Multi-Modal Mechanical Response of Self-Healing Double-Network Hydrogel Coatings Based on Schiff Base Bond
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Mussel Chemical Self-Assembly Prepared on a Titanium Alloy Surface
2.3. Preparation of Core–Shell Nanoparticles (PDCS)
2.4. Synthesis of the SA/VA/AM and PDCS/SA/VA/AM Hydrogels
2.5. Analysis Methods
2.6. Adhesion Strength of Coatings and Film Thickness Test
2.7. Swelling Test
2.8. Dynamic Oscillatory Rheometric and Mechanics Performance Testing
2.9. Tribological Properties
3. Results and Discussion
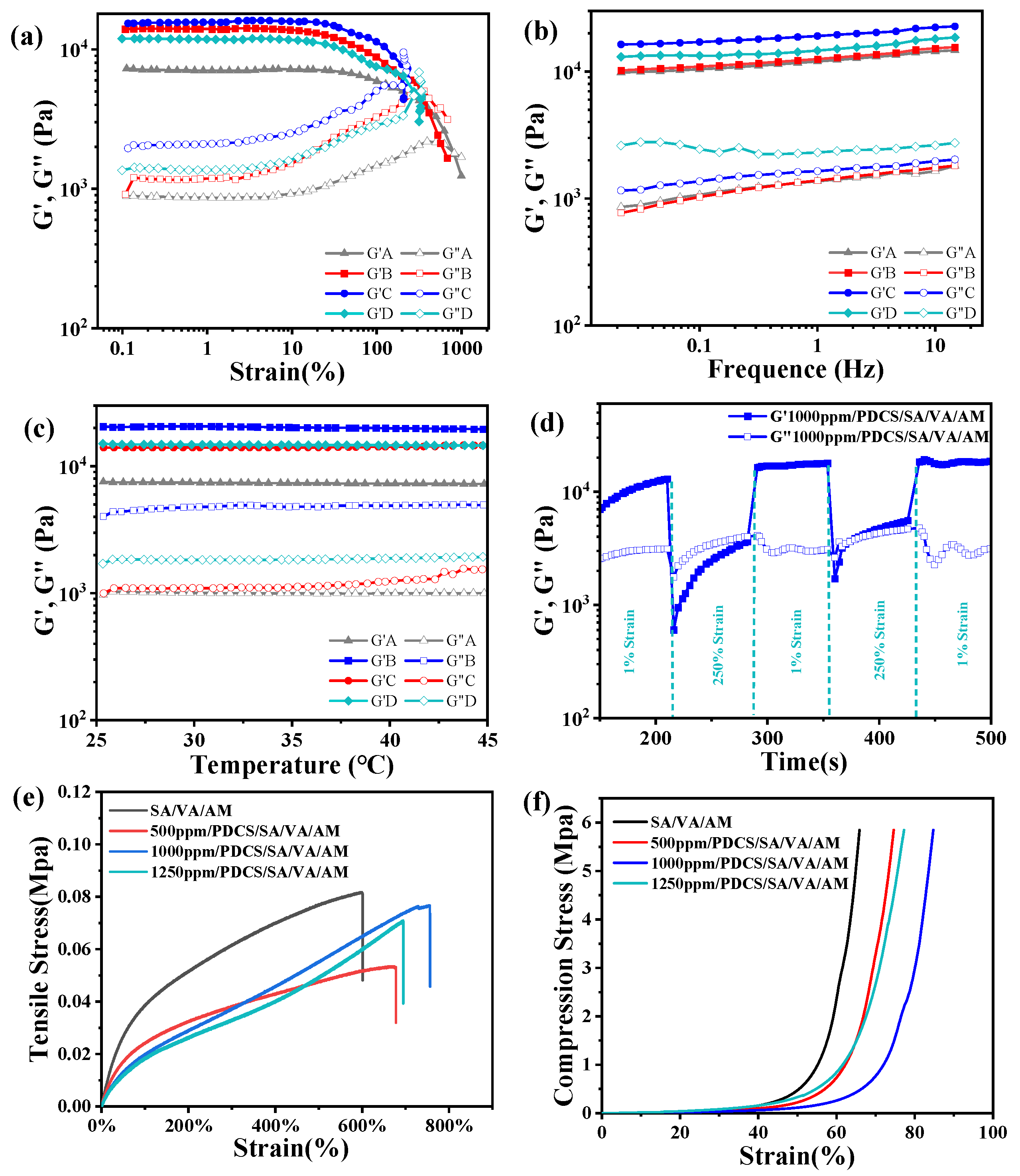
3.1. Design Rationale and Characterization of PDCS/SA/VA/AM Hydrogel
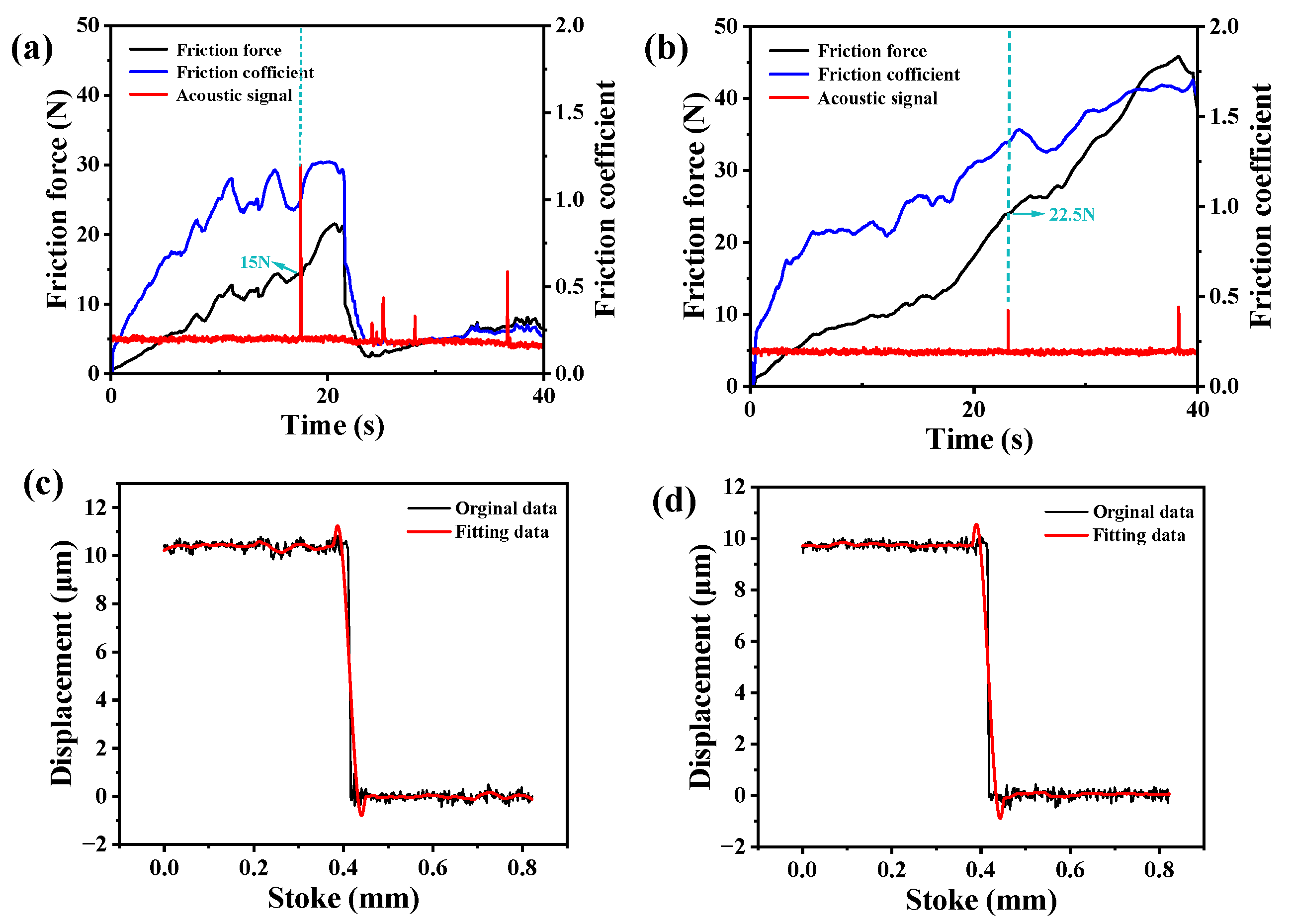
3.2. Bonding Strength and Film Thickness Test of Hydrogels
3.3. Mechanics Performance Testing
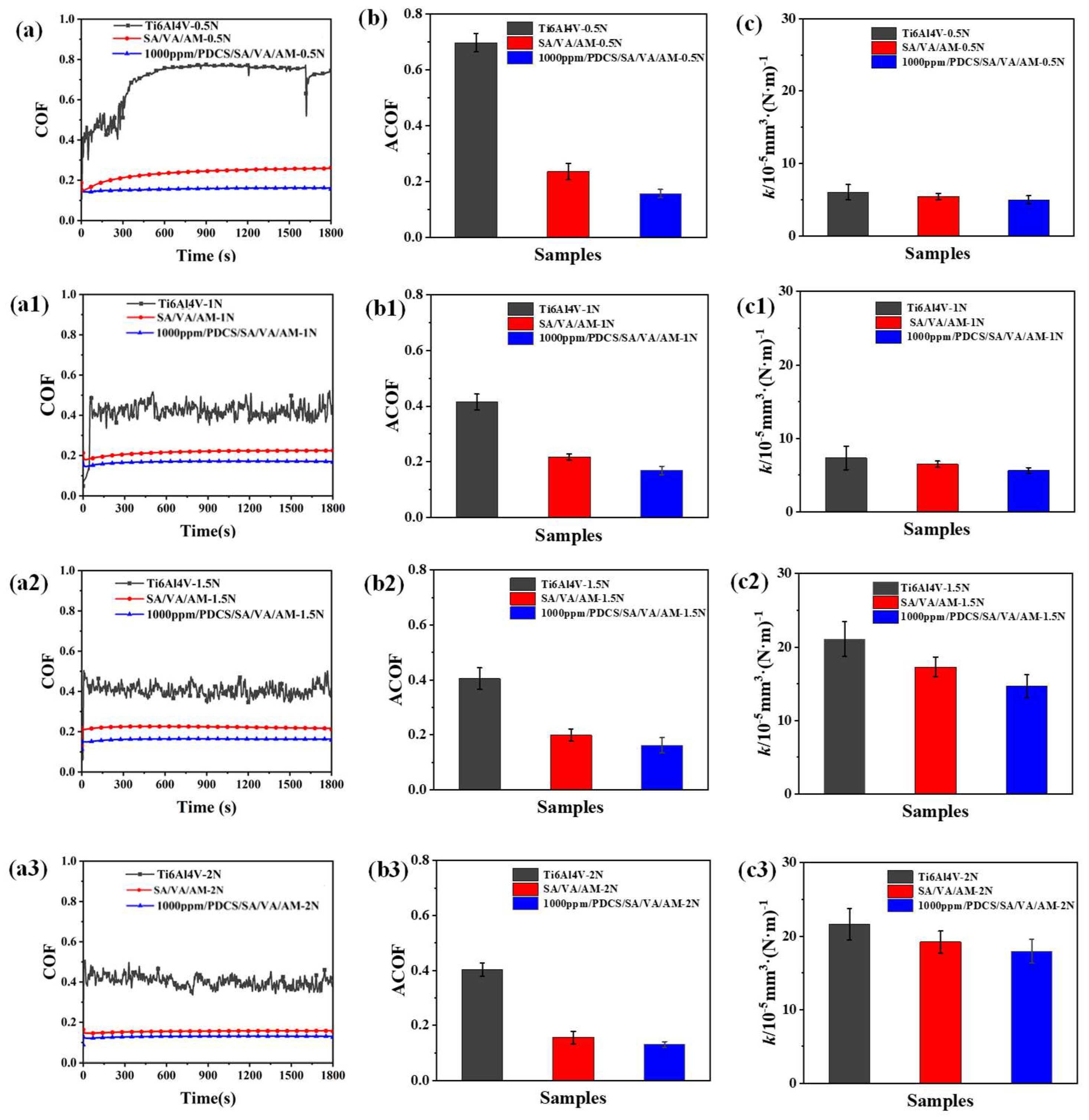
3.4. Biotribological Response Under Different Loads
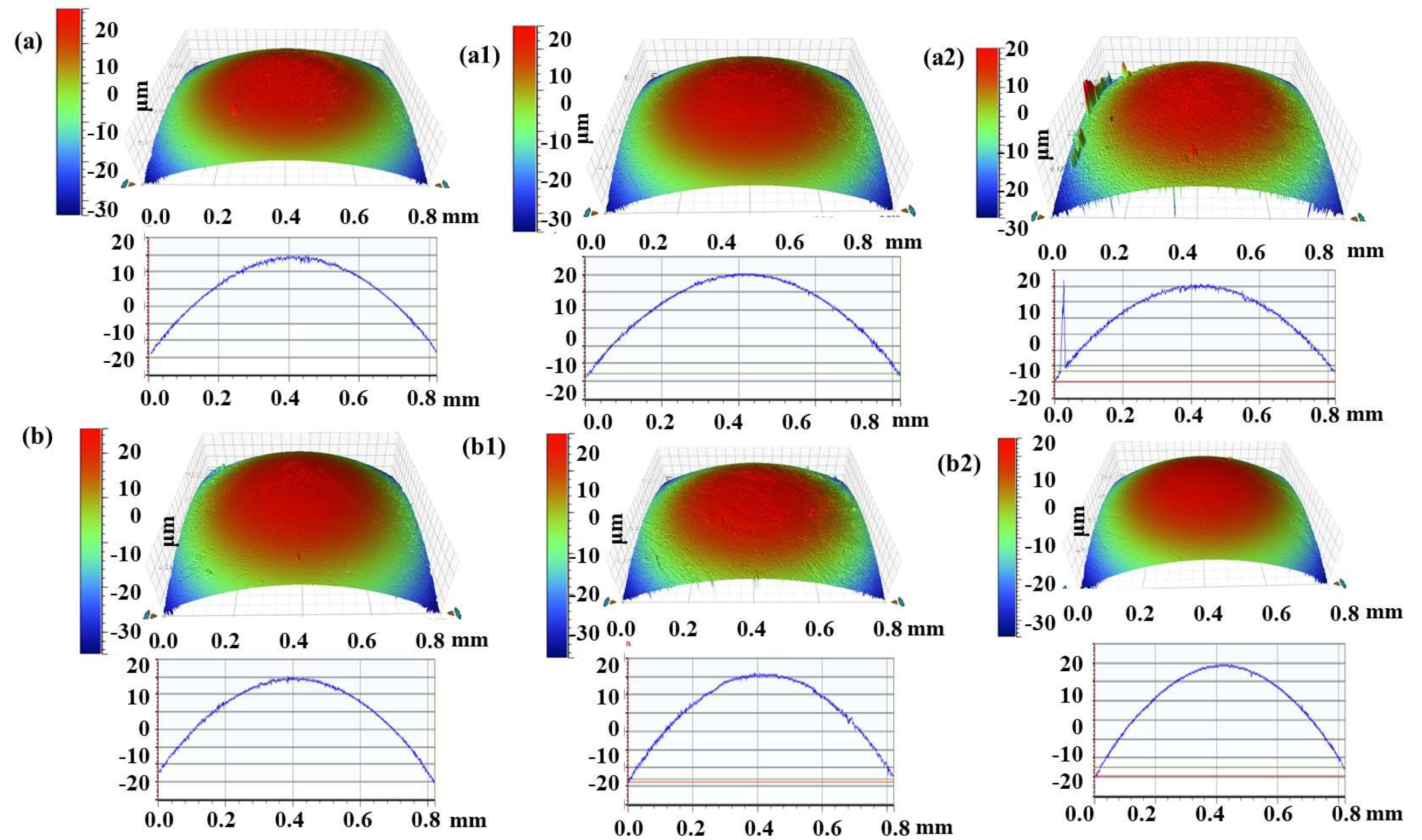
3.5. Wear Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Zhang, X.; Liu, S.; Chen, K.; Feng, C.; Li, X.; Qi, J.; Luo, Y.; Liu, H.; Zhang, D. Cartilage-bone inspired the construction of soft-hard composite material with excellent interfacial binding performance and low friction for artificial joints. Friction 2022, 11, 1177–1193. [Google Scholar] [CrossRef]
- Cui, L.; Chen, J.; Yan, C.; Xiong, D. Articular Cartilage Inspired the Construction of LTi–DA–PVA Composite Structure with Excellent Surface Wettability and Low Friction Performance. Tribol. Lett. 2021, 69, 41. [Google Scholar] [CrossRef]
- Kanca, Y.; Milner, P.; Dini, D.; Amis, A.A. Tribological properties of PVA/PVP blend hydrogels against articular cartilage. J. Mech. Behav. Biomed. Mater. 2018, 78, 36–45. [Google Scholar] [CrossRef]
- Taheridoustabad, I.; Khosravi, M.; Yaghoubinezhad, Y. Fabrication of GO/RGO/TiC/TiB2 nanocomposite coating on Ti–6Al–4V alloy using electrical discharge coating and exploring its tribological properties. Tribol. Int. 2021, 156, 106860. [Google Scholar] [CrossRef]
