A Contribution of XPS and Electrochemistry to the Understanding of Hydrogen Diffusion in X60 Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
- Procedure 1: Five solvent cleaning.
- Procedure 2: Five solvents cleaning and mechanical polishing
2.3. XPS Analysis
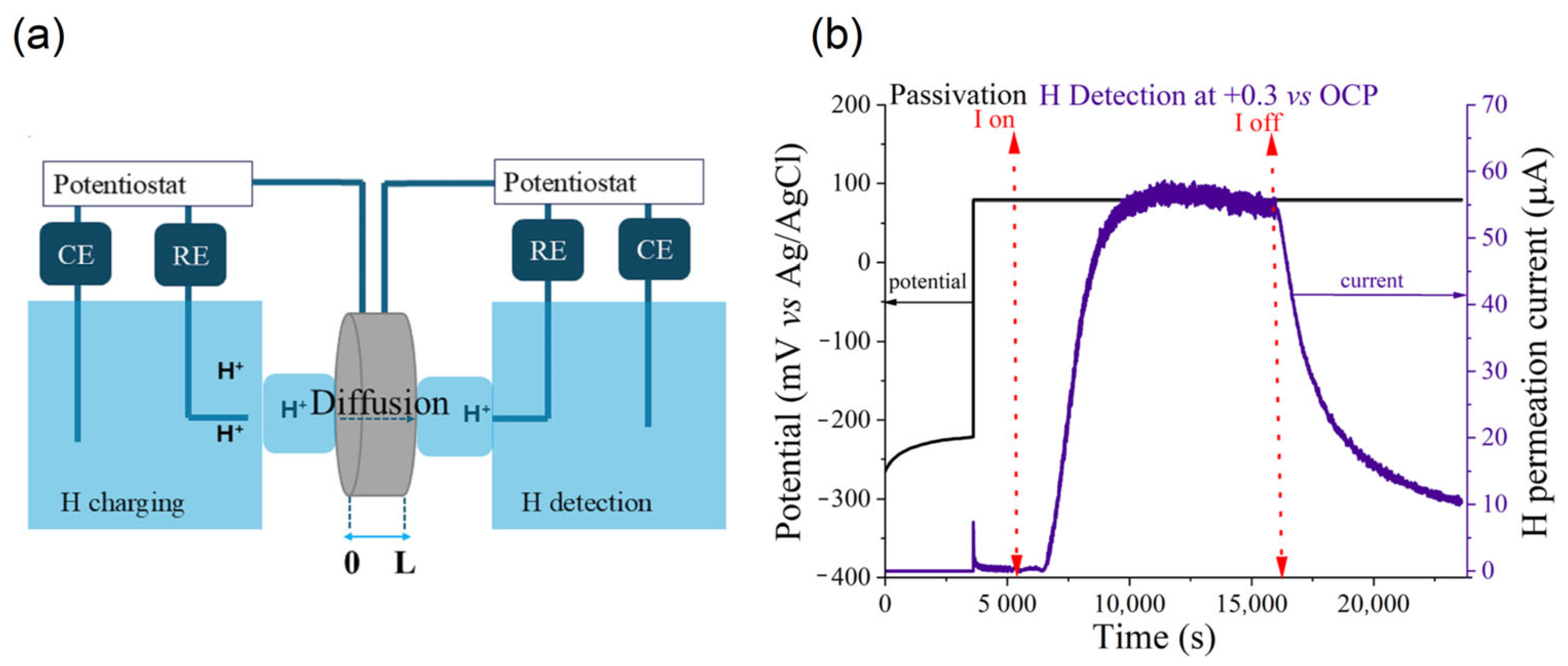
2.4. Electrochemical Permeation Test
- tlag method:
- Breakthrough method:where L is the thickness in meters of the sample, tlag is the time (s) required to achieve 63% of steady state current density (iss), and tb is the breakthrough time (s) that corresponds to the intersection of the tangent line at the inflection point and the x-axis in the permeation curve.
