Enhancing the Corrosion Resistance of Passivation Films via the Synergistic Effects of Graphene Oxide and Epoxy Resin
Abstract
1. Introduction
2. Experimental Procedures
2.1. Sample Preparation
2.2. Surface Analysis
2.3. Anticorrosion Performance Test
2.3.1. Electrochemical Testing
2.3.2. Neutral Salt Spray Test
2.3.3. Adhesion Test of Passivation Film
3. Results and Discussions
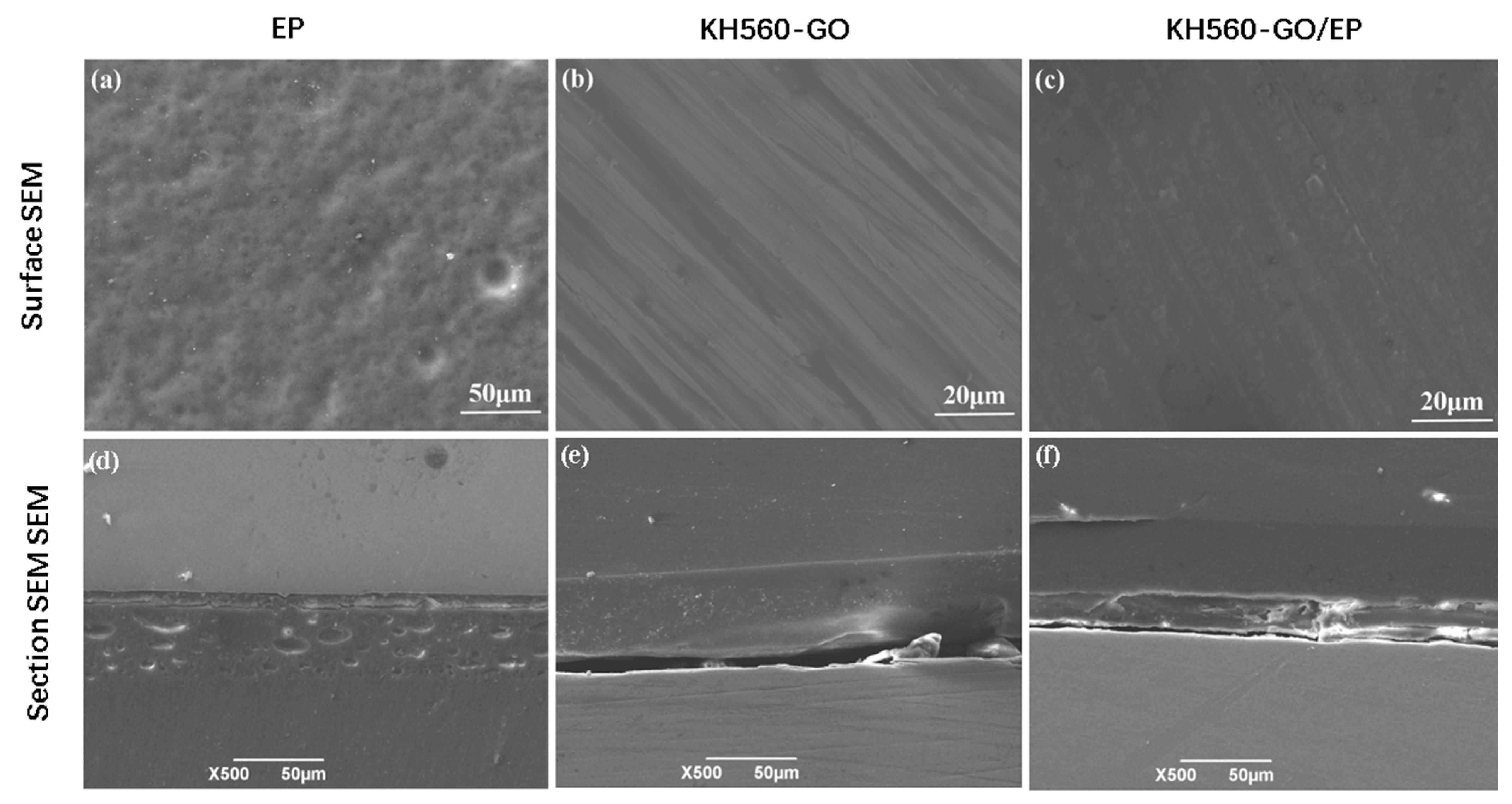
3.1. Micromorphology
3.2. FTIR Analysis
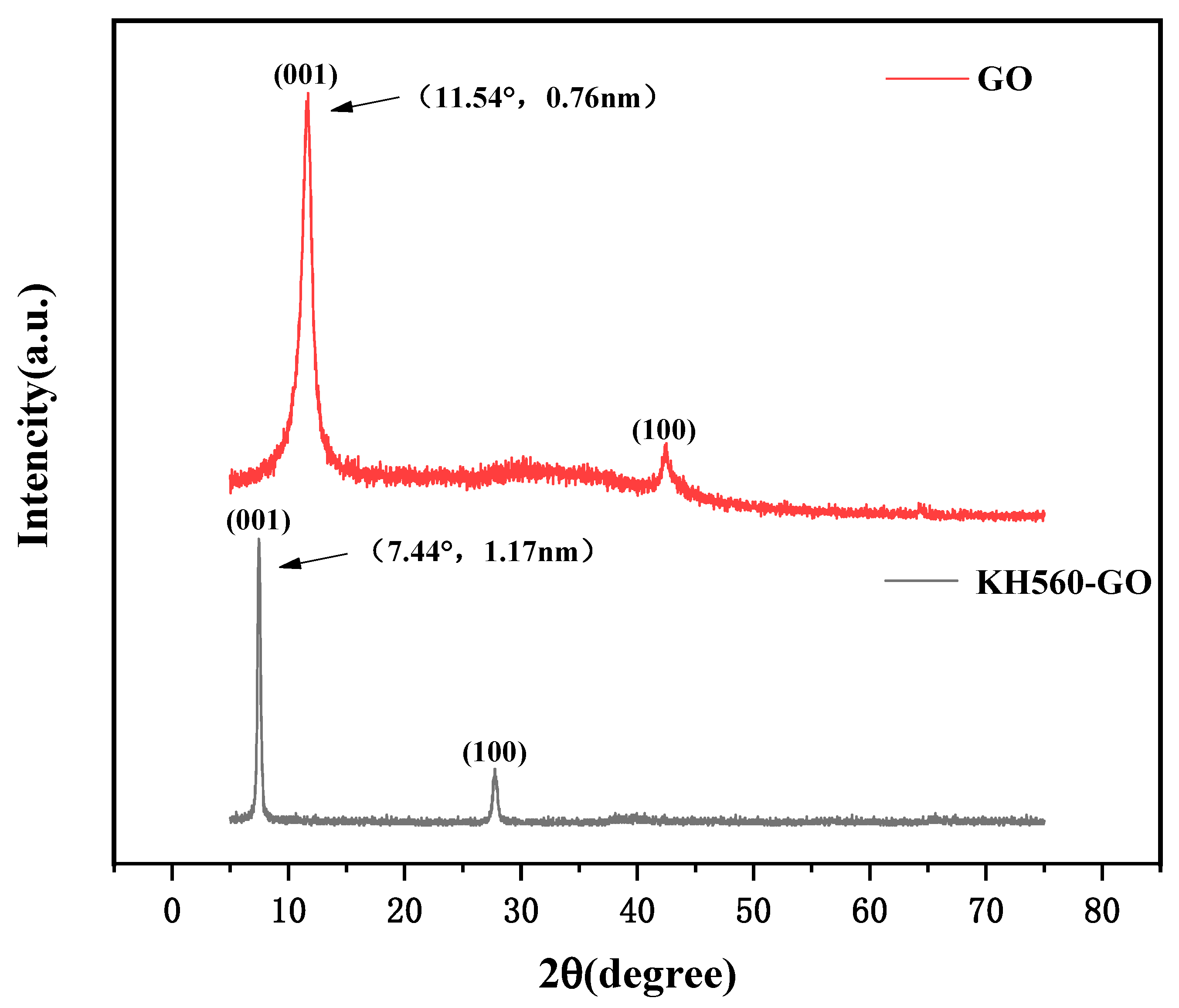
3.3. XRD Analysis
3.4. Electrochemical Analysis
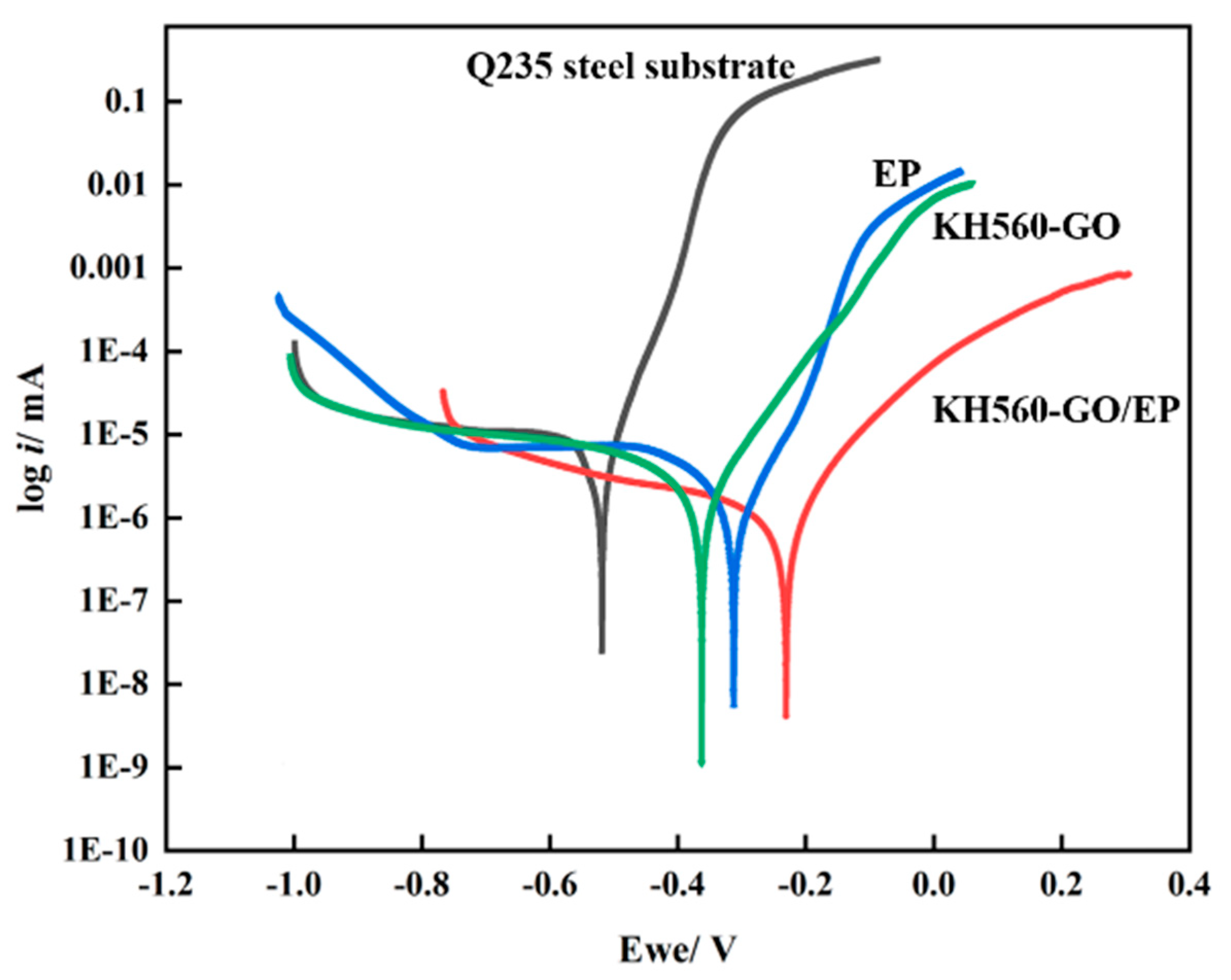
3.4.1. Electrochemical Polarization Curve Analysis
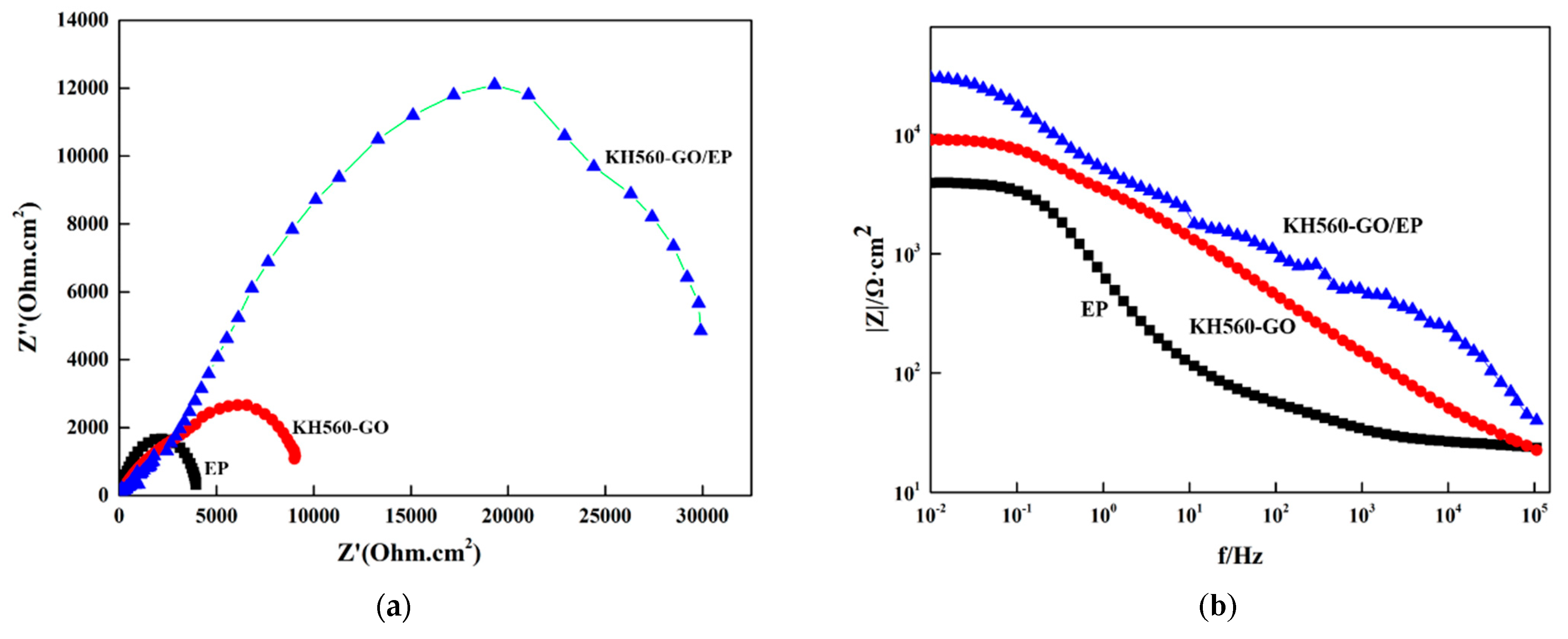
3.4.2. Electrochemical Impedance of Film Layer (EIS)
3.5. Analysis of Neutral Hydrochloric Acid Experiment
3.6. Adhesion Analysis of Passivation Film
4. Exploration of Anti-Corrosion Mechanism
5. Conclusions
- (1)
- By comparing the GO analysis with the SEM analysis of the surface morphology of the composite film layers, it was evident that the surface of the KH560-GO/EP film layer is smooth, without noticeable small pores or GO aggregation. This result is significantly better than those observed for the pure EP, GO, and KH560-GO film layers;
- (2)
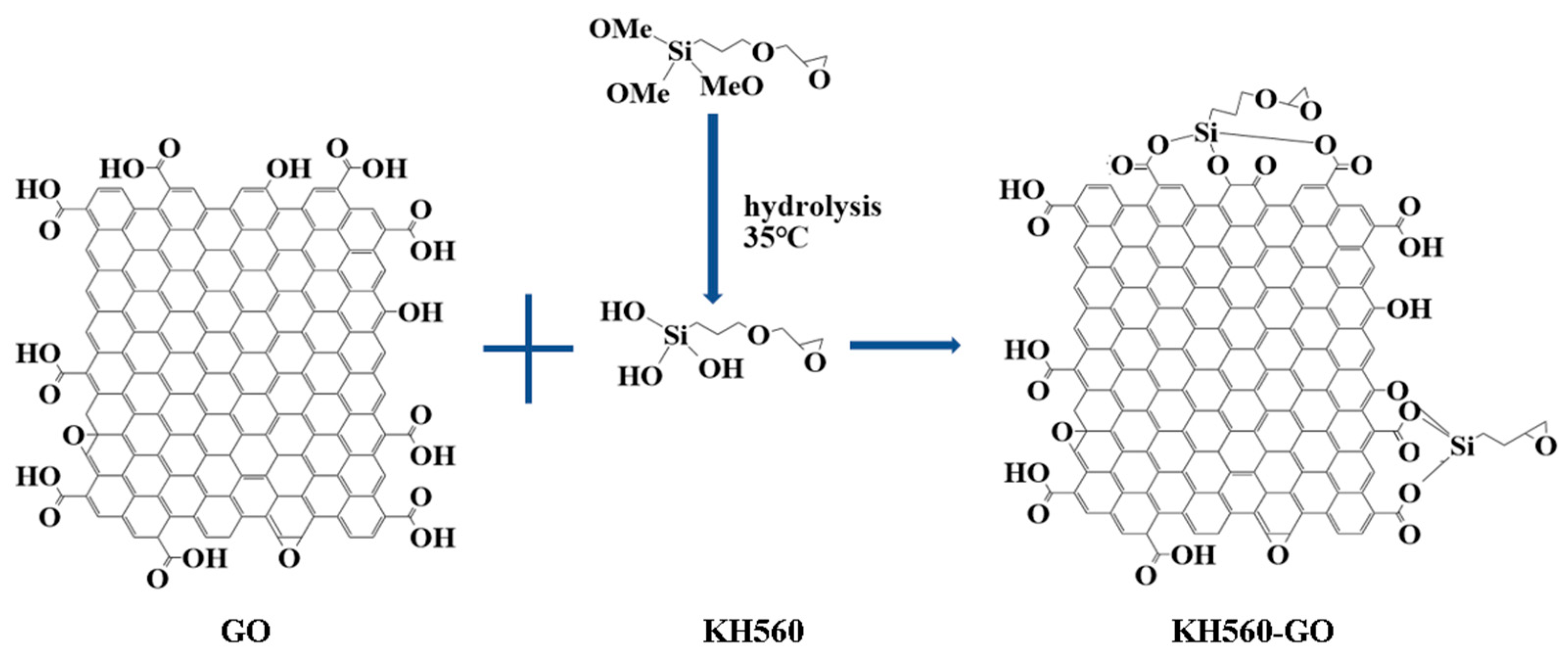
- GO contains a large number of functional groups. The characteristic absorption peaks of these functional groups confirm the successful introduction of GO into the composite material, where it bonds with Si-OH generated by silane hydrolysis to form Si-O-C. The Si-O-C structure covers the surface of the matrix and fills the micropores, thereby enhancing the corrosion resistance;
- (3)
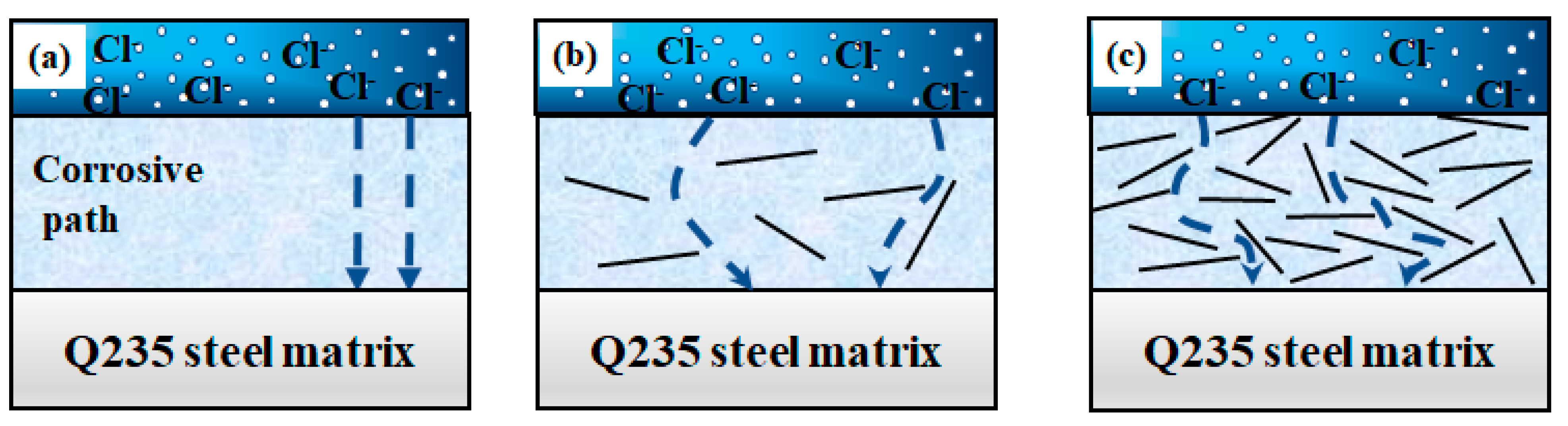