- Alvi, S.; Neikter, M.; Antti, M.-L.; Akhtar, F. Tribological performance of Ti6Al4V at elevated temperatures fabricated by electron beam powder bed fusion. Tribol. Int. 2021, 153, 106658. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Li, X.; Zhang, X.; Gong, X.; Zhu, Y.; Ren, Z.; Zhang, B.; Cheng, J. Biocompatibility and osseointegration properties of a novel high strength and low modulus beta- Ti10Mo6Zr4Sn3Nb alloy. Front. Bioeng. Biotechnol. 2023, 11, 1127929. [Google Scholar] [CrossRef]
- Qin, M.; Yuan, W.; Zhang, X.; Cheng, Y.; Xu, M.; Wei, Y.; Chen, W.; Huang, D. Preparation of PAA/PAM/MXene/TA hydrogel with antioxidant, healable ability as strain sensor. Colloids Surf. B Biointerfaces 2022, 214, 112482. [Google Scholar] [CrossRef]
- Park, J.; Kim, T.Y.; Kim, Y.; An, S.; Kim, K.S.; Kang, M.; Kim, S.A.; Kim, J.; Lee, J.; Cho, S.W.; et al. A Mechanically Resilient and Tissue-Conformable Hydrogel with Hemostatic and Antibacterial Capabilities for Wound Care. Adv. Sci. 2023, 10, e2303651. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Wang, J.; Kang, Y.; Wang, Z.; Cao, W.; Ye, J.; Gao, C. A tough, antibacterial and antioxidant hydrogel dressing accelerates wound healing and suppresses hypertrophic scar formation in infected wounds. Bioact. Mater. 2024, 34, 269–281. [Google Scholar] [CrossRef]
- Talodthaisong, C.; Patramanon, R.; Thammawithan, S.; Lapmanee, S.; Maikaeo, L.; Sricharoen, P.; Khongkow, M.; Namdee, K.; Jantimaporn, A.; Kayunkid, N.; et al. A Shear-Thinning, Self-Healing, Dual-Cross Linked Hydrogel Based on Gelatin/Vanillin/Fe3+ /AGP-AgNPs: Synthesis, Antibacterial, and Wound-Healing Assessment. Macromol. Biosci. 2023, 23, e2300250. [Google Scholar] [CrossRef]
- Yasar, M.; Oktay, B.; Dal Yontem, F.; Haciosmanoglu Aldogan, E.; Kayaman Apohan, N. Development of self-healing vanillin/PEI hydrogels for tissue engineering. Eur. Polym. J. 2023, 188, 111933. [Google Scholar] [CrossRef]
