Abstract
The growing concern over the transmission of pathogens, particularly in high-risk environments such as healthcare facilities and public spaces, necessitates the development of effective and sustainable antimicrobial solutions. Traditional coatings often rely on metals, which despite their efficacy, pose significant environmental and economic challenges. This study explores the potential of bio-based alkyd resins, supplemented with natural antimicrobial bioadditives, as an eco-friendly alternative to traditional antibacterial and antiviral coatings. Specifically, alkyd formulations incorporating thymol and soft resins extracted from hops were evaluated for antimicrobial and antiviral efficacy, following ISO standards (ISO 22196:2007 and ISO 21702:2019, respectively). The coating formulations showed significant activity against Gram-negative (Escherichia coli) and Gram-positive (Staphylococcus aureus), and Influenza A (H3N2) virus, proving their potential for mitigating pathogen spread. These bio-based coatings not only reduce reliance on harmful chemicals but also align with circular economy principles by repurposing industrial by-products. This innovative approach represents a significant step toward greener antimicrobial technologies, with broad applications in healthcare, public infrastructure, and beyond, especially considering the rising zoonotic disease outbreaks.
1. Introduction
The discovery of microorganisms by Robert Hooke and Antoni van Leeuwenhoek in the mid-17th century marked a pivotal point in scientific history. Inconceivable at that point, they laid the groundwork for modern microbiology while underscoring the significant impact of microorganisms on human history [1]. Over the centuries, pathogens have driven major historical events. The most recent example is the COVID-19 pandemic caused by SARS-CoV-2, which resulted in over 74 million cases by the end of 2020 [2]. This pattern of pathogen-driven crises underscores the critical need to control microbial spread. Since the 1970s, over 1500 new pathogens have been identified, some of which have significantly impacted global public health, including MERS-CoV, Zaire ebolavirus, and Zika virus. These examples highlight the persistent threat of emerging infectious diseases and the necessity for vigilance in combating microbial threats [3].
Recently, disinfection methods have played a crucial role in controlling local transmissions [3]. Although the primary mode of transmission during the COVID-19 pandemic was through person-to-person contact with an infected individual, the World Health Organization (WHO) emphasised the potential for surface-mediated transmission of pathogens [4,5], as some bacterial and viral pathogens can persist on surfaces from hours to days [3,6]. This is particularly concerning in hospital environments, where surfaces such as door handles, washbasins, and elevator buttons have been identified as potential reservoirs for nosocomial pathogens such as Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, or Streptococcus pneumoniae [7,8,9,10,11,12]. In fact, a study conducted across 27 intensive care units using a fluorescent tracer revealed that standard cleaning protocols disinfected only 48.1% of all surfaces. Alarmingly, common touch points such as room doorknobs (25%) and bathroom light switches (26%) were even less thoroughly cleaned, highlighting critical gaps in current disinfection practices [13]. Given that disinfecting these surfaces is laborious, costly, and often inefficient, the development of antimicrobial coatings has emerged in recent years as a promising alternative, with potential applications in diverse settings such as healthcare facilities, public transport, and educational institutions [6,14,15].
In simple terms, antimicrobial coatings operate through two different, but not mutually exclusive, strategies: (i) passive surfaces that prevent microorganism adhesion, such as anti-biofouling surfaces [16] or (ii) active surfaces with intrinsic antimicrobial activity [3,17]. However, according to the data provider company BCC Research (https://www.bccresearch.com/market-research/chemicals/antimicrobial-coating-market.html, accessed on 25 January 2025), most traditional coatings contain environmentally harmful metals like silver and copper, which, although effective, are not sustainable both environmentally and economically [3]. As a result, the increasing demand for eco-friendly materials has driven interest in alternative coatings derived from renewable sources, such as lignin, cellulose, and vegetable oils [18,19,20].
The transition toward a bio-based circular economy has brought significant innovation to the polymer and coating industries. Plastics, indispensable in modern life, are largely produced from petroleum-based feedstocks. However, growing emphasis on reducing carbon footprints and achieving sustainable production has catalysed a shift toward renewable raw materials. In the surface coating industry, this trend has prioritised the development of polymer-based products to minimise dependence on petrochemicals [21,22].
Alkyd resins, synthesised from vegetable oils, represent a cornerstone of eco-friendly coatings due to their excellent adhesion, durability, and gloss [23,24]. First produced in the 1920s by Kienle, and subsequently commercialised in the 1930s by General Electric, alkyd resins are part of a broader class of modified condensation polymers known as polyesters [21,22]. They are widely employed as binders in architectural, marine, and industrial coatings, offering low cost, versatility, and high performance, including resistance to ageing, weather, chemicals, and heat. These attributes, combined with their adaptability to chemical modifications, have established alkyd resins as a dominant material in the paint and coating industries alongside acrylic, epoxy, and polyurethane resins [21,22,25]. Currently, global production of alkyd resins exceeds 200,000 tons annually, with market projections estimating growth to USD 5.3 billion by 2030 [25] or even more [21].
Additionally, alkyd-based systems offer a versatile platform for incorporating different additives to achieve new and desirable traits [21,22]. One of the earliest examples of the alkyd hybrid system was the synthesis of silicone–alkyd resin, first reported in 1947. Subsequently, urethane hybridisation was developed to enhance the drying time of alkyd resins while improving abrasion resistance, toughness, chemical durability, and UV resistance [22]. More recently, advances have focused on the incorporation of antimicrobial agents, presenting an innovative approach to reducing surface-mediated pathogen transmission [23,26,27,28]. One promising additive is thymol (Figure 1) (also referred as 2-isopropyl-5-methylphenol or IPMP), a monoterpenoid phenol from thyme oil. Thymol is a white crystalline substance with antiseptic, antibacterial, antifungal, and antiviral properties, making it suitable for antimicrobial applications. Toxicological studies indicate that thymol is safe and non-genotoxic [29,30]. Furthermore, recent studies conducted by our group have demonstrated the antimicrobial properties of hop soft resins (data under publication), a fraction obtained from hop extraction that contains bitter acids such as humulones and lupulones (Figure 1). These compounds are well documented for their effectiveness against both Gram-positive and Gram-negative bacteria, as well as select actinomycetes and yeast and fungal species [31].
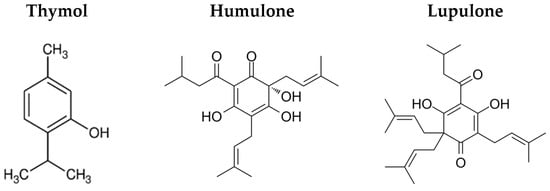
Figure 1.
Molecular structures of thymol, humulone, and lupulone.
Despite their extensive use, the integration of lignin into alkyd resins remains underexplored [25], even when this approach offers a dual opportunity: increasing the bio-based content of alkyd resins while utilising lignin as a renewable resource to develop more sustainable coatings.