- The KH560-GO/EP film layer exhibits significantly superior anti-corrosion performance compared to both the EP and KH560-GO film layers. The KH560-GO/EP film layer has the highest polarization resistance (6.8763 × 103 Ω·cm2) and the lowest corrosion rate (7.3702 × 10−4 mm/a). This improvement is primarily attributed to the formation of the “labyrinth effect” in graphene oxide, which isolates the pathways for corrosion medium invasion into the substrate and delays the penetration of the corrosion medium;
- (4)
- The average corrosion rate of the KH560-GO/EP film layer is 0.365 g/(m2/h), which is significantly lower than those of the GO and KH560-GO film layers. This result demonstrates the excellent corrosion resistance of the KH560-GO/EP composite film layer. After wiping, the average corrosion rate of the KH560-GO/EP film layer increases to 0.729 g/(m2/h). These findings suggest that the wiped composite coating sample retains a certain level of corrosion resistance, indicating that the composite passivation film has not completely peeled off.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, R.; Liu, L.; Wang, F. The role of hydrostatic pressure on the metal corrosion in simulated deep-sea environments—A review. J. Mater. Sci. Technol. 2022, 112, 230–238. [Google Scholar] [CrossRef]
- Ko, C.L.; Kuo, Y.L.; Chen, S.H.; Chen, S.Y.; Guo, J.Y.; Wang, Y.J. Formation of aluminum composite passive film on magnesium alloy by integrating sputtering and anodic aluminum oxidation processes. Thin Solid Film. 2020, 709, 138151. [Google Scholar] [CrossRef]
- Li, F.; Zhao, M.; Zhan, Y.; Wu, C.; Zhang, Y.; Jiang, X.; Sun, Z. Facile fabrication of novel superhydrophobic Al2O3/polysiloxane hybrids coatings for aluminum alloy corrosion protection. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128444. [Google Scholar] [CrossRef]