- Iftime, M.M.; Rosca, I.; Sandu, A.I.; Marin, L. Chitosan crosslinking with a vanillin isomer toward self-healing hydrogels with antifungal activity. Int. J. Biol. Macromol. 2022, 205, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, C.; Li, Z.; Gu, Z.; Yang, J.; Luo, K. Chitosan-based hydrogel dressings for diabetic wound healing via promoting M2 macrophage-polarization. Carbohydr. Polym. 2024, 331, 121873. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, J.; Koshut, W.J.; Watt, J.; Riboh, J.C.; Gall, K.; Wiley, B.J. A Synthetic Hydrogel Composite with the Mechanical Behavior and Durability of Cartilage. Adv. Funct. Mater. 2020, 30, 2003451. [Google Scholar] [CrossRef]
- Ni, Y.; Chen, J.; Chen, K. Flexible vanillin-polyacrylate/chitosan/mesoporous nanosilica-MXene composite film with self-healing ability towards dual-mode sensors. Carbohydr. Polym. 2024, 335, 122042. [Google Scholar] [CrossRef]
- Cui, J.; Tian, Y.; Zhang, B.; Zhang, R.; Zhao, X.; Li, J.; Chen, L. Injectable antibacterial hydrogels based on oligolysines for wound healing. Biomater. Adv. 2024, 164, 213981. [Google Scholar] [CrossRef]
- Xu, C.; Zhan, W.; Tang, X.; Mo, F.; Fu, L.; Lin, B. Self-healing chitosan/vanillin hydrogels based on Schiff-base bond/hydrogen bond hybrid linkages. Polym. Test. 2018, 66, 155–163. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, Y.; Kong, X.; Li, Y.; Cao, S.; Hu, W.; Zhang, G.; Wang, C. Improved Tribological Properties of Epoxy Cement Reinforced with Impact-Resistant Core-Shell Structured Polymer Nanoparticles. Lubricants 2024, 12, 267. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, K.; Gao, Y.; Han, S.; Ju, J.; Ren, T.; Zhao, X. Multifunctional GO-based hydrogel coating on Ti-6Al-4 V Alloy with enhanced bioactivity, anticorrosion and tribological properties against cortical bone. Tribol. Int. 2023, 184, 108423. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, W.; Jiang, H.; Xiang, Z.; Li, J.; Cao, D.; Zeng, J.; Wang, B.; Xu, J. Sensitive and Stable Detection of Pesticide Residues Using Flexible 3D Nanocellulose-Based SERS Substrates. J. Agric. Food Chem. 2025, 73, 8026–8039. [Google Scholar] [CrossRef]