This study aims to develop sustainable antimicrobial coatings based on alkyd resins by incorporating bio-based antimicrobial additives such as thymol and soft hop resins, as alternatives to conventional metal-based substances. Additionally, we investigate the incorporation of lignin into alkyd resins to further increase the bio-based content of these coatings. It is hypothesised that these modifications will not only improve the antimicrobial performance of the coatings but also contribute to reducing pathogen transmission through surfaces. By aligning with the growing demand for bio-based materials, this approach contributes to the advancement of the circular bioeconomy and offers a promising strategy for the development of more sustainable antimicrobial coatings.
2. Materials and Methods
2.1. Materials and Strains
Two bioadditives were selected as antimicrobial agents: (i) thymol (CAS 89-83-8, BORDAS, Sevilla, Spain) and (ii) hop soft resins extracted from the Nugget variety (Órbigo Valley S.L., Villamor de Órbigo, León, Spain) using an optimised sequential extraction procedure [32].
The antimicrobial activity of these bioadditives was tested on two resins: (i) a commercial alkyd resin (Resydrol AY 6150w, ALLNEX, solids 46.5%, Biocontent 35%, Werndorf, Austria) and (ii) a bio-alkyd resin synthetised from lignin by our group (V004/2023-80%EtOH, solids 47.6%, Biocontent 52%, BARPIMO Coatings, Nájera, Spain) [25].
The final paint formulations included the following additives: (i) cobalt drier (Additol VXW 6206), (ii) anti-skinning (Additol XL 297), (iii) wetting agent (Additol VXW 6503), (iv) dispersing additive (Additol VXW 6208), (v) VOC-free defoamer additive (Additol VXW 6393), (vi) levelling additive (Modaflow AQ 3025), and (vii) anti-foam air releaser (Additol VXW 4973), all from ALLNEX (Germany, Werndorf, Austria). Additionally, a VOC-free thickener or rheological modifier (Acrysol RM 5000, DOW, Shwalbach A.T., Germany), titanium dioxide (TiO2, Kronos 2300, TiO2 content ≥94%, KRONOS, Leverkusen, Germany) for pigmentation (white colour and opacity), and 30% ammonia solution (SCHARLAU, Cham, Germany) for pH adjustment (recommended pH > 8) were incorporated into the final formulations.
For antimicrobial efficacy testing, two bioindicator bacterial strains (E. coli CECT 515 and S. aureus CECT 239, both from Colección Española de Cultivos Tipo, Paterna, Spain), as well as the Influenza A (H3N2) virus (Wesel, Germany) (strain A/Hong Kong/8/68, propagated in MDCK cells (Wesel, Germany), a canine kidney cell line) were employed.
2.2. Paint Formulation
The initial stage of paint formulation involved preparing two water-based coatings: (i) A-REF, derived from the commercial alkyd resin (based on ALLNEX’s guide formulation) and (ii) BA-REF, based on the synthesised bio-alkyd resin [25]. Each formulation incorporated only the resin and essential additives to ensure homogeneous mixtures (Table 1). To impart antimicrobial properties to the final products, bioadditives were subsequently introduced.

Table 1.
Base paints formulations for both commercial alkyd and bio-alkyd resins.
The mixing process was conducted using a DISPERMAT® CV3 Plus mechanical stirrer (VMA-GETZMANN GmbH, Reichshof, Germany) at 2000 rpm for 50 min at room temperature (RT). The solid content was determined by measuring the total mass of solids in a 100 g sample of paint.
After preparing the base paints (Table 1), antimicrobial bioadditives (thymol or soft hop resins) were incorporated to create antimicrobial formulations (Table 2). Thymol (T) was added at concentrations of 0.01%, 0.03%, 0.05%, and 0.10%, while soft hop resins (H) were added at 0.15% and 0.25%.

Table 2.
Antimicrobial paint formulations.
2.3. Application and Physical Characterisation of Paints
The paints were applied to various substrates, including glass, B/N Leneta charts and stainless steel. Multiple tests were conducted to evaluate properties such as adhesion, colour, gloss, hardness, water resistance, and contact angle.
Viscosity (in centipoise, cP) was determined using a Brookfield DV-II+ Pro Viscometer (Brookfield Engineering Laboratories Inc., Middleboro, MA, USA) at 25 °C and 100 rpm, with spindle sizes of #4 and #5 selected for accuracy.
Paint colour was assessed by comparing the films on substrates with the original substrate colour. Colorimetric measurements based on the CIE colour system (Equation (1)) were performed with a Konica Minolta CM-2600d spectrophotometer (Osaka, Japan). Films were applied on Leneta test charts (Neurtek Instruments, Eibar, Spain) with a 120 μm Baker Applicator using an ATX Automatic Applicator (Neurtek Instruments, Eibar, Spain) at 40 mm/min; this was followed by a two-week drying period. Each analysis was based on colour measurements compared to a white calibration plate, with the corresponding average and standard deviation derived from ten individual measurements automatically performed by the spectrophotometer.
where L* refers to lightness, a* refers to red–green chromatic coordinates, and b* refers to yellow–blue chromatic coordinates [33,34]. According to this system, the colour difference is considered undetectable by the human eye when the ΔE*ab (D65) is smaller than 2. Colour was measured on the white part of the films and compared to the white calibration reference.
Gloss was measured using a BYK GARDNER micro-TRI-gloss glossmeter (Geretsried, Germany), compatible with 20°, 60°, and 85° geometries, according to ISO 2813:2014 [35]. An incident angle of 60° was used initially; if gloss values exceeded 70 units, the angle was adjusted to 20°, and for values below 10 units, it was set to 85°. Paint films were applied at a thickness of 120 μm on dark glass and allowed to dry for two weeks at RT. The results are averages of ten measurements.
Persoz pendulum hardness was assessed using a TQC Sheen Pendulum Hardness Tester (Capelle aan den IJssel, the Netherlands), in line with the EN ISO 1522:2022 standard [36]. This test measures the number of oscillations before the pendulum comes to rest; higher oscillation counts indicate greater hardness. Samples were prepared with a 120 μm paint layer on dark glass and dried for one month at RT, with results averaged from three replicates.
Adhesion on stainless steel substrates (without and with primer) was evaluated using a cross-cut test, according to EN ISO 2409:2020 [37]. A 9 mm wide NT edge cutter, 25 mm wide adhesive tape, and a ZCT 2160.2 cross-cut template (Zehntner GmbH Testing Instruments, Sissach, Switzerland) were used. A single 120 µm wet paint layer was applied to the metal, with samples dried for two weeks at RT.
Sample surface morphology was examined using a JEOL IT700HR field-emission scanning electron microscope, revealing detailed topographical features and providing high-resolution images essential for analysing the material’s microstructure (FE-SEM, Tokyo, Japan). Non-conductive samples were coated with a ~20 nm gold layer using a cathodic sputter coater to improve conductivity and image quality. Samples were prepared by applying a 120 μm paint layer on dark glass for assessment of levelling and general film appearance.
Water resistance was evaluated by placing a cotton pad with 1 mL of water on the paint film, covered by a watch glass for 24 h. Changes in the coatings were observed at two intervals: (i) after 24 h in water contact and (ii) after 24 h of recovery at RT. Paint films were applied to B/N test charts with a 120 μm Baker Applicator at 40 mm/min and dried for two weeks at RT.