- Nejad, S.A.T.; Ramezanzadeh, M.; Alibakhshi, E.; Ramezanzadeh, B.; Olivier, M.-G.; Mahdavian, M. Fabrication of 8-hydroxyquinoline loaded in an aluminum-based metal-organic framework for strengthening anti-corrosion behavior of silane primer coating. Prog. Org. Coat. 2023, 174, 107280. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, L.; Hua, Y.; Wang, J.; Dong, N.; Li, X.; Xu, S.; Neville, A. Revealing the superior corrosion protection of the passive film on selective laser melted 316L SS in a phosphate-buffered saline solution. Appl. Surf. Sci. 2020, 529, 147170. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Li, W.; Zhou, R.; Chou, T.; Wang, X.; Huang, J. Passivation evolution of Ti-Ta-Nb medium-entropy sputtered thin films in sulfuric acid solution. Appl. Surf. Sci. 2022, 576, 151824. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, G.; Li, D. A pre-passive state observed for the passive film formed on Alloy 625 in a hydrochloric acid solution. Appl. Surf. Sci. 2018, 431, 197–201. [Google Scholar] [CrossRef]
- Safaruddin, A.S.; Bermundo, J.P.S.; Jallorina, M.P.A.; Yamamoto, A.; Uraoka, Y. Spray pyrolyzed fluorinated inorganic-organic passivation for solution-processed a-InZnO thin-film transistors. Mater. Sci. Semicond. Process. 2022, 146, 106669. [Google Scholar] [CrossRef]
- Ahn, J.; Ryu, T.; Park, J. Composite membranes with ultrathin and conformal passivation for universal microfiltration compatible with organic solvents. J. Membr. Sci. 2022, 651, 120455. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Kustov, L. Polymeric ionic liquids: Here, there and everywhere. Eur. Polym. J. 2023, 203, 112657. [Google Scholar] [CrossRef]
- Li, E.; Liu, S.L.; Luo, F.; Pei, Y. Amino acid imidazole ionic liquids as green corrosion inhibitors for mild steel in neutral media: Synthesis, electrochemistry, surface analysis and theoretical calculations. J. Electroanal. Chem. 2023, 944, 117650. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Q.; Yin, L.; Yang, G.; Xie, J.; Deng, L. Surface passivation of applying an organic-inorganic hybrid coatings toward significantly chemically stable iron powder. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125910. [Google Scholar] [CrossRef]