- Wang, W.; Han, S.; Ren, J.; Xiao, X.; Chen, J.; You, R.; Zhang, G.; Lu, Y. Flexible 2D S-CNF/Au NSs substrate for detection of malondialdehyde in serum of gastric cancer patients. Cellulose 2024, 31, 3717–3728. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z. Tailoring the Swelling-Shrinkable Behavior of Hydrogels for Biomedical Applications. Adv. Sci. 2023, 10, e2303326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, J.; Xu, J.; Zhang, L.; Luo, Y. Preparation and biotribological properties of PSBMA polyelectrolyte brush on PEEK surface. J. Appl. Polym. Sci. 2023, 140, e54698. [Google Scholar] [CrossRef]
- Mahaninia, M.H.; Wang, Z.; Rajabi-Abhari, A.; Yan, N. Self-healing, flame-retardant, and antimicrobial chitosan-based dynamic covalent hydrogels. Int. J. Biol. Macromol. 2023, 252, 126422. [Google Scholar] [CrossRef]
- Suneetha, M.; Hemalatha, D.; Kim, H.; Rao, K.; Han, S.S. Vanillin/fungal-derived carboxy methyl chitosan/polyvinyl alcohol hydrogels prepared by freeze-thawing for wound dressing applications. Int. J. Biol. Macromol. 2024, 266, 130910. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Cheng, M.; Hu, L.; Zhang, Z.; Sun, Q.; Wang, S.; Fan, Y.; Pan, P.; Chen, J. Self-Healing Conductive Hydrogels with Dynamic Dual Network Structure Accelerate Infected Wound Healing via Photothermal Antimicrobial and Regulating Inflammatory Response. ACS Appl. Mater. Interfaces 2024, 16, 30776–30792. [Google Scholar] [CrossRef]
- Yang, K.; Yang, J.; Chen, R.; Dong, Q.; Yang, H.; Gu, S.; Zhou, Y. Antibacterial hyaluronic acid hydrogels with enhanced self-healing properties via multiple dynamic bond crosslinking. Int. J. Biol. Macromol. 2024, 256, 128320. [Google Scholar] [CrossRef]
- Qin, Y.; Mo, J.; Liu, Y.; Zhang, S.; Wang, J.; Fu, Q.; Wang, S.; Nie, S. Stretchable Triboelectric Self-Powered Sweat Sensor Fabricated from Self-Healing Nanocellulose Hydrogels. Adv. Funct. Mater. 2022, 32, 2201846. [Google Scholar] [CrossRef]
