Interfacial Adsorption Mechanisms of Arginine, Glutamic Acid, Aspartic Acid, and Valine on Magnesium and Magnesium Alloy Surfaces: A First-Principles Investigation
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. Adsorption Properties of Amino Acids on Mg(0001) Surfaces
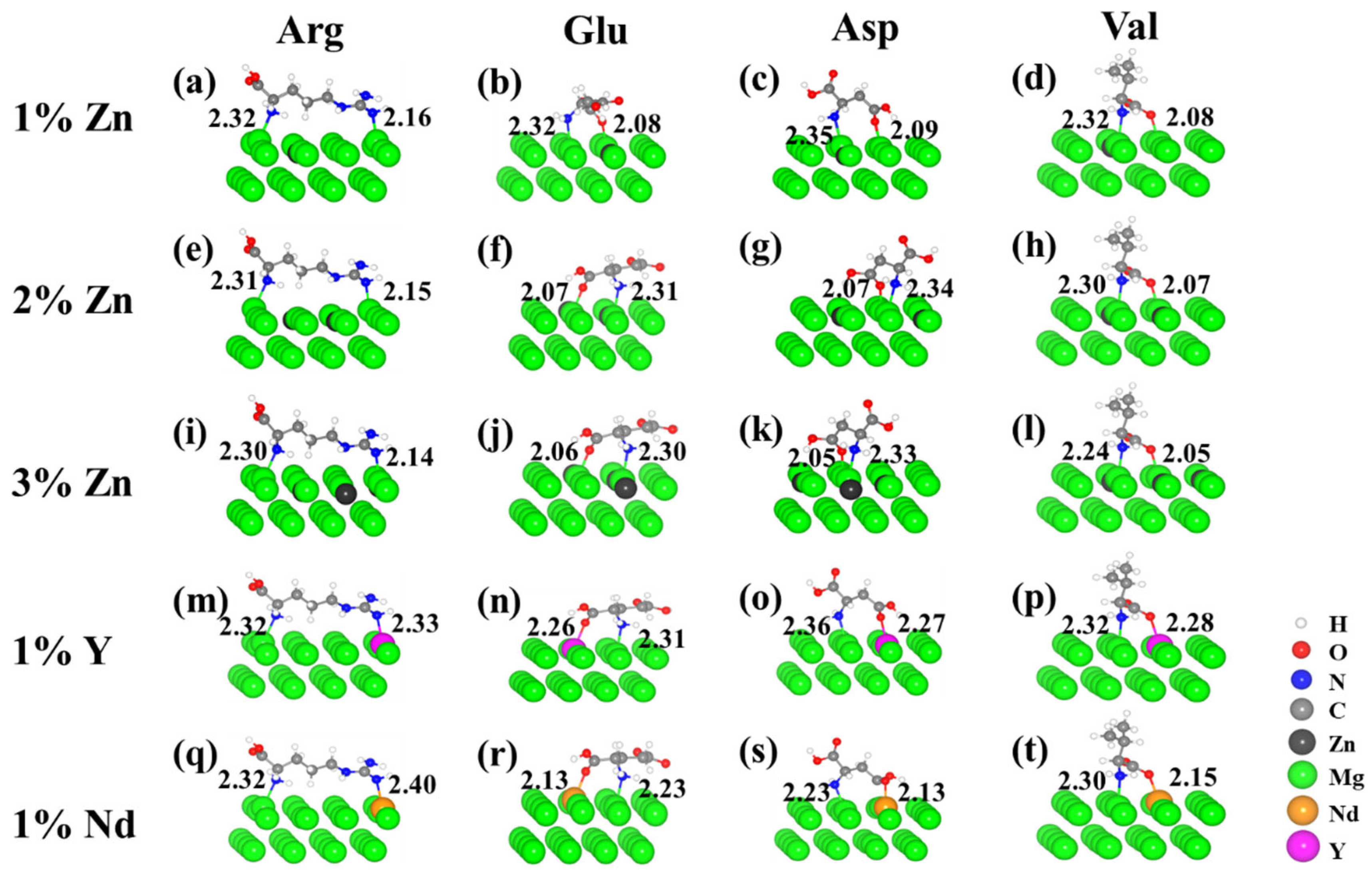
3.2. Effects of Alloying Elements on the Adsorption Properties
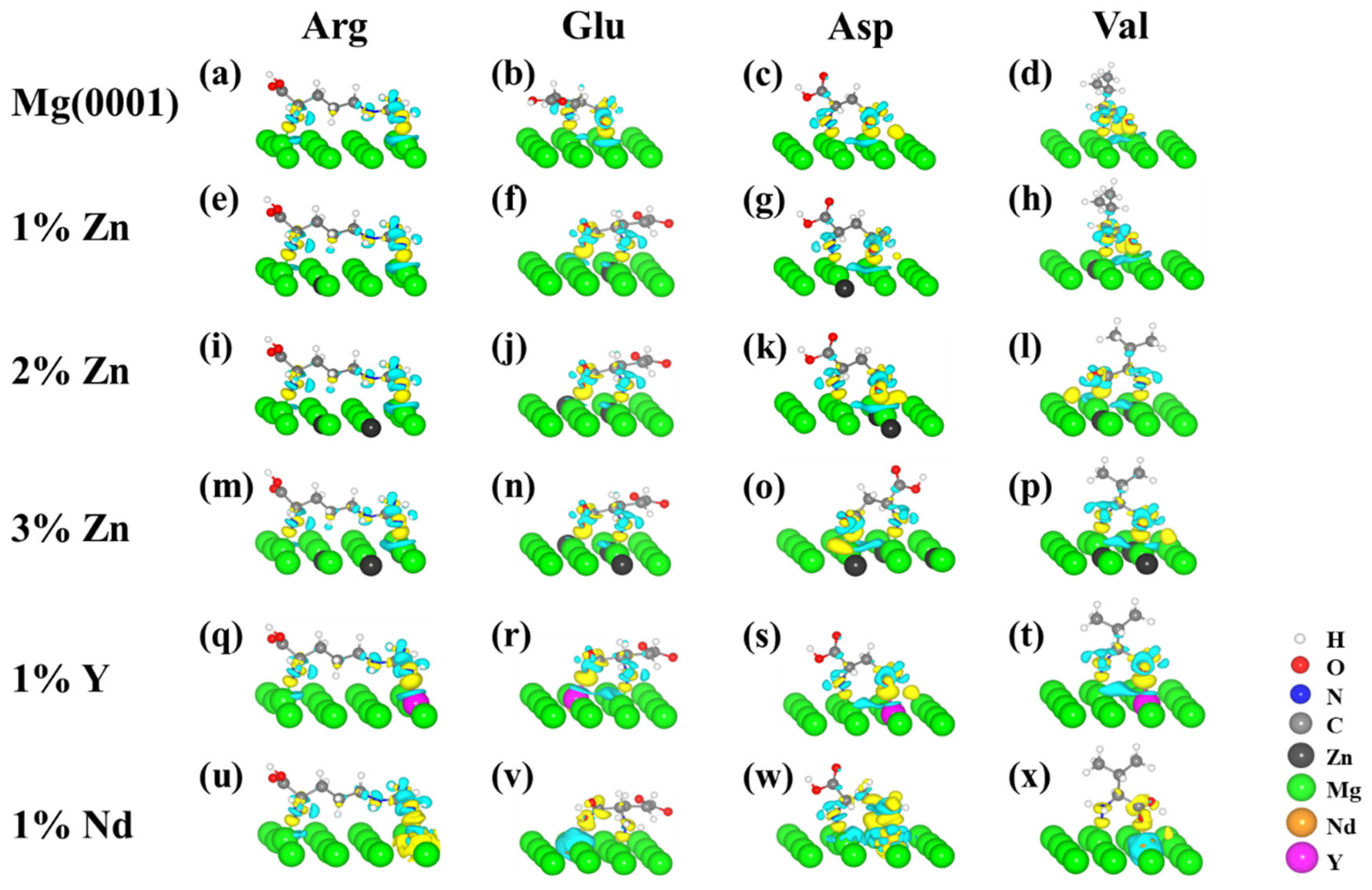
3.3. Electronic Structure Properties of Amino Acids on the Mg and Mg Alloy Surfaces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janisse, S.E.; Fernandez, R.L.; Heffern, M.C. Characterizing metal-biomolecule interactions by mass spectrometry. Trends Biochem. Sci. 2023, 48, 815–825. [Google Scholar] [CrossRef]
- Xu, W.J.; Lin, Z.X.; Kim, C.J.; Wang, Z.R.; Wang, T.Z.; Jugo, C.; Caruso, F. Assembly and biological functions of metal-biomolecule network nanoparticles formed by metal-phosphonate coordination. Sci. Adv. 2024, 10, eads9542. [Google Scholar] [CrossRef] [PubMed]
- Alvisi, N.; Vries, R. Biomedical applications of solid-binding peptides and proteins. Mater. Today Bio. 2023, 19, 100580. [Google Scholar] [CrossRef] [PubMed]
- Giovana, C.C.; Carlos, R.G.; Julietta, V.R. Comprehensive review of PEO coatings on titanium alloys for biomedical implants. J. Mater. Res. Technol. 2024, 31, 311–328. [Google Scholar]
- Nassar, M.F.; Taban, T.Z.; Obaid, R.F.; Shadhar, M.H.; Almashhadani, H.A.; Kadhim, A.M.; Liu, P. Study to amino acid-based inhibitors as an effective anti-corrosion material. J. Mol. Liq. 2022, 360, 119449. [Google Scholar] [CrossRef]
- Zhu, L.W.; Mori, Y.; Song, J.Q.; Kuroda, K.; Okido, M.; Peng, C. Surface modification by pre-adsorption of proteins and polypeptides on Ti substrate with controlled hydrophilicity to improve biocompatibility. Mater. Today Commun. 2023, 37, 107124. [Google Scholar] [CrossRef]