- Aziz, T.; Ullah, A.; Fan, H.; Jamil, M.I.; Khan, F.U.; Ullah, R.; Iqbal, M.; Ali, A.; Ullah, B. Recent progress in silane coupling agent with its emerging applications. J. Polym. Environ. 2021, 29, 3427–3443. [Google Scholar] [CrossRef]
- Wen, Z.; Xu, C.; Qian, X.; Zhang, Y.; Wang, X.; Song, S.; Dai, M.; Zhang, C. A two-step carbon fiber surface treatment and its effect on the interfacial properties of CF/EP composites: The electrochemical oxidation followed by grafting of silane coupling agent. Appl. Surf. Sci. 2019, 486, 546–554. [Google Scholar] [CrossRef]
- Gou, J.; Wang, G.; Ning, Y.; Guan, L.; Zhang, Y.; Liao, J.; Wang, Y. Preparation and corrosion resistance of chromium-free Zn-Al coatings with two different silane coupling agents. Surf. Coat. Technol. 2019, 366, 1–6. [Google Scholar] [CrossRef]
- Feng, Y.L.; Chen, X.Y.; Ma, Y.C.; Peng, S.S. Fabrication of an orderly layered nanostructure coating via cathodic EPD of silanized GO nanosheet for anti-corrosion protection. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125754. [Google Scholar] [CrossRef]
- Jena, G.; Anandkumar, B.; Sofia, S.; George, R.; Philip, J. Fabrication of silanized GO hybrid coating on 316L SS with enhanced corrosion resistance and antibacterial properties for marine applications. Surf. Coat. Technol. 2020, 402, 126295. [Google Scholar] [CrossRef]
- Shi, Y.; Nie, Y.; Xu, H.; Bi, P.; Chen, B.; Hou, X.; Ma, H.; Cao, M. Fabrication of silane-based surface coating with Ag/TiO2 hybridization and its anti-corrosion performance on metal surface. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132241. [Google Scholar] [CrossRef]