3. Results
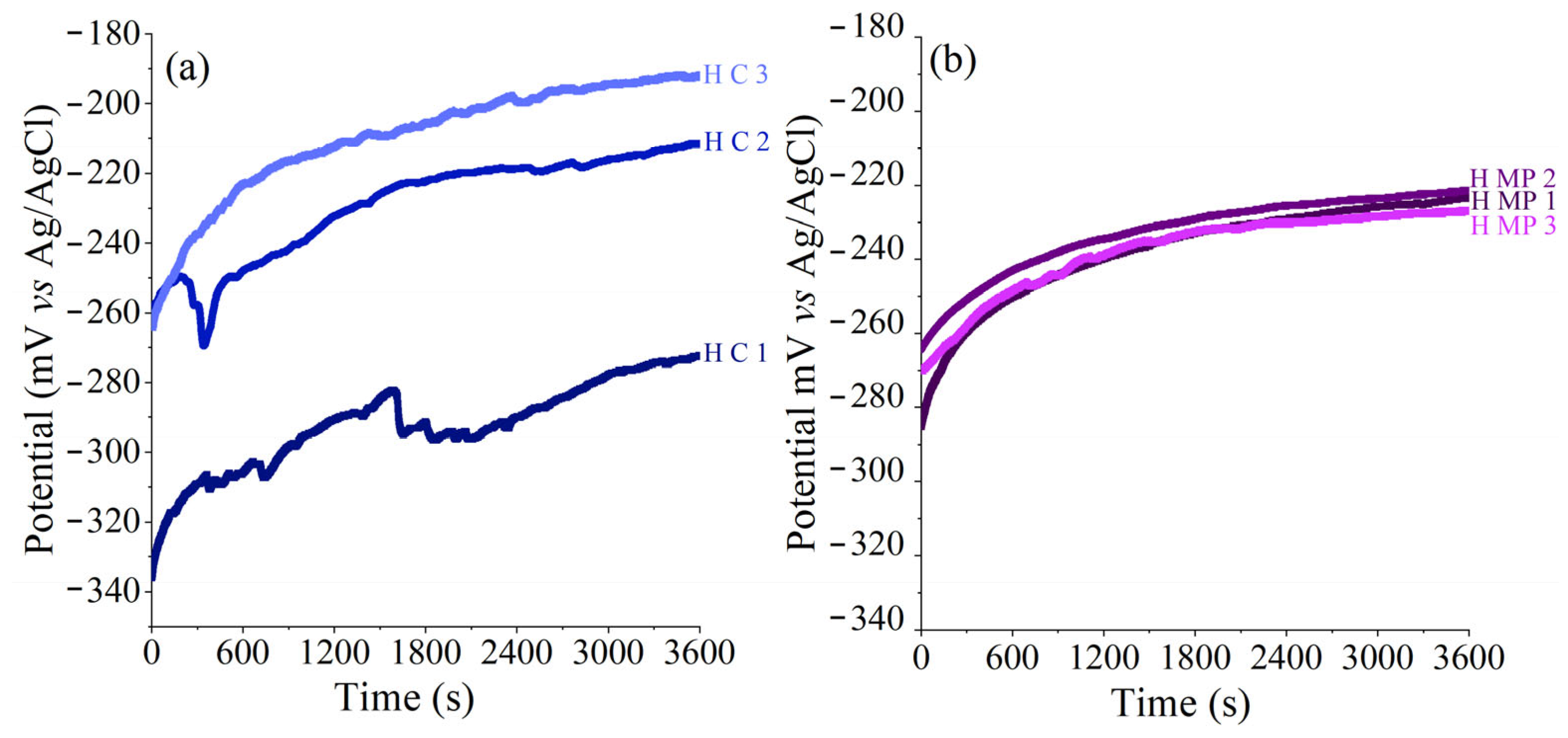
3.1. Conditioning of the Sample Surface Exposed to the Detection Side
3.2. Conditioning of the Sample Surface Exposed to the Production Side
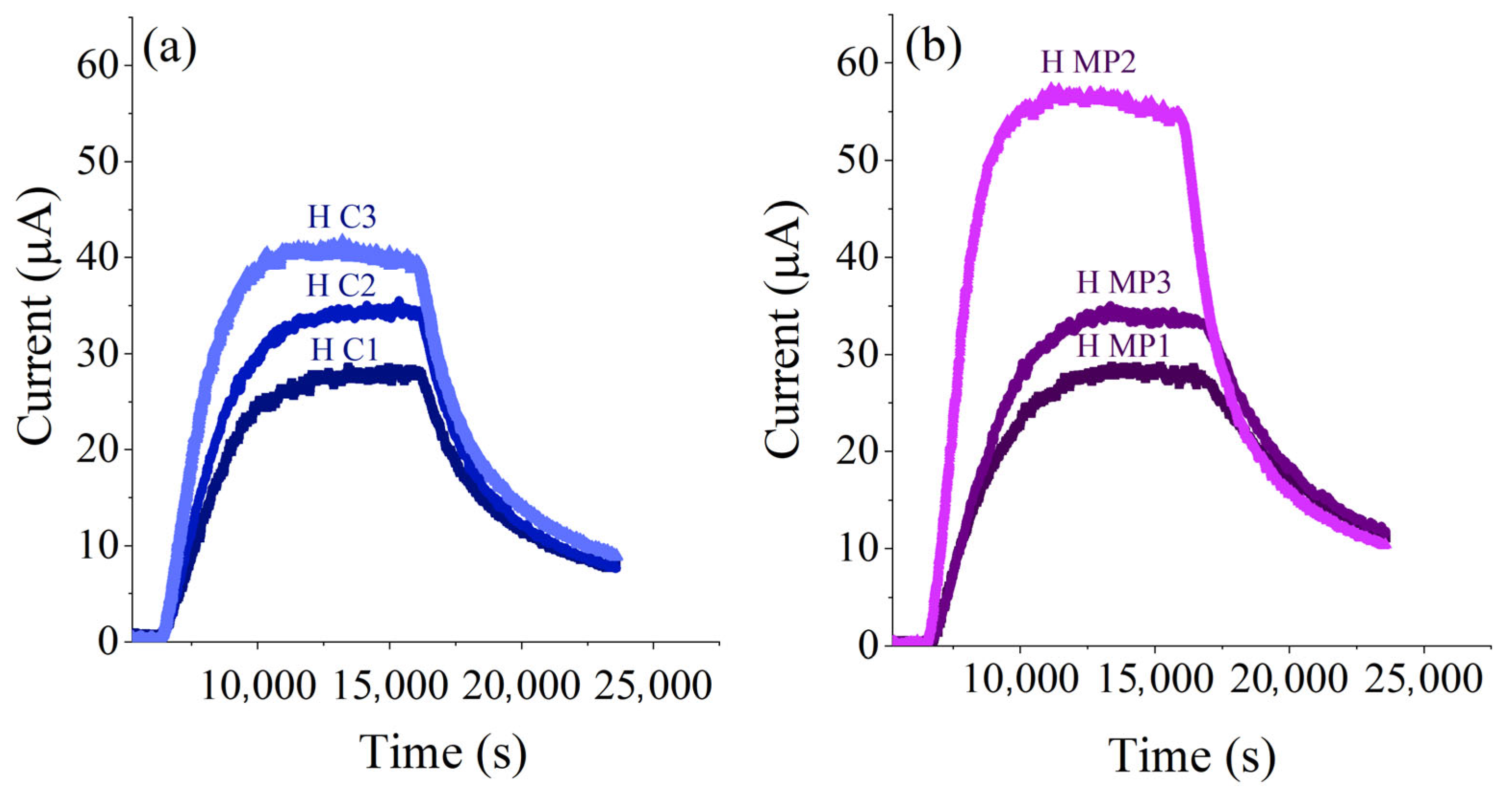
3.3. Hydrogen Permeation Results
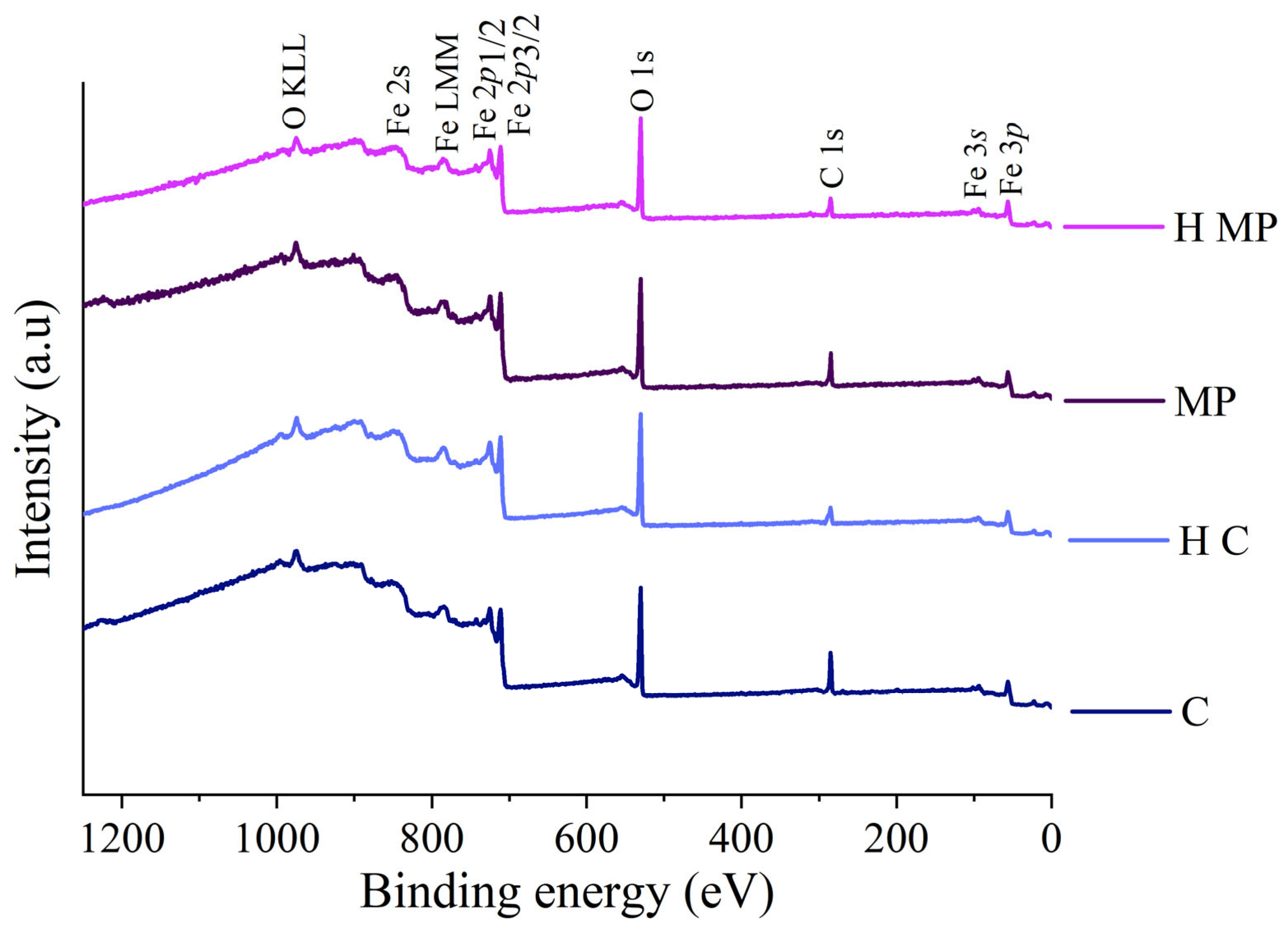
3.4. XPS Results
4. Discussion
4.1. Surface Composition and Homogeneity
4.2. Hydrogen Diffusion Coefficient of X60
4.3. Hydrogen Sub-Surface Concentration (C0) of X60 Steel
4.4. Influence of Steel Microstructure and Composition
5. Conclusions
- XPS analysis revealed the presence of a film composed of Fe (II) oxide, Fe (III) oxide, and Fe (III) oxyhydroxide on the steel substrate on both organic-solvents cleaned and mechanically polished X60 steel samples. The composition and the film thickness formed upon heating at 80 °C for 15 h at the surface of mechanically polished samples are more reproducible than in the case of films present on the steels following the cleaning procedure with organic solvents. The reproducibility of the sample preparation ensures the reproducibility of the electrochemical results.
- The effective diffusion coefficients Deff of both H C X60 and H MP X60 steels, calculated using tlag and tb methods, are in agreement with the literature data. Deff depends on the bulk properties of the steel; indeed, Deff of X60 steel seems to be unaffected by the presence of a nanometric thick film constituted of iron oxy-hydroxides. The lower Deff value of X60 steel compared to pure iron might be explained by the presence of higher carbon content or other alloying elements changing the microstructure.
