Abstract
Microbial fuel cell (MFC) is a bioelectrochemical device for biomass power generation, and the anode material determines the performance of the MFC. In this study, a novel anode material, which is a combination of graphite oxide/polythiophene (GO/Pth), was prepared on a carbon felt (CF) substrate and exhibited excellent capacitive performance. The MFC equipped with the CF/GO/Pth anode achieved a significant increase in power density, reaching a maximum value of 2.9 W/m3, which is a 3.32-fold increase in power density compared to that of the CF anode. Meanwhile, the CF/GO/Pth anode stored charge Qt value was as high as 11,258.68 C/m2, which was 4.13 times higher than that of the CF anode (2727.66 C/m2). High-throughput analysis showed that the percentage of charge-producing bacteria on the surface of the CF/GO/Pth anode was more than 90%, which was significantly higher than that of the charge-producing bacteria attached to the CF anode. This further confirms the significant enhancement of MFC performance by materials such as GO and Pth coated on the CF surface. In this study, CF/GO/Pth anode materials were prepared to successfully enhance the power output and charge storage capacity of MFC, and they also showed broad application prospects in the degradation of polluted waste liquids.
1. Introduction
Against the background of the current increasingly severe water pollution situation, the efficient conversion of abundant pollutants in wastewater into valuable resources has become a hot topic in the research field. In this context, Microbial Fuel Cell (MFC) technology, as an innovative biological treatment technology, is gradually emerging and showing broad prospects for treating polluted waste liquids [1,2,3,4]. Compared with traditional methods, microbial fuel cells have the advantages of high material adaptability, no secondary pollution, high energy conversion rate, easy operation, etc., and show great potential for application in the field of waste liquid treatment. At the same time, microbial fuel cells have some drawbacks: the current output power of MFC is often difficult to reach the standard of large-scale application, and there are stability problems during operation, which limits its wide application in the field of industrial wastewater treatment and other fields, and secondly, the electricity generated by MFC is instantaneous and has no built-in storage mechanism, so it is difficult to meet the continuous power supply needs. These shortcomings limit the use of MFCs in applications that require a stable power output. There are many reasons that affect the power output, among which the anode is one of the most important parts that affect the performance of MFC power production. During the operation of MFC, microorganisms are responsible for decomposing organic matter and generating electrons, while the anode acts as the main acceptor of these electrons and is responsible for receiving the electrons released by microbial metabolism [5]. In addition, after attaching to the anode surface, electroproducing bacteria secrete extracellular polymers to form a biofilm, which is essential for the stable production of electricity in MFC. At the same time, the biocompatibility and structural design of the anode material will directly affect the stability of the biofilm; therefore, the use of highly biocompatible anode materials can more effectively maintain the stability of the biofilm, thus ensuring that the MFC can continue to produce electricity stably. The anode material determines the performance and cost of MFC [6].
Among many materials, carbon materials have the characteristics of low price, large specific surface area, and good electrochemical properties; therefore they are often used as the anode material in MFC. Carbon materials include carbon paper, carbon cloth, carbon felt, and carbon brush [7]. Carbon felt is favored because of its loose structure and large pores, which enable the charge to be distributed uniformly, and also because it has the characteristics of good biocompatibility. The carbon felt is widely favored because of its loose structure and large pores, which make the charge distributed uniformly, and also has the characteristics of good biocompatibility. Xu et al. used continuous carbon fiber as the anode of microbial fuel cell, which improved the power density of MFC and the internal electron transfer efficiency, and at the same time, shortened the distance between the cathode and anode electrodes, and increased the maximum power density to 5.81 mW/m3 [8]. Aelterman et al. obtained a maximum output power of 386 Wm−3 by utilizing carbon felts (CF) as an anode for MFC [9]. Nonetheless, the conductivity of the MFC anode using only a single carbon material as the anode is still low, which may lead to a certain resistance when electrons are transported inside the anode material, thus causing energy loss and reducing the output power and efficiency of the battery. Graphite oxide has good electrical conductivity [10] and the advantages of high plasticity and low cost. This makes graphite oxide become a very popular research focus recently, especially because it has great application prospects in the field of new energy. Huang et al. [11] loaded graphene and polyaniline on the surface of the carbon cloth as the anode, and obtained the output voltage of the MFC was 573 mV, and the maximum power density was 884 mW/m2. Liu et al. [12] electrochemically reduced graphene oxide to the surface of carbon cloth in the laboratory and prepared graphene-modified carbon cloth electrodes for MFC anode application with remarkable results. The maximum power density increased by 270%. Yang et al. [13] used graphene-coated sponge as MFC anode and the power density was 4.1 times higher than that of carbon cloth under the same condition. Hou et al. [14] used graphene and polyaniline composite modification of carbon cloth as an MFC anode, and the output power was three times higher than that of carbon cloth alone. Lv et al. [15] used a one-step deposition method to synthesize a polypyrrole/reduced graphite oxide composite electrode as an MFC anode with an output power of up to 1100 mW/m2 Although graphite oxide can enhance the performance of the MFC, graphite oxide stores charge mainly through the functional groups on its surface and interlayer structure. However, the relatively limited number of surface functional groups, such as hydroxyl and carboxyl groups, and the limited degree of redox reactions that these functional groups participate in during the charging and discharging process lead to a specific capacitance that cannot be compared with that of some specialized high-performance energy storage materials; therefore, it is necessary to add pseudo-capacitive materials on top of the graphite oxide to improve the energy storage characteristics of MFC. In order to improve the energy storage and power production performance of MFCs, the anode is often modified with conductive polymers, among which polypyrrole (PPy), polyaniline (PANI), and polythiophene (Pth) are commonly used modified anode conductive polymers. These conductive polymers can significantly improve the output power density and stability of MFCs by modifying them on the anode surface using specific methods. Roh and Woo [16] used in situ chemical polymerization method to dope PPy on CNT, and subsequently deposited CNT-PPy complexes on CF electrodes, which were then applied as anodes in MFC. The results showed that the polymer composite modified anode had lower resistance and the power density of the MFC increased by 36% compared to that of the unmodified CF electrode. Kang et al. [17] electrochemically polymerized PEDOT on CC electrode and the polymer helped to increase the redox activity of the anode and decrease the anode resistance, which resulted in 43% increase in power density. Jayesh M. Sonawane et al. [18] modified two conductive polymers, polypyrrole (PPy) and polyaniline (PANi), on the surface of stainless steel mesh to be used as microbial The maximum current densities and maximum power densities of the pristine SS-W, SS/PPy-W and SS/PANi-W anodes in the MFC were found to be around 0.30, 0.56 and 0.67 mW/cm2 and 0.127, 0.187 and 0.288 mA/cm2, respectively. Among many polymers, polythiophene (Pth) is considered to be one of the MFC electrode materials with outstanding performance, and the molecular chain structure of polythiophene and its derivatives is easy to regulate and process compared with other conjugated polymers, such as polypyrrole (PPy), and polyaniline (PANI). The polythiophene electrodes obtained by chemical polymerization by the Laforgue group had a specific capacitance value of 40 mAhg−1, and the capacitance retention was still high after 500 charging and discharging cycles [19]. Ambade [20] obtained a fibrous polythiophene electrode via electrochemical polymerization, which was assembled into a supercapacitor with a specific capacitance of 1357.31 mFg−1. The capacitance retention was 97% after 3000 charge and discharge cycles. Nejati [21] obtained a polythiophene electrode using oxidative vapor deposition. The research group synthesized ultrathin thiophene films by oxidative vapor deposition and tested the electrochemical properties of the films by loading them onto different substrates. The polythiophene film loaded on an activated carbon electrode showed a 50% higher specific capacitance value than the self-supported film and a capacitance retention of 90% after 5000 charging and discharging cycles.