- Dubey, A.; Ghosh, S.; Jaiswal, S.; Roy, P.; Lahiri, D. Assessment of protein adhesion behaviour and biocompatibility of magnesium/Co-substituted HA-based composites for orthopaedic application. Int. J. Biol. Macromol. 2022, 208, 707–719. [Google Scholar] [CrossRef]
- Liu, K.P.; Cheng, A.Y.; You, J.L.; Chang, Y.H.; Tseng, C.C.; Ger, M.D. Biocompatibility and corrosion resistance of drug coatings with different polymers for magnesium alloy cardiovascular stents. Colloids Surf. B Biointerfaces 2025, 245, 114202. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Huang, Q.X.; Chi, Y.; Yan, J.H. Adsorption and thermal degradation of microplastics from aqueous solutions by Mg/Zn modified magnetic biochars. J. Hazard. Mater. 2021, 419, 126486. [Google Scholar] [CrossRef]
- Fang, Z.; Xu, S.; Wang, Z.; Sun, Y. Effect of Solution and Aging Treatment on the Microstructure and Properties of LAZ931 Mg-Li Alloy by Friction Stir Processing. Metals 2025, 15, 314. [Google Scholar] [CrossRef]
- Cazzola, R.; Della Porta, M.; Piuri, G.; Maier, J.A. Magnesium: A defense line to mitigate inflammation and oxidative stress in adipose tissue. Antioxidants 2024, 13, 893. [Google Scholar] [CrossRef]
- Fattah, A.; Chaharmahali, R.; Askari, A.; Alizad, S.; Kaseem, M. Unraveling the impact of purification and alloying elements on corrosion performance and passivation of magnesium alloys. J. Magnes. Alloys 2024, 12, 4808–4827. [Google Scholar] [CrossRef]
- Ventura, E.; Martinez, P.M.; Pérez, R.; Vilhena, J.G. Quantum mechanical derived (VdW-DFT)transferable Lennard–Jones and morse potentials to model cysteine and alkanethiol adsorption on Au(111). Adv. Mater. Interfaces 2024, 11, 30. [Google Scholar]
- Xu, M.L.; Liu, S.H.; Vijay, S.; Bligaard, T.; Kastlunger, G. Benchmarking water adsorption on metal surfaces with ab initio molecular dynamics. J. Chem. Phys. 2024, 160, 244707. [Google Scholar] [CrossRef]
- Weber, I.; Penschke, C.; Michaelides, A.; Morgenstern, K. Importance of ion size on the dominance of water-ion versus water-water interactions in Au-supported solvatomers. Nano Lett. 2025, 25, 6. [Google Scholar] [CrossRef]