- Vembathurajesh, A.; Selvakumar, S.; Ramakrishnan, T.; Sundaram, M. Graphene applications in Unconventional Machining Processes—A review. Mater. Today Proc. 2022, 52, 1326–1330. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, X.; Yang, Q.; Cui, G.; Li, Z. The role of graphene in anti-corrosion coatings: A review. Constr. Build. Mater. 2021, 294, 123613. [Google Scholar] [CrossRef]
- Ding, R.; Chen, S.; Lv, J.; Zhang, W.; Zhao, X.D.; Liu, J.; Wang, X.; Gui, T.J.; Li, B.J.; Tang, Y.Z. Study on graphene modified organic anti-corrosion coatings: A comprehensive review. J. Alloys Compd. 2019, 806, 611–635. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R. Enhancing corrosion and wear performance of PEO coatings on Mg alloys using graphene and graphene oxide additions: A review. FlatChem 2021, 27, 100241. [Google Scholar] [CrossRef]
- Tang, S.; Lei, B.; Feng, Z.; Guo, H.; Zhang, P.; Meng, G. Progress in the Graphene Oxide-Based Composite Coatings for Anticorrosion of Metal Materials. Coatings 2023, 13, 1120. [Google Scholar] [CrossRef]
- Geng, Y.; Zhou, P.; Li, S.; Cao, J.; Zhou, Z.; Wu, Z.; Liu, A. Superior corrosion resistance of mild steel coated with graphene oxide modified silane coating in chlorinated simulated concrete solution. Prog. Org. Coat. 2022, 164, 106716. [Google Scholar] [CrossRef]
- Dun, Y.; Zuo, Y. Preparation and characterization of a GPTMS/graphene coating on AA-2024 alloy. Appl. Surf. Sci. 2017, 416, 492–502. [Google Scholar] [CrossRef]
- Obaid, A.N.; Al-Bermany, E. Performance of functionalized graphene oxide to improve anti-corrosion of epoxy coating on 2024-T3 aluminium alloy. Mater. Chem. Phys. 2023, 305, 127849. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.W.; Xie, Y.; Zhang, H.H.; Du, X.Q.; Zhang, Z. Preparation of hydrophobic silane/graphene oxide composite coating implanted with benzotriazole to improve the anti-corrosion performance of copper. J. Alloys Compd. 2022, 893, 162305. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y. Fabrication of dual self-healing silane nanocomposite for efficient corrosion protection of aluminum alloy. J. Alloys Compd. 2024, 983, 173890. [Google Scholar] [CrossRef]