Water contact angle (CA) was determined using a DataPhysics OCA 15 Plus goniometer (Filderstadt, Germany), with image analysis performed by DataPhysics Instruments GmbH software (Version 3.11.11 Build 160). Paints were applied on metal substrates with a 120 μm Baker Applicator and dried for two weeks at RT. A 3 μL water droplet was used for each reading, and the results are averages of ten measurements. The evolution of the contact angle was determined at 0, 1, 3, and 6 min.
2.4. Antimicrobial Tests
The antimicrobial properties of the paints were evaluated with metal samples (50 mm2), coated with a 125 μm wire bar coater. The painted samples were air-dried for two weeks at RT and 50% ± 5% relative humidity. To ensure successful antimicrobial testing, it was necessary to apply a primer to enhance paint adhesion on the metal substrate, as initial testing showed detachment of the coating. Therefore, an acrylic primer (Oroprimer 446, supplied by IRURENA GROUP, Azpeitia, Spain) was applied using a 75 μm wire bar coater and left to dry for one day at RT.
The antibacterial activity of the coatings was evaluated in accordance with ISO 22196:2007 [38]. Each antimicrobial agent was incorporated separately into a reference alkyd paint (0.05% or 0.10% for thymol and 0.15% for soft hop resins) or a bio-alkyd paint (0.01%, 0.03%, or 0.05% for thymol and 0.15% or 0.25% for soft hop resins). The coated metal surfaces (50 ± 2 mm x 50 ± 2 mm) were then compared to untreated metal surfaces (negative control) in terms of antibacterial activity against E. coli CECT 515 and S. aureus CECT 239.
Pre-cultures of each bacterial strain were prepared by transferring a single colony from a stock culture to a screw-capped tube containing slant nutrient agar (NA, Condalab, Madrid, Spain) using a sterile inoculation loop. The cultures were incubated at 37 °C for 24 h. To prepare the test inoculum, a loopful of bacteria from the slant culture was transferred to a small volume of nutrient broth (NB, Condalab, Madrid, Spain), obtaining a bacterial concentration ranging from 2.5 × 105 to 10.0 × 105 cells/mL.
Subsequently, the metal sample (coated or untreated) was placed in a sterile Petri dish, and 400 µL of the test inoculum was added to its surface. The inoculum was then covered with a 40 × 40 mm film, the dish was sealed with its lid, and incubation was carried out at 37 °C and ~90% relative humidity for 24 h.
To determine the number of viable cells, the samples were recovered by washing the surface with 10 mL of sterile soybean casein digest broth supplemented with lecithin and polyoxyethylene sorbitan monooleate (SCDLP, Condalab, Madrid, Spain). To validate the bacterial recovery process, additional untreated metal surface samples were washed with 10 mL of SCDLP at 0 h (immediately after inoculum addition) and at 24 h.
The number of viable cells was determined by preparing 10-fold serial dilutions of the wash solutions in phosphate-buffered physiological saline (Thermo Fisher Scientific, Waltham, MA, USA). Then, 1 mL of each dilution was plated on Plate Count Agar (PCA, Biokar, Paris, France) using the pour plate method with 15 mL of molten medium. The plates were incubated at 37 °C for 24 h. All tests were performed in triplicate. The number of viable bacteria per cm2 (N) was calculated using Equation (2):
where C is the average plate count for the duplicate plates; D is the dilution factor; V is the volume (mL) of SCDLP added (10 mL); A is the surface area (mm2) of the cover film (in this case, 40 mm × 40 mm = 1600 mm2). In the event that no colonies were recovered in any of the Petri dishes for a dilution series, the number of viable bacteria was assumed to be V (10 mL), and the value of N was calculated based in that value.
The reduction in the number of viable bacteria (R) was calculated according to Equation (3):
where U0 is the average logarithm of the viable bacteria count from untreated samples (negative control) immediately after inoculation (0 h), UT is the average logarithm of the viable bacteria count from untreated samples (negative control) after 24 h, and AT is the average logarithm of the viable bacteria count from treated samples after 24 h. A reduction of two orders of magnitude (R ≥ 2) was considered indicative of a bactericidal effect.
R = (UT − U0) − (AT − U0) = UT − AT
2.5. Antiviral Bioassay
The antiviral activity of the coated samples was evaluated in accordance with ISO 21702:2019 [39], “Measurement of antiviral activity on plastics and other non-porous surfaces”. Metal-coated samples (5 cm2) were cut, sterilised using UV light, and then inoculated with a virus suspension. A total of six samples of the bio-alkyd formulation without bioadditives (BA-REF) were prepared, along with three samples containing 0.1% thymol (BA-T010) and three with 0.15% hop soft resin (BA-H015). Following application of the virus suspension, each sample was covered with a sterile 4 cm2 film (Seward, Stomacher) and incubated at 25 °C and > 90% relative humidity for 24 h.
MDCK cells were grown in Eagle’s Minimum Essential Medium (EMEM, Gibco, Paisley UK) supplemented with 10% foetal bovine serum (Sigma-Aldrich, Oslo, Norway) at 37 °C and 5% CO2 until a confluent monolayer formed. The cells were infected with Influenza A (H3N2) at 103 plaque-forming units per millilitre (PFUs/mL) and stored at −80 °C. Dilutions of the virus were applied to MDCK cells, incubated for 1 h, overlaid with agarose (Sigma-Aldrich, Oslo, Norway), and incubated at 37 °C and 5% CO2 for 3 days. Plaques were visualised microscopically (Nikon, Amstelveen, the Netherlands) after fixation and staining, enabling the calculation of viral concentrations.
Residual viral material on the samples was neutralised using soybean casein digest broth with lecithin and polyoxyethylene sorbitan monooleate (SCDLP, Sigma-Aldrich, Oslo, Norway). To confirm the absence of cytotoxic effects, MDCK cells were exposed to the neutralised sample broth and then incubated, washed, and analysed via a plaque assay.
To establish control measures, virus suspensions were also applied to untreated metal surfaces and bio-alkyd controls (BA-REF) and MDCK cells were inoculated to measure viral concentrations in PFUs/mL, ensuring consistency in cell responses. The viral infectivity titre (S) was calculated using Equation (4):
where S is the infectivity titre and P is the average plaque count. Infectivity difference conditions (|Sn − Su| ≤ 0.5) were met for the control and untreated samples.
S = (10·P)
Immediately post-inoculation, untreated and treated samples were washed with SCDLP, and virus recovery was quantified via a plaque assay. After a 24 h incubation, treated samples were processed similarly to determine the antiviral effect. Antiviral activity (R) was quantified based on PFUs.
The viral recovery data were analysed using one-way ANOVA to determine significant differences between groups, followed by pairwise t-tests for specific comparisons. Statistical significance was set at p < 0.05.