- The subsurface hydrogen concentration C0 in X60 steel was found to be equal to 0.66 (0.02) ppm for C X60 steel and to 0.8 (0.1) ppm for MP X60 steel is lower than in high-carbon steels and other high-strength pipeline steels such as X80. This may be attributed to its specific microstructure, which includes bainite and possible martensite with globular carbides.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HE | Hydrogen Embrittlement |
| HPB | Hydrogen Permeation Barrier |
| HPT | Hydrogen Permeation Test |
| tlag | Time lag |
| tb | Breakthrough time |
| Deff | Effective hydrogen diffusion coefficient |
| C0 | Subsurface hydrogen concentration |
| OCP | Open Circuit Potential |
| MP | Mechanically Polished |
| C | Cleaned |
| H MP | Heated MP |
| H C | Heated Cleaned |
| BCC | Body-centered cubic |
| XRD | X ray Diffraction |
| SEM | Scanning Electron Microscopy |
| XPS | X ray Photoelectron Spectroscopy |
| FWHM | Full Width at Half-Maximum |
| BE | Binding Energy |
References
- Heiniger, S.P.; Fan, Z.; Lustenberger, U.B.; Stark, W.J. Safe Seasonal Energy and Hydrogen Storage in a 1: 10 Single-Household-Sized Pilot Reactor Based on the Steam-Iron Process. Sustain. Energy Fuels 2024, 8, 125–132. [Google Scholar] [CrossRef]
- Haller, M.Y.; Carbonell, D.; Dudita, M.; Zenhäusern, D.; Häberle, A. Seasonal Energy Storage in Aluminium for 100 Percent Solar Heat and Electricity Supply. Energy Convers. Manage X 2020, 5, 100017. [Google Scholar] [CrossRef]
- Mahajan, D.; Tan, K.; Venkatesh, T.; Kileti, P.; Clayton, C.R. Hydrogen Blending in Gas Pipeline Networks—A Review. Energies 2022, 15, 3582. [Google Scholar] [CrossRef]
- Ohaeri, E.; Eduok, U.; Szpunar, J. Hydrogen Related Degradation in Pipeline Steel: A Review. Int. J. Hydrogen Energy 2018, 43, 14584–14617. [Google Scholar] [CrossRef]
- Negi, A.; Elkhodbia, M.; Barsoum, I.; AlFantazi, A. Coupled Analysis of Hydrogen Diffusion, Deformation, and Fracture: A Review. Int. J. Hydrogen Energy 2024, 82, 281–310. [Google Scholar] [CrossRef]
- He, Y.; Su, Y.; Yu, H.; Chen, C. First-Principles Study of Hydrogen Trapping and Diffusion at Grain Boundaries in γ-Fe. Int. J. Hydrogen Energy 2021, 46, 7589–7600. [Google Scholar] [CrossRef]
- Faucon, L.E.; Boot, T.; Riemslag, T.; Scott, S.P.; Liu, P.; Popovich, V. Hydrogen-Accelerated Fatigue of API X60 Pipeline Steel and Its Weld. Metals 2023, 13, 563. [Google Scholar] [CrossRef]
- Contreras, A.; Hernández, S.L.; Galvan-Martinez, R.; Vega-Becerra, O. Tension Tests Behavior of API 5L X60 Pipeline Steel in a Simulated Soil Solution to Evaluate SCC Susceptibility. MRS Online Proc. Libr. 2012, 1481, 1–10. [Google Scholar] [CrossRef]
- Walallawita, R.; Hinchliff, M.C.; Sediako, D.; Quinn, J.; Chou, V.; Walker, K.; Hill, M. Evaluating the Effect of Blended and Pure Hydrogen in X60 Pipeline Steel for Low-Pressure Transmission Using Hollow-Specimen Slow-Strain-Rate Tensile Testing. Metals 2024, 14, 1132. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Eskandari, M.; Rahman, K.M.M.; Ouellet, R.; Szpunar, J.A. An Extensive Study of Hydrogen-Induced Cracking Susceptibility in an API X60 Sour Service Pipeline Steel. Int. J. Hydrogen Energy 2016, 41, 4185–4197. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, H.; Dong, X.; Teng, C.; Zhang, A.; Kong, S.; Zhou, J.; Zhang, X.-C.; Tu, S.-T. Current Challenges in the Utilization of Hydrogen Energy-a Focused Review on the Issue of Hydrogen-Induced Damage and Embrittlement. Adv. Appl. Energy 2024, 14, 100168. [Google Scholar] [CrossRef]
- Hoschke, J.; Chowdhury, M.F.W.; Venezuela, J.; Atrens, A. A Review of Hydrogen Embrittlement in Gas Transmission Pipeline Steels. Corros. Rev. 2023, 41, 277–317. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Huang, C.; Liu, P.-Y.; Yen, H.-W.; Niu, R.; Burr, P.; Moore, K.L.; Martínez-Pañeda, E.; Atrens, A.; Cairney, J.M. Hydrogen Trapping and Embrittlement in Metals—A Review. Int. J. Hydrogen Energy 2024, in press. [CrossRef]
- Young, G. Hydrogen Embrittlement in Nuclear Power Systems. In Gaseous Hydrogen Embrittlement of Materials in Energy Technologies; Woodhead Publishing: Sawston, UK, 2011; ISBN 978-1-84569-673-3. [Google Scholar]
- Devanathan, M.A.V.; Stachurski, Z.; Tompkins, F.C. The Adsorption and Diffusion of Electrolytic Hydrogen in Palladium. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1997, 270, 90–102. [Google Scholar] [CrossRef]
- Zakroczymski, T. Adaptation of the Electrochemical Permeation Technique for Studying Entry, Transport and Trapping of Hydrogen in Metals. Electrochim. Acta 2006, 51, 2261–2266. [Google Scholar] [CrossRef]
- API 5L X60 Pipe Specifications (PSL1 & PSL2)—Octal Steel. Available online: https://www.octalsteel.com/resources/api-5l-gr-x60-pipe/ (accessed on 18 March 2025).