In this paper, capacitive composites of CF/Go/Pth were prepared to utilize composite electrode materials to provide more active sites, thus promoting electron transfer between microorganisms and electrodes, and at the same time enhancing the conductivity and stability of the electrodes, which significantly improved the power density and energy storage capacity of the MFC. In addition, the constructed MFC can operate realize intermittently, storing electric charge when it is not in operation and releasing the stored charge when it is in operation, thus releasing a larger current. The improved MFC combines both power generation and energy storage capacity and realizes the resourceful use of wastewater and energy recovery, contributing to environmental protection and sustainable development.
2. Materials and Methods
2.1. Preparation of CF/Go/Pth Electrode
First, 0.2 g GO (carbon content: 40% Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China) was added to 40 mL of glucose solution (1 mg/mL) (Shandong Keyuan Biochemical Co., Ltd., Laizhou, China), and then the mixture was transferred to a PTFE-lined stainless steel autoclave and placed in a blower drying oven at 190 °C for 6 h. The treated carbon mats were added to the mixture and ultrasonicated for 2 h. The final ultrasonicated carbon mats were rinsed repeatedly with distilled water and dried in a blower drying oven at 60 °C.
Thiophene (0.05 mol purity 99%, Shanghai Yi En Chemical Technology Co., Ltd., Shanghai, China) was added to 50 mL of chloroform chloroform (analytical purity, Guangzhou Size Chemical Co., Ltd., Guangzhou, China) at a temperature of 25 °C under the conditions of the magnetic stirrer on the stirring for 2 h, so that the thiophene monomer was uniformly dispersed in the chloroform, and then 7.73 g of anhydrous ferric chloride (analytical purity, Guangzhou Hoying Chemical Technology Co., Ltd., Guangzhou, China) was added to initiate the polymerization reaction. The polymerization reaction was initiated, and then the reaction mixture was magnetically stirred at 20 °C for 12 h. The solution was then placed in a fume hood, and the solvent was evaporated at room temperature to obtain a polythiophene solid. Subsequently, 0.5 g of the powder was placed in a beaker and stirred for 40 min at 25 °C. The prepared CF/GO was then added to the solution and ultrasonicated for 1 h, after which it was washed repeatedly with anhydrous ethanol (purity 99.5%; Shanghai Aladdin Biochemical Science and Technology Co., Ltd.) and deionized water until the liquid became colorless. The finished product was then dried at 60 °C for 24 h to obtain CF/Go/Pth.
2.2. MFC Construction and Operation
The anode and cathode chambers of the MFC device were arranged opposite each other and separated by a proton exchange membrane (Suzhou Yilongsheng Energy Technology Co., Ltd., Shanghai, China). Go/Pth modified carbon felt was selected as the electrode material for the anode, and carbon rods (Carbon Energy Technology Co., Ltd., Beijing, China) were selected as the electrode material for the cathode. The anode and cathode chambers of the MFC have two symmetrical holes, which are symmetrically distributed through the proton exchange membrane, and the electrode materials for the cathode and anode are suspended in the anode and cathode chambers through the holes in the upper part of the MFC device. The cathode liquid of MFC is mainly 10 g/L potassium ferrocyanide (A.R.P.). A 10 g/L solution of potassium ferricyanide (A.R. 99.5%; Tianjin Guangfu Fine Chemical Co., Ltd., Tianjin, China) was used. The inoculated strains were obtained from anaerobic sludge of the wastewater treatment plant, and the microbial strains were domesticated by adjusting the temperature and external resistance. The specific composition of the anode liquid is provided in Table S1, and sodium acetate (2.5 g/L) (A.R.; Tianjin Komeo Chemical Reagent Co., Ltd., Tianjin, China) was used as the carbon source. The MFC device was placed under room temperature conditions. The performance of the MFC was tested after it was stabilized.
2.3. Characterization and Measurement
All electrochemical tests were performed using a three-electrode system. In this study, the voltage of the MFC in the optimal state was determined by adjusting the external resistance value, and the corresponding current value was calculated accordingly, and then the anodic polarization curves, power density curves and polarization curves of the MFC were plotted. In this experiment, the Shanghai Chenhua electrochemical workstation CHI760E (Chenhua Instrument Co., Ltd. Shanghai, China) was chosen as the main measurement equipment, and constant current charge/discharge tests, cyclic voltammetry tests and AC impedance measurements of the electrode were carried out. In order to test the performance enhancement of the electrode modification, the CF electrode and CF/GO electrode were used as comparative references. In addition, a Hitachi SU5000 scanning electron microscope (Hitachi, Ltd., Tokyo, Japan) was used to observe the morphology of the synthesized materials and to analyze the composition in combination with EDS energy spectrum analysis. Meanwhile, the functional group information of the samples in the wavelength range of 4000–400 cm−1 was recorded using an IRAffinity-1S Fourier transform infrared spectrometer from Shimadzu Co., Ltd. (Suzhou, China).
2.4. Microbial Characterization Techniques
High-throughput sequencing was performed according to a standardized protocol provided by Shanghai Shengong Biological Company (Shanghai, China). The main processes were gDNA extraction, library construction and sequencing. gDNA was extracted using extraction kits, the target sequences were enriched using highly specific primers, and the data were sequenced for bioinformatics analysis. By analyzing the test results, the taxonomic information of the dominant strains in the samples was clarified, including the rank of which kingdom, phylum, order, order, family, genus, and species they belonged to, respectively, to study the characteristics and functions of microorganisms in depth [22].