- Xia, Y.F.; Hong, Z.J.; Zhou, L.M.; Chen, S.; Luo, Z.; Jin, S.T.; Huang, Y.H.; Jiang, Y.Z.; Wu, Y.J. Multiscale simulations of surface adsorption characteristics of amino acids on zinc metal anode. J. Energy Chem. 2023, 87, 153–161. [Google Scholar] [CrossRef]
- Meng, X.Z.; Zhang, Q.R.; Lv, X.T.; Chen, J.B.; Peng, Y.Z.; Dong, P.X.; Sun, Q.Q. Corrosion inhibition effect of several amino acids on an Al-Zn-Mg-Cu alloy: Electrochemical study, DFT and MD simulation. Mater. Today Commun. 2024, 41, 110529. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhao, Y.; Wang, X.Y.; Xie, Y.D.; Bai, L.C.; Guan, S.K. Fucoidan/collagen composite coating on magnesium alloy for better corrosion resistance and pro-endothelialization potential. Int. J. Biol. Macromol. 2024, 255, 128044. [Google Scholar] [CrossRef]
- Kasprzhitskii, A.; Lazorenko, G. Corrosion inhibition potential of sulfur-containing and aromatic amino acids on magnesium in Hank’s solution. Colloids Surf. A Physicochem. Eng. Asp. 2024, 703, 135267. [Google Scholar] [CrossRef]
- Pei, K.M.; Ying, Y.D.; Chu, C.C. Molecular dynamic simulations of a new family of synthetic biodegradable amino acid-based poly(ester amide) biomaterials: Glass transition temperature and adhesion behavior. Mater. Today Chem. 2017, 4, 90–96. [Google Scholar] [CrossRef]
- Ma, C.; Liu, J.Y.; Zhang, Z.Y.; Wu, F.; Wen, Y.Q.; Shang, W. Preparation and Properties of Micro-Arc Oxidation/Self-Assembly Coatings with Different Hydrophobicities on Magnesium Alloy. Adv. Eng. Mater. 2022, 24, 2200741. [Google Scholar] [CrossRef]
- Xie, T.; Zhao, P.Y.; Chen, Y.Y.; Zhang, M.Y.; Wang, Y.W.; Ying, T.; Zhu, H.; Zeng, X.Q. Investigation on the corrosion behavior of single-phase and binary-phase Mg-Sc alloys: An experimental and first-principles study. Mater. Charact. 2021, 179, 111294. [Google Scholar] [CrossRef]
