Advanced Electrochromic Functionality via Layered Cobalt Oxide Deposition on Tungsten Oxide Electrodes
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Materials
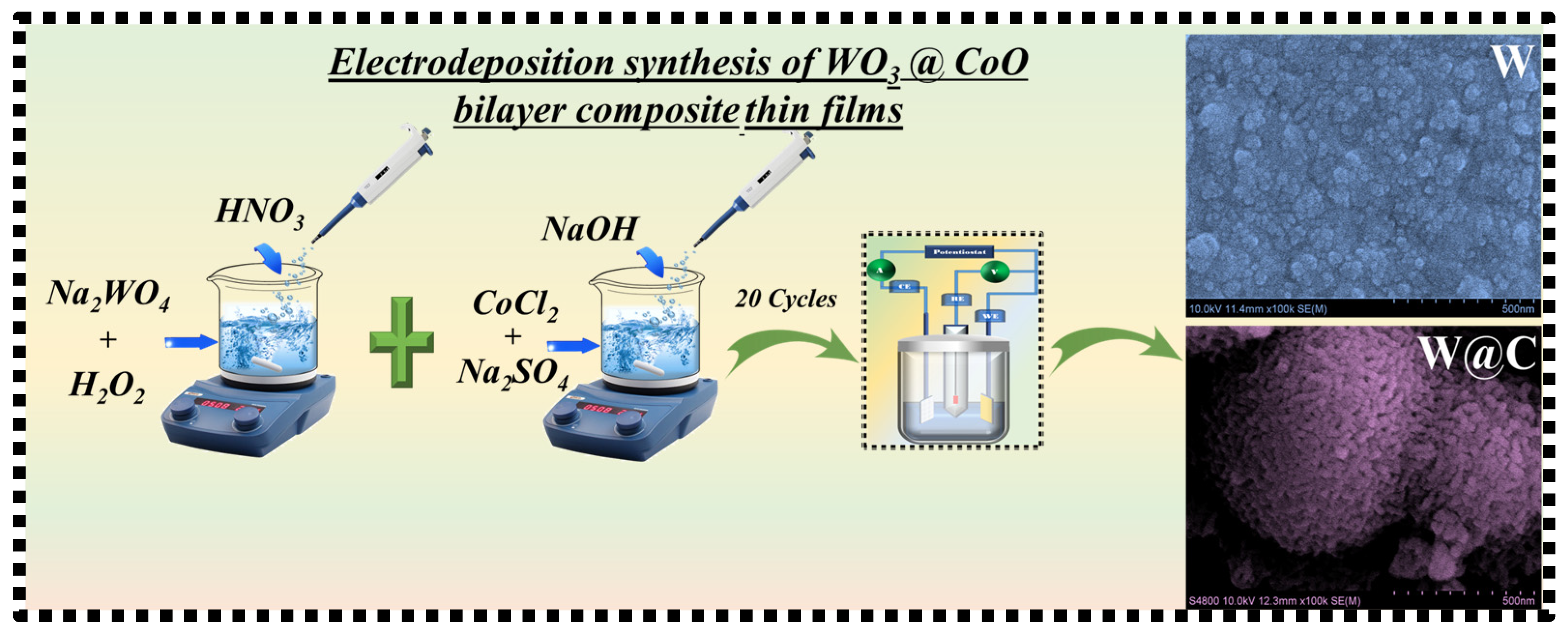
2.2. Synthesis of WO3 and W@C Bilayer Composite Thin Films
3. Electrochromic Device Fabrication
4. Material Characterization
5. Results and Discussions
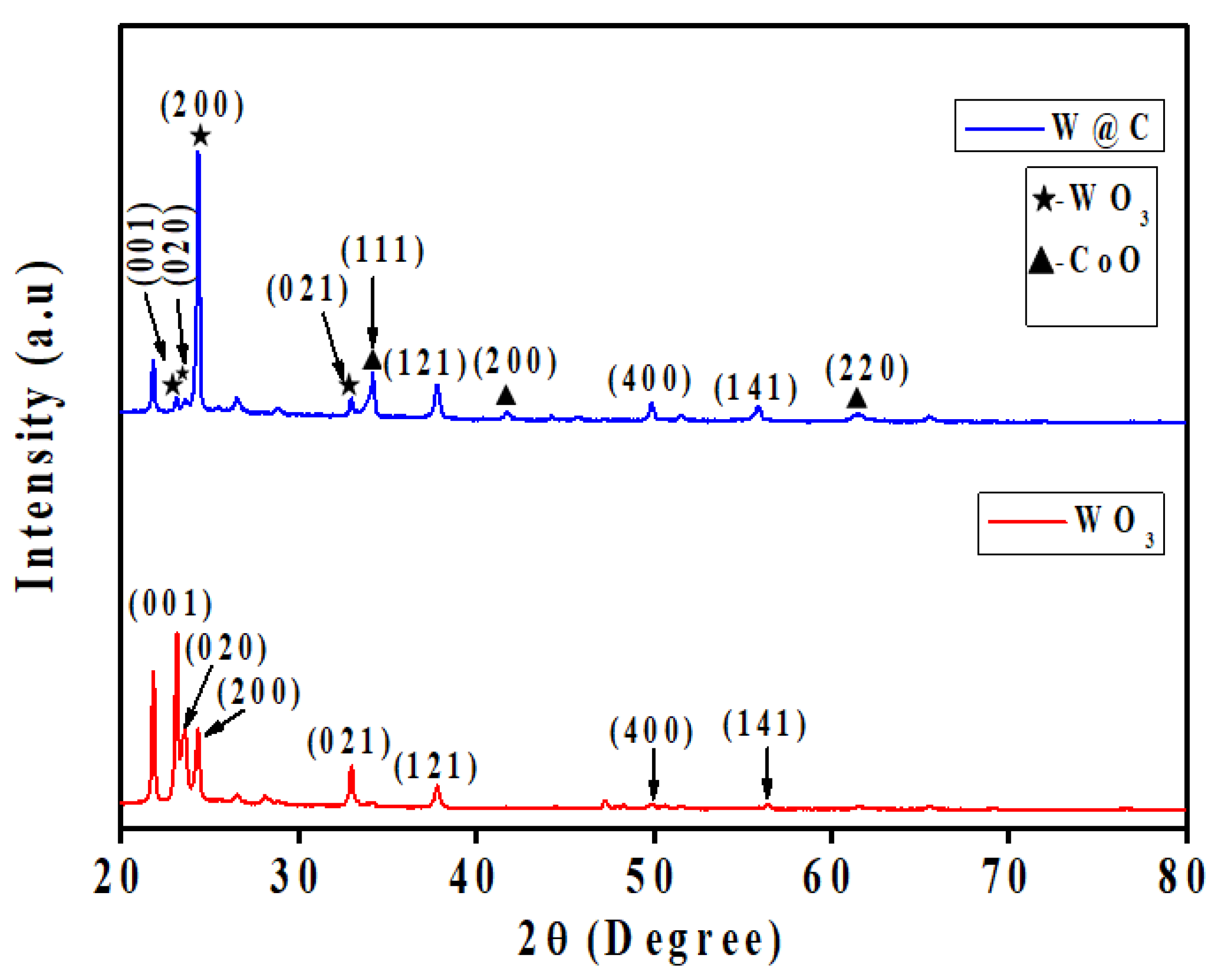
5.1. XRD Elucidation
5.2. XPS Analysis
5.3. Morphological and Elemental Composition Study
6. Electrochromic Analysis
7. W@C Based Electrochromic Device
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, B.; Dang, J.; Zhuang, Q.; Lv, Z. Recent advances in inorganic electrochromic materials from synthesis to applications: Critical review on functional chemistry and structure engineering. Chem. Asian J. 2022, 17, e202200022. [Google Scholar] [CrossRef]
- Afik, N.; Murugesan, S.; Shreteh, K.; Fridman, H.; Hijaze, Y.; Volokh, M.; Mokari, T. Synthesis of Ultrathin Alloy (Mo, V)-Tungsten-Oxide Nanowires: Implications for Electrochromic and Supercapacitor Applications. ACS Appl. Nano Mater. 2024, 7, 5878–5888. [Google Scholar] [CrossRef]
- Reyes-Gil, K.R.; Stephens, Z.D.; Stavila, V.; Robinson, D.B. Composite WO3/TiO2 nanostructures for high electrochromic activity. ACS Appl. Mater. Interfaces 2015, 7, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-K.; Sahu, D.; Wang, S.-C.; Huang, J.-L. Electrochromic Nb-doped WO3 films: Effects of post annealing. Ceram. Int. 2012, 38, 2829–2833. [Google Scholar] [CrossRef]
- Prasad, A.K.; Kim, J.-Y.; Kang, S.-H.; Ahn, K.-S. Molybdenum induced defective WO3 multifunctional nanostructure as an electrochromic energy storage device: Novel assembled photovoltaic-electrochromic Mo–WO3 film. J. Ind. Eng. Chem. 2024, 135, 388–396. [Google Scholar] [CrossRef]
- Wen, R.-T.; Granqvist, C.G.; Niklasson, G.A. Eliminating degradation and uncovering ion-trapping dynamics in electrochromic WO3 thin films. Nat. Mater. 2015, 14, 996–1001. [Google Scholar] [CrossRef]
- Tang, C.-J.; He, J.-L.; Jaing, C.-C.; Liang, C.-J.; Chou, C.-H.; Han, C.-Y.; Tien, C.-L. An all-solid-state electrochromic device based on WO3–Nb2O5 composite films prepared by fast-alternating bipolar-pulsed reactive magnetron sputtering. Coatings 2018, 9, 9. [Google Scholar] [CrossRef]