- Ahangaran, F.; Navarchian, A.H.J.A.i.C.; Science, I. Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: A review. Adv. Colloid Interface Sci. 2020, 286, 102298. [Google Scholar] [CrossRef]
- Zhang, H.H.; Liu, Y.W.; Bian, H.; Zhang, Y.; Yang, Z.N.; Zhang, Z.; Chen, Y. Electrodeposition of silane/reduced graphene oxide nanocomposite on AA2024-T3 alloy with enhanced corrosion protection, chemical and mechanical stability. J. Alloys Compd. 2022, 911, 165058. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Zheng, X.; Lai, X.; Jia, R.; Yuan, X. Graphene oxide modified by zirconium dioxide to enhance the corrosion resistance of Zinc/Aluminum coatings. Diam. Relat. Mater. 2020, 108, 107868. [Google Scholar] [CrossRef]
- Du, X.Q.; Liu, Y.W.; Chen, D.C.; Zhang, Z.; Chen, Y. Co-electrodeposition of silane and graphene oxide on copper to enhance the corrosion protection performance. Surf. Coat. Technol. 2022, 436, 128279. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Xie, Y.; Zhang, H.H.; Zhang, Z. Preparation and anti-corrosion performance of superhydrophobic silane/graphene oxide composite coating on copper. Surf. Coat. Technol. 2021, 423, 127622. [Google Scholar] [CrossRef]
- Pandey, U.; Kumar, A.; Sharma, C. Comprehensive anti-corrosion study of electrodeposited graphene oxide-alumina composite with nickel matrix in NaCl solution. Diam. Relat. Mater. 2024, 141, 110653. [Google Scholar] [CrossRef]
- Du, W.C.; Wu, H.G.; Chen, H.W.; Xu, G.C.; Li, C. Graphene oxide in aqueous and nonaqueous media: Dispersion behaviour and solution chemistry. Carbon 2020, 158, 568–579. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.M. Electrophoretic deposition of graphene oxide on mild carbon steel for anti-corrosion application. Surf. Coat. Technol. 2014, 254, 167–174. [Google Scholar] [CrossRef]