3. Results and Discussion
3.1. Paints’ Physical Characterisation
Viscosity of paint formulations plays a critical role in film formation and surface characteristics. Both the developed A-REF alkyd and BA-REF bio-alkyd paints exhibited high viscosities at RT. Specifically, A-REF paint had a viscosity of 1500 ± 100 cP at 23 °C and at 100 rpm, while BA-REF paint displayed a higher viscosity of 2500 ± 100 cP under the same test conditions. This increase in viscosity for the BA-REF formulation aligns with previous reports, attributing the effect to the inclusion of lignin during resin production [25,40]. Lignin’s branched molecular structure, rich in polar functional groups such as hydroxyl and phenolic groups, facilitates strong intermolecular interactions, thereby increasing viscosity [40]. Despite this notable rise in viscosity, application and uniform coating formation were not impeded.
Colour of the paint films was analysed on a Leneta chart substrate using colorimetric testing (Table 3). Measurements were obtained via the CIE colour system, revealing that A paints appeared white, while BA paints exhibited a beige to brown hue (Figure 2). This difference is reflected in the colorimetric data. A-REF showed a high L* value (>95), indicating a nearly white appearance, with neutral a* and b*, meaning the resin maintained the properties of titanium dioxide. In contrast, BA-REF, despite its high titanium dioxide content, exhibited a lower L* (<83), reflecting a darker beige to brown hue. The high b* value indicates the yellow hue in BA-REF paints. This colour shift is due to lignin, which contains chromophore groups such as quinones that reflect yellow or brown tones, leading to paint darkening [41]. Consequently, ΔE*ab (D65) values in comparison to white plate exceeded 20 for BA paints, whereas A paints had values below 15. The yellowing effect, commonly observed in alkyd resins due to oil content [23,42,43], was further intensified in BA paints, likely due to lignin. Regarding the effect of antimicrobial compounds on colour, thymol did not induce any changes. However, the inclusion of soft resins, which contain phenolic structures and conjugated double bonds [44] similar to lignin, further contributed to paint darkening [41].

Table 3.
Physical characterisation: colour, gloss in gloss units, and Persoz hardness results in oscillation number.

Figure 2.
Visual colour comparison of the alkyd (left)- and bio-alkyd (right)-based paints.
Gloss measurements at a 60° angle, applied on dark glass, are presented in Table 3. Alkyd paints showed high gloss values, close to 70 gloss units, typical for alkyd resins [45]. The bio-alkyd (BA) paints exhibited even higher gloss values, suggesting that the lignin enhances a smoother surface finish.
Persoz pendulum hardness was assessed after one month of air drying at RT (Table 3). The BA formulations demonstrated fewer oscillations, indicating a slower drying process compared to the conventional alkyd formulations. This trend was also observed in BA variants with bioadditives like thymol and hop soft resin, in contrast to the faster drying of A paints with or without bioadditives. Paint hardness is influenced by factors such as chain flexibility and the degree of crosslinking within the polymer network. Additionally, external factors, including substrate type, coating–substrate adhesion, and coating heterogeneity, affect hardness measurements [28].
Paint adhesion to metal substrates was evaluated using a cross-cut adhesion test (Table 4). The results indicated a significant improvement in adhesion when a primer was applied, as evidenced by smooth cut edges (class 0). Samples without primer exhibited minor edge defects (class 1).

Table 4.
Adhesion results on metal substrates (cross-cut test). Class 0 indicates that the edges of the cut are completely smooth, with no detachment of the coating squares, whereas class 1 indicates the detachment of small flakes at the intersections of the cuts, affecting up to 5% of the cross-cut area.
Both A-REF and BA-REF paints displayed good levelling and appearance, confirmed via FE-SEM analysis. SEM images (Figure 3) revealed detailed nanometric structures with a smooth and homogeneous film surface, devoid of notable defects such as blisters, craters, or cracks.
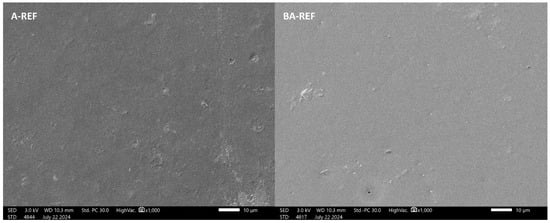
Figure 3.
SEM images of A-REF (left) and BA-REF (right) paints.
Water sensitivity was assessed by immersing samples in water for 24 h, and their appearance was recorded (Figure 4). The BA paint exhibited slight bleaching. This suggests that after 24 h of immersion in water, the lignin in the paint interacts with water through its polar areas, such as the hydroxyl and carbonyl groups. Upon drying, the areas where bleaching has occurred release the absorbed water, recovering their original appearance, indicating that the effect is reversible after drying (after 24 h in air).

Figure 4.
Water sensibility evaluation results of paints.
Water contact angle measurements (Table 5) indicated increased hydrophilicity in BA-REF paint due to lignin incorporation, with contact angles of 83° ± 3°, compared to 65° ± 3° for A-REF paint (Figure 5). According to Thomas Young’s classical equation, surface wettability is classified as hydrophilic (CA < 90°), hydrophobic (CA > 90°), or superhydrophobic (CA > 150°). The lower contact angle of BA-REF confirms its higher affinity for water.

Table 5.
Water contact angle results.
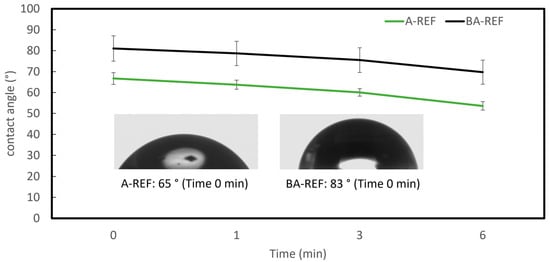
Figure 5.
Contact angle results of paints (water drops).
Overall, A-REF and BA-REF alkyd paints revealed comparable physicochemical properties suitable for application on metal surfaces. However, the BA-REF paint displayed a brownish colouration, higher viscosity, and slightly increased water sensitivity, all attributed to lignin.
3.2. Antimicrobial Activity of the Paints
The antimicrobial activity of bioadditive-enriched conventional alkyd and bio-alkyd coatings was evaluated against E. coli and S. aureus. Control coatings without antimicrobial additives were used to isolate the effect of the tested bioadditives. Based on MIC (minimum inhibitory concentration) and MBC (minimum bactericidal concentration) values, different concentrations were analysed [46]. Preliminary antimicrobial tests identified effective doses at 0.05% thymol and 0.15% soft hop resins in the reference alkyd paint. Given their antimicrobial nature, low concentrations were used. Reduced thymol doses were tested in the bio-alkyd paint, while the concentration of soft hop resins in the same formulation was increased to 0.25% due to insufficient efficacy at lower levels. This approach allowed for a direct comparison of antimicrobial effects across both paints while minimising replication.