- Cai, G.; Zhou, D.; Xiong, Q.; Zhang, J.; Wang, X.; Gu, C.; Tu, J. Efficient electrochromic materials based on TiO2@ WO3 core/shell nanorod arrays. Sol. Energy Mater. Sol. Cells 2013, 117, 231–238. [Google Scholar] [CrossRef]
- Safdar, B.; Prasad, A.K.; Ahn, K.-S. NiCo-mixed hydroxide nanosheets as a new electrochromic material with fast optical response. Chem. Phys. Lett. 2021, 783, 139024. [Google Scholar] [CrossRef]
- Morankar, P.J.; Amate, R.U.; Teli, A.M.; Beknalkar, S.A.; Chavan, G.T.; Ahir, N.A.; Jeon, C.-W. Nanogranular advancements in molybdenum-doped tungsten oxide for superior electrochromic energy storage. J. Energy Storage 2024, 84, 110978. [Google Scholar] [CrossRef]
- Cossari, P.; Pugliese, M.; Simari, C.; Mezzi, A.; Maiorano, V.; Nicotera, I.; Gigli, G. Simplified all-solid-state WO3 based electrochromic devices on single substrate: Toward large area, low voltage, high contrast, and fast switching dynamics. Adv. Mater. Interfaces 2020, 7, 1901663. [Google Scholar] [CrossRef]
- Kim, J.; Ong, G.K.; Wang, Y.; LeBlanc, G.; Williams, T.E.; Mattox, T.M.; Helms, B.A.; Milliron, D.J. Nanocomposite architecture for rapid, spectrally-selective electrochromic modulation of solar transmittance. Nano Lett. 2015, 15, 5574–5579. [Google Scholar]
- Bathe, S.R.; Patil, P. Influence of Nb doping on the electrochromic properties of WO3 films. J. Phys. D Appl. Phys. 2007, 40, 7423. [Google Scholar]
- Chavan, G.; Sikora, A.; Pawar, R.; Warycha, J.; Morankar, P.; Jeon, C.-W. Hierarchical framework of CoZnS as a high-performance electrode material for supercapacitors. Ceram. Int. 2023, 49, 282–293. [Google Scholar]
- Yu, C.; Ma, D.; Wang, Z.; Zhu, L.; Guo, H.; Zhu, X.; Wang, J. Solvothermal growth of Nb2O5 films on FTO coated glasses and their electrochromic properties. Ceram. Int. 2021, 47, 9651–9658. [Google Scholar]
- Amate, R.U.; Morankar, P.J.; Chavan, G.T.; Teli, A.M.; Desai, R.S.; Dalavi, D.S.; Jeon, C.-W. Bi-functional electrochromic supercapacitor based on hydrothermal-grown 3D Nb2O5 nanospheres. Electrochim. Acta 2023, 459, 142522. [Google Scholar]
- Wu, W.; Wang, M.; Ma, J.; Cao, Y.; Deng, Y. Electrochromic metal oxides: Recent progress and prospect. Adv. Electron. Mater. 2018, 4, 1800185. [Google Scholar]