3. Results and Discussion
3.1. Morphological Characteristics of CF/GO/Pth Anodes
The surface morphologies of the different anodes were examined using scanning electron microscopy (SEM), as shown in Figure 1, where GO and oxidized polymerized Pth prepared by hydrothermal treatment were covered on the surface of the smooth CF fibers (a, b, c). In Figure 1d–f, it can be clearly observed that the graphite oxide is uniformly coated on the carbon fibers in the form of a film, which proves the uniform loading of graphite oxide on the carbon mats. Further analysis showed that the introduction of graphite oxide significantly enhanced the conductivity of the electrode and effectively increased the surface area of the carbon fiber. The expansion of the surface area provides more potential reaction sites for bacterial attachment. In Figure 1g–i, it can be seen that after the addition of polythiophene on top of the CF/GO composite structure, the polythiophene on the surface of CF showed a spherical nanostructure, which is consistent with the description in the related literature. In addition, the thickness of the CF fibers increased, and their surface area further increased. A further increase in the surface area not only provided more space for bacterial attachment, but also, due to the good biocompatibility and electrical conductivity of polythiophene, it was able to accelerate the rate of electron transfer between microorganisms, thus accommodating more electroproducing bacteria for attachment and propagation. Together, these properties contribute to the optimization and enhancement of the MFC performance.
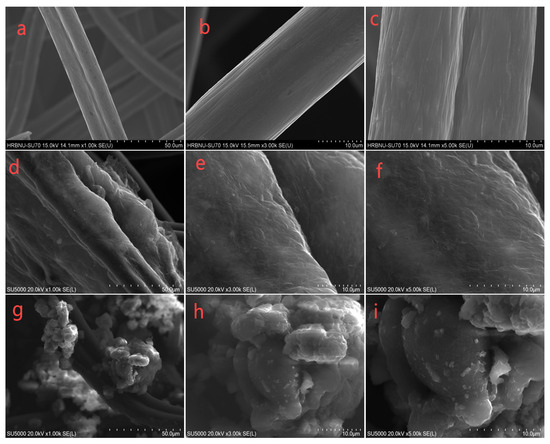
Figure 1.
SEM images of the CF electrode (a–c), CF/GO electrode (d–f), and CF/GO/Pth electrode (g–i) magnified 1000×, 3000×, and 5000×, respectively.
Figure 2 shows the infrared spectra of the three electrodes in the range of wave number from 400 to 4000 cm−1. The absorption peak at 1972 cm−1 for the CF electrode corresponded to the carbonyl (C=O) functional group, and the absorption peak at 2154 cm−1 corresponded to the O-H bond in the phenol group. After the modification of graphite oxide, the overall peak shape changed and some of the characteristic peaks were more obvious, and a peak appeared at 1690 cm−1, which corresponded to the stretching vibration of the carbonyl-oxygen double bond and corresponded to the carboxylic acid group and ester group in the graphite oxide, and at 1810 cm−1, which corresponded to the carboxylic acid group in the graphite oxide. When polythiophene was added, the overall peak shape changed again, where the absorption peak at 1621 cm−1 indicates that this is caused by the trans stretching vibration of the thiophene ring, indicating that the thiophene ring did not change when the composite underwent polymerization [23] and is still present. The absorption peak at 1367 cm−1 corresponds to the symmetric stretching vibration of the thiophene ring. The peak at 1120 cm−1 is caused by the stretching vibration of the molecular chain of polythiophene. The peak at 1120 cm−1 is caused by the stretching vibration of the polythiophene molecular chain, the bending vibration peak caused by the polythiophene molecular chain occurs at 1091 cm−1, and the peak at 3056 cm−1 reflects the presence of unsaturated hydrocarbons in the polythiophene. By analyzing the infrared spectra, it was found that the characteristic peaks of graphite oxide and polythiophene appeared in the graphs, which indicated that graphite oxide and polythiophene had been successfully modified on the surface of the carbon mats.
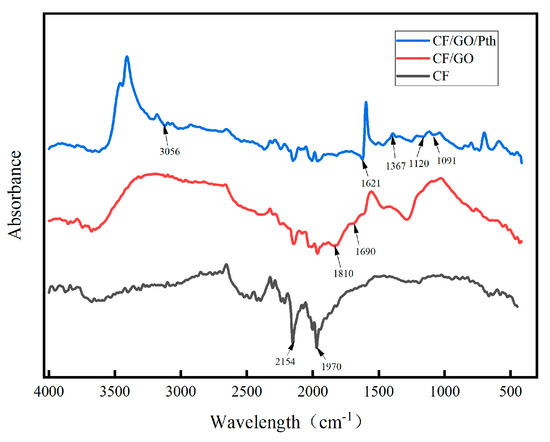
Figure 2.
IR spectra of the CF, CF/GO, and CF/GO/Pth electrodes.
From Figure 3a, it can be clearly observed that the CF electrode is mainly composed of C and O elements, in which the mass ratio of C element is as high as 88.24%, which is 7.5 times higher than the mass ratio of the oxygen element (11.76%), which fully demonstrates the dominance of C element in the CF electrode. When the CF electrode was loaded with graphite oxide (as shown in Figure 3b), its elemental composition changed. The main elements of graphite oxide include carbon (C), oxygen (O), and hydrogen (H), and this change led to a significant increase in the mass ratio of elemental oxygen in the electrode from the original 11.76% to 21.29%, which suggests that graphite oxide is a major component of the electrode. Further, when polythiophene was added on top of graphite oxide, the elemental composition of the electrode changed again. At this time, the element sulfur was added to the electrode with a mass fraction of 25.58%. The main elements of polythiophene are carbon (C) and sulfur (S), which form the thiophene ring, and hydrogen (H) atoms, which connect the thiophene ring. Therefore, the above changes prove the successful preparation and loading of polythiophene. Due to the addition of polythiophene, the mass fraction of carbon in the electrode increased accordingly from 54.06% to 67.11%, while the mass fraction of oxygen decreased to 7.31%. The EDS analysis shows that the mass percentage of each element in the electrode in the figure corresponds to the main elements contained in the graphite oxide and polythiophene, indicating that the graphite oxide and polythiophene have been successfully modified on the surface of the carbon mats.
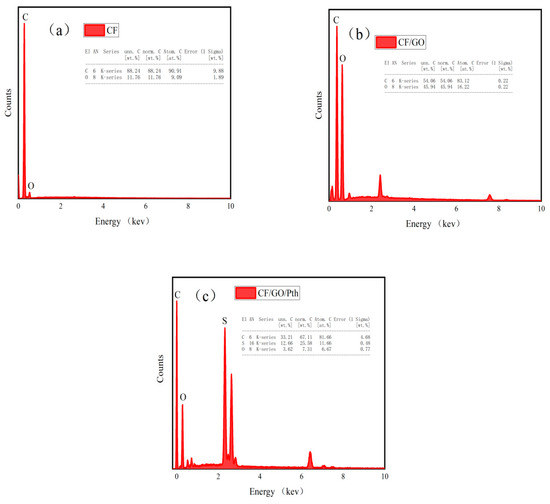
Figure 3.
(a–c) EDS of the CF, CF/GO, and CF/GO/Pth electrodes.