Unexpectedly, the untreated alkyd resin base (A-REF) exhibited antimicrobial activity against S. aureus but not against E. coli (Table 6). This effect may be linked to the presence of TiO2 in the paint, known to generate reactive oxygen species (ROS) under UV light, which can degrade organic compounds [47]. The difference in bacterial susceptibility may be due to the cell wall structure: Gram-positive bacteria such as S. aureus lack an outer membrane, making them more susceptible ROS-induced oxidative damage. Conversely, Gram-negative bacteria such as E. coli have an additional outer membrane that limits molecule permeability [48], including ROS. However, further research is needed to confirm the role of TiO2 in the observed antimicrobial activity. Additionally, other studies report the opposite effect, with higher inhibitory efficacy of TiO2 against E. coli than S. aureus under certain conditions [49,50,51]. This discrepancy highlights the need for further analysis to elucidate the factors influencing this selective antimicrobial activity. As a result, antibacterial testing of the remaining paints was finalised exclusively against S. aureus.

Table 6.
Antibacterial activity of metal surface samples coated with conventional alkyd paints (A) or bio-alkyd paints (BA) with or without antibacterial agents (thymol and hop soft resins) against E. coli and S. aureus. Log (CFU/cm2) is the logarithm of the number of viable bacteria found on a test metal surface, based on triplicate measurements, with the standard deviation (SD) included. R represents the antibacterial activity, calculated as R = UT − AT. UT represents the average logarithm of viable bacteria recovered from untreated samples after 24 h. AT represents the average logarithm of viable bacteria recovered from treated samples after 24 h. A value of R ≥ 2 is considered as grown inhibition. NA: not analysed.
Thymol addition was effective in both alkyd and bio-alkyd resins at concentrations of 0.05% and above, completely inhibiting E. coli growth within 24 h (Table 6). This suggests that thymol maintains its antimicrobial efficacy across resin types, with 0.05% identified as the minimum bactericidal concentration. Lower concentrations (0.01% and 0.03%) lacked activity in the bio-alkyd resin, indicating a threshold concentration for inhibition. This is in agreement with the previously described MIC for thymol [46]. Thymol-rich essential oils have been investigated for their antimicrobial properties, attributed to its membrane-disrupting and DNA-binding properties, which likely underpins its efficacy against E. coli [52].
In contrast, hop-derived soft resins showed antibacterial activity only in the conventional alkyd paint at 0.15%, achieving an R value of 3.00 against E. coli. Higher concentrations of hop resins are likely required to exert significant antibacterial activity compared to thymol, as several compounds in hop extracts (such as xanthohumol, humulone, lupulone, bitter acids, and essential oils) have shown potent antibacterial effects, particularly against Gram-positive bacteria and fungi. However, these compounds are generally less effective against Gram-negative bacteria and yeasts [53,54]. Given that E. coli is a Gram-negative bacterium, the lack of efficacy at lower hop resin concentrations is not unexpected [55,56,57,58,59]. Indeed, some studies suggest that hop fractions may require concentrations exceeding 1000 mg/L to effectively inhibit Gram-negative bacterial strains [60]. It would therefore be reasonable to anticipate stronger antimicrobial effects against other Gram-positive bacterial species, though further analysis is necessary.
Nevertheless, no biocidal effect was observed in the bio-alkyd resin, even when the concentration was increased to 0.25%. This result is somewhat unexpected, as the presence of lignin might suggest an increase in the antibacterial activity of the bio-alkyd resin. In the late 1970s, Zemek et al. [61] reported the antibacterial activity of guaiacyl and syringyl lignin models against both Gram-negative and Gram-positive bacteria, likely due to the structure of their side chains. Since then, the antimicrobial effects of lignin have been controversial, varying by plant source and bacterial strain analysed [62].
It is also possible that lignin, a complex polymer rich in phenolic structures, interacts chemically with the alpha and beta acids present in hops, thereby reducing their antimicrobial efficacy. Alternatively, lignin may act as a physical barrier within the bio-alkyd matrix, restricting the dispersion of hop resins and potentially encapsulating them, further diminishing their effectiveness. These findings suggest that while hop resins exhibit promising antimicrobial properties, their efficacy may be compromised in bio-based formulations due to matrix interactions, highlighting the need for further chemical analysis using spectroscopy to determine the release rate.
Given the increasing interest in bio-alkyd resins enriched with antimicrobial agents [23,26,27], these results open new possibilities in sustainable coatings. The successful incorporation of thymol highlights its potential as an eco-friendly antimicrobial additive, aligning with circular economy and sustainability principles. Similarly, hop resins, derived as a waste product of the beer industry, offer another avenue for repurposing industrial by-products in the development of sustainable coatings.
3.3. Antiviral Activity of the Paints
Based on the results obtained in the previous sections, bio-based alkyd resin samples supplemented with 0.10% thymol and 0.15% hop resins were selected to evaluate their virucidal activity against Influenza A (H3N2).
All the test conditions described in the ISO standard were met (Table 7), ensuring that the test setup accurately reflected the antiviral activity of the samples studied, thereby confirming the test as valid. In the first condition, a value of < 0.2 indicated that the maximum and minimum number of plaques counts from the specimens were closely aligned. The initial viral titre (U0) was 1.1 × 106 PFUs/cm2, which fell within acceptable range. While the viral titre at 24 h (Ut) was naturally lower than U0, it remained well above the minimum threshold, confirming that the control surface exhibited no antiviral activity, allowing the virus to remain viable over the 24 h period and validating the test conditions. Additionally, no cytotoxic effect on the host cell, no reduction in cell sensitivity to the virus, and effective inactivation of the antiviral activity in the SCDLP broth were confirmed.

Table 7.
Summary of validity conditions for ISO 21702:2019. All four conditions were met, which established reliability in the assay results. * Lmax is the common log of the maximum number of plaques recovered from a specimen, Lmin is the common log of the minimum number of plaques recovered from a specimen, Lmean is the common log of the mean number of plaques recovered from the 3 specimens, ** U0 is the average number of plaques recovered immediately after inoculation from the untreated specimens, and *** Ut is the number of plaques recovered from each untreated test specimen with 24 h of contact. **** Supplementary Materials (Table S1: Raw_data_antiviral).
The viral titre of the negative control at 0 h was 1 × 108 PFUs/mL, corresponding to an average logarithmic titre of 6.03 PFUs/cm2 (Table 8). After 24 h, the control samples showed a decrease to a mean logarithmic titre of 5.04 PFUs/cm2. In contrast, for the treated samples (BA-T010 and BA-H015), minimal or no plaques were observed across all tested dilutions. The average logarithmic viral titres for BA-T010 and BA-H015 after 24 h were 1.62 and 1.80 PFUs/cm2, respectively. Both thymol and hop resin demonstrated significant antiviral activity effects, achieving reductions of 3.42 and 3.24 logs, equivalent to a viral load reduction of over 99.99% compared to the untreated controls (Table 8). Statistical analysis confirmed a significant reduction in viral recovery for both formulations compared to the reference (ANOVA: F = 148.15, p = 7.82 × 10−6). Pairwise t-tests showed that thymol and hop resin were both significantly different from the reference (p = 0.0067 for both), whereas no significant difference was observed between the two treatments (p = 0.23). A table summarising the ANOVA and t-test results, along with the raw data, is provided in Supplementary Materials (Table S1).