- Teli, A.M.; Beknalkar, S.A.; Amte, R.U.; Morankar, P.J.; Yewale, M.A.; Burungale, V.V.; Jeon, C.-W.; Efstathiadis, H.; Shin, J.C. Investigating into the intricacies of charge storage kinetics in NbMn-oxide composite electrodes for asymmetric supercapacitor and HER applications. J. Alloys Compd. 2023, 965, 171305. [Google Scholar]
- Richardson, T.J.; Slack, J.L.; Rubin, M.D. Electrochromism in copper oxide thin films. Electrochim. Acta 2001, 46, 2281–2284. [Google Scholar] [CrossRef]
- Amate, R.U.; Morankar, P.J.; Teli, A.M.; Chavan, G.T.; Beknalkar, S.A.; Ahir, N.A.; Jeon, C.-W. Exploring the electrochemical performance of niobium phosphate electrode for supercapacitor application. Surf. Interfaces 2023, 41, 103265. [Google Scholar] [CrossRef]
- Morankar, P.J.; Amate, R.U.; Teli, A.M.; Beknalkar, S.A.; Jeon, C.-W. Exploring electrochromic performance via layered deposition of tungsten oxide on niobium oxide composite electrode. J. Power Sources 2024, 613, 234930. [Google Scholar] [CrossRef]
- Memar, A.; Phan, C.M.; Tade, M.O. Controlling particle size and photoelectrochemical properties of nanostructured WO3 with surfactants. Appl. Surf. Sci. 2014, 305, 760–767. [Google Scholar]
- Poongodi, S.; Kumar, P.S.; Mangalaraj, D.; Ponpandian, N.; Meena, P.; Masuda, Y.; Lee, C. Electrodeposition of WO3 nanostructured thin films for electrochromic and H2S gas sensor applications. J. Alloys Compd. 2017, 719, 71–81. [Google Scholar]
- Venugopal, R.; Dinakaran, A.; Nair, M.C.; Balachandran, A.C.; Madhavan, N.D.; Deb, B. Electrochromic properties of MnO2/WO3 bilayered electrodes for enhanced charge storage and superior stability. RSC Appl. Interfaces 2024, 1, 1382–1394. [Google Scholar]
- Lee, Y.-H.; Park, J.-Y.; Ahn, K.-S.; Sung, Y.-E. MnO2 nanoparticles advancing electrochemical performance of Ni(OH)2 films for application in electrochromic energy storage devices. J. Alloys Compd. 2022, 923, 166446. [Google Scholar]
- Zhao, L.; Cai, Z.; Wang, X.; Liao, W.; Huang, S.; Ye, L.; Fang, J.; Wu, C.; Qiu, H.; Miao, L. Constructed TiO2/WO3 heterojunction with strengthened nano-trees structure for highly stable electrochromic energy storage device. J. Adv. Ceram. 2023, 12, 634–648. [Google Scholar]
- Mishra, S.; Yogi, P.; Sagdeo, P.; Kumar, R. TiO2–Co3O4 core–shell nanorods: Bifunctional role in better energy storage and electrochromism. ACS Appl. Energy Mater. 2018, 1, 790–798. [Google Scholar]
