Influence of Ultrasonic Rolling Extrusion Static Pressure on Corrosion Resistance of GCr15 Bearing Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Test
2.3. Microstructure Analysis
3. Results
3.1. Analysis of Electrochemical Test Results
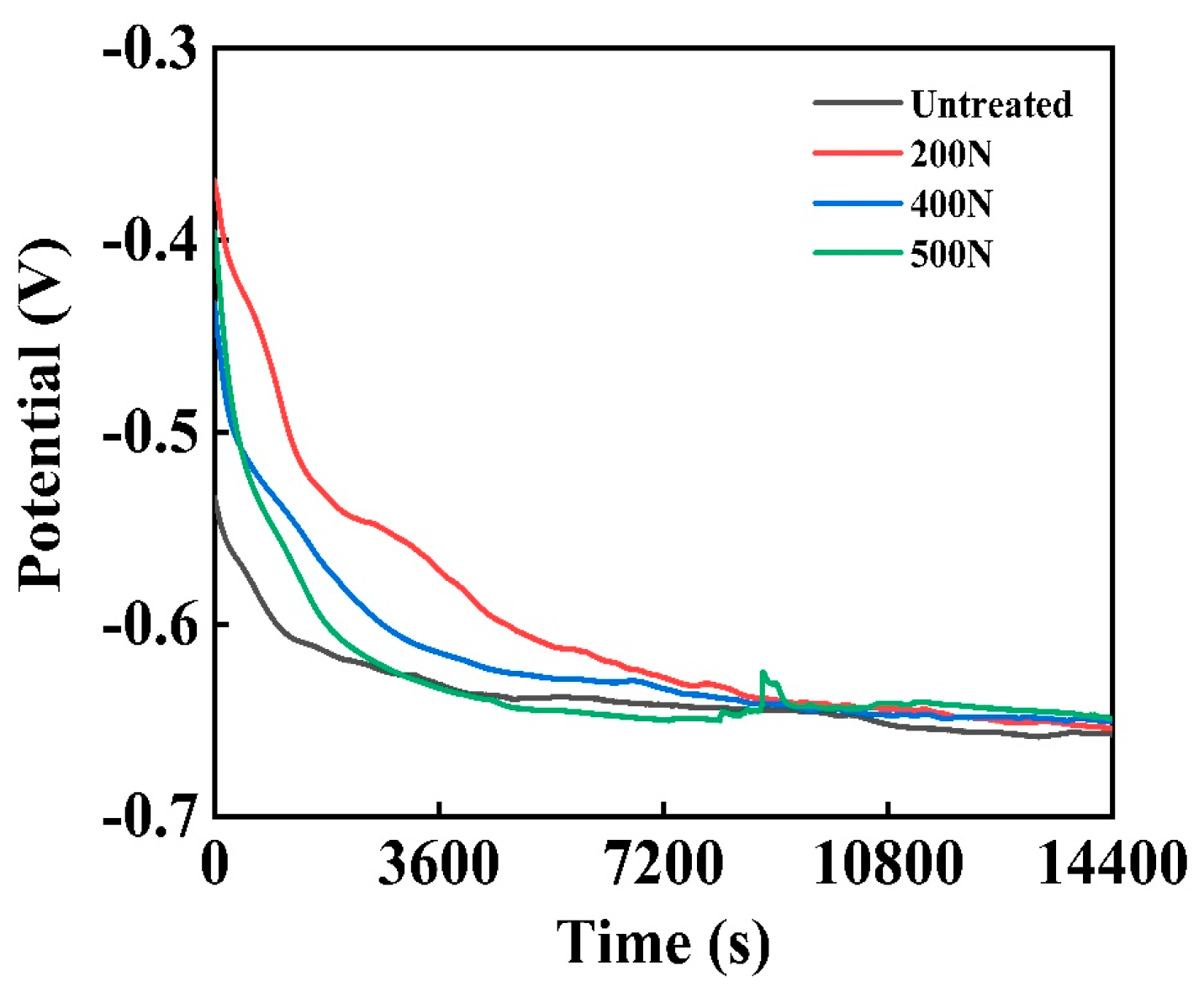
3.1.1. OCPT
3.1.2. Tafel
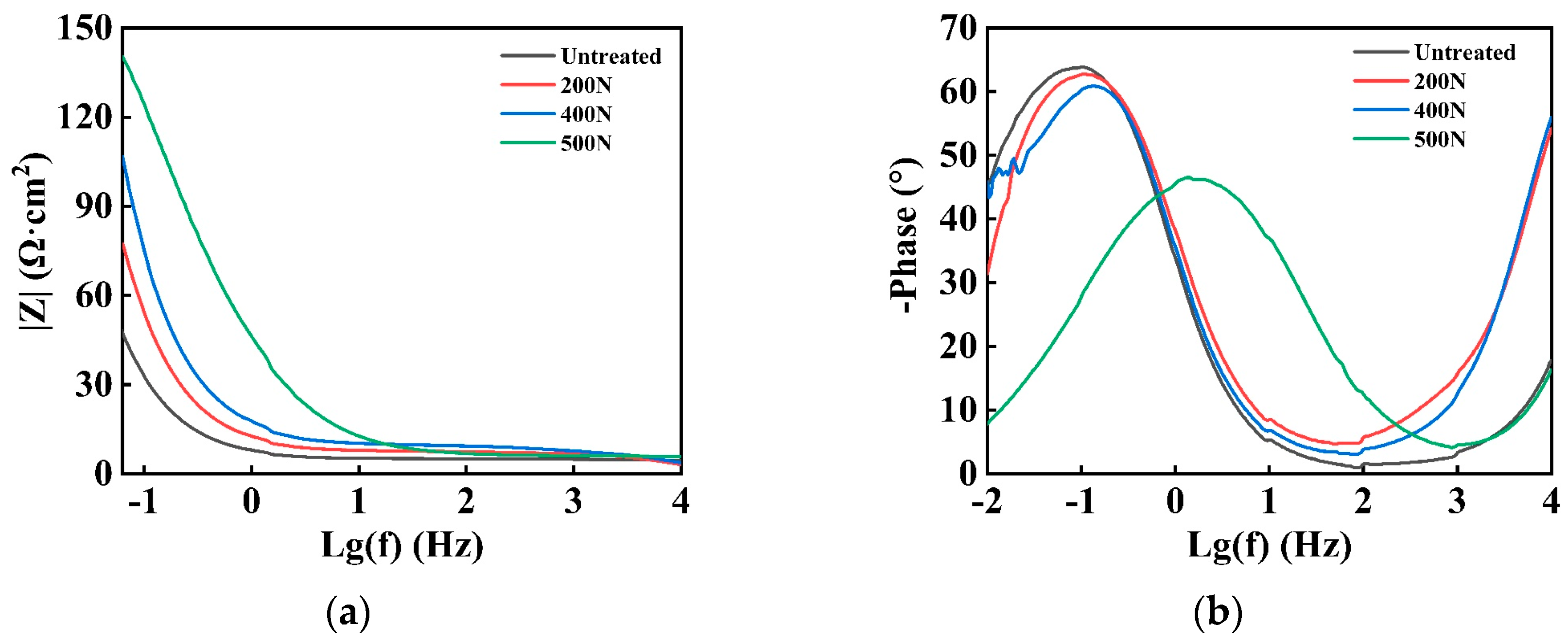
