Metal Forming. Friction 2021, 9, 665–685. [Google Scholar] [CrossRef]
- Budinski, K.G. Tribological Properties of Titanium Alloys; Elsevier: Amsterdam, The Netherlands, 1991; Volume 151, pp. 203–217. [Google Scholar]
- Lee, H.-J.; Park, Y.U.; Kim, S.J.; Kim, H.N. Screw Stripping and Its Prevention in the Hexagonal Socket of 3.5-Mm Titanium Locking Screws. Sci. Rep. 2021, 11, 21324. [Google Scholar] [CrossRef] [PubMed]
- Babaei, K.; Fattah-alhosseini, A.; Molaei, M. The Effects of Carbon-Based Additives on Corrosion and Wear Properties of Plasma Electrolytic Oxidation (PEO) Coatings Applied on Aluminum and Its Alloys: A Review. Surf. Interfaces 2020, 21, 100677. [Google Scholar] [CrossRef]
- Tang, M.; Li, W.; Liu, H.; Zhu, L. Influence of Titania Sol in the Electrolyte on Characteristics of the Microarc Oxidation Coating Formed on 2A70 Aluminum Alloy. Surf. Coat. Technol. 2011, 205, 4135–4140. [Google Scholar] [CrossRef]
- Norgate, T.E.; Jahanshahi, S.; Rankin, W.J. Assessing the Environmental Impact of Metal Production Processes. J. Clean. Prod. 2007, 15, 838–848. [Google Scholar] [CrossRef]
- Froes, F.H.; Friedrich, H.; Kiese, J.; Bergoint, D. Titanium in the Family Automobile: The cost challenge. JOM 2004, 56, 40–44. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Koltun, P. Global Warming Impact of the Magnesium Produced in China Using the Pidgeon Process. Resour. Conserv. Recycl. 2004, 42, 49–64. [Google Scholar] [CrossRef]
- Musfirah, A.H.; Jaharah, A.G. Magnesium and Aluminum Alloys in Automotive Industry. J. Appl. Sci. Res. 2012, 8, 4865–4875. [Google Scholar]
- Wang, K.; Zhang, Z.; Dandu, R.S.B.; Cai, W. Understanding Tribocorrosion of Aluminum at the Crystal Level. Acta Mater. 2023, 245, 118639. [Google Scholar] [CrossRef]
- Youssef, A.A.; Budzynski, P.; Filiks, J.; Surowiec, Z. Improvement of Tribological Properties of Aluminum by Nitrogen Implantation. Vacuum 2005, 78, 599–603. [Google Scholar] [CrossRef]
- Matyunin, V.M.; Marchenkov, A.Y.; Agafonov, R.Y.; Danilin, V.V.; Karimbekov, M.A.; Goryachkina, M.V.; Volkov, P.V.; Zhgut, D.A. Correlation between the Ultimate Tensile Strength and the Brinell Hardness of Ferrous and Nonferrous Structural Materials. Russ. Metall. 2021, 2021, 1719–1724. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (Peo) Process—Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef]
- Souza, M.T.; Simão, L.; Montedo, O.R.K.; Raupp Pereira, F.; de Oliveira, A.P.N. Aluminum Anodizing Waste and Its Uses: An Overview of Potential Applications and Market Opportunities. Waste Manag. 2019, 84, 286–301. [Google Scholar] [CrossRef]
- Yerokhin, A.; Khan, R.H.U. Anodizing of Light Alloys. In Surface Engineering of Light Alloys; Dong, H., Ed.; Woodhead Publishing: Boca Raton, FL, USA, 2010. [Google Scholar]
- Talib, R.J.; Saad, S.; Toff, M.R.M.; Hashim, H. Thermal Spray Coating Technology: A review. Solid State Sci. Technol. 2003, 11, 109–117. [Google Scholar]
- Li, C.J. Thermal Spraying of Light Alloys. In Surface Engineering of Light Alloys; Dong, H., Ed.; Woodhead Publishing: Boca Raton, FL, USA, 2010. [Google Scholar]
- Qadir, D.; Sharif, R.; Nasir, R.; Awad, A.; Mannan, H.A. A Review on Coatings through Thermal Spraying. Chem. Pap. 2024, 78, 71–91. [Google Scholar] [CrossRef]
- Li, W.; Liao, H.; Wang, H. Cold Spraying of Light Alloys. In Surface Engineering of Light Alloys; Dong, H., Ed.; Woodhead Publishing: Boca Raton, FL, USA, 2010. [Google Scholar]