3.2. Electrochemical Testing of CF Electrode, CF/GO Electrode, CF/GO/Pth Electrode
The CF, CF/GO, and CF/GO/Pth electrodes were charged and discharged at a constant current, and the change in potential over time was recorded to calculate the specific capacitance (as shown in Equation (1)), which is shown in Table 1 for the different electrodes. Figure 4 shows the charging and discharging test graphs of the electrodes at a constant current of 1 mA. The length of the discharge time of different electrodes can be used to measure the size of their specific capacitance. Then the area-specific capacities of the above three electrodes in descending order are as follows: CF/GO/Pth electrode (1.30 F/cm2), CF/GO electrode (0.642 F/cm2), and CF electrode (0.389 F/cm2). This is because the high specific surface area and excellent electrical conductivity of graphite oxide, combined with the stability and electrochemical activity of polythiophene, can work together to promote the effective transfer of electrons between the electrodes and microorganisms and reduce the internal resistance to charge transfer. In addition, the graphite oxide polythiophene composite improves the cycling stability of the MFC by slowing down the shrinkage and swelling phenomena of the active material during charging and discharging. This effect helps minimize the performance degradation of the battery during long-term use and extends the service life of the MFC.

Table 1.
Specific capacitance of CF/GO/Pth electrodes with different reaction times.
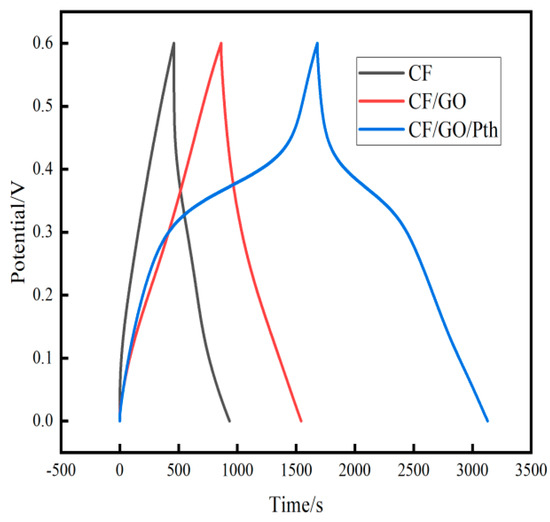
Figure 4.
Charge-discharge test plots of the CF, CF/GO, and CF/GO/Pth electrodes at a constant current of 1 mA.
- —Area-specific capacitance, F/cm2
- I—constant current constant, A
- t—discharge time, s
- A—surface area of the electrode, cm2
- V—Scanning potential window, V
3.3. Output Performance of MFC
Figure 5 shows the cyclic voltammograms of the CF, CF/GO, and CF/GO/Pth electrodes at a sweep rate of 5 mV/s. From the figure, it can be seen that the CF/GO/Pth electrode has obvious redox peaks, and the area of the CV curve is related to the electrochemical surface area of the electrode, the larger the area surrounded by the CV curve, it means that the number of oxidized or reduced substances in the experiment is larger, i.e., the reaction produces a larger amount of charge, which indicates a larger electrochemical surface area of the electrode, and it can be seen that when the electrode is loaded with GO, the area of CV curve is larger than CF, which indicates that when the oxidized stone is loaded with oxidized stone, the area is larger than CF. area is larger than CF, indicating that the charge generated by the electrode is enhanced when loaded with graphite oxide, which is due to the fact that the layered structure of graphite oxide as well as the surface functional groups are able to increase the pseudocapacitance of the interface between the biofilm and the electrode, thus improving the electrochemical performance of the electrode. When Pth was added, the area of the curve increased again, which indicated that the power production capacity of the electrode was further enhanced when loaded with polythiophene. This phenomenon occurred because polythiophene, as a conductive polymer material, has a high specific capacity and can store more charge, thus improving the capacity of the electrode. This implies that graphite oxide polythiophene composites are able to perform charge transfer and storage more efficiently in MFC, thus improving the power generation capacity and energy utilization efficiency of MFC.
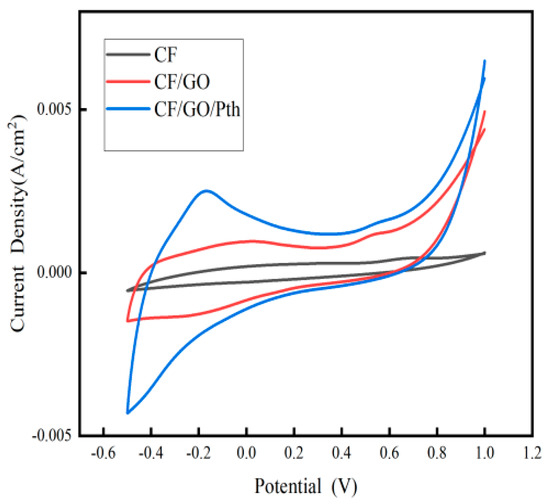
Figure 5.
Cyclic voltammograms of the CF, CF/GO, and CF/GO/Pth electrodes at a sweep rate of 5 mV/s.
As can be seen in Figure 6, the AC impedance spectrogram consists of a semicircle in the high-frequency region and a straight line in the low-frequency region; the semicircle is determined by the electrochemical reaction, while the straight line is determined by the diffusion of the solution. The semicircle radius in the high-frequency region reflects the resistance to charge transfer (Rct) at the interface between the electrolyte and active material [24]. The fitted data of the electrode impedance for the different electrodes are listed in Table 2.
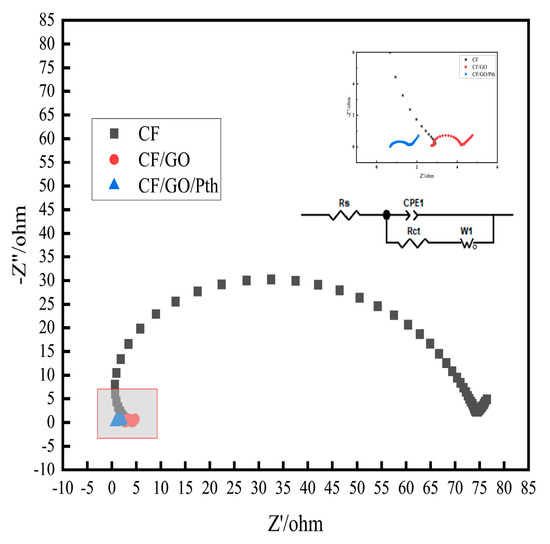
Figure 6.
EIS plots of the CF, CF/GO, and CF/GO/Pth electrodes.

Table 2.
Fitted data for different electrode impedances.