Table 8.
Summary of results for the antiviral activity tests, showing the percentage of viral load reduction in the two coated surfaces and the untreated control specimens. The data shown correspond to the average of 3 replicates.
The consistent antiviral efficacy of both bioadditives contrasts with their differences in antimicrobial effectiveness. Both ISO standards employ similar test conditions with regards to humidity and contact time, which ensures that the active compound behaves consistently in both resins. Yet, different factors likely influence the activity against bacteria versus viruses. Bacteria possess rigid cell walls composed of peptidoglycan and lipopolysaccharides, which act as a structural barrier and influence the way antimicrobial agents penetrate or disrupt the cell. However, antiviral activity follows a distinct mechanism. Unlike bacteria, viruses lack cell walls, so antiviral efficacy may depend less on bioadditive release or diffusion and more on direct interactions with viral envelopes. Influenza A (H3N2), the virus employed in the assay, is an enveloped virus of the Orthomyxoviridae family. Enveloped viruses are known to be more sensitive than their non-enveloped counterparts. They depend on their lipid bilayer for structural integrity and host cell entry, making them more susceptible to compounds that target membrane stability [63].
Both thymol [64] and hop resins [55] are known to integrate into lipid membranes, affecting, via different mechanisms, both membrane fluidity and protein conformation. Their virucidal activity is likely linked to this ability to interact with the viral envelope, destabilising viral proteins required for infection. In the case of hop extracts, β-acids and oil extracts have been found to lack antiviral activity, whereas bitter acids, particularly those present in hop soft resins, exhibit strong antiviral properties [55,65]. This suggests that specific components within the hop extract selectively target viral envelopes, further reinforcing the mechanistic distinction between antiviral and antibacterial modes of action.
4. Conclusions
The increasing concern over the spread of pathogens, particularly in high-risk environments such as hospitals and public spaces, underscores the urgent need for innovative antimicrobial coatings. Developing bio-based alternatives aligns with global efforts to reduce ecological impact and improve sustainability. In this context, the exploration of alkyd resins enriched with antimicrobial bioadditives offers a promising solution to control microbial dissemination effectively.
The incorporation of natural compounds such as thymol and soft hop resins into bio-based alkyd formulations not only provides potent antimicrobial properties but also supports circular economy principles. Thymol, derived as a by-product of the cosmetic industry, and hop resins, a waste product from beer production, exemplify the transformation of industrial residues into high-value materials. This approach fosters resource efficiency and environmental sustainability while addressing the pressing demand for eco-friendly and functional coatings to mitigate pathogen transmission, at a time when outbreaks of zoonotic diseases are increasingly frequent.
The newly developed antimicrobial biocoatings demonstrated good and glossy appearance when applied to metal surfaces, with beige to brown colouration (derived from lignin presence in the alkyd resin), strong adhesion to metal when a primer is applied beforehand (“class 0” rating in the cross-cut adhesion test), lower hardness (Persoz hardness exceeding 50 oscillations after 1 month at RT), and slight water sensitivity (although it recovers when water contact is removed) compared to a reference white coating containing a conventional alkyd resin.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings15040445/s1. Table S1: Raw_data_antiviral.
Author Contributions
Writing (original draft preparation, review, and editing), A.I., I.E., J.G. and A.G.-M.; technical support, A.I.P.-G., R.D.-A. and Y.D.; conceptualisation, A.B., I.E. and J.G. All authors have read and agreed to the published version of the manuscript.
Funding
The LIGNICOAT project has received funding from the Bio-based Industries Joint Undertaking (JU) under the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 101023342. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and the Bio-based Industries Consortium.
Data Availability Statement
Data available in a publicly accessible repository.
Acknowledgments
(i) Ana Ibáñez (A.I.) is supported by a “Margarita Salas” modality postdoctoral grant (reference no.: UP2021-025) through the University of León awarded by the Spanish Ministry of Universities within the Recovery, Transformation and Resilience Plan (Modernization and digitalization of the Educational System), whose funding comes from the European Recovery Instrument European Union-NextGenerationEU. Special thanks to: (ii) the BioBIVE project (BIOdegradable delivery systems for plant pathogens control of horticultural crops through BIoactiVE agents) (Project no.: 101130442) funded by the European Union through the Horizon Europe Framework Programme (HORIZON-CL4-2023-RESILIENCE-01-34); and (iii) We gratefully acknowledge BARPIMO for providing the bio-alkyd resin used in this study, which was instrumental in conducting this research, and ALLNEX for providing raw materials (conventional alkyd resin and additives) and recommendations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gest, H. The Discovery of Microorganisms by Robert Hooke and Antoni van Leeuwenhoek, Fellows of The Royal Society. Notes Rec. R. Soc. Lond. 2004, 58, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Boivin, G. Pandemics Throughout History. Front. Microbiol. 2021, 11, 631736. [Google Scholar] [CrossRef]
- Cassidy, S.S.; Sanders, D.J.; Wade, J.; Parkin, I.P.; Carmalt, C.J.; Smith, A.M.; Allan, E. Antimicrobial Surfaces: A Need for Stewardship? PLoS Pathog. 2020, 16, e1008880. [Google Scholar] [CrossRef]
- Marquès, M.; Domingo, J.L. Contamination of Inert Surfaces by SARS-CoV-2: Persistence, Stability and Infectivity. A Review. Environ. Res. 2021, 193, 110559. [Google Scholar] [CrossRef] [PubMed]
- Brief, W.S. Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Correa, J.d.S.; Primo, J.d.O.; Balaba, N.; Pratsch, C.; Werner, S.; Toma, H.E.; Anaissi, F.J.; Wattiez, R.; Zanette, C.M.; Onderwater, R.C.A.; et al. Copper(II) and Cobalt(II) Complexes Based on Abietate Ligands from Pinus Resin: Synthesis, Characterization and Their Antibacterial and Antiviral Activity against SARS-CoV-2. Nanomaterials 2023, 13, 1202. [Google Scholar] [CrossRef]
- Susan Muthoni, M. Hospitals Surfaces and Sites as a Reservoir for Pathogenic Bacteria That Play a Role in Transmission of Infectious Diseases. J. Health Environ. Res. 2021, 7, 139. [Google Scholar] [CrossRef]