- Yu, T.F.; Xu, Z.J.; Liu, S.Y.; Liu, H.; Yang, X.N. Enhanced hydrophilicity and water-permeating of functionalized graphene-oxide nanopores: Molecular dynamics simulations. J. Membr. Sci. 2018, 550, 510–517. [Google Scholar] [CrossRef]
- Marzo, F.; Alberro, M.; Manso, A.; Garikano, X.; Alegre, C.; Montiel, M.; Lozano, A.; Barreras, F. Evaluation of the corrosion resistance of Ni (P) Cr coatings for bipolar plates by electrochemical impedance spectroscopy. Int. J. Hydrogen Energy 2020, 45, 20632–20646. [Google Scholar] [CrossRef]
- Pantoja, M.; Díaz-Benito, B.; Velasco, F.; Abenojar, J.; Del Real, J.C. Analysis of hydrolysis process of γ-methacryloxypropyltrimethoxysilane and its influence on the formation of silane coatings on 6063 aluminum alloy. Appl. Surf. Sci. 2009, 255, 6386–6390. [Google Scholar] [CrossRef]
- Wan, C.Y. Corrosion assessment of Sn–Ni alloy coatings using neutral salt spray tests and electrochemical methods. Int. J. Electrochem. Sci. 2020, 15, 26–38. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Gao, M.; Kang, K.H.; Kim, C.L.; Kim, D.E. Selective release of less defective graphene during sliding of an incompletely reduced graphene oxide coating on steel. Carbon 2018, 134, 411–422. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coatings. Prog. Org. Coat. 2017, 111, 47–56. [Google Scholar] [CrossRef]

| Ecorr (V) | Icorr (A/cm2) | Rp (Ω·cm2) | CR (mm/year) | Ba (mV/dec) | Bc (mV/dec) | |
|---|---|---|---|---|---|---|
| Q235 steel substrate | −0.499 | 5.030 × 10−5 | 1.765 × 10−4 | 7.372 × 10−3 | 120 | −85 |
| Epoxy resin coating | −0.391 | 3.609 × 10−6 | 4.191 × 10−4 | 1.12 8 × 10−3 | 115 | −78 |
| KH560-GO | −0.315 | 5.855 × 10−6 | 2.844 × 10−4 | 1.918 × 10−3 | 105 | −70 |
| KH560-GO/EP | −0.239 | 6.157 × 10−7 | 6.876 × 10−3 | 7.370 × 10−4 | 95 | −65 |
| Rct (Ω·cm2) | Cdl (F/cm2) | n | |Z| 0.01 Hz (Ω·cm2) | |