- Liu, F.; Chen, X.; Xia, Q.; Tian, L.; Chen, X. Ultrathin tungsten oxide nanowires: Oleylamine assisted nonhydrolytic growth, oxygen vacancies and good photocatalytic properties. RSC Adv. 2015, 5, 77423–77428. [Google Scholar]
- Deori, K.; Deka, S. Morphology oriented surfactant dependent CoO and reaction time dependent Co3O4 nanocrystals from single synthesis method and their optical and magnetic properties. CrystEngComm 2013, 15, 8465–8474. [Google Scholar]
- Li, Y.; Chopra, N. Structural evolution of cobalt oxide–tungsten oxide nanowire heterostructures for photocatalysis. J. Catal. 2015, 329, 514–521. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Q.; Liu, J.; Luo, H.A. Enhanced photoelectrochemical water oxidation of WO3/R-CoO and WO3/B-CoO photoanodes with a type II heterojunction. J. Mater. Sci. 2021, 56, 8079–8090. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, X.; Zhu, K.; Wang, J.; Cheng, Z.; Li, G.; Yang, L.; Bai, Z. Hybrid Co/CoO/Ce-Doped WO3 Nanoparticles on a ZIF-L Framework as Bifunctional Oxygen Electrocatalysts for Rechargeable Zinc–Air Batteries. ACS Appl. Nano Mater. 2023, 6, 14353–14363. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Thuy, U.T.D.; Truong, Q.D.; Honma, I.; Nguyen, Q.L.; Tran, P.D. Electrodeposited Amorphous Tungsten-doped Cobalt Oxide as an Efficient Catalyst for the Oxygen Evolution Reaction. Chem. Asian J. 2018, 13, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-C.; Chang, C.-W. Preparation of orthorhombic WO3 thin films and their crystal quality-dependent dye photodegradation ability. Coatings 2019, 9, 90. [Google Scholar] [CrossRef]
- Ziakhodadadian, S.; Ren, T. Structural and tribological properties of tungsten oxide thin film on a silicon substrate. J. Chem. Res. 2020, 44, 744–749. [Google Scholar]
- Xiao, T.D.; Tan, X.; Yi, M.; Peng, S.; Peng, F.; Yang, J.; Dai, Y. Synthesis of commercial-scale tungsten carbide-cobalt (WC/Co) nanocomposite using aqueous solutions of tungsten (W), cobalt (Co), and carbon (C) precursors. J. Chem. Eng. 2014, 2, 1–15. [Google Scholar]
- Sonpir, R.B.; Dake, D.V.; Raskar, N.D.; Mane, V.A.; Shinde, S.S.; Ingole, S.S.; Tak, M.S.; Dole, B.N. Effect of Enhancement in Surface Area of Sn-Doped Cobalt Oxide Nanoflakes for Supercapacitor Application. Phys. Status Solidi 2025, 222, 2400502. [Google Scholar]