- Ngonda, F. Assessment of Bacterial Contamination of Toilets and Bathroom Doors Handle/Knobs at Daeyang Luke Hospital. Pharm. Biol. Eval. 2017, 4, 193. [Google Scholar] [CrossRef]
- Saka, K.; Akanbi II, A.; Obasa, T.; Raheem, R.; Oshodi, A. Bacterial Contamination of Hospital Surfaces According to Material Make, Last Time of Contact and Last Time of Cleaning/Disinfection. J. Bacteriol. Parasitol. 2017, 8, 8–11. [Google Scholar] [CrossRef]
- Odigie, A.B.; Ekhiase, F.O.; Orjiakor, P.I.; Omozuwa, S. The Role of Door Handles in the Spread of Microorganisms of Public Health Consequences in University of Benin Teaching Hospital (UBTH), Benin City, Edo State. Pharm. Sci. Technol. 2017, 2, 15–21. [Google Scholar]
- Weber, D.J.; Rutala, W.A.; Miller, M.B.; Huslage, K.; Sickbert-Bennett, E. Role of Hospital Surfaces in the Transmission of Emerging Health Care-Associated Pathogens: Norovirus, Clostridium Difficile, and Acinetobacter Species. Am. J. Infect. Control 2010, 38, S25–S33. [Google Scholar] [CrossRef]
- Otter, J.A.; Yezli, S.; Salkeld, J.A.G.; French, G.L. Evidence That Contaminated Surfaces Contribute to the Transmission of Hospital Pathogens and an Overview of Strategies to Address Contaminated Surfaces in Hospital Settings. Am. J. Infect. Control 2013, 41, S6–S11. [Google Scholar] [CrossRef] [PubMed]
- Carling, P.C.; Parry, M.F.; Bruno-Murtha, L.A.; Dick, B. Improving Environmental Hygiene in 27 Intensive Care Units to Decrease Multidrug-Resistant Bacterial Transmission. Crit. Care Med. 2010, 38, 1054–1059. [Google Scholar] [CrossRef]
- Deng, Y.; Song, G.-L.; Zheng, D.; Zhang, Y. Fabrication and Synergistic Antibacterial and Antifouling Effect of an Organic/Inorganic Hybrid Coating Embedded with Nanocomposite Ag@TA-SiO Particles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126085. [Google Scholar] [CrossRef]
- Klein, S.E.; Alzagameem, A.; Rumpf, J.; Korte, I.; Kreyenschmidt, J.; Schulze, M. Antimicrobial Activity of Lignin-Derived Polyurethane Coatings Prepared from Unmodified and Demethylated Lignins. Coatings 2019, 9, 494. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Murthy, N.S. Bio-Inspired Strategies for Designing Antifouling Biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Su, B.; Dalby, M.J. Multifunctional Coatings and Nanotopographies: Toward Cell Instructive and Antibacterial Implants. Adv. Healthc. Mater. 2019, 8, e1801103. [Google Scholar] [CrossRef]
- de Haro, J.C.; Allegretti, C.; Smit, A.T.; Turri, S.; D’Arrigo, P.; Griffini, G. Biobased Polyurethane Coatings with High Biomass Content: Tailored Properties by Lignin Selection. ACS Sustain. Chem. Eng. 2019, 7, 11700–11711. [Google Scholar] [CrossRef]
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable Oil Based Eco-Friendly Coating Materials: A Review Article. Arab. J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Dastpak, A.; Yliniemi, K.; De Oliveira Monteiro, M.C.; Höhn, S.; Virtanen, S.; Lundström, M.; Wilson, B.P. From Waste to Valuable Resource: Lignin as a Sustainable Anti-Corrosion Coating. Coatings 2018, 8, 454. [Google Scholar] [CrossRef]
- Dizman, C.; Kaçakgil, E.C. Alkyd Resins Produced from Bio-Based Resources for More Sustainable and Environmentally Friendly Coating Applications. Turk. J. Chem. 2023, 47, 1. [Google Scholar] [CrossRef]
- Chardon, F.; Denis, M.; Negrell, C.; Caillol, S. Hybrid Alkyds, the Glowing Route to Reach Cutting-Edge Properties? Prog. Org. Coat. 2021, 151, 106025. [Google Scholar] [CrossRef]
- Abd El-Wahab, H.; El-Eisawy, R.A. Preparation of New Modified Antimicrobial Alkyd Resin Based on Benzo [b] Thiophene Derivative as Source of Polyol for Surface Coating Applications. Pigment. Resin. Technol. 2023, 52, 661–670. [Google Scholar] [CrossRef]
- Kızılkonca, E.; Erim, F.B. Development of Anti-Aging and Anticorrosive Nanoceria Dispersed Alkyd Coating for Decorative and Industrial Purposes. Coatings 2019, 9, 610. [Google Scholar] [CrossRef]
- Ghosh, A.; Fearon, O.; Agustin, M.; Alonso, S.; Balda, E.C.; Franco, S.; Kalliola, A. Fractionation of Kraft Lignin for Production of Alkyd Resins for Biobased Coatings with Oxidized Lignin Dispersants as a Co-Product. ACS Omega 2024, 9, 46276–46292. [Google Scholar] [CrossRef]
- El-Mosallamy, E.-S.H.; Gabr, M.Y. Preparation and Evaluation of the Antibacterial Alkyd Resin Modified with 2,2-Di-Thiosalicylic Acid. Macromol. Indian J. 2014, 10, 53–59. [Google Scholar]
- Musa, H.; Usman, S.N. Preparation and Antimicrobial Evaluation of Neem Oil Alkyd Resin and Its Application as Binder in Oil-Based Paint. Environ. Nat. Resour. Res. 2016, 6, 92. [Google Scholar] [CrossRef][Green Version]
- İşeri-Çağlar, D.; Baştürk, E.; Oktay, B.; Kahraman, M.V. Preparation and Evaluation of Linseed Oil Based Alkyd Paints. Prog. Org. Coat. 2014, 77, 81–86. [Google Scholar] [CrossRef]
- Villa, R.E.; Azimonti, G.; Bonos, E.; Christensen, H.; Durjava, M.; Dusemund, B.; Gehring, R.; Glandorf, B.; Kouba, M.; López-Alonso, M.; et al. Safety and Efficacy of a Feed Additive Consisting of an Essential Oil Derived from the Flowering Tops of Thymbra capitata L. Cav. (Spanish Type Origanum Oil) for Use in All Animal Species (FEFANA Asbl). EFSA J. 2024, 22, e9017. [Google Scholar] [CrossRef]
- Maisanaba, S.; Prieto, A.I.; Puerto, M.; Gutiérrez-Praena, D.; Demir, E.; Marcos, R.; Cameán, A.M. In Vitro Genotoxicity Testing of Carvacrol and Thymol Using the Micronucleus and Mouse Lymphoma Assays. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2015, 784, 37–44. [Google Scholar] [CrossRef]
- Lewis, J.C.; Alderton, G.; Carson, J.F.; Reynolds, D.M.; Maclay, W.D. Lupulon and Humulon—Antibiotic Constituents of Hops. J. Clin. Investig. 1949, 28, 916–919. [Google Scholar] [CrossRef]
- Paniagua-García, A.I.; Ruano-Rosa, D.; Díez-Antolínez, R. Fractionation of High-Value Compounds from Hops Using an Optimised Sequential Extraction Procedure. Antioxidants 2023, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Ly, B.C.K.; Dyer, E.B.; Feig, J.L.; Chien, A.L.; Del Bino, S. Research Techniques Made Simple: Cutaneous Colorimetry: A Reliable Technique for Objective Skin Color Measurement. J. Investig. Dermatol. 2020, 140, 3–12.e1. [Google Scholar] [CrossRef]
- McGrath, J.R.; Beck, M.; Hill, M.E. Replicating Red: Analysis of Ceramic Slip Color with CIELAB Color Data. J. Archaeol. Sci. Rep. 2017, 14, 432–438. [Google Scholar] [CrossRef]
- ISO ISO 2813: 2014; Paints and Varnishes—Determination of Gloss Value at 20°, 60° and 85°. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO EN ISO 1522: 2022; Paints and Varnishes—Pendulum Damping Test. International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO EN ISO 2409: 2020; Paints and Varnishes—Cross-Cut Test. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO ISO 22196: 2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO ISO 21702: 2019; Measurement of Antiviral Activity on Plastics and Other Non-Porous Surfaces. International Organization for Standardization: Geneva, Switzerland, 2020.