- Sun, W.; Chu, X.; Lan, H.; Huang, R.; Huang, J.; Xie, Y.; Huang, J.; Huang, G. Current Implementation Status of Cold Spray Technology: A Short Review. J. Therm. Spray Technol. 2022, 31, 848–865. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, O.C.; Matthews, N.; Helfritch, D.; Glass, S.W.; Ferguson, G. Application, Qualification, and Standardization of Cold Spray; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar]
- Jiang, B.L.; Wang, Y.M. Plasma Electrolytic Oxidation Treatment of Aluminium and Titanium Alloys. In Surface Engineering of Light Alloys; Dong, H., Ed.; Woodhead Publishing: Boca Raton, FL, USA, 2010. [Google Scholar]
- Karbasi, M.; Nikoomanzari, E.; Hosseini, R.; Bahramian, H.; Chaharmahali, R.; Giannakis, S.; Kaseem, M.; Fattah-alhosseini, A. A Review on Plasma Electrolytic Oxidation Coatings for Organic Pollutant Degradation: How to Prepare Them and What to Expect of Them? J. Environ. Chem. Eng. 2023, 11, 110027. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma Electrolysis for Surface Engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, T.; Jiang, B.; Guo, L. Growth, Microstructure and Mechanical Properties of Microarc Oxidation Coatings on Titanium Alloy in Phosphate-Containing Solution. Appl. Surf. Sci. 2004, 233, 258–267. [Google Scholar] [CrossRef]
- Nie, X.; Cai, R.; Zhao, C.; Sun, J.; Zhang, J.; Matthews, D.T.A. Advancement of Plasma Electrolytic Oxidation towards Non-Valve Metals. Surf. Coat. Technol. 2022, 442, 128403. [Google Scholar] [CrossRef]
- Lugovskoy, A.; Zinigr, M. Plasma Electrolytic Oxidation of Valve Metals. In Materials Science—Advanced Topics; InTech: London, UK, 2013. [Google Scholar]
- Molaei, M.; Babaei, K.; Fattah-alhosseini, A. Improving the Wear Resistance of Plasma Electrolytic Oxidation (PEO) Coatings Applied on Mg and Its Alloys under the Addition of Nano- and Micro-Sized Additives into the Electrolytes: A Review. J. Magnes. Alloys 2021, 9, 1164–1186. [Google Scholar] [CrossRef]
- Gim, H.G.; Kim, Y.T.; Choi, J. Polydimethylsiloxane-Assisted Plasma Electrolytic Oxidation of Ti for Synthesizing SiO2-TiO2 Composites for Application as Li-Ion Battery Anodes. Electrochem. Commun. 2023, 148, 107455. [Google Scholar] [CrossRef]
- Li, N.; Ling, N.; Fan, H.; Wang, L.; Zhang, J. Self-Healing and Superhydrophobic Dual-Function Composite Coating for Active Protection of Magnesium Alloys. Surf. Coat. Technol. 2023, 454, 129146. [Google Scholar] [CrossRef]
- Li, N.; Ling, N.; Fan, H.; Liang, K.; Zhang, J.; Wang, L. Smart Functionalization of PEO Coating on AZ31B Magnesium Alloy by a Novel Facile One-Step Sealing Method. Surf. Coat. Technol. 2023, 467, 129674. [Google Scholar] [CrossRef]
- Mortazavi, G.; Jiang, J.; Meletis, E.I. Investigation of the Plasma Electrolytic Oxidation Mechanism of Titanium. Appl. Surf. Sci. 2019, 488, 370–382. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O.; Nie, X. Coating Growth Behavior during the Plasma Electrolytic Oxidation Process. J. Vac. Sci. Technol. A Vac. Surf. Film. 2010, 28, 766–773. [Google Scholar] [CrossRef]
- Friedemann, A.E.R.; Thiel, K.; Haßlinger, U.; Ritter, M.; Gesing, T.M.; Plagemann, P. Investigations into the Structure of PEO-Layers for Understanding of Layer Formation. Appl. Surf. Sci. 2018, 443, 467–474. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. Influence of Process Parameters on Electrolytic Plasma Discharging Behaviour and Aluminum Oxide Coating Microstructure. Surf. Coat. Technol. 2010, 205, 1659–1667. [Google Scholar] [CrossRef]
- Hussein, R.O.; Zhang, P.; Nie, X.; Xia, Y.; Northwood, D.O. The Effect of Current Mode and Discharge Type on the Corrosion Resistance of Plasma Electrolytic Oxidation (PEO) Coated Magnesium Alloy AJ62. Surf. Coat. Technol. 2011, 206, 1990–1997. [Google Scholar] [CrossRef]