- SPECTRO xSORT XHH03 XRF Handheld Analyzer. Available online: https://www.spectro.com/products/xrf-handheld-analyzer/www.spectro.com/products/xrf-handheld-analyzer/xsort-xhh03 (accessed on 18 March 2025).
- ISO 15472:2010; Surface Chemical Analysis—X-Ray Photoelectron Spectrometers—Calibration of Energy Scales. International Organization for Standardization: Geneva, Switzerland, 2010. Available online: https://www.iso.org/standard/55796.html (accessed on 30 January 2025).
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and Collaborative Approach to Problem Solving Using X-Ray Photoelectron Spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Hannachi, R.; Biggio, D.; Elsener, B.; Fantauzzi, M.; Rossi, A. X-Ray Photoelectron Spectroscopy Investigation of X60 Steel. Surf. Sci. Spectra 2024, 31, 024014. [Google Scholar] [CrossRef]
- Rossi, A.; Eisener, B. XPS Analysis of Passive Films on the Amorphous Alloy Fe70 Cr10 P13 C7: Effect of the Applied Potential. Surf. Interface Anal. 1992, 18, 499–504. [Google Scholar] [CrossRef]
- Ypma, T.J. Historical Development of the Newton-Raphson Method. SIAM Rev. 1995, 37, 531–551. [Google Scholar] [CrossRef]
- Koren, E.; Hagen, C.M.H.; Wang, D.; Lu, X.; Johnsen, R.; Yamabe, J. Experimental Comparison of Gaseous and Electrochemical Hydrogen Charging in X65 Pipeline Steel Using the Permeation Technique. Corros. Sci. 2023, 215, 111025. [Google Scholar] [CrossRef]
- Van den Eeckhout, E.; De Baere, I.; Depover, T.; Verbeken, K. The Effect of a Constant Tensile Load on the Hydrogen Diffusivity in Dual Phase Steel by Electrochemical Permeation Experiments. Mater. Sci. Eng. A 2020, 773, 138872. [Google Scholar] [CrossRef]
- Ellison, S.L.R.; Williams, A. Eurachem/CITAC Guide: Quantifying Uncertainty in Analytical Measurement, 3rd ed.; Eurachem/CITAC: Teddington, UK, 2012; ISBN 978-0-948926-30-3. [Google Scholar]
- Casanova, T.; Crousier, J. The Influence of an Oxide Layer on Hydrogen Permeation through Steel. Corros. Sci. 1996, 38, 1535–1544. [Google Scholar] [CrossRef]
- Biggio, D.; Elsener, B.; Usai, G.; Fantauzzi, M.; Rossi, A. Surface Chemistry of Passive Films on Ni-Free Stainless Steel: The Effect of Organic Components in Artificial Saliva. Langmuir 2024, 40, 6824–6833. [Google Scholar] [CrossRef] [PubMed]
- Elsener, B.; Pisu, M.; Fantauzzi, M.; Addari, D.; Rossi, A. Electrochemical and XPS Surface Analytical Study on the Reactivity of Ni-Free Stainless Steel in Artificial Saliva. Mater. Corros. 2016, 67, 591–599. [Google Scholar] [CrossRef]
- Rossi, A.; Elsener, B.; Hähner, G.; Textor, M.; Spencer, N.D. XPS, AES and ToF-SIMS Investigation of Surface Films and the Role of Inclusions on Pitting Corrosion in Austenitic Stainless Steels. Surf. Interface Anal. 2000, 29, 460–467. [Google Scholar] [CrossRef]