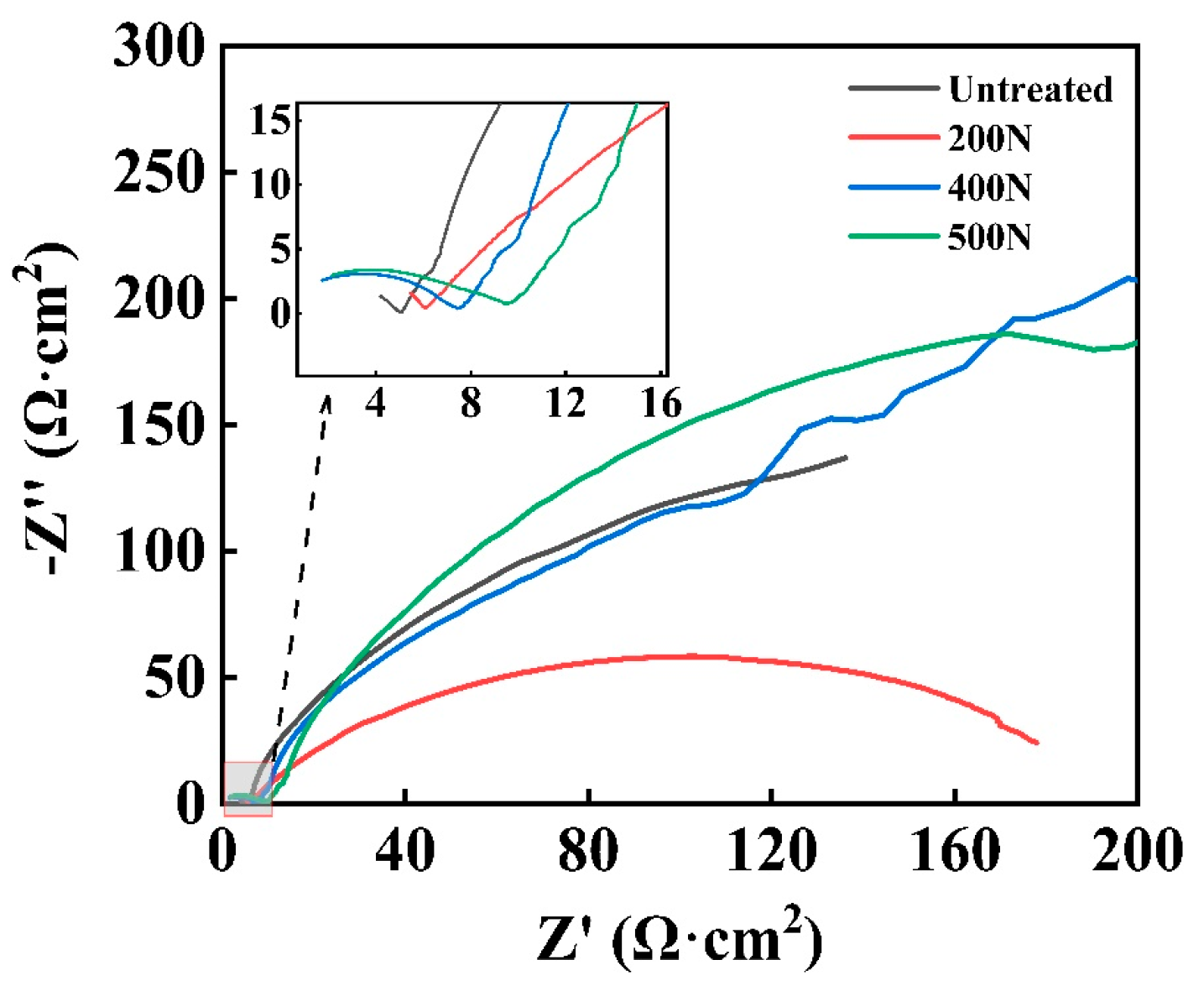
3.1.3. EIS
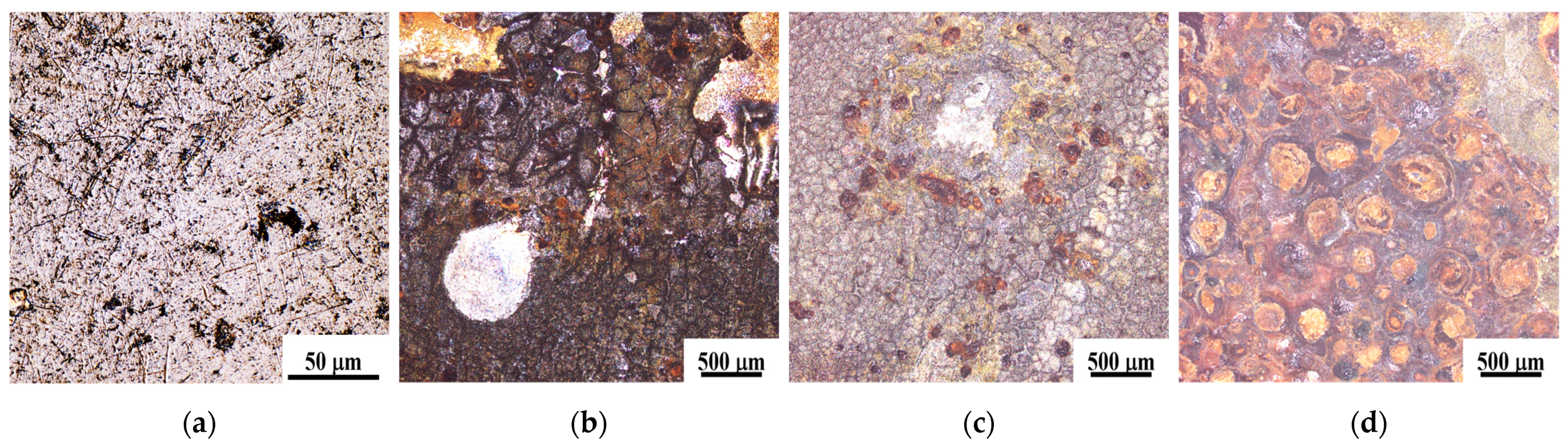
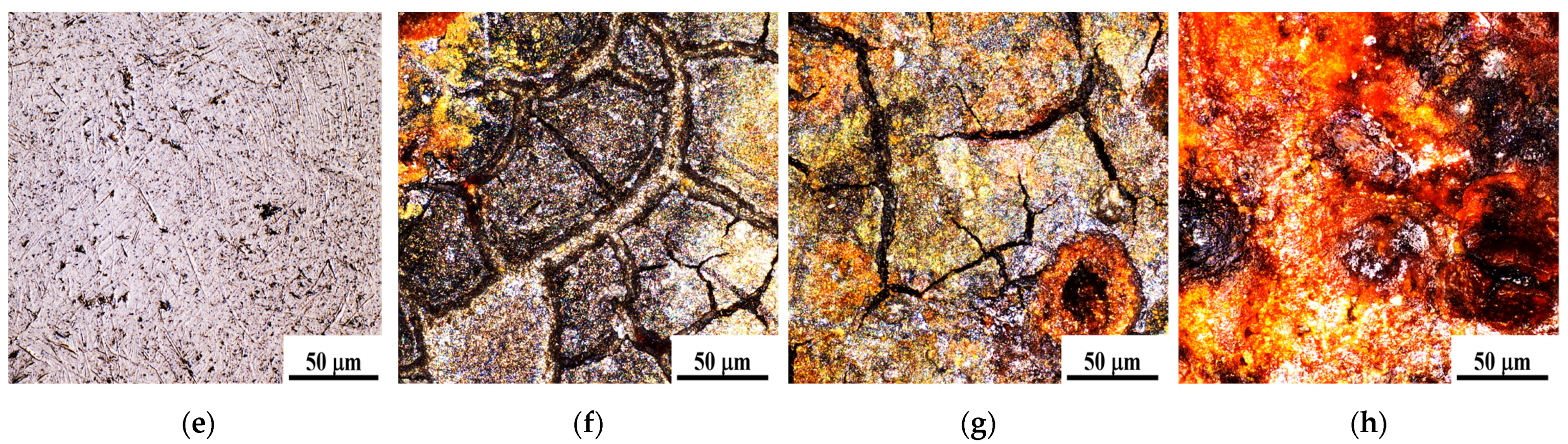
3.2. Microstructure and Chemical Characterization
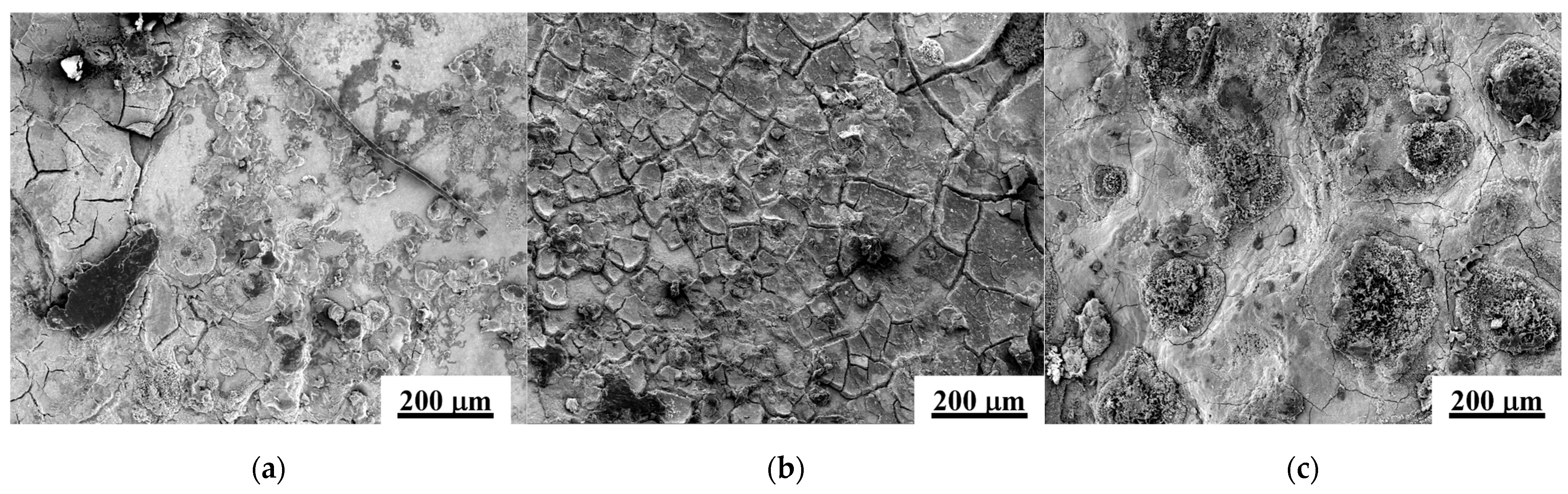
3.2.1. Microstructure
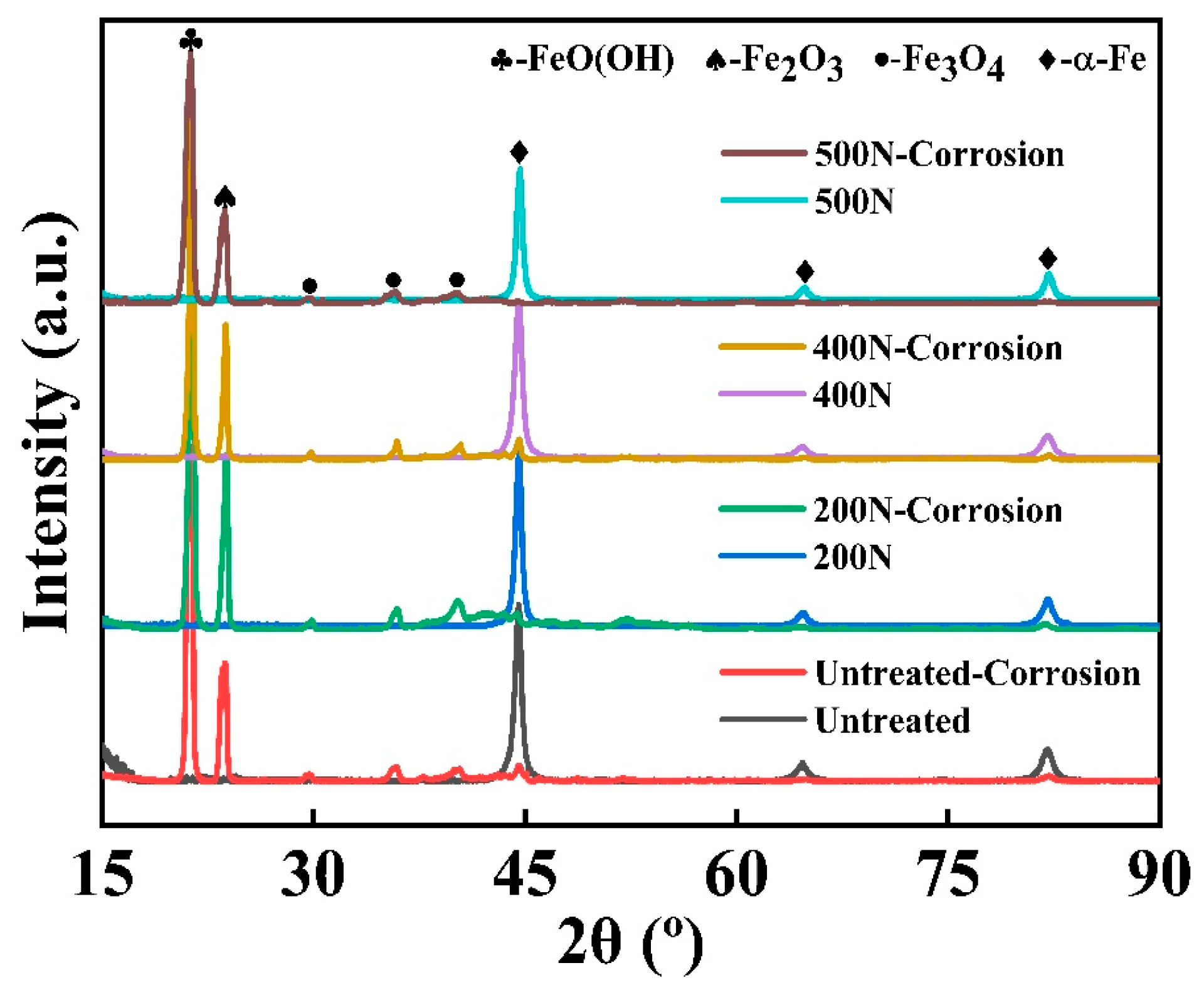
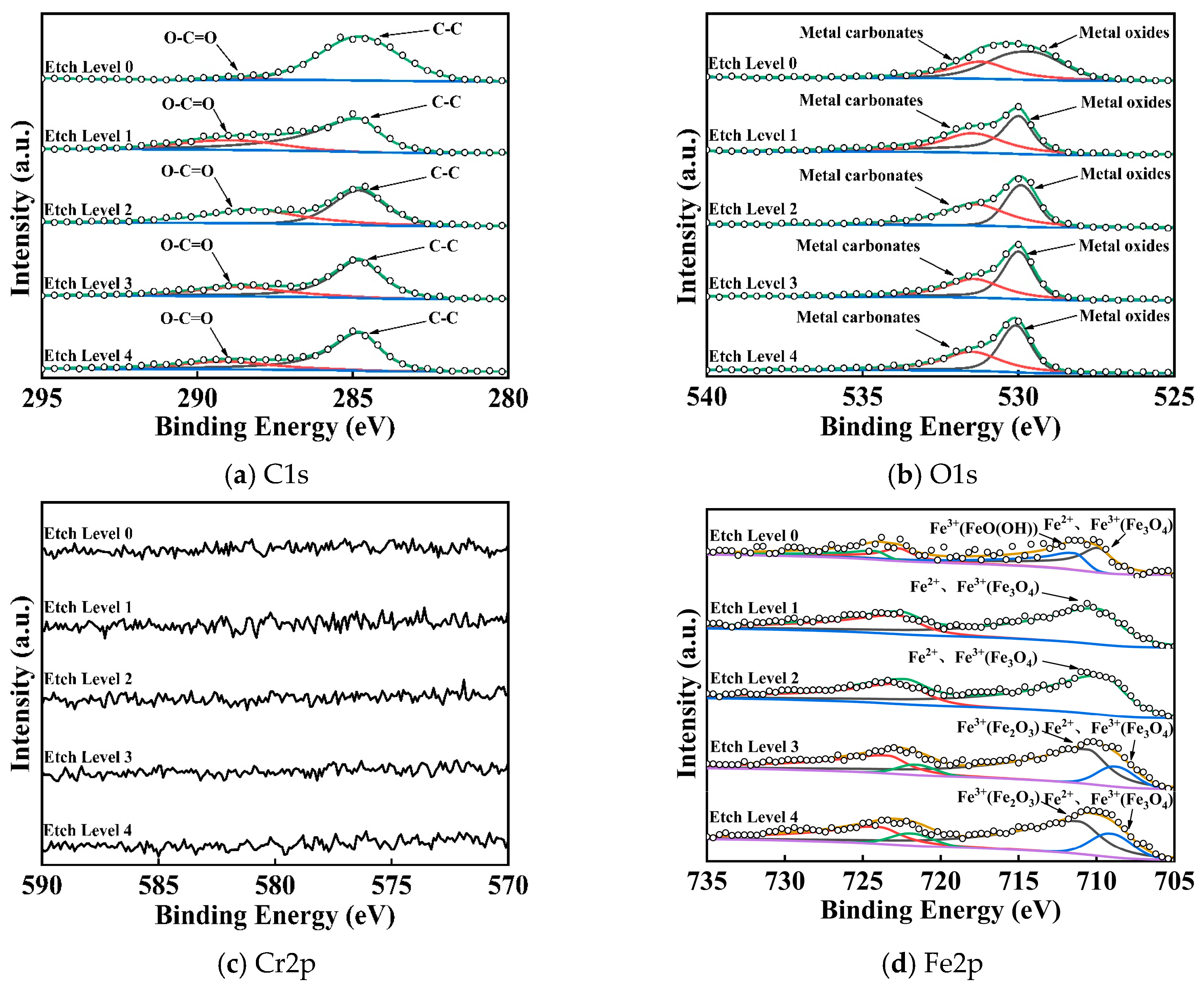
3.2.2. Chemical Characterization
4. Conclusions
- (1)
- Electrochemical corrosion: an increase in static pressure during ultrasonic rolling extrusion results in a marked enhancement of linear polarization resistance, a substantial reduction in corrosion current, a forward shift in corrosion potential, and a decrease in corrosion susceptibility. Additionally, both the low-frequency impedance modulus and charge transfer resistance exhibit an upward trend with increasing static pressure. Following the application of ultrasonic rolling extrusion treatment, there was a marked reduction in the corrosion rate of the samples. Furthermore, it was observed that, as static pressure increased, the corrosion rate continued to decline progressively.
- (2)
- Microstructure and surface quality: the enhancement of static pressure during ultrasonic rolling extrusion leads to improved surface flatness of GCr15 bearing steel, significantly reducing defects such as cracks, spalling, and voids, while continuously optimizing the microstructure. At a static pressure of 200 N, the surface exhibits considerable roughness and numerous defects. In contrast, at 400 N, there is a notable improvement in surface quality and, at 500 N, the surface becomes denser and smoother, effectively impeding the penetration of external corrosive agents, thereby augmenting the material’s corrosion resistance.