- Sela, S.; Borodianskiy, K. Synthesis of Ceramic Surface on Zr Alloy Using Plasma Electrolytic Oxidation in Molten Salt. Surf. Interfaces 2023, 36, 102533. [Google Scholar] [CrossRef]
- Li, Q.; Yang, W.; Liu, C.; Wang, D.; Liang, J. Correlations between the Growth Mechanism and Properties of Micro-Arc Oxidation Coatings on Titanium Alloy: Effects of Electrolytes. Surf. Coat. Technol. 2017, 316, 162–170. [Google Scholar] [CrossRef]
- Wu, T.; Blawert, C.; Serdechnova, M.; Karlova, P.; Dovzhenko, G.; Wieland, D.C.F.; Stojadinovic, S.; Vasilic, R.; Wang, L.; Wang, C.; et al. Role of Phosphate, Silicate and Aluminate in the Electrolytes on PEO Coating. Appl. Surf. Sci. 2022, 595, 153523. [Google Scholar] [CrossRef]
- Mingo, B.; Arrabal, R.; Mohedano, M.; Llamazares, Y.; Matykina, E.; Yerokhin, A.; Pardo, A. Influence of Sealing Post-Treatments on the Corrosion Resistance of PEO Coated AZ91 Magnesium Alloy. Appl. Surf. Sci. 2018, 433, 653–667. [Google Scholar] [CrossRef]
- Bryzhin, A.A.; Tarkhanova, I.G.; Gantman, M.G.; Rudnev, V.S.; Vasilyeva, M.S.; Lukiyanchuk, I.V. Titanium-Supported W-Containing PEO Layers Enriched with Mn or Zn in Oxidative Desulfurization and the Zwitterionic Liquid Effect. Surf. Coat. Technol. 2020, 393, 125746. [Google Scholar] [CrossRef]
- Lee, G.; Kim, S.; Kim, S.; Choi, J. SiO2/TiO2 Composite Film for High Capacity and Excellent Cycling Stability in Lithium-Ion Battery Anodes. Adv. Funct. Mater. 2017, 27, 1703538. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Yin, X.; Du, N. Influence of Electrolyte Components on the Microstructure and Growth Mechanism of Plasma Electrolytic Oxidation Coatings on 1060 Aluminum Alloy. Surf. Coat. Technol. 2020, 381, 125214. [Google Scholar] [CrossRef]
- Sun, M.; Wu, J.; Lu, P.; Zhang, Z.; Zhang, Y.; Li, D. Sphere-like MoS2 and Porous TiO2 Composite Film on Ti Foil as Lithium-Ion Battery Anode Synthesized by Plasma Electrolytic Oxidation and Magnetron Sputtering. J. Alloys Compd. 2021, 892, 162075. [Google Scholar] [CrossRef]
- Wei, B.; Yan, S.; Jia, D.; Feng, C.; Yin, J.; Wang, Z. Preparation of SnO2@TiO2/Graphene by Micro-Arc Oxidation as an Anode Material for Lithium Ion Batteries. Inorg. Chem. Commun. 2022, 145, 110048. [Google Scholar] [CrossRef]
- Hutsaylyuk, V.; Student, M.; Posuvailo, V.; Student, O.; Hvozdets’kyi, V.; Maruschak, P.; Zakiev, V. The Role of Hydrogen in the Formation of Oxide-Ceramic Layers on Aluminum Alloys during Their Plasma-Electrolytic Oxidation. J. Mater. Res. Technol. 2021, 14, 1682–1696. [Google Scholar] [CrossRef]
- Sobolev, A.; Kossenko, A.; Zinigrad, M.; Borodianskiy, K. An Investigation of Oxide Coating Synthesized on an Aluminum Alloy by Plasma Electrolytic Oxidation in Molten Salt. Appl. Sci. 2017, 7, 889. [Google Scholar] [CrossRef]
- Sobolev, A.; Kossenko, A.; Zinigrad, M.; Borodianskiy, K. Comparison of Plasma Electrolytic Oxidation Coatings on Al Alloy Created in Aqueous Solution and Molten Salt Electrolytes. Surf. Coat. Technol. 2018, 344, 590–595. [Google Scholar] [CrossRef]
- Sobolev, A.; Peretz, T.; Borodianskiy, K. Fabrication and Characterization of Ceramic Coating on Al7075 Alloy by Plasma Electrolytic Oxidation in Molten Salt. Coatings 2020, 10, 993. [Google Scholar] [CrossRef]
- Sobolev, A.; Wolicki, I.; Kossenko, A.; Zinigrad, M.; Borodianskiy, K. Coating Formation on Ti-6Al-4V Alloy by Micro Arc Oxidation in Molten Salt. Materials 2018, 11, 1611. [Google Scholar] [CrossRef]
- Sobolev, A.; Zinigrad, M.; Borodianskiy, K. Ceramic Coating on Ti-6Al-4V by Plasma Electrolytic Oxidation in Molten Salt: Development and Characterization. Surf. Coat. Technol. 2021, 408, 126847. [Google Scholar] [CrossRef]