- Chen, T.; Yuan, Y.; Mi, X.X.; Wu, J.J.; Tang, A.T.; Wang, J.F.; Moelans, N.; Pan, F.S. Interaction of elements in dilute Mg alloys: A DFT and machine learning study. J. Mater. Res. Technol. 2022, 21, 4512–4525. [Google Scholar] [CrossRef]
- Zhou, R.Y.; Wu, Y.M.; Chen, K.; Zhang, D.T.; Chen, Q.; Zhang, D.H.; She, Y.R.; Zhang, W.J.; Liu, L.Q.; Zhu, Y.Q.; et al. A polymeric strategy empowering vascular cell selectivity and potential application superior to extracellular matrix peptides. Adv. Mater. 2022, 34, 2200464. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, Y.M.; Wang, Y.B. A nitric oxide-eluting and REDV peptide-conjugated coating promotes vascular healing. Biomaterials 2022, 284, 121478. [Google Scholar] [CrossRef]
- Bai, L.C.; Wang, Y.H.; Chen, L.; Wang, J.; Li, J.A.; Zhu, S.J.; Wang, L.G.; Guan, S.K. Preparation of functional coating on magnesium alloy with hydrophilic polymers and bioactive peptides for improved corrosion resistance and biocompatibility. J. Magnes. Alloys 2022, 10, 1957–1971. [Google Scholar] [CrossRef]
- Pan, C.J.; Liu, X.H.; Hong, Q.X.; Chen, J.; Cheng, Y.X.; Zhang, Q.Y.; Meng, L.J.; Dai, J.; Yang, Z.M.; Wang, L.R. Recent advances in surface endothelialization of the magnesium alloy stent materials. J. Magnes. Alloys 2023, 11, 48–77. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Berland, K.; Cooper, V.R.; Lee, K.; Schröder, E.; Thonhauser, T.; Hyldgaard, P.; Lundqvist, B.I. van der Waals forces in density functional theory: A review of the vdW-DF method. Rep. Prog. Phys. 2015, 78, 066501. [Google Scholar] [CrossRef]
- Kennedy, P.S.B.; Emily, A.C.; Maria, A.P.; Erin, V.I. Extreme atomic-scale surface roughening: Amino acids on Ag on Au(111). J. Vac. Sci. Technol. A 2021, 39, 060404. [Google Scholar]
- Mosaddeghi Amini, P.; Subbotina, J.; Lobaskin, V. Milk Protein Adsorption on Metallic Iron Surfaces. Nanomaterials 2023, 13, 1857. [Google Scholar] [CrossRef]