- (3)
- Passivation film thickness and densification: X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) analyses reveal that the static pressure applied during ultrasonic rolling extrusion significantly influences the composition, thickness, and structure of the passivation film on the surface of GCr15 bearing steel. As static pressure increases, both the thickness and densification of the passivation films improve markedly. The passivation films, primarily composed of Fe2O3 and Fe3O4, exhibit enhanced densification and stability at a static pressure of 500 N.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Su, G.; Fan, Q.; Zhang, Y.; Chen, H.; Zhang, C. Investigation of the Surface Characteristics of GCr15 in Electrochemical Machining. Micromachines 2024, 15, 1062. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Zhang, H.; Wu, F.; Qiao, S.; Dai, C.; Zhang, X.; Sun, M.; Liao, B.K.; Shen, Y.; Hao, L.; et al. Rare earth addition powered corrosion resistance of the surface oxide film on GCr15 bearing steel substrate. Corros. Sci. 2024, 240, 112490. [Google Scholar]
- Liu, J.; Liu, Q.; Zhao, L.; Yang, W.; Wang, X. Reducing Pitting Corrosion Trend of Cast GCr15 Steel by Inoculation: An In Situ Corrosion Morphology Study. Coatings 2024, 14, 836. [Google Scholar] [CrossRef]
- Bisma, P.; Wani, M.F. Tribological behaviour of nano-zirconia reinforced iron-based self-lubricating composites for bearing applications. Tribol. Int. 2021, 159, 106969. [Google Scholar]
- Łach, Ł. Recent Advances in Laser Surface Hardening: Techniques, Modeling Approaches, and Industrial Applications. Crystals 2024, 14, 726. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Tian, Y.; Ling, Y. Study on surface residual stress of 42CrMo steel treated by ultrasonic rolling extrusion. Sci. Rep. 2023, 13, 6956. [Google Scholar]
- Wang, X.; Wang, H.; Wang, P.; Zhu, Q. Multi-objective optimization of process parameters for ultrasonic rolling extrusion of 42CrMo material. Mech. Ind. 2023, 24, 6. [Google Scholar]
- Yang, Y.; Huang, T.; Ye, C.; Ding, H. Recent Progress in Ultrasonic Surface Rolling: A Comprehensive Overview. Adv. Eng. Mater. 2024, 26, 2401100. [Google Scholar] [CrossRef]
- Wang, M.; Shang, Y.; Liu, C.; Wang, J.; Zheng, J.; Xu, X. 3D multiphysic simulations of energy field and material process in radial ultrasonic rolling electrochemical micromachining. Chin. J. Aeronaut. 2022, 35, 494–508. [Google Scholar]
- Tang, J.; Shi, Y.; Zhao, J.; Chen, L.; Wu, Z. Numerical modeling considering initial gradient mechanical properties and experiment verification of residual stress distribution evolution of 12Cr2Ni4A steel generated by ultrasonic surface rolling. Surf. Coat. Technol. 2023, 452, 129127. [Google Scholar]
- Liu, Z.; Liu, H.; Li, J.; Xu, K.; Li, Z.; Qin, S. Ultrasonic rolling surface roughness study of shaft parts based on three-dimensional morphology reconstruction. J. Mech. Sci. Technol. 2024, 38, 6267–6278. [Google Scholar]
- Liu, Z.; Niu, Z.; Liu, H.; Xu, K.; Qin, S. Simulation of Residual Stress of V-Notch Specimen Treated by Ultrasonic Rolling. J. Mater. Eng. Perform. 2024, 34, 1232–1242. [Google Scholar]
- Wang, H.; Wang, X.; Tian, Y.; Ling, Y. Study on theory and finite element simulation of ultrasonic rolling extrusion process. Int. J. Adv. Manuf. Technol. 2024, 134, 1091–1109. [Google Scholar]
- Zhang, K.M.; Liu, S.; Wang, J.; Sun, Z.X.; Liu, W.J.; Zhang, C.C.; Zhang, X.C. Effect of high-frequency dynamic characteristics in the ultrasonic surface rolling process on the surface properties. J. Mater. Process. Tech. 2024, 327, 118353. [Google Scholar]
- Zheng, Z.; Feng, Y.C.; Liang, T.; Ya, Z.; Yi, F. Experimental study on surface integrity refactoring changes of Ti-17 under milling-ultrasonic rolling composite process. Adv. Manuf. 2023, 11, 492–508. [Google Scholar]
- Su, C.J.; Xu, T.T.; Zhang, K.; Lou, S.M.; Wang, Q. Plastic deformation of magnesium alloy with different forming parameters during ultrasonic vibration-assisted single-point incremental forming. Rare Met. 2018, 41, 3878–3886. [Google Scholar]
- Su, C.; Shi, Y.; Liu, Z. Strengthening Principle and Process Parameter Optimization of Ultrasonic Rolling of GCr15 Steel Bearing Roller. J. Mater. Eng. Perform. 2023, 33, 13079–13094. [Google Scholar]