- Lai, Y.; Kuang, X.; Zhu, P.; Huang, M.; Dong, X.; Wang, D. Colorless, Transparent, Robust, and Fast Scratch-Self-Healing Elastomers via a Phase-Locked Dynamic Bonds Design. Adv. Mater. 2018, 30, e1802556. [Google Scholar] [CrossRef]
- Cheng, L.; Zhao, X.; Zhao, J.; Liu, S.; Yu, W. Structure and Dynamics of Associative Exchange Dynamic Polymer Networks. Macromolecules 2022, 55, 6598–6608. [Google Scholar] [CrossRef]
- Martorana, A.; Lenzuni, M.; Contardi, M.; Palumbo, F.S.; Cataldo, S.; Pettignano, A.; Catania, V.; Schillaci, D.; Summa, M.; Athanassiou, A.; et al. Schiff Base-Based Hydrogel Embedded with In Situ Generated Silver Nanoparticles Capped by a Hyaluronic Acid-Diethylenetriamine Derivative for Wound Healing Application. ACS Appl. Mater. Interfaces 2024, 16, 20186–20201. [Google Scholar] [CrossRef]
- Li, F.; Liu, T.; Liu, X.; Han, C.; Li, L.; Zhang, Q.; Sui, X. Ganoderma lucidum polysaccharide hydrogel accelerates diabetic wound healing by regulating macrophage polarization. Int. J. Biol. Macromol. 2024, 260, 129682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, X.L.; Yang, Q.; Guo, Y.; Cui, Y.; Xiang, Y.; Hu, B.; Wei, J.; Tu, P. In situ forming of PEG-NH2/dialdehyde starch Schiff-base hydrogels and their application in slow-release urea. Int. J. Biol. Macromol. 2024, 256, 128355. [Google Scholar] [CrossRef]
- Ge, M.; Zhang, L. Ultrastretchable hydrogels with strong damping effects. Polym. J. 2024, 56, 599–607. [Google Scholar] [CrossRef]
- Ye, C.; Wei, C.; Liu, J.; Wong, T.H.; Liu, X.; Song, Z.; Wu, C.; Li, Z.; Lin, S. Mechano-diffusion of particles in stretchable hydrogels. Soft Matter 2025, 21, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Z.; Li, H. Ultrastretchable Luminescent Nanocomposite Hydrogel with Self-Healing Behavior. ACS Appl. Polym. Mater. 2022, 4, 2329–2336. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, J.; Gui, Y.; Lv, Y.; Feng, H.; Zhao, X.; Qiu, J.; Ma, X.; Yang, Y. Highly stretchable, stable MXene-based hydrogel for human motion monitoring. Mater. Today Commun. 2024, 38, 108240. [Google Scholar] [CrossRef]