- Rossi, A.; Elsener, B.; Spencer, N.D. XPS Surface Analysis: Imaging and Spectroscopy of Metal and Polymer Surfaces; Spectroscopy Europe/World: West Sussex, UK, 2004; Volume 16. [Google Scholar]
- Samanta, S.; Kumari, P.; Mondal, K.; Dutta, M.; Singh, S.B. An Alternative and Comprehensive Approach to Estimate Trapped Hydrogen in Steels Using Electrochemical Permeation Tests. Int. J. Hydrogen Energy 2020, 45, 26666–26687. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Zhang, H.X. A New Perspective on Hydrogen Diffusion and Hydrogen Embrittlement in Low-Alloy High Strength Steel. Corros. Sci. 2020, 174, 108800. [Google Scholar] [CrossRef]
- Frappart, S.; Feaugas, X.; Creus, J.; Thebault, F.; Delattre, L.; Marchebois, H. Study of the Hydrogen Diffusion and Segregation into Fe–C–Mo Martensitic HSLA Steel Using Electrochemical Permeation Test. J. Phys. Chem. Solids 2010, 71, 1467–1479. [Google Scholar] [CrossRef]
- Fallahmohammadi, E.; Bolzoni, F.; Lazzari, L. Measurement of Lattice and Apparent Diffusion Coefficient of Hydrogen in X65 and F22 Pipeline Steels. Int. J. Hydrogen Energy 2013, 38, 2531–2543. [Google Scholar] [CrossRef]
- Hadam, U.; Zakroczymski, T. Absorption of Hydrogen in Tensile Strained Iron and High-Carbon Steel Studied by Electrochemical Permeation and Desorption Techniques. Int. J. Hydrogen Energy 2009, 34, 2449–2459. [Google Scholar] [CrossRef]
- Drexler, A.; Siegl, W.; Ecker, W.; Tkadletz, M.; Klösch, G.; Schnideritsch, H.; Mori, G.; Svoboda, J.; Fischer, F.D. Cycled Hydrogen Permeation through Armco Iron—A Joint Experimental and Modeling Approach. Corros. Sci. 2020, 176, 109017. [Google Scholar] [CrossRef]
- Yao, C.; Ming, H.; Chen, J.; Wang, J.; Han, E.-H. Effect of Cold Deformation on the Hydrogen Permeation Behavior of X65 Pipeline Steel. Coatings 2023, 13, 280. [Google Scholar] [CrossRef]
- Xue, H.B.; Cheng, Y.F. Hydrogen Permeation and Electrochemical Corrosion Behavior of the X80 Pipeline Steel Weld. J. Mater. Eng Perform 2013, 22, 170–175. [Google Scholar] [CrossRef]




| Fe | Mn | Cr | Mo | Ni | Cu | S | P | |
|---|---|---|---|---|---|---|---|---|
| Wt.% | 98.2 (0.1) | 1.20 (0.04) | 0.20 (0.02) | 0.10 (0.01) | 0.10 (0.01) | 0.10 (0.01) | <LoD | <LoD |
| OCP (1 h, 0.1 M NaOH) (mV vs. Ag/AgCl) | Passivation Current (After 1 h) (µA) | |
|---|---|---|
| H C X60 steel | −226 (42) | 0.4 (0.3) |
| H MP X60 steel | −224 (3) | 0.5 (0.2) |
| Deff-tlag Method (10−10 m2/s) | Deff-tb Method (10−10 m2/s) | C0 (ppm a) | |
|---|---|---|---|
| H C X60 steel | 2.4 (0.4) | 2.8 (0.2) | 0.66 (0.02) |
| H MP X60 steel | 2.0 (0.4) | 2.9 (0.5) | 0.8 (0.1) |
| Elements | C X60 Steel Peak Energy (eV) | Heated C X60 Steel Peak Energy (eV) | MP X60 Steel Peak Energy (eV) | Heated C X60 Steel Peak Energy (eV) |