- Xue, X.; Xu, S.; Pan, Y.; Zheng, W.; Han, J.; Li, J. Finite Element Analysis and Surface Performance Study of High-Speed Precision Bearing Ultrasonic Surface Rolling Processing. J. Mater. Eng. Perform. 2023, 33, 11924–11936. [Google Scholar]
- Ma, X.; Xu, S.; Xue, X.; Zhao, X.; Pan, Y.; Li, J.; Zheng, W. Effect of Multi-pass Ultrasonic Surface Rolling Process on Surface Properties and Microstructure of GCr15 Steel. J. Mater. Eng. Perform. 2024, 1–13. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X. Experimental study on surface roughness processed by ultrasonic surface rolling process. J. Phys. Conf. Ser. 2024, 2760, 012085. [Google Scholar]
- Cong, J.; Gao, J.; Zhou, S.; Zhang, Z.; Wang, J.; Wang, N. Effect of ultrasonic rolling on the fatigue performance of laser-welded TC4 titanium alloy joints. Int. J. Fract. 2024, 247, 87–105. [Google Scholar]
- Kang, J.; Zhang, H.; Zhang, J.; Zhang, Z.; Gui, T. Enhancement of the tool performance of circular saw blades by improving the microstructure and residual stress field using an ultrasonic rolling tensioning process. Int. J. Adv. Manuf. Technol. 2024, 135, 1517–1532. [Google Scholar]
- Zhang, H.; Yang, X.; Ma, X.; Jin, D.; You, J. Effect of Ultrasonic Rolling on Surface Properties of GCr15 Spherical Joint Bearing. Lubricants 2024, 12, 208. [Google Scholar] [CrossRef]
- Wang, P.; Pan, Y.; Liu, Y.; Fu, X.; Li, H. Research on surface properties of Ti-6Al-4V alloy by multi-ultrasonic rolling. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2021, 235, 5594–5602. [Google Scholar]
- Zheng, K.; Zhao, X.; Pan, L.; Ren, Z. Ultrasonic rolling strengthening of TC11 titanium alloy surface: Corrosion and wear properties under extreme conditions. Wear 2024, 550–551, 205415. [Google Scholar]
- Yan, L.; Gang, L.; Ni, A.; Chuanqi, Q.; Yi, W.; Guanzhen, Z.; Shengchuan, W. Effect of ultrasonic rolled material layer on the corrosion fatigue resistance of railway axle EA4T alloy steel. Eng. Fail. Anal. 2024, 157, 107895. [Google Scholar]
- Feng, H.; Li, H.-B.; Jiang, Z.-H.; Zhang, T.; Dong, N.; Zhang, S.-C.; Han, P.-D.; Zhao, S.; Chen, Z.-G. Designing for high corrosion-resistant high nitrogen martensitic stainless steel based on DFT calculation and pressurized metallurgy method. Corros. Sci. 2019, 158, 108081. [Google Scholar]




| Ingredient | C | Mn | Si | S | P | Cr |
|---|---|---|---|---|---|---|
| Proportion (wt%) | 0.95~1.05 | 0.20~0.40 | 0.15~0.35 | ≤0.02 | ≤0.027 | 1.30~1.65 |
| Number | Static Pressure (N) | Speed (r/min) | Feed Speed (mm/min) | Amplitude (μm) |
|---|---|---|---|---|
| 1 | 200 | 200 | 30 | 10 |
| 2 | 400 | 200 | 30 | 10 |
| 3 | 500 | 200 | 30 | 10 |
| Sample | Untreated | 200 N | 400 N | 500 N |
|---|---|---|---|---|
| Open-Circuit Potential (V) | −0.657 | −0.654 | −0.650 | −0.649 |
| Sample | Polarization Resistance (Ω) | Corrosion Current (A) | Corrosion Potential (V) | Corrosion Rate (g/m2·a) |
|---|---|---|---|---|
| Untreated | 169.5 | −1.074 | 51.90838422 | |
| 200 N | 535.3 | −1.077 | 15.68546983 | |
| 400 N | 2244.5 | −0.964 | 4.933485458 | |
| 500 N | 11360.2 | −0.675 | 1.008556627 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.; Huang, J.; Du, J.; Ma, X.; Jin, D. Influence of Ultrasonic Rolling Extrusion Static Pressure on Corrosion Resistance of GCr15 Bearing Steel. Coatings 2025, 15, 413. https://doi.org/10.3390/coatings15040413
Cheng M, Huang J, Du J, Ma X, Jin D. Influence of Ultrasonic Rolling Extrusion Static Pressure on Corrosion Resistance of GCr15 Bearing Steel. Coatings. 2025; 15(4):413. https://doi.org/10.3390/coatings15040413
Chicago/Turabian StyleCheng, Maolin, Jian Huang, Jiaran Du, Xiqiang Ma, and Dongliang Jin. 2025. "Influence of Ultrasonic Rolling Extrusion Static Pressure on Corrosion Resistance of GCr15 Bearing Steel" Coatings 15, no. 4: 413. https://doi.org/10.3390/coatings15040413
APA StyleCheng, M., Huang, J., Du, J., Ma, X., & Jin, D. (2025). Influence of Ultrasonic Rolling Extrusion Static Pressure on Corrosion Resistance of GCr15 Bearing Steel. Coatings, 15(4), 413. https://doi.org/10.3390/coatings15040413