It can be seen that the solution impedance (2.91 Ω) and transfer impedance (77.8 Ω) of the CF electrode are much larger than those of the other two electrodes, indicating that when the electrodes are added with GO and Pth, the charge transfer ability of the electrodes are both improved to some extent. The solution impedance of the CF/GO electrode is (2.73 Ω) and the transfer impedance is (1.37 Ω), which is attributed to the presence of many oxygen-containing functional groups on the surface of GO, such as hydroxyl and carboxyl groups, which are able to enhance the electron transfer effect. This enhancement effect enables graphite oxide to promote effective charge transfer when used as an electrode material in MFCs, thus improving the power production performance and energy utilization of MFCs. When Pth was added, the solution impedance of CF/GO/Pth was (1.01 Ω) and the transfer impedance (0.68 Ω), which were both better than that of CF/GO electrode (2.73 Ω) (1.37 Ω), which was due to the fact that Pth, as a kind of conductive polymer, could interact with microorganisms and enhance the microorganisms’ surface of the electrodes when modified on the electrodes of MFC. attachment ability of the microorganisms. Pth promotes the transfer of electrons from microorganisms to the electrode through the extracellular pathway by providing abundant electrically active sites, thus completing the charge transfer process. This suggests that graphite oxide polythiophene can reduce the internal resistance to charge transfer at the interface between the biofilm and electrode in MFC.
3.4. The Storage Performance of MFC
Figure 7 shows the anodic polarization curves of the CF/GO/Pth, CF/GO, and CF electrodes in the MFC. As can be seen from Figure 7, relative to the blank CF anode, the modified CF/GO/Pth electrode and CF/GO electrode showed a slow change in anodic potential, i.e., a smaller anodic polarization, when the current density was increased. The anodic potentials of the CF/GO/Pth, CF/GO, and CF electrodes were polarized by 9, 69, and 94 mV, respectively, when the current density was increased from 1 A/m2 to 2 A/m2, and the CF/GO/Pth electrode was the least polarized. When the current density was 1.5 A/m2, the anodic potentials of the CF/GO/Pth, CF/GO, and CF electrodes were −0.28, −0.494, and −0.508 V, respectively, which indicated that the CF/GO/Pth electrode had the highest anodic potential. This is because the modification of graphite oxide may also change the microenvironment on the anode surface to be more favorable for microbial growth and metabolic activities, which indirectly promotes the polarization of the anode. The biocompatibility of the anode was further improved when Pth was added, allowing the microorganisms to attach more tightly to the anode surface, thus increasing the efficiency of electron transfer. This suggests that when the electrode was loaded with graphite oxide polythiophene, the metabolic ability of the electroproducing bacteria was effectively enhanced in the MFC, which improved the biocompatibility of the electrode, and, thus, the electrode’s ability to produce electricity.
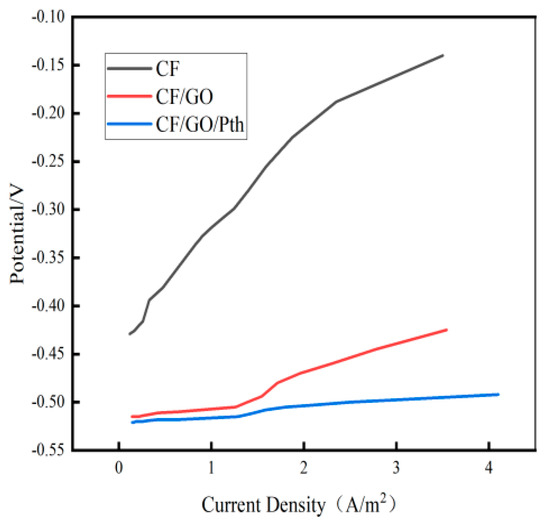
Figure 7.
Anodic polarization curves of CF, CF/GO, and CF/GO/Pth electrodes as MFC anodes.
Figure 8 shows the MFC polarization and power density curves for the CF/GO/Pth, CF/GO, and CF electrodes, where (a) shows the cell polarization curves of the three anodes and (b) shows the MFC power density curves of the three anodes. As shown in Figure 8a, there was no significant difference in the MFC open-circuit voltage of the CF electrode, CF/GO electrode, and CF/GO/Pth anode. When the cell output voltage was the same, the modified anode MFC exhibited a greater current density. The voltages corresponding to the three electrodes at different current densities are shown in Figure 8. It can be seen that the voltage reduction of the CF/GO/Pth electrode is smaller than that of the other two electrodes when the current density is increased, which indicates that the MFC of CF/GO/Pth has the smallest degree of polarization in the same current density interval. When the three electrodes were subjected to the same voltage, the CF/GO/Pth electrode exhibited the maximum current density. When the output voltage of the MFC was 500 mV, the current densities of the MFC with the CF/GO/Pth and CF/GO electrodes were 3.23 and 2.62 A/m2, respectively, which were 2.01 and 1.63 times higher than that of the blank CF anode (1.6 A/m2), respectively. The MFC polarization of CF/GO/Pth was minimized in the same current density interval as that of CF/GO. This is due to the synergistic effect of graphite oxide and polythiophene, which enhances the conductivity of the electrode surface while maintaining excellent biocompatibility. This creates an optimal environment for the survival of microorganisms and facilitates the formation of a stable biofilm on the electrode surface, which improves the efficiency of electron transfer to the electrode. When graphite oxide is modified on the anode of the MFC, it enhances the hydrophilicity and biocompatibility of the anode, enabling the microorganisms to attach more tightly to the anode surface and transfer electrons from the body to the electrode more efficiently through the process of extracellular electron transfer (EET). In addition, the electrochemical reaction rate on the surface of the anode may be enhanced by the introduction of graphite, which smoothens the electron transfer on the anode surface. Meanwhile, Pth has high capacitance characteristics, which improve the polarization performance of MFC. This improves the overall conductivity of the electrode, reduces the internal resistance, lowers the resistance to the extracellular electron transfer of the electroproducing bacteria, and reduces the polarization of the MFC.
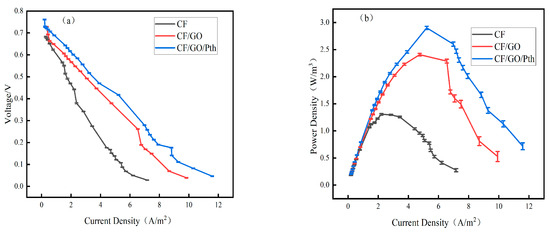
Figure 8.
(a) Polarization and (b) power density curves for the CF, CF/GO, and CF/GO/Pth electrodes.
As shown in Figure 8b, the MFC power densities of the above three anodes (as shown in Equation (2)) show a trend of increasing and then decreasing with the increase in current density. The power density of the modified electrode was higher than that of the blank CF. The maximum power densities of the modified CF/GO/Pth electrode and CF/GO/electrode MFCs were 2.9 W/m3 and 2.4 W/m3, respectively, which were 2.217 and 1.83 times higher than the maximum power density of the blank CF anode MFC (1.3 W/m3). When the cells with CF/GO/Pth electrodes and CF/GO/Pth electrodes obtained the maximum power densities, the corresponding current densities were 5.21 A/m2 and 4.75 A/m2, which were 2.35 and 2.14 times higher than those of the blank CF anode (2.21 A/m2), respectively. This indicates that graphite oxide has good biocompatibility and can effectively promote microbial power generation and reduce energy loss during MFC operation. Meanwhile, graphite oxide and polythiophene exhibited a good synergistic effect. When graphite oxide and polythiophene were modified on the MFC anode, they could provide more electrically active sites, which was favorable for microbial attachment and growth and promoted the electron transfer process between the microorganisms and the electrode. This facilitation of electron transfer helps accelerate the electrochemical reaction rate on the anode surface and reduce the internal resistance of charge transfer, thus improving the utilization of electrical energy.