- Sobolev, A.; Peretz, T.; Borodianskiy, K. Synthesis and Growth Mechanism of Ceramic Coatings on an Al-Cu Alloy Using Plasma Electrolytic Oxidation in Molten Salt. J. Alloys Compd. 2021, 869, 159309. [Google Scholar] [CrossRef]
- Hanjun, H.; Zhen, C.; Xingguang, L.; Xingguo, F.; Yugang, Z.; Kaifeng, Z.; Hui, Z. Effects of Substrate Roughness on the Vacuum Tribological Properties of Duplex PEO/Bonded-MoS2 Coatings on Ti6Al4V. Surf. Coat. Technol. 2018, 349, 593–601. [Google Scholar] [CrossRef]
- Wei-Chao, G.; Guo-Hua, L.; Huan, C.; Guang-Liang, C.; Wen-Ran, F.; Gu-Ling, Z.; Si-Ze, Y. Investigation of Morphology and Composition of Plasma Electrolytic Oxidation Coatings in Systems of Na2SiO3-NaOH and (NaPO3)6-NaOH. J. Mater. Process. Technol. 2007, 182, 28–33. [Google Scholar] [CrossRef]
- Sun, W.; Liu, Y.; Li, T.; Cui, S.; Chen, S.; Yu, Q.; Wang, D. Anti-Corrosion of Amphoteric Metal Enhanced by MAO/Corrosion Inhibitor Composite in Acid, Alkaline and Salt Solutions. J. Colloid Interface Sci. 2019, 554, 488–499. [Google Scholar] [CrossRef]
- Habazaki, H.; Tsunekawa, S.; Tsuji, E.; Nakayama, T. Formation and Characterization of Wear-Resistant PEO Coatings Formed on β-Titanium Alloy at Different Electrolyte Temperatures. Appl. Surf. Sci. 2012, 259, 711–718. [Google Scholar] [CrossRef]
- Franz, S.; Perego, D.; Marchese, O.; Lucotti, A.; Bestetti, M. Photoactive TiO2 Coatings Obtained by Plasma Electrolytic Oxidation in Refrigerated Electrolytes. Appl. Surf. Sci. 2016, 385, 498–505. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Wang, S.; Zhang, Z.; Wen, C.; Chen, F. Enhancing Corrosion Resistance in Mg Li Alloy through Plasma Electrolytic Oxidation Coatings—Exploring the Impact of Ionic Liquids (BmimBF4). Surf. Coat. Technol. 2023, 477, 130329. [Google Scholar] [CrossRef]
- Molaei, M.; Fattah-Alhosseini, A.; Gashti, S.O. Sodium Aluminate Concentration Effects on Microstructure and Corrosion Behavior of the Plasma Electrolytic Oxidation Coatings on Pure Titanium. Met. Mater. Trans. A Phys. Met. Mater. Sci. 2018, 49, 368–375. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Keshavarz, M.K.; Molaei, M.; Gashti, S.O. Plasma Electrolytic Oxidation (PEO) Process on Commercially Pure Ti Surface: Effects of Electrolyte on the Microstructure and Corrosion Behavior of Coatings. Met. Mater. Trans. A Phys. Met. Mater. Sci. 2018, 49, 4966–4979. [Google Scholar] [CrossRef]
- Malinovschi, V.; Marin, A.; Ducu, C.; Andrei, V.; Coaca, E.; Craciun, V.; Lungu, M. Influence of Sodium Aluminate Concentration and Process Duration on Microstructure, Mechanical and Electrochemical Behavior of PEO Coatings Formed on CP-Ti. Surf. Coat. Technol. 2021, 418, 127240. [Google Scholar] [CrossRef]
- Stojadinović, S.; Tadić, N.; Radić, N.; Grbić, B.; Vasilić, R. MgO/ZnO Coatings Formed on Magnesium Alloy AZ31 by Plasma Electrolytic Oxidation: Structural, Photoluminescence and Photocatalytic Investigation. Surf. Coat. Technol. 2017, 310, 98–105. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Sinebryukhov, S.L.; Imshinetskiy, I.M.; Gnedenkov, A.S.; Nadaraia, K.V.; Ustinov, A.Y.; Gnedenkov, S.V. Hard Wearproof PEO-Coatings Formed on Mg Alloy Using TiN Nanoparticles. Appl. Surf. Sci. 2020, 503, 144062. [Google Scholar] [CrossRef]
- Lim, T.S.; Ryu, H.S.; Hong, S.H. Electrochemical Corrosion Properties of CeO2-Containing Coatings on AZ31 Magnesium Alloys Prepared by Plasma Electrolytic Oxidation. Corros. Sci. 2012, 62, 104–111. [Google Scholar] [CrossRef]
- Mohedano, M.; Blawert, C.; Zheludkevich, M.L. Silicate-Based Plasma Electrolytic Oxidation (PEO) Coatings with Incorporated CeO2 Particles on AM50 Magnesium Alloy. Mater. Des. 2015, 86, 735–744. [Google Scholar] [CrossRef]