|---|---|---|---|---|
| O 1s–Oxide | 530.1 (0.1) | 530.2 (0.1) | 530.1 (0.1) | 530.2 (0.1) |
| O 1s–Hydroxide | 531.4 (0.1) | 531.4 (0.1) | 531.4 (0.1) | 531.5 (0.1) |
| O 1s–Water | 532.2 (0.2) | 532.2 (0.1) | 532.2 (0.1) | 532.3 (0.1) |
| O 1s–Organic | 533.5(0.1) | 533.4 (0.1) | 533.5 (0.1) | 533.5 (0.1) |
| contamination | ||||
| Fe 2p3/2–Fe (0) | 706.8 (0.1) | 707.0 (0.1) | 706.8 (0.1) | 707.0 (0.1) |
| Fe 2p3/2–FeO | 709.4 (0.1) | 709.4 (0.2) | 709.3 (0.1) | 709.4 (0.1) |
| Fe 2p3/2–FeO sat | 714.9 (0.1) | 714.9 (0.2) | 714.8 (0.1) | 714.9 (0.1) |
| Fe 2p3/2–Fe2O3 | 710.6 (0.1) | 710.7 (0.1) | 710.6 (0.1) | 710.6 (0.1) |
| Fe 2p3/2–Fe (III)-OOH | 712.5 (0.1) | 712.6 (0.1) | 712.5 (0.1) | 712.4 (0.1) |
| C X60 Steel | Heated C X60 Steel | MP X60 Steel | Heated MP X60 Steel | |
|---|---|---|---|---|
| lc (nm) | 3.5 (0.8) | 2.7 (0.3) | 2.9 (0.5) | 3.3 (0.2) |
| t (nm) | 4.1 (0.5) | 6.8 (0.9) | 5.5 (0.7) | 7.7 (0.5) |
| Atom % FeO | 5 (2) | 0.5 (0.7) | 4 (3) | 0.9 (0.9) |
| Atom % Fe2O3 | 60 (2) | 64 (3) | 62 (2) | 64 (2) |
| Atom % FeOOH | 35 (3) | 36 (3) | 34 (3) | 35 (2) |
| * C0 (ppm) | * C0 mol H/m3 | Ref. | |
|---|---|---|---|
| Heated C X60 steel | 0.66 (0.02) | 5.2 (1.4) | This work |
| Heated MP X60 steel | 0.8 (0.1) | 6.3 (0.8) | This work |
| LAHSS steel T640 | 0.21 | 1.65 | [33] |
| LAHSS steel T600 | 0.87 | 6.85 | [33] |
| LAHSS steel T560 | 1.52 | 11.96 | [33] |
| High carbon steel | 6.09 | 47.5 | [36] |
| Weld X80 steel | 2.21 | 17.43 | [39] |
| HAZ X80 steel | 2.12 | 16.71 | [39] |
| Base X80 steel | 1.54 | 12.12 | [39] |
| Annealed iron | 0.022 | 0.17 | [37] |
| Armco Iron | 0.085 | 0.662 | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannachi, R.; Biggio, D.; Elsener, B.; Fantauzzi, M.; Zacchetti, N.; Rossi, A. A Contribution of XPS and Electrochemistry to the Understanding of Hydrogen Diffusion in X60 Steel. Coatings 2025, 15, 442. https://doi.org/10.3390/coatings15040442
Hannachi R, Biggio D, Elsener B, Fantauzzi M, Zacchetti N, Rossi A. A Contribution of XPS and Electrochemistry to the Understanding of Hydrogen Diffusion in X60 Steel. Coatings. 2025; 15(4):442. https://doi.org/10.3390/coatings15040442
Chicago/Turabian StyleHannachi, Raouaa, Deborah Biggio, Bernhard Elsener, Marzia Fantauzzi, Nicoletta Zacchetti, and Antonella Rossi. 2025. "A Contribution of XPS and Electrochemistry to the Understanding of Hydrogen Diffusion in X60 Steel" Coatings 15, no. 4: 442. https://doi.org/10.3390/coatings15040442
APA StyleHannachi, R., Biggio, D., Elsener, B., Fantauzzi, M., Zacchetti, N., & Rossi, A. (2025). A Contribution of XPS and Electrochemistry to the Understanding of Hydrogen Diffusion in X60 Steel. Coatings, 15(4), 442. https://doi.org/10.3390/coatings15040442