- Merv, A.; Guglielmo, A.C.; Marie, V.G.; Tomohiko, Y.; Manuela, S.K.; Rob, L.; Erik, V.E.; Hans, P.S.; Annabel, B. Influence of polydopamine functionalization on the rapid protein immobilization by alternating current electrophoretic deposition. Surf. Interfaces 2022, 34, 102347. [Google Scholar]
- Sudhakar, H.R.; Renner, J.N.; Warburton, R.E. Interfacial Electric Fields Drive Rearrangement of Adsorbed Cysteine and Electrolyte Ions on Au Electrodes. J. Phys. Chem. C 2024, 128, 18063–18073. [Google Scholar] [CrossRef]
- Fang, Z.; Ma, B.W.; Liang, E.J.; Jia, Y.; Guan, S.K. Interaction Regularity of Biomolecules on Mg and Mg-Based Alloy Surfaces: A First-Principles Study. Coatings 2024, 14, 25. [Google Scholar] [CrossRef]
- Swanson, H.E.; McMurdie, H.F.; Morris, M.C.; Evans, E.H. Standard X-Ray Diffraction Powder Patterns. In National Bureau of Standards Monograph 25-Section 6; U.S. Department of Commerce, National Bureau of Standards: Gaithersburg, MD, USA, 1968. [Google Scholar]
- Sargent, W. Table of Periodic Properties of the Elements; Sargent-Welch Scientific: Skokie, IL, USA, 1980. [Google Scholar]
- Xi, T.F.; Wei, L.N.; Liu, J.; Liu, X.L.; Zhen, Z.; Zheng, Y.F. Research Progress in Bioresorbable Magnesium Scaffolds. Acta Metall. Sin. 2017, 53, 1153–1167. [Google Scholar]
- Yu, Y.; Zhu, S.J.; Dong, H.T.; Zhang, X.Q.; Li, J.A.; Guan, S.K. A novel MgF2/PDA/S-HA coating on the bio-degradable ZE21B alloy for better multi-functions on cardiovascular application. J. Magnes. Alloys 2023, 11, 480–492. [Google Scholar] [CrossRef]
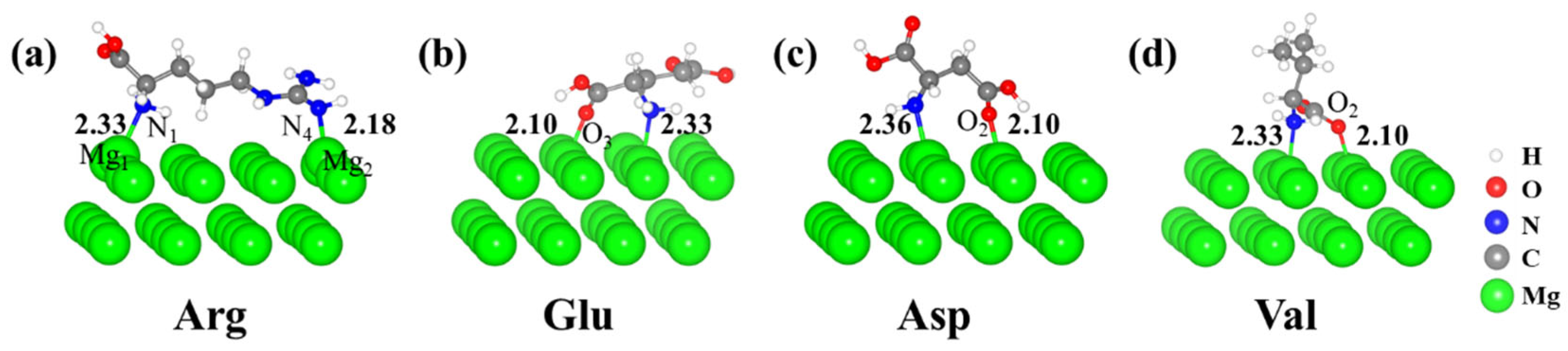

| Arg | Glu | Asp | Val | |
|---|---|---|---|---|
| Eads (eV) | −1.67 | −1.31 | −1.23 | −1.16 |
| Distance from N/O Atom to Nearest Mg Atom (Å) | ||
|---|---|---|
| Arg | N1 | 2.33 |
| N4 | 2.18 | |
| Glu | N | 2.33 |
| O3 | 2.10 | |
| Asp | N | 2.36 |
| O2 | 2.10 | |
| Val | N | 2.33 |
| O2 | 2.10 | |
| Eads (eV) | |||||
|---|---|---|---|---|---|
| 1% Zn | 2% Zn | 3% Zn | 1% Y | 1% Nd | |
| Arg | −1.88 | −1.91 | −1.99 | −2.41 | −2.43 |