- Atapour, M.; Blawert, C.; Zheludkevich, M.L. The Wear Characteristics of CeO2 Containing Nanocomposite Coating Made by Aluminate-Based PEO on AM 50 Magnesium Alloy. Surf. Coat. Technol. 2019, 357, 626–637. [Google Scholar] [CrossRef]
- Yin, B.; Peng, Z.; Liang, J.; Jin, K.; Zhu, S.; Yang, J.; Qiao, Z. Tribological Behavior and Mechanism of Self-Lubricating Wear-Resistant Composite Coatings Fabricated by One-Step Plasma Electrolytic Oxidation. Tribol. Int. 2016, 97, 97–107. [Google Scholar] [CrossRef]
- Štrbák, M.; Kajánek, D.; Knap, V.; Florková, Z.; Pastorková, J.; Hadzima, B.; Goraus, M. Effect of Plasma Electrolytic Oxidation on the Short-Term Corrosion Behaviour of AZ91 Magnesium Alloy in Aggressive Chloride Environment. Coatings 2022, 12, 566. [Google Scholar] [CrossRef]
- Hadzima, B.; Kajánek, D.; Jambor, M.; Drábiková, J.; Březina, M.; Buhagiar, J.; Pastorková, J.; Jacková, M. Peo of Az31 Mg Alloy: Effect of Electrolyte Phosphate Content and Current Density. Metals 2020, 10, 1521. [Google Scholar] [CrossRef]
- Chu, C.L.; Han, X.; Xue, F.; Bai, J.; Chu, P.K. Effects of Sealing Treatment on Corrosion Resistance and Degradation Behavior of Micro-Arc Oxidized Magnesium Alloy Wires. Appl. Surf. Sci. 2013, 271, 271–275. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, D.; Gong, Y.; Yang, C.; Zhu, H.; Wang, M.; Chen, D.; Wang, H. Improved Corrosion Resistance of PEO-Coated 7085Al Alloy via a Novel Organic and Inorganic Sealing-Treatment Combination. Surf. Coat. Technol. 2022, 441, 128566. [Google Scholar] [CrossRef]
- Sopchenski, L.; Robert, J.; Touzin, M.; Tricoteaux, A.; Olivier, M.G. Improvement of Wear and Corrosion Protection of PEO on AA2024 via Sol-Gel Sealing. Surf. Coat. Technol. 2021, 417, 127195. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Ling, N.; Zhang, J.; Wang, L. Enhanced Long-Term Corrosion Resistance of Mg Alloys by Superhydrophobic and Self-Healing Composite Coating. Chem. Eng. J. 2022, 449, 137778. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Q.; Zhang, C.; Liu, Y.; Liang, J.; Wang, D.; Zhou, F. Synergistic Effect of Hydrophobic Film and Porous MAO Membrane Containing Alkynol Inhibitor for Enhanced Corrosion Resistance of Magnesium Alloy. Surf. Coat. Technol. 2019, 357, 515–525. [Google Scholar] [CrossRef]
- Zehra, T.; Fattah-alhosseini, A.; Kaseem, M. Surface Properties of Plasma Electrolytic Oxidation Coating Modified by Polymeric Materials: A Review. Prog. Org. Coat. 2022, 171, 107053. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, G.; Wang, X.; Qian, H.; Yi, J.; Huang, L.; Liu, P. Bio-Functionalization of Micro-Arc Oxidized Magnesium Alloys via Thiol-Ene Photochemistry. Surf. Coat. Technol. 2015, 269, 191–199. [Google Scholar] [CrossRef]
- Alabbasi, A.; Liyanaarachchi, S.; Kannan, M.B. Polylactic Acid Coating on a Biodegradable Magnesium Alloy: An in Vitro Degradation Study by Electrochemical Impedance Spectroscopy. Thin Solid Film 2012, 520, 6841–6844. [Google Scholar] [CrossRef]
- Lu, P.; Cao, L.; Liu, Y.; Xu, X.; Wu, X. Evaluation of Magnesium Ions Release, Biocorrosion, and Hemocompatibility of MAO/PLLA-Modified Magnesium Alloy WE42. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96B, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Alabbasi, A.; Mehjabeen, A.; Kannan, M.B.; Ye, Q.; Blawert, C. Biodegradable Polymer for Sealing Porous PEO Layer on Pure Magnesium: An in Vitro Degradation Study. Appl. Surf. Sci. 2014, 301, 463–467. [Google Scholar] [CrossRef]