- Johnson, C.L.; Dunn, A.C. Tribological Characterization of Gradient-density Polyacrylamide Hydrogel Surfaces. Exp. Mech. 2021, 61, 829–842. [Google Scholar] [CrossRef]
- Feng, H.; Wang, S.; Chen, K.; Zhang, X.; Feng, C.; Li, X.; Zhang, D. Dual-network nanocomposite robust hydrogel with excellent durability properties as cartilage replacement. Tribol. Int. 2024, 194, 109518. [Google Scholar] [CrossRef]
- Rong, M.; Liu, H.; Scaraggi, M.; Bai, Y.; Bao, L.; Ma, S.; Ma, Z.; Cai, M.; Dini, D.; Zhou, F. High Lubricity Meets Load Capacity: Cartilage Mimicking Bilayer Structure by Brushing Up Stiff Hydrogels from Subsurface. Adv. Funct. Mater. 2020, 30, 2004062. [Google Scholar] [CrossRef]
- Van Meter, K.E.; Pitenis, A.A.; Harris, K.L.; Sawyer, W.G.; Krick, B.A. Contact pressure dependent mechanisms of ultralow wear PTFE composites. Wear 2023, 522, 204715. [Google Scholar] [CrossRef]
- Xu, D.; Harvey, T.; Martínez, J.; Begiristain, E.; Domínguez-Trujillo, C.; Sánchez-Abella, L.; Browne, M.; Cook, R.B. Mechanical and tribological characterisations of PEG-based hydrogel coatings on XLPE surfaces. Wear 2023, 522, 204699. [Google Scholar] [CrossRef]
- Li, Y.; Tian, P.; Cao, H.; Wang, Y.; Zhao, X.; Han, S.; Wang, C. Remarkable enhancement of corrosion resistance and tribological properties of chitosan-MXene based hydrogel coating on the surface of Ti6Al4V alloy. Tribol. Int. 2024, 192, 109229. [Google Scholar] [CrossRef]
- Gombert, Y.; Simič, R.; Roncoroni, F.; Dübner, M.; Geue, T.; Spencer, N.D. Structuring Hydrogel Surfaces for Tribology. Adv. Mater. Interfaces 2019, 6, 1901320. [Google Scholar] [CrossRef]
- Zhao, Y.; Yi, G.; Cui, J.; Zhao, Z.; Yan, Y.; Wei, L.; Shao, J.; Zeng, H.; Huang, J. Probing the intriguing frictional behavior of hydrogels during alternative sliding velocity cycles. Friction 2023, 11, 2329–2341. [Google Scholar] [CrossRef]