- P—Power density, W/m3
- U—Output voltage, V
- I—Current, A
- R—Load resistance, Ω
- V—Anode chamber volume, mL
The maximum power densities and corresponding current densities that can be obtained from the three anode cells are listed in Table 3.

Table 3.
Maximum power densities and corresponding current densities of the three different anodes.
The power density of the CF/GO/Pth anodes compared to other anode materials is shown in Table 4.

Table 4.
Comparison of maximum power density of anodes with different materials.
Figure 9 shows the time-voltage variation curves of the MFC equipped with three electrodes, charged for 295 s under a load condition of a 100 Ω external resistor. The obtained data were organized into Table 5. The starting potential (−0.463 V) and steady-state potential (−0.55 V) of CF (from Table 5) were lower than those of CF/GO electrodes (starting potential of −0.325 V, steady-state potential of −0.458 V) and CF/GO/Pth electrodes (starting potential of −0.216 V, steady-state potential of −0.266 V), during which the voltage drop of CF/GO/Pth electrodes was the slowest (voltage drop of −0.266 V). Pth (starting potential of −0.216 V, steady-state potential of −0.266 V), and the voltage of the CF/GO/Pth electrode decreased the slowest in this process (voltage decrease of 0.05 V). The experimental results show that the graphite oxide and polythiophene composite electrodes have a slower decrease in potential and a better charge storage capacity (See Table 5).
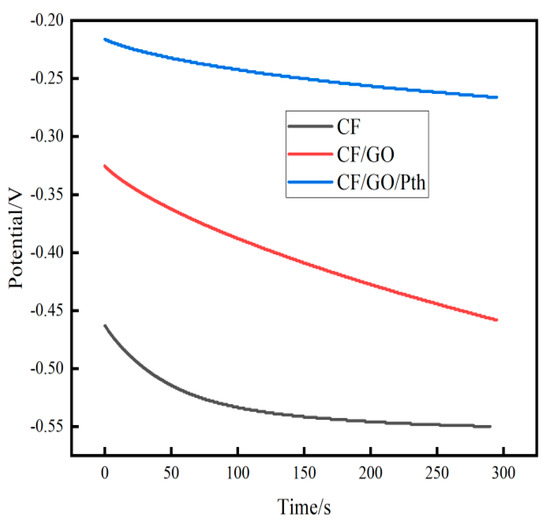
Figure 9.
Timing potential curves of the CF, CF/GO, and CF/GO/Pth electrodes.

Table 5.
Starting potentials of the three different anodes and their corresponding steady-state potentials.
Figure 10 shows the anode timing current test curves (Charging/Discharging, C/D) after charging and discharging different anode MFC for different times. The discharge curves (C295/D295) were obtained for 295 s of charging and 295 s of discharging. As shown in the figure, the discharge curves of both electrodes showed a peak current, then decreased rapidly, and finally reached a steady state. The specific data are shown in Table 6 below. The peak current density (ih) and steady-state current density (is) of the CF anode were 67 A/m2 and 5.025 A/m2, respectively, which were much lower than those of the CF/GO anode (ih = 125.25 A/m2, is = 8.125 A/m2) and CF/GO/Pth electrodes (ih = 154.45 A/m2, is = 21.91 A/m2). When C295/D295, the total power Qt of the CF/GO/Pth, CF/GO, and CF anodes were 11,258.68 C/m2, 5483.39 C/m2, and 2727.66 C/m2 in that order, and the CF/GO and CF/GO/Pth anodes were 2.01 and 4.13 times more than the CF anodes, respectively. When C295/D295, the stored power Qs of the CF/GO/Pth, CF/GO, and CF anodes were 4795.23 C/m2, 3086.51 C/m2, and 1245.28 C/m2, respectively, and the stored power of the CF/GO, and CF/GO/Pth anodes were 2.47 and 3.85 times that of the CF anode, respectively. This indicates that graphite oxide has excellent electrical conductivity and electrochemical stability and can be used as a modifying material for electrodes in MFC to improve the electron transfer efficiency of the electrodes. This improvement contributed to a more stable and higher current response in the timed-current test. When polythiophene is added, the charge transfer ability of the electrode can be significantly improved because of the excellent conductivity and electrochemical stability of polythiophene. Secondly, the modification of polythiophene may also change the interaction between the electrode and microorganisms. In MFC, effective contact between microorganisms and electrodes is crucial for electricity production. Polythiophene modification can increase the biocompatibility of the electrode surface and promote the attachment and growth of microorganisms on it. This improvement contributes to the enhancement of the MFC’s ability to produce electricity and demonstrates a higher current output in the current test. The experimental results illustrate that the capacitive properties of graphite oxide polythiophene positively affected the response to the timed-current test by increasing the pseudocapacitance of the electrode in the MFC, which contributes to the improvement of the energy utilization of the MFC during the intermittent discharge process. (See Table 6).
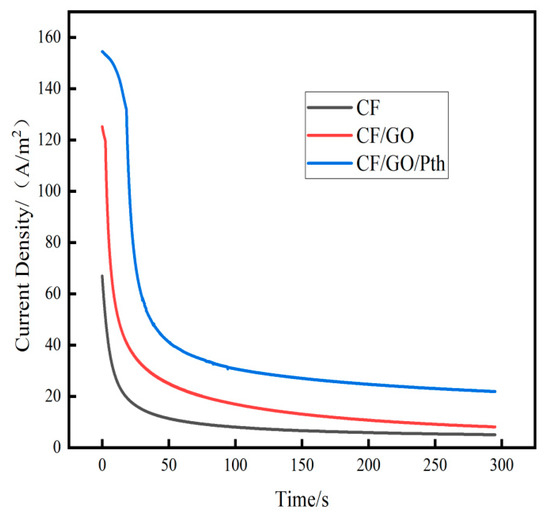
Figure 10.
Discharge curves of the CF, CF/GO, and CF/GO/Pth electrodes.

Table 6.
Parameters of anode timing current curves after charging and discharging different anode MFC for different times.
3.5. Analysis of Anode Biodiversity and Microbial Community Structure
The power generation performance of MFCs depends largely on the number of microorganisms attached to the surface of the anode material and the proportion of electricity-generating bacteria attached to the microorganisms [29]. The content and composition of the microorganisms attached to the anode were analyzed using high-throughput sequencing. 16sDNA high-throughput sequencing was used to analyze the composition of microbial communities on anodes inoculated in wastewater plants, as well as to analyze the structure of microbial colonies on modified and control anodes. For clarity and comparison, the CF electrode is A1, CF/GO electrode is A2, and CF/GO/Pth electrodes are noted as A3.