- Li, Z.; Yang, W.; Yu, Q.; Wu, Y.; Wang, D.; Liang, J.; Zhou, F. New Method for the Corrosion Resistance of AZ31 Mg Alloy with a Porous Micro-Arc Oxidation Membrane as an Ionic Corrosion Inhibitor Container. Langmuir 2019, 35, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Z.; Yu, Q.; Qi, Y.; Peng, Z.; Liang, J. Dual Self-Healing Composite Coating on Magnesium Alloys for Corrosion Protection. Chem. Eng. J. 2021, 424, 13055. [Google Scholar] [CrossRef]
- Tikhov, S.F.; Chernykh, G.V.; Sadykov, V.A.; Salanov, A.N.; Alikina, G.M.; Tsybulya, S.V.; Lysov, V.F. Honeycomb Catalysts for Clean-up of Diesel Exhausts Based upon the Anodic-Spark Oxidized Aluminum Foil. Catal. Today 1999, 53, 639–646. [Google Scholar] [CrossRef]
- Shtefan, V.V.; Smirnova, A.Y. Synthesis of Ce-, Zr-, and Cu-Containing Oxide Coatings on Titanium Using Microarc Oxidation. Russ. J. Electrochem. 2015, 51, 1168–1175. [Google Scholar] [CrossRef]
- Rudnev, V.S.; Tyrina, L.M.; Ustinov, A.Y.; Vybornova, S.; Lukiyanchuk, I.V. Comparative Analysis of the Composition, Structure, and Catalytic Activity of the NiO-CuO-TiO2 on Titanium and NiO-CuO-Al2O3 on Aluminum Composites. Kinet. Catal. 2010, 51, 266–272. [Google Scholar] [CrossRef]
- Lin, G.W.; Huang, Y.H.; Tseng, W.; Lu, F.H. Production of N-Doped Anatase TiO2 on TiN-Coated Ti Substrates by Plasma Electrolytic Oxidation for Visible-Light Photocatalysts. Ceram. Int. 2019, 45, 22506–22512. [Google Scholar] [CrossRef]
- Lee, J.H.; Youn, J.I.; Kim, Y.J.; Kim, I.K.; Jang, K.W.; Oh, H.J. Photocatalytic Characteristics of Boron and Nitrogen Doped Titania Film Synthesized by Micro-Arc Oxidation. Ceram. Int. 2015, 41, 11899–11907. [Google Scholar] [CrossRef]
- Franz, S.; Arab, H.; Chiarello, G.L.; Bestetti, M.; Selli, E. Single-Step Preparation of Large Area TiO2 Photoelectrodes for Water Splitting. Adv. Energy Mater. 2020, 10, 2000652. [Google Scholar] [CrossRef]
- Shin, S.; Choi, Y.W.; Choi, J. Water Splitting by Dimensionally Stable Anode Prepared through Micro-Arc Oxidation. Mater. Lett. 2013, 105, 117–119. [Google Scholar] [CrossRef]
- Woo, S.-R.; Sung, Y.-M. Enhanced Photoelectrochemical Water Splitting of Micro-Arc Oxidized TiO2 via Anatase/Rutile Phase Control and Nitrogen Doping. J. Electrochem. Soc. 2016, 163, H278–H285. [Google Scholar] [CrossRef]
- Bayati, M.R.; Zargar, H.; Molaei, R.; Golestani-Fard, F.; Kajbafvala, E.; Zanganeh, S. One Step Growth of WO3-Loaded Al2O3 Micro/Nano-Porous Films by Micro Arc Oxidation. Colloids Surf. A Physicochem. Eng. Asp. 2010, 355, 187–192. [Google Scholar] [CrossRef]
- Bayati, M.R.; Zargar, H.R.; Molaei, R.; Golestani-Fard, F.; Zanganeh, N.; Kajbafvala, A. MAO-Synthesized Al2O3-Supported V2 O5 Nano-Porous Catalysts: Growth, Characterization, and Photoactivity. Appl. Surf. Sci. 2010, 256, 3806–3811. [Google Scholar] [CrossRef]
- Bayati, M.R.; Moshfegh, A.Z.; Golestani-Fard, F. In Situ Growth of Vanadia-Titania Nano/Micro-Porous Layers with Enhanced Photocatalytic Performance by Micro-Arc Oxidation. Electrochim. Acta 2010, 55, 3093–3102. [Google Scholar] [CrossRef]
- Bayati, M.R.; Golestani-Fard, F.; Moshfegh, A.Z. Photo-Degradation of Methelyne Blue over V2O5-TiO2 Nano-Porous Layers Synthesized by Micro Arc Oxidation. Catal. Lett. 2010, 134, 162–168. [Google Scholar] [CrossRef]
- Bayati, M.R.; Golestani-Fard, F.; Moshfegh, A.Z. Visible Photodecomposition of Methylene Blue over Micro Arc Oxidized WO3-Loaded TiO2 Nano-Porous Layers. Appl. Catal. A Gen. 2010, 382, 322–331. [Google Scholar] [CrossRef]
- Bayati, M.R.; Golestani-Fard, F.; Moshfegh, A.Z.; Molaei, R. A Photocatalytic Approach in Micro Arc Oxidation of WO3-TiO2 Nano Porous Semiconductors under Pulse Current. Mater. Chem. Phys. 2011, 128, 427–432. [Google Scholar] [CrossRef]