| Glu | −1.43 | −1.47 | −1.50 | −2.23 | −2.18 |
| Asp | −1.30 | −1.45 | −1.42 | −1.72 | −1.98 |
| Val | −1.21 | −1.28 | −1.33 | −1.79 | −1.85 |
| Distance from N or O Atom to Nearest Mg Atom (Å) | ||||||
|---|---|---|---|---|---|---|
| 1% Zn | 2% Zn | 3% Zn | 1% Y | 1% Nd | ||
| Arg | N1 | 2.32 | 2.31 | 2.30 | 2.32 | 2.32 |
| N4 | 2.36 | 2.15 | 2.14 | 2.33 | 2.40 | |
| Glu | N | 2.32 | 2.31 | 2.30 | 2.31 | 2.23 |
| O3 | 2.08 | 2.07 | 2.06 | 2.26 | 2.13 | |
| Asp | N | 2.35 | 2.34 | 2.33 | 2.36 | 2.23 |
| O2 | 2.09 | 2.07 | 2.05 | 2.27 | 2.13 | |
| Val | N | 2.32 | 2.30 | 2.24 | 2.32 | 2.30 |
| O2 | 2.08 | 2.07 | 2.05 | 2.28 | 2.15 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Xu, S.; Cao, R.; Jiao, M.; Liu, K.; Diao, Q.; Guan, S.; Jia, Y. Interfacial Adsorption Mechanisms of Arginine, Glutamic Acid, Aspartic Acid, and Valine on Magnesium and Magnesium Alloy Surfaces: A First-Principles Investigation. Coatings 2025, 15, 586. https://doi.org/10.3390/coatings15050586
Fang Z, Xu S, Cao R, Jiao M, Liu K, Diao Q, Guan S, Jia Y. Interfacial Adsorption Mechanisms of Arginine, Glutamic Acid, Aspartic Acid, and Valine on Magnesium and Magnesium Alloy Surfaces: A First-Principles Investigation. Coatings. 2025; 15(5):586. https://doi.org/10.3390/coatings15050586
Chicago/Turabian StyleFang, Zhe, Shuaiwei Xu, Rui Cao, Mingli Jiao, Keyi Liu, Quan Diao, Shaokang Guan, and Yu Jia. 2025. "Interfacial Adsorption Mechanisms of Arginine, Glutamic Acid, Aspartic Acid, and Valine on Magnesium and Magnesium Alloy Surfaces: A First-Principles Investigation" Coatings 15, no. 5: 586. https://doi.org/10.3390/coatings15050586
APA StyleFang, Z., Xu, S., Cao, R., Jiao, M., Liu, K., Diao, Q., Guan, S., & Jia, Y. (2025). Interfacial Adsorption Mechanisms of Arginine, Glutamic Acid, Aspartic Acid, and Valine on Magnesium and Magnesium Alloy Surfaces: A First-Principles Investigation. Coatings, 15(5), 586. https://doi.org/10.3390/coatings15050586