The abundance of microbial species on the three electrodes shown in Figure 11 indicates that more than 90% of the electrogenic bacteria on the surface of the CF/GO/Pth anode were significantly higher than those on the CF and CF/GO electrodes, suggesting that the graphite oxide polythiophene had a positive effect on the MFC anode colonies. Specifically, the main bacterial colonies on A1 (CF electrode) consisted of Alcaligenes (9.37%) and Trichomonas (7.12%). Both of these bacteria are electroproducing bacteria [30,31], but their contents were relatively low under these conditions. On A2 (CF/GO electrode), the contents of Alcaligenes and Trichomonas were elevated to 13.87% and 9.46%, respectively. This indicates that the loading of graphite oxide on the electrode favored the growth of these two types of electroproducing bacteria. The main flora on electrode A3 (CF/GO/Pth electrode) consisted of Arcobacter (83.28%) and Alcaligenes (8.97%), which accounted for 92.25% of electrode A3. Arcobacter usually adheres to the surface of the electrode. by transferring electrons to the electrode surface in situ, thus slowing down product inhibition and increasing the electron transfer rate, which in turn dramatically increases the microbial degradation efficiency of organic matter and simultaneously recovers electrical energy [32]. This indicates that when polythiophene was added, the graphite oxide polythiophene-modified anode surface formed a stable electricity-producing biofilm, which was conducive to power generation [33]. The experimental results showed that the proportion of electroproducing bacteria on the surface of the CF/GO/Pth anode was more than 90%, which was significantly higher than that of the electroproducing bacteria attached to the surface of the CF anode and CF/GO. This suggests that graphite polythiophene oxide has a positive effect on MFC anode colonies, which promotes the attachment and growth of electroproducing bacteria and improves the electroproduction performance of MFC by providing a favorable growth environment and improving the electron transfer pathway. In this study, the MFC anode was continuously connected to an external resistor, microorganisms on the surface of the anode were cultured into electroproducing bacteria, and CF/GO/Pth electrodes were placed in the anode, which promoted the predominance of the electroproducing microorganisms on the surface of the anode, resulting in an effective decomposition of organic waste.
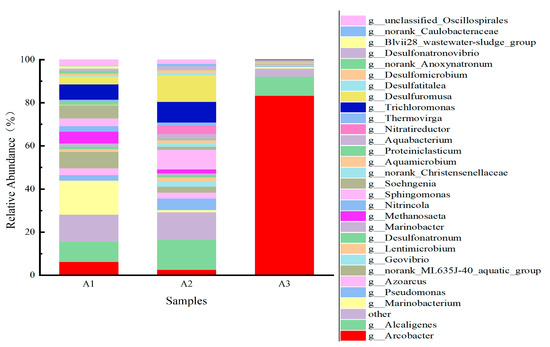
Figure 11.
Community distribution of strains on anodes A1, A2, and A3 at the genus level.
4. Conclusions
In this study, graphite oxide polythiophene (CF/GO/Pth) electrodes on carbon felt (CF) substrates were successfully prepared and applied to microbial fuel cells (MFCs) to enhance their power production and energy storage capacity. The experimental results showed that the maximum power density of the MFC with the CF/GO/Pth anode was 3.32-fold higher compared to that of the conventional CF electrode. In the 295-s discharge test, the charge Qt of the CF/GO/Pth anode reached 11,258.68 C/m2, which was 4.13 times higher than that of the CF electrode (2727.66 C/m2). High-throughput tests further confirmed that the percentage of electricity-producing microorganisms on the modified anode exceeded 90%. In this paper, by preparing a graphite oxide/polythiophene capacitive composite bioanode on carbon felt, the biocompatibility of graphite oxide and the capacitive properties of polythiophene were combined to confer the advantage of anode energy storage. The anode not only has a large specific capacity and specific surface area, but also exhibits excellent electron transfer capability and biocompatibility. These properties enable the improved MFC to possess the functions of simultaneously generate power and store energy, which significantly enhances its current output capability and provides an effective way to solve the problem of low MFC output power. Therefore, this technology shows broad application prospects for the degradation of polluted waste liquids.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings15040412/s1, Table S1: The formula of anode chamber nutrient solution.
Author Contributions
Conceptualization, Y.W.; Methodology, Z.W.; Software, Y.S.; Validation, D.Z.; Investigation, X.K. and A.V.; Resources, S.M.; Supervision, Y.D. and V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the Opening Project of Shanxi Province Key Laboratory of Chemical Process Intensification, North University of China (No. 2024-CPI07), and the Provincial Foreign Expert Program for 2024 (NO. G2024050); and the State Key Laboratory of Microbial Technology Open Projects Fund (Project NO. M2024-19).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, P.; Yin, D.; Song, P.; Liu, Y.; Cai, L.; Wang, H.; Zhang, L. Demulsification and oil recovery from oil-in-water cutting fluid wastewater using electrochemical micromembrane technology. J. Clean. Prod. 2020, 244, 118698. [Google Scholar]
- Jiang, W.-M.; Chen, Y.-M.; Chen, M.-C.; Liu, X.-L.; Liu, Y.; Wang, T.; Yang, J. Removal of emulsified oil from polymer-flooding sewage by an integrated apparatus including EC and separation process. Sep. Purif. Technol. 2019, 211, 259–268. [Google Scholar]
- Ardakani, M.N.; Gholikandi, G.B. Microbial fuel cells (MFCs) in integration with anaerobic treatment processes (AnTPs) and membrane bioreactors (and MBRs) for simultaneous efficient wastewater/sludge treatment and energy recovery-A state-of-the-art review. Biomass Bioenergy 2020, 141, 105726. [Google Scholar]
- Du, Z.; Li, H.; Gu, T. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar]
- Logan, B.E. Microbial Fuel Cells; John Wiely & Sons Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Liu, Z.; Zhou, L.; Chen, Q.; Zhou, W.; Liu, Y. Advances in graphene/graphene composite based microbial fuel/electrolysis cells. Electroanalysis 2017, 29, 652–661. [Google Scholar]
- Ahn, Y.; Logan, B. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475. [Google Scholar] [PubMed]