- Tarkhanova, I.G.; Bryzhin, A.A.; Gantman, M.G.; Yarovaya, T.P.; Lukiyanchuk, I.V.; Nedozorov, P.M.; Rudnev, V.S. Ce-, Zr-Containing Oxide Layers Formed by Plasma Electrolytic Oxidation on Titanium as Catalysts for Oxidative Desulfurization. Surf. Coat. Technol. 2019, 362, 132–140. [Google Scholar] [CrossRef]
- Tao, W.; Wang, M.; Zhu, B.; Huo, W.; Yang, R.; Xiong, H.; Tang, H.; Wei, Z.; Wang, Y. In-Situ Synthesized Binder-Free Flocculent TiO2−x Film as Anode for Lithium-Ion Batteries. Electrochim. Acta 2020, 334, 135569. [Google Scholar] [CrossRef]
- Lu, P.; Wu, J.; Zhao, H.; Zhao, M.; Dong, L.; Xue, W.; Li, D. One-Step Plasma Electrolytic Oxidation for TiO2/SnO2 Film as LIB Anode. Surf. Eng. 2021, 37, 918–925. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S. Surface Modification of Titanium and Titanium Alloy by Plasma Electrolytic Oxidation Process for Biomedical Applications: A Review. Mater. Today 2021, 46, 594–602. [Google Scholar] [CrossRef]
- Acquesta, A.; Russo, P.; Monetta, T. Plasma Electrolytic Oxidation Treatment of AZ31 Magnesium Alloy for Biomedical Applications: The Influence of Applied Current on Corrosion Resistance and Surface Characteristics. Crystals 2023, 13, 510. [Google Scholar] [CrossRef]
- Mishra, S.; Sundaram, B. A Review of the Photocatalysis Process Used for Wastewater Treatment. Mater. Today Proc. 2023, 102, 393–409. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Clerc, J.C. Catalytic Diesel Exhaust Aftertreatment. Appl. Catal. B Environ. 1996, 10, 99–115. [Google Scholar] [CrossRef]
- Lebukhova, N.V.; Rudnev, V.S.; Chigrin, P.G.; Lukiyanchuk, I.V.; Pugachevsky, M.A.; Ustinov, A.Y.; Kirichenko, E.A.; Yarovaya, T.P. The Nanostructural Catalytic Composition CuMoO4/TiO2+SiO2/Ti for Combustion of Diesel Soot. Surf. Coat. Technol. 2013, 231, 144–148. [Google Scholar] [CrossRef]
- Caero, L.C.; Hernández, E.; Pedraza, F.; Murrieta, F. Oxidative Desulfurization of Synthetic Diesel Using Supported Catalysts: Part I. Study of the Operation Conditions with a Vanadium Oxide Based Catalyst. Catal. Today 2005, 107–108, 564–569. [Google Scholar] [CrossRef]
- Armandsefat, F.; Hamzehzadeh, S.; Azizi, N. Efficient and Promising Oxidative Desulfurization of Fuel Using Fenton like Deep Eutectic Solvent. Sci. Rep. 2024, 14, 12614. [Google Scholar] [CrossRef]
- Rudnev, V.S.; Lukiyanchuk, I.V.; Vasilyeva, M.S.; Morozova, V.P.; Zelikman, V.M.; Tarkhanova, I.G. W-Containing Oxide Layers Obtained on Aluminum and Titanium by PEO as Catalysts in Thiophene Oxidation. Appl. Surf. Sci. 2017, 422, 1007–1014. [Google Scholar] [CrossRef]
- Rakhmanov, E.V.; Tarakanova, A.V.; Valieva, T.; Akopyan, A.V.; Litvinova, V.V.; Maksimov, A.L.; Anisimov, A.V.; Vakarin, S.V.; Semerikova, O.L.; Zaikov, Y.P. Oxidative Desulfurization of Diesel Fraction with Hydrogen Peroxide in the Presence of Catalysts Based on Transition Metals. Pet. Chem. 2014, 54, 48–50. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, J. Nanoengineering Titania for High Rate Lithium Storage: A Review. J. Mater. Sci. Technol. 2013, 29, 97–122. [Google Scholar] [CrossRef]
- Makurat-Kasprolewicz, B.; Ossowska, A. Recent Advances in Electrochemically Surface Treated Titanium and Its Alloys for Biomedical Applications: A Review of Anodic and Plasma Electrolytic Oxidation Methods. Mater. Today Commun. 2023, 34, 105425. [Google Scholar] [CrossRef]
- Park, S.Y.; Jo, C.I.; Choe, H.C.; Brantley, W.A. Hydroxyapatite Deposition on Micropore-Formed Ti-Ta-Nb Alloys by Plasma Electrolytic Oxidation for Dental Applications. Surf. Coat. Technol. 2016, 294, 15–20. [Google Scholar] [CrossRef]