- Huang, S.; Wang, B.; Zhao, X.; Li, S.; Liang, X.; Zeng, R.; Li, W.; Wang, X. Phospholipid reinforced P(AAm-co-AAc)/Fe3+ hydrogel with ultrahigh strength and superior tribological performance. Tribol. Int. 2022, 168, 107436. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Zhao, X.; Ji, Z.; Ma, Z.; Gao, X.; Ma, S.; Wang, X.; Zhou, F. Bioinspired Design of a Cartilage-like Lubricated Composite with Mechanical Robustness. ACS Appl. Mater. Interfaces 2022, 14, 9899–9908. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, B.; Li, D.; Yuan, W.; Huang, Y.; Hu, Z. Catechol-modified epoxy backbones for multifunctional and ultra-tough thermoset. Chem. Eng. J. 2023, 455, 140889. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, M.; Chen, K.; Sun, H.; Xing, H.; Liu, X.; Liu, W.; Guo, H. MXene-Based Pressure Sensor with a Self-Healing Property for Joule Heating and Friction Sliding. Small 2024, 20, e2400593. [Google Scholar] [CrossRef]
- da Silva, R.; Bernardinelli, O.D.; Frachini, E.C.G.; Ulrich, H.; Sabadini, E.; Petri, D.F.S. Vanillin crosslinked chitosan films: The states of water and the effect of carriers on curcumin uptake. Carbohydr. Polym. 2022, 292, 119725. [Google Scholar] [CrossRef]
- Song, G.; Zhao, Z.; Peng, X.; He, C.; Weiss, R.A.; Wang, H. Rheological Behavior of Tough PVP-in Situ-PAAm Hydrogels Physically Cross-Linked by Cooperative Hydrogen Bonding. Macromolecules 2016, 49, 8265–8273. [Google Scholar] [CrossRef]
- Zou, J.; Wang, G.; Tang, Y.; Jing, X. Investigation on the Dynamic Rheological Behavior of the Highly Elastic Organohydrogel. Ind. Eng. Chem. Res. 2023, 62, 21802–21810. [Google Scholar] [CrossRef]
- Cho, Y.E.; Park, J.M.; Song, W.J.; Lee, M.G.; Sun, J.Y. Solvent Engineering of Thermo-Responsive Hydrogels Facilitates Strong and Large Contractile Actuations. Adv. Mater. 2024, 36, e2406103. [Google Scholar] [CrossRef]