- Xu, H.; Zhang, M.; Ma, Z.; Zhao, N.; Zhang, K.; Song, H.; Li, X. Improving electron transport efficiency and power density by continuous carbon fibers as anode in the microbial fuel cell. J. Electroanal. Chem. 2020, 857, 113743. [Google Scholar]
- Aelterman, P.; Versichele, M.; Marzorati, M.; Boon, N.; Verstraete, W. Loading rate and external resistance control the electricity generation of microbial fuel cells with different three-dimensional anodes. Bioresour. Technol. 2008, 99, 8895–8902. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, Y.; Guo, C.X.; Lim, S.; Song, H.; Li, C.M. Graphene/carbon cloth anode for high-performance mediatorless microbial fuel cells. Bioresour. Technol. 2012, 114, 275–280. [Google Scholar]
- Huang, L.; Li, X.; Ren, Y.; Wang, X. In-situ modified carbon cloth with polyaniline/graphene as anode to enhance performance of microbial fuel cell. Int. J. Hydrogen Energy 2016, 41, 11369–11379. [Google Scholar]
- Prabakar, S.J.R.; Narayanan, S.S. Amperometric determination of paracetomol by a surface modified cobalt hexacyanoferrate graphite wax composite electrode. Talanta 2007, 72, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, S.; Peng, S.; Jiang, H.; Zhang, Y.; Deng, W.; Tan, Y.; Ma, M.; Xie, Q. Facile Fabrication of Graphene-Containing Foam as a High-Performance Anode for Microbial Fuel Cells. Chem. Eur. J. 2015, 21, 10634–10638. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liu, Z.; Zhang, P. A new method for fabrication of graphene/polyaniline nanocomplex modified microbial fuel cell anodes. J. Power Sources 2013, 224, 139–144. [Google Scholar] [CrossRef]
- Lv, Z.; Chen, Y.; Wei, H.; Li, F.; Hu, Y.; Wei, C.; Feng, C. One-step electrosynthesis of polypyrrole/graphene oxide composites for microbial fuel cell application. Electrochim. Acta 2013, 111, 366–373. [Google Scholar] [CrossRef]
- Roh, S.H.; Woo, H.G. Carbon nanotube composite electrode coated with polypyrrole for microbial fuel cell application. J. Nanosci. Nanotechnol. 2015, 15, 484–487. [Google Scholar] [CrossRef]
- Kang, Y.L.; Ibrahim, S.; Pichiah, S. Synergetic effect of conductive polymer poly (3, 4-ethylenedioxythiophene) with different structural configuration of anode for microbial fuel cell application. Bioresour. Technol. 2015, 189, 364–369. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Patil, S.A.; Ghosh, P.C.; Adeloju, S.B. Low-cost stainless-steel wool anodes modified with polyaniline and polypyrrole for high-performance microbial fuel cells. J. Power Sources 2018, 379, 103–114. [Google Scholar] [CrossRef]
- Laforgue, A.; Simon, P.; Sarrazin, C.; Fauvarque, J.F. Polythiophene-based supercapacitors. J. Power Sources 1999, 80, 142–148. [Google Scholar] [CrossRef]
- Ambade, R.B.; Ambade, S.B.; Salunkhe, R.R.; Malgras, V.; Jin, S.-H.; Yamauchi, Y.; Lee, S.-H. Flexible-wire shaped all-solid-state supercapacitors based on facile electropolymerization of polythiophene with ultra-high energy density. J. Mater. Chem. A 2016, 4, 7406–7415. [Google Scholar] [CrossRef]
- Nejati, S.; Minford, T.E.; Smolin, Y.Y.; Lau, K.K. Enhanced charge storage of ultrathin polythiophene films within porous nanostructures. ACS Nano 2014, 8, 5413–5422. [Google Scholar] [CrossRef]
- Sun, G.; Kang, K.; Qiu, L.; Guo, X.; Zhu, M. Electrochemical performance and microbial community analysis in air cathode microbial fuel cells fuelled with pyroligneous liquor. Bioelectrochemistry 2019, 126, 12–19. [Google Scholar] [PubMed]
- Sakthivel, S.; Boopathi, A. Synthesis and preparation of poly-thiophene thin film by spin coating method. J. Chem. Chem. Sci. 2014, 4, 150–155. [Google Scholar]
- Sumisha, A.; Ashar, J.; Asok, A.; Karthick, S.; Haribabu, K. Reduction of copper and generation of energy in double chamber microbial fuel cell using Shewanella putrefaciens. Sep. Sci. Technol. 2019, 55, 1–9. [Google Scholar]
- Ajit, K.; Anand, V.D.; Niharika, R.; Harikrishna, B.; Kumar, Y.A.; Krishnan, H. Scaling up of MFC technology using cost effective electrodes for treatment of kitchen wastewater. Mater. Today Proc. 2024, 111, 22–27. [Google Scholar]
- Pandit, S.; Patel, V.; Ghangrekar, M.; Das, D. Wastewater as anolyte for bioelectricity generation in graphite granule anode single chambered microbial fuel cell: Effect of current collector. Int. J. Environ. Technol. Manag. 2014, 17, 252–267. [Google Scholar] [CrossRef]
- Jayashree, C.; Tamilarasan, K.; Rajkumar, M.; Arulazhagan, P.; Yogalakshmi, K.; Srikanth, M.; Banu, J.R. Treatment of seafood processing wastewater using upflow microbial fuel cell for power generation and identification of bacterial community in anodic biofilm. J. Environ. Manag. 2016, 180351–180358. [Google Scholar]
- Wang, Y.; Wen, Q.; Chen, Y.; Yin, J.; Duan, T. Enhanced performance of a microbial fuel cell with a capacitive bioanode and removal of Cr (VI) using the intermittent operation. Appl. Biochem. Biotechnol. 2016, 180, 1372–1385. [Google Scholar]
- Ghasemi, B.; Yaghmaei, S.; Ghaderi, S.; Bayat, A.; Mardanpour, M.M. Effects of chemical, electrochemical, and electrospun deposition of polyaniline coatings on surface of anode electrodes for evaluation of MFCs’ performance. J. Environ. Chem. Eng. 2020, 8, 104039. [Google Scholar]
- Shahabivand, S.; Mortazavi, S.S.; Mahdavinia, G.R.; Darvishi, F. Phenol biodegradation by immobilized Rhodococcus qingshengii isolated from coking effluent on Na-alginate and magnetic chitosan-alginate nanocomposite. J. Environ. Manag. 2022, 307, 114586. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Y.; Xu, K.; Long, X.; Zhang, Y.; Liu, H.; Chen, T.; Li, J. Distinguishing anaerobic digestion from electrochemical anaerobic digestion: Metabolic pathways and the role of the microbial community. Chemosphere 2023, 326, 138492. [Google Scholar]
- Fedorovich, V.; Knighton, M.C.; Pagaling, E.; Ward, F.B.; Free, A.; Goryanin, I. Novel electrochemically active bacterium phylogenetically related to Arcobacter butzleri, isolated from a microbial fuel cell. Appl. Environ. Microbiol. 2009, 75, 7326–7334. [Google Scholar] [PubMed]
- Wang, Y.; Zhao, C.E.; Sun, D.; Zhang, J.R.; Zhu, J.J. A graphene/poly (3,4-ethylenedioxythiophene) hybrid as an anode for high-performance microbial fuel cells. ChemPlusChem 2013, 78, 823–829. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).