- Sheng, Y.; Tian, L.; Wu, C.; Qin, L.; Ngai, T. Biodegradable Poly(l-Lactic Acid) (PLLA) Coatings Fabricated from Nonsolvent Induced Phase Separation for Improving Corrosion Resistance of Magnesium Rods in Biological Fluids. Langmuir 2018, 34, 10684–10693. [Google Scholar] [CrossRef] [PubMed]
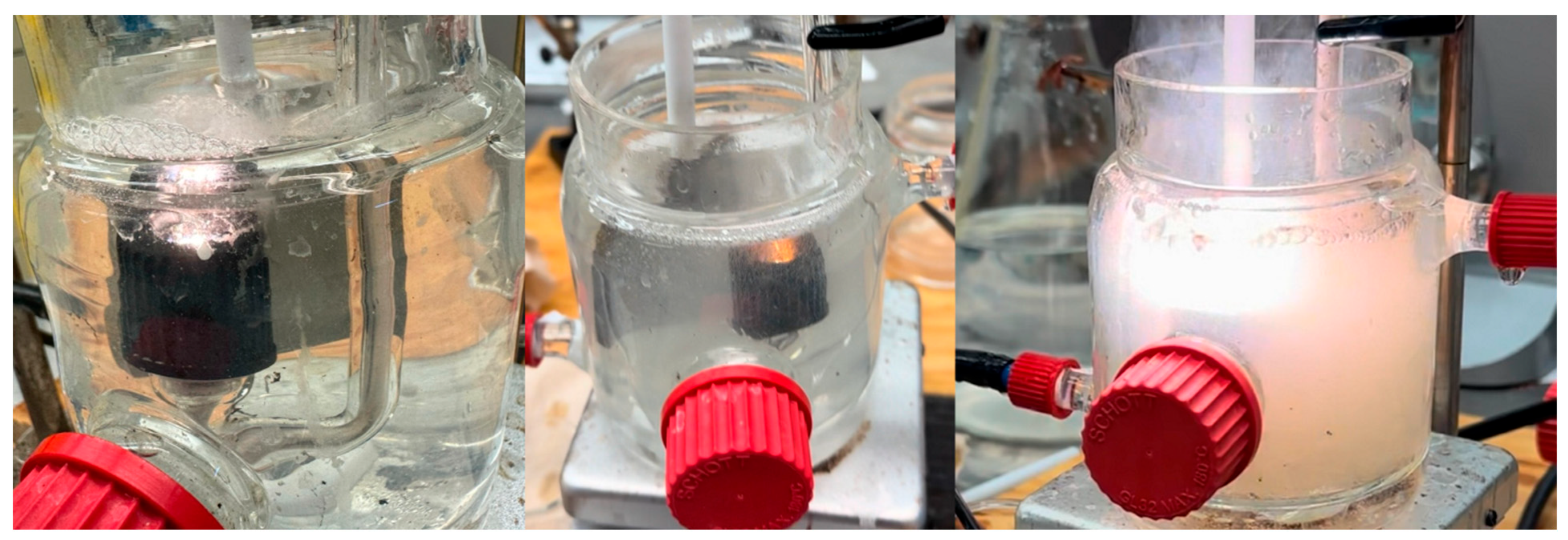
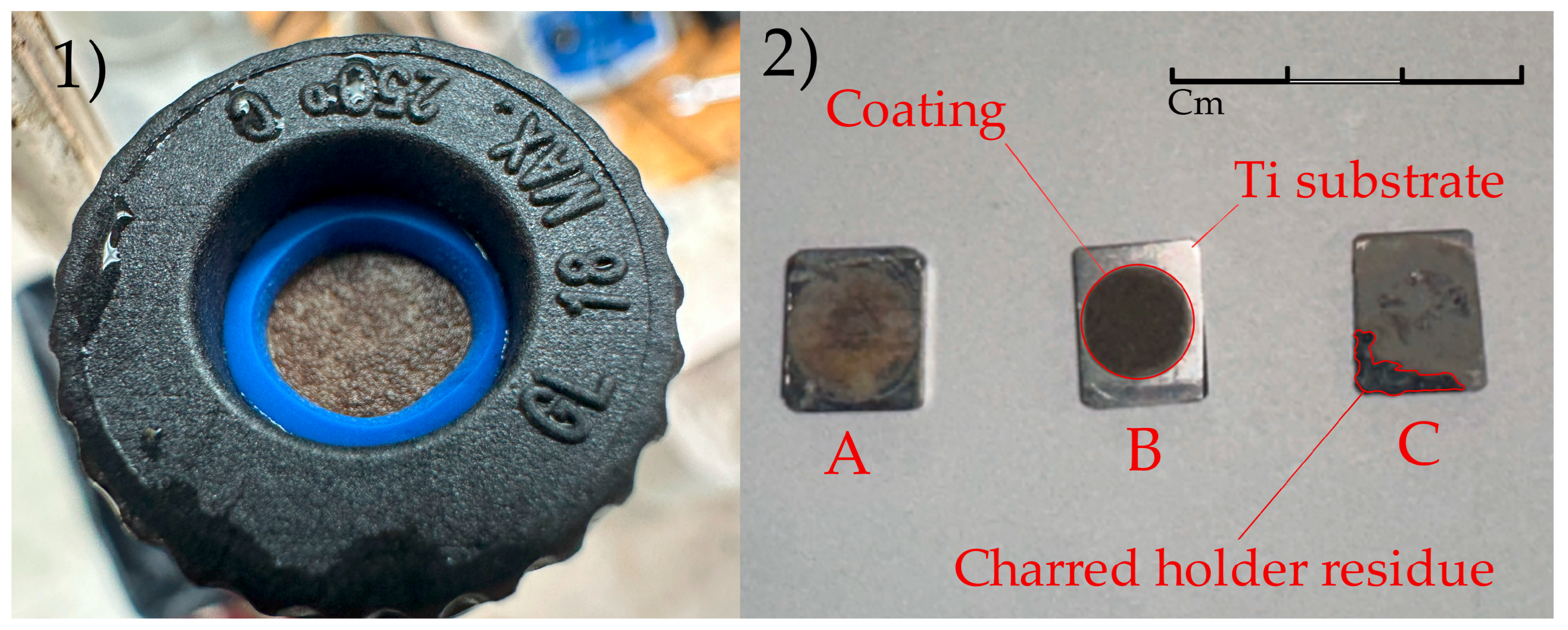
| Parameter | Effect | Reference |
|---|---|---|
| Current mode | Bipolar current lowers plasma temperatures, lowers porosity, improves corrosion resistance | [49,50] |
| Higher negative current vs. positive reduces porosity | ||
| Current frequency | Higher frequencies reduce strong discharges, improving coating quality | [47] |
| Short pulses result in better bonding but higher porosity | ||
| Processing time | Longer processing produces thicker coating, phase composition changes as coating develops | [51] |
| Electrolyte composition | Different electrolyte components result in different oxide phases | [52,53,54,55] |
| High electrolyte conductivity reduces operating voltages and improves efficiency, but process can fail if conductivity is too high | [56] |
| Substrate | Coating | Contaminant | Conversion % | Ref. |
|---|---|---|---|---|
| Ti | WO3 ± SiO2 ± TiO2 | Thiophene, 1% | 55 | [121] |
| Al-Mn | WO3 ± SiO2 ± −Al2O3 | 34 | ||
| Al | WO3 Al2O3 | 59 | ||
| Al-Mn | WO3 Al2O3 | 46 | ||
| Mn-WO2.9 Al2O3 | 47 | |||
| Al2(WO4)3 Al2O3 | 45 | |||
| WO3 Al2(WO4)3 | 51 | |||
| Ti | ZrO2, TiO2, CeOx | Thioanizole, 1% | 100 | [110] |
| Thiophene, 1% | 67 | |||
| Thiophene, 0.5% | 61 | |||
| Dibenzothiophene, 0.5% | 48 | |||
| ZrO2, TiO2, CeOx, Ionic liquid | Thioanizole, 1% | 100 | ||
| Thiophene, 1% | 89 | |||
| Thiophene, 0.5% | 83 | |||
| Dibenzothiophene, 0.5% | 65 | |||
| Ti | Ti, WO3 | Thiophene | 40 | [55] |
| TiO2, WO3, MnWO4 | 30 | |||
| TiO2 (anatase), WO3, Na0.28WO3 * | 25 | |||
| WO3, Zn0.3WO3 * | 82 | |||
| Thioanizole | 100 | |||
| Dibenzothiophene | 91 | |||
| Diesel fuel | 97 | |||
| WO3, Zn0.3WO3 *, Ionic liquid | Thiophene | 93 | ||
| Thioanizole | 100 | |||
| Dibenzothiophene | 98 | |||
| Diesel fuel | 99.6 |
| Coating | Capacity | Ref. |
|---|---|---|
| TiO2, SiO2 | 700 μAh/cm2 | [56] |
| TiO2−x (self-doped) | 520 mAh/cm3 | [111] |
| TiO2, MoS2 sputtered | 560 mAh/g | [58] |
| SnO2, TiO2 | 200 mAh/g | [59] |
| SnO2, TiO2, graphene | 482 mAh/g | |
| SiO2, TiO2 | 758 μAh/cm2 | [43] |
| SiO2, TiO2, PDMS | 1072 μAh/cm2 |
| PEO Modification | Ecorr % Reduction | Icorr % Reduction | Source |
|---|---|---|---|
| Boiling water immersion | 35% | 76% | [84] |
| Zirconia sol-gel | 14% | 92% | |
| Gelatin-hydroxyapatite coating | 75% | 98% | |
| Poly(L-lactide) spin coating | 14% | 99.87% | [93] |
| MPTS sulfhydration | 5.8% | 99.96% | [90] |
| PEGMA coating | 6.1% | 99.98% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCarroll, A.G.; Menezes, P.L. Modern Innovations and Applications in Plasma Electrolytic Oxidation Coatings on Aluminum, Magnesium, and Titanium. Coatings 2025, 15, 592. https://doi.org/10.3390/coatings15050592
McCarroll AG, Menezes PL. Modern Innovations and Applications in Plasma Electrolytic Oxidation Coatings on Aluminum, Magnesium, and Titanium. Coatings. 2025; 15(5):592. https://doi.org/10.3390/coatings15050592
Chicago/Turabian StyleMcCarroll, Angus G., and Pradeep L. Menezes. 2025. "Modern Innovations and Applications in Plasma Electrolytic Oxidation Coatings on Aluminum, Magnesium, and Titanium" Coatings 15, no. 5: 592. https://doi.org/10.3390/coatings15050592
APA StyleMcCarroll, A. G., & Menezes, P. L. (2025). Modern Innovations and Applications in Plasma Electrolytic Oxidation Coatings on Aluminum, Magnesium, and Titanium. Coatings, 15(5), 592. https://doi.org/10.3390/coatings15050592