|---|---|---|---|---|
| Q235 steel substrate | 1.2 × 103 | 2.5 × 10−5 | 0.78 | 8.6 × 102 |
| Epoxy resin coating | 5.8 × 103 | 1.3 × 10−6 | 0.82 | 9.2 × 103 |
| KH560-GO | 1.5 × 104 | 6.4 × 10−7 | 0.85 | 2.7 × 104 |
| KH560-GO/EP | 6.9 × 104 | 2.1 × 10−7 | 0.89 | 9.4 × 104 |
| Quantity Before Corrosion (g) | Quality After Corrosion (g) | Area (cm2) | Time (h) | Rate (g/(m2/h)) | |
|---|---|---|---|---|---|
| 1 | 12.997 | 12.942 | 0.08 | 48 | 1.432 ± 0.121 |
| 2 | 11.846 | 11.799 | 0.08 | 48 | 1.224 ± 0.098 |
| 3 | 12.769 | 12.755 | 0.08 | 48 | 0.365 ± 0.034 |
| Quantity Before Corrosion (g) | Quality After Corrosion (g) | Area (cm2) | Time (h) | Rate (g/(m2/h)) | |
|---|---|---|---|---|---|
| unwiped | 12.77 | 12.755 | 0.08 | 48 | 0.365 ± 0.029 |
| wiped | 12.77 | 12.741 | 0.08 | 48 | 0.729 ± 0.067 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.-R.; Yao, J.-T.; Dong, H.; Chen, Z.-L.; Liu, X.-G. Enhancing the Corrosion Resistance of Passivation Films via the Synergistic Effects of Graphene Oxide and Epoxy Resin. Coatings 2025, 15, 444. https://doi.org/10.3390/coatings15040444
Wu B-R, Yao J-T, Dong H, Chen Z-L, Liu X-G. Enhancing the Corrosion Resistance of Passivation Films via the Synergistic Effects of Graphene Oxide and Epoxy Resin. Coatings. 2025; 15(4):444. https://doi.org/10.3390/coatings15040444
Chicago/Turabian StyleWu, Bo-Rui, Jian-Tao Yao, Hui Dong, Ze-Lin Chen, and Xiao-Gang Liu. 2025. "Enhancing the Corrosion Resistance of Passivation Films via the Synergistic Effects of Graphene Oxide and Epoxy Resin" Coatings 15, no. 4: 444. https://doi.org/10.3390/coatings15040444
APA StyleWu, B.-R., Yao, J.-T., Dong, H., Chen, Z.-L., & Liu, X.-G. (2025). Enhancing the Corrosion Resistance of Passivation Films via the Synergistic Effects of Graphene Oxide and Epoxy Resin. Coatings, 15(4), 444. https://doi.org/10.3390/coatings15040444