- Pang, B.; Lam, S.S.; Shen, X.; Cao, X.; Liu, S.; Yuan, T.; Sun, R. Valorization of Technical Lignin for the Production of Desirable Resins with High Substitution Rate and Controllable Viscosity. ChemSusChem 2020, 13, 4446–4454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, S.; Chen, Y. Basic Understanding of the Color Distinction of Lignin and the Proper Selection of Lignin in Color-Depended Utilizations. Int. J. Biol. Macromol. 2020, 147, 607–615. [Google Scholar] [CrossRef]
- Saravari, O.; Phapant, P.; Pimpan, V. Synthesis of Water-reducible Acrylic–Alkyd Resins Based on Modified Palm Oil. J. Appl. Polym. Sci. 2005, 96, 1170–1175. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Maliki, M.; Odiachi, I.J.; Aghedo, O.N.; Ohiocheoya, E.B. Review on Solvents Based Alkyd Resins and Water Borne Alkyd Resins: Impacts of Modification on Their Coating Properties. Chem. Afr. 2022, 5, 211–225. [Google Scholar] [CrossRef]
- Tursun, E.; Li, Z.; Aisa, H.A. Isolation and Identification of Soft Resins from Humulus lupulus L. Ind. Crops Prod. 2021, 172, 114014. [Google Scholar] [CrossRef]
- Denis, M.; Le Borgne, D.; Sonnier, R.; Caillol, S.; Negrell, C. Improvement of the Flame Retardant Properties of Alkyd Resins through Incorporation of Phosphorus-Containing Furan Derivative. Green. Mater. 2023, 12, 168–182. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of Antibacterial Action of Three Monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Hwang, G.B.; Patir, A.; Allan, E.; Nair, S.P.; Parkin, I.P. Superhydrophobic and White Light-Activated Bactericidal Surface through a Simple Coating. ACS Appl. Mater. Interfaces 2017, 9, 29002–29009. [Google Scholar] [CrossRef]
- Tortora, G.J.; Funke, B.R.; Case, C.L. Microbiology: An Introduction, 9th ed.; Pearson Education: London, UK, 2013. [Google Scholar]
- Salman, G. A Comparative Study of Antibacterial Activity of ZnO and TiO2 Nanoparticles Against Gram-Positive and Gram-Negative Bacteria. Eng. Technol. J. 2023, 41, 1232–1240. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Abdullah, N.; Yasin, F.M. Toxicity Assessment of Reduced Graphene Oxide and Titanium Dioxide Nanomaterials on Gram-Positive and Gram-Negative Bacteria under Normal Laboratory Lighting Condition. Toxicol. Rep. 2020, 7, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Lashgari, A.; Ghamami, S.; Golzani, M. Gram-Negative and Gram-Positive Bacteria; Antibacterial Activity of a Clay-TiO2 Nanocomposits. Bull. Environ. Pharmacol. Life Sci. 2016, 5, 53–59. [Google Scholar]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and Antifungal Activities of Thymol: A Brief Review of the Literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, P.; Katta, S.; Andrei, I.; Babu Rao Ambati, V.; Leonida, M.; Haas, G.J. Positive Antibacterial Co-Action between Hop (Humulus lupulus) Constituents and Selected Antibiotics. Phytomedicine 2008, 15, 194–201. [Google Scholar] [CrossRef]
- Fahle, A.; Bereswill, S.; Heimesaat, M.M. Antibacterial Effects of Biologically Active Ingredients in Hop Provide Promising Options to Fight Infections by Pathogens Including Multi-Drug Resistant Bacteria. Eur. J. Microbiol. Immunol. 2022, 12, 22–30. [Google Scholar] [CrossRef]
- Olšovská, J.; Boštíková, V.; Dušek, M.; Jandovská, V.; Bogdanová, K.; Čermák, P.; Boštík, P.; Mikyska, A.; Kolář, M. Humulus lupulus L. (Hops)—A Valuable Source of Compounds with Bioactive Effects for Future Therapies. Mil. Med. Sci. Lett. 2016, 85, 19–30. [Google Scholar] [CrossRef]
- Astray, G.; Gullón, P.; Gullón, B.; Munekata, P.E.S.; Lorenzo, J.M. Humulus lupulus L. as a Natural Source of Functional Biomolecules. Appl. Sci. 2020, 10, 5074. [Google Scholar] [CrossRef]
- Srinivasan, V.; Goldberg, D.; Haas, G.J. Contributions to the Antimicrobial Spectrum of Hop Constituents. Econ. Bot. 2004, 58, S230–S238. [Google Scholar]
- Erzinger, G.S.; Lopes, P.C.; del Ciampo, L.F.; Zimath, S.C.; Vicente, D.; Martins de Albuquerque, F.; Prates, R.C. Bioactive Compounds of Hops Resulting from the Discarding of the Beer Industry in the Control of Pathogenic Bacteria. In Natural Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2021; pp. 41–55. [Google Scholar]
- Sun, S.; Wang, X.; Yuan, A.; Liu, J.; Li, Z.; Xie, D.; Zhang, H.; Luo, W.; Xu, H.; Liu, J.; et al. Chemical Constituents and Bioactivities of Hops (Humulus lupulus L) and Their Effects on Beer-related Microorganisms. Food Energy Secur. 2022, 11, e367. [Google Scholar] [CrossRef]
- Bogdanova, K.; Kolar, M.; Langova, K.; Dusek, M.; Mikyska, A.; Bostikova, V.; Bostik, P.; Olsovska, J. Inhibitory Effect of Hop Fractions Against Gram-Positive Multi-Resistant Bacteria. Pilot Study Biomed. Pap. 2018, 162, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Zemek, J.; Košíková, B.; Augustín, J.; Joniak, D. Antibiotic Properties of Lignin Components. Folia Microbiol. 1979, 24, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Y.; Liu, H.; Zhang, D.; Shi, Q.-S.; Zhong, X.-Q.; Guo, Y.; Xie, X.-B. High Value Valorization of Lignin as Environmental Benign Antimicrobial. Mater. Today Bio 2023, 18, 100520. [Google Scholar] [CrossRef]
- Lin, Q.; Lim, J.Y.C.; Xue, K.; Yew, P.Y.M.; Owh, C.; Chee, P.L.; Loh, X.J. Sanitizing Agents for Virus Inactivation and Disinfection. View 2020, 1, e16. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A Story That Begs to Be Told. A Review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).