- Liang, C.; Dudko, V.; Khoruzhenko, O.; Hong, X.; Lv, Z.P.; Tunn, I.; Umer, M.; Timonen, J.V.I.; Linder, M.B.; Breu, J.; et al. Stiff and self-healing hydrogels by polymer entanglements in co-planar nanoconfinement. Nat. Mater. 2025, 24, 599–606. [Google Scholar] [CrossRef]
- Wang, Z.J.; Li, W.; Li, X.; Nakajima, T.; Rubinstein, M.; Gong, J.P. Rapid self-strengthening in double-network hydrogels triggered by bond scission. Nat. Mater. 2025, 24, 607–614. [Google Scholar] [CrossRef]
- Song, X.; Man, J.; Qiu, Y.; Wang, J.; Liu, J.; Li, R.; Zhang, Y.; Li, J.; Li, J.; Chen, Y. High-density zwitterionic polymer brushes exhibit robust lubrication properties and high antithrombotic efficacy in blood-contacting medical devices. Acta Biomater. 2024, 178, 111–123. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Avestro, A.-J.; McGonigal, P.R.; Zhang, H. Supramolecular repair of hydration lubrication surfaces. Chem 2022, 8, 480–493. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hu, W.; Gao, Q.; Yan, J.; Wang, G.; Han, S.; Wang, C.; Hou, X. Multi-Modal Mechanical Response of Self-Healing Double-Network Hydrogel Coatings Based on Schiff Base Bond. Coatings 2025, 15, 552. https://doi.org/10.3390/coatings15050552
Li Y, Hu W, Gao Q, Yan J, Wang G, Han S, Wang C, Hou X. Multi-Modal Mechanical Response of Self-Healing Double-Network Hydrogel Coatings Based on Schiff Base Bond. Coatings. 2025; 15(5):552. https://doi.org/10.3390/coatings15050552
Chicago/Turabian StyleLi, Yanan, Wenbin Hu, Qike Gao, Jincan Yan, Guan Wang, Sheng Han, Chenchen Wang, and Xiaozheng Hou. 2025. "Multi-Modal Mechanical Response of Self-Healing Double-Network Hydrogel Coatings Based on Schiff Base Bond" Coatings 15, no. 5: 552. https://doi.org/10.3390/coatings15050552
APA StyleLi, Y., Hu, W., Gao, Q., Yan, J., Wang, G., Han, S., Wang, C., & Hou, X. (2025). Multi-Modal Mechanical Response of Self-Healing Double-Network Hydrogel Coatings Based on Schiff Base Bond. Coatings, 15(5), 552. https://doi.org/10.3390/coatings15050552