- Morankar, P.J.; Amate, R.U.; Chavan, G.T.; Teli, A.M.; Dalavi, D.S.; Jeon, C.-W. Improved electrochromic performance of potentiostatically electrodeposited nanogranular WO3 thin films. J. Alloys Compd. 2023, 945, 169363. [Google Scholar]
- Amate, R.U.; Morankar, P.J.; Teli, A.M.; Beknalkar, S.A.; Chavan, G.T.; Ahir, N.A.; Dalavi, D.S.; Jeon, C.-W. Versatile electrochromic energy storage smart window utilizing surfactant-assisted niobium oxide thin films. Chem. Eng. J. 2024, 484, 149556. [Google Scholar]
- Dalavi, D.S.; Desai, R.S.; Patil, P.S. Nanostructured materials for electrochromic energy storage systems. J. Mater. Chem. A 2022, 10, 1179–1226. [Google Scholar]
- Dalavi, D.S.; Devan, R.S.; Patil, R.A.; Patil, R.S.; Ma, Y.-R.; Sadale, S.B.; Kim, I.; Kim, J.-H.; Patil, P.S. Efficient electrochromic performance of nanoparticulate WO3 thin films. J. Mater. Chem. C 2013, 1, 3722–3728. [Google Scholar] [CrossRef]
- More, A.J.; Patil, R.S.; Dalavi, D.S.; Mali, S.S.; Hong, C.K.; Gang, M.G.; Kim, J.H.; Patil, P.S. Electrodeposition of nano-granular tungsten oxide thin films for smart window application. Mater. Lett. 2014, 134, 298–301. [Google Scholar] [CrossRef]
- Guo, J.; Jia, H.; Shao, Z.; Jin, P.; Cao, X. Fast-Switching WO3-Based Electrochromic Devices: Design, Fabrication, and Applications. Acc. Mater. Res. 2023, 4, 438–447. [Google Scholar] [CrossRef]
- Zeb, S.; Sun, G.; Nie, Y.; Xu, H.; Cui, Y.; Jiang, X. Advanced developments in nonstoichiometric tungsten oxides for electrochromic applications. Mater. Adv. 2021, 2, 6839–6884. [Google Scholar] [CrossRef]
- An, F.H.; Yuan, Y.Z.; Liu, J.Q.; He, M.D.; Zhang, B. Enhanced electrochromic properties of WO3/ITO nanocomposite smart windows. RSC Adv. 2023, 13, 13177–13182. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Sen, S.; Samanta, S.; Kundu, S. Study on the role of rGO in enhancing the electrochromic performance of WO3 film. Electrochim. Acta. 2022, 427, 140820. [Google Scholar] [CrossRef]
- Arvizu, M.A.; Niklasson, G.A.; Granqvist, C.G. Electrochromic W1−x−yTixMoyO3 Thin Films Made by Sputter Deposition: Large Optical Modulation, Good Cycling Durability, and Approximate Color Neutrality. Chem. Mat. 2017, 29, 2246–2253. [Google Scholar] [CrossRef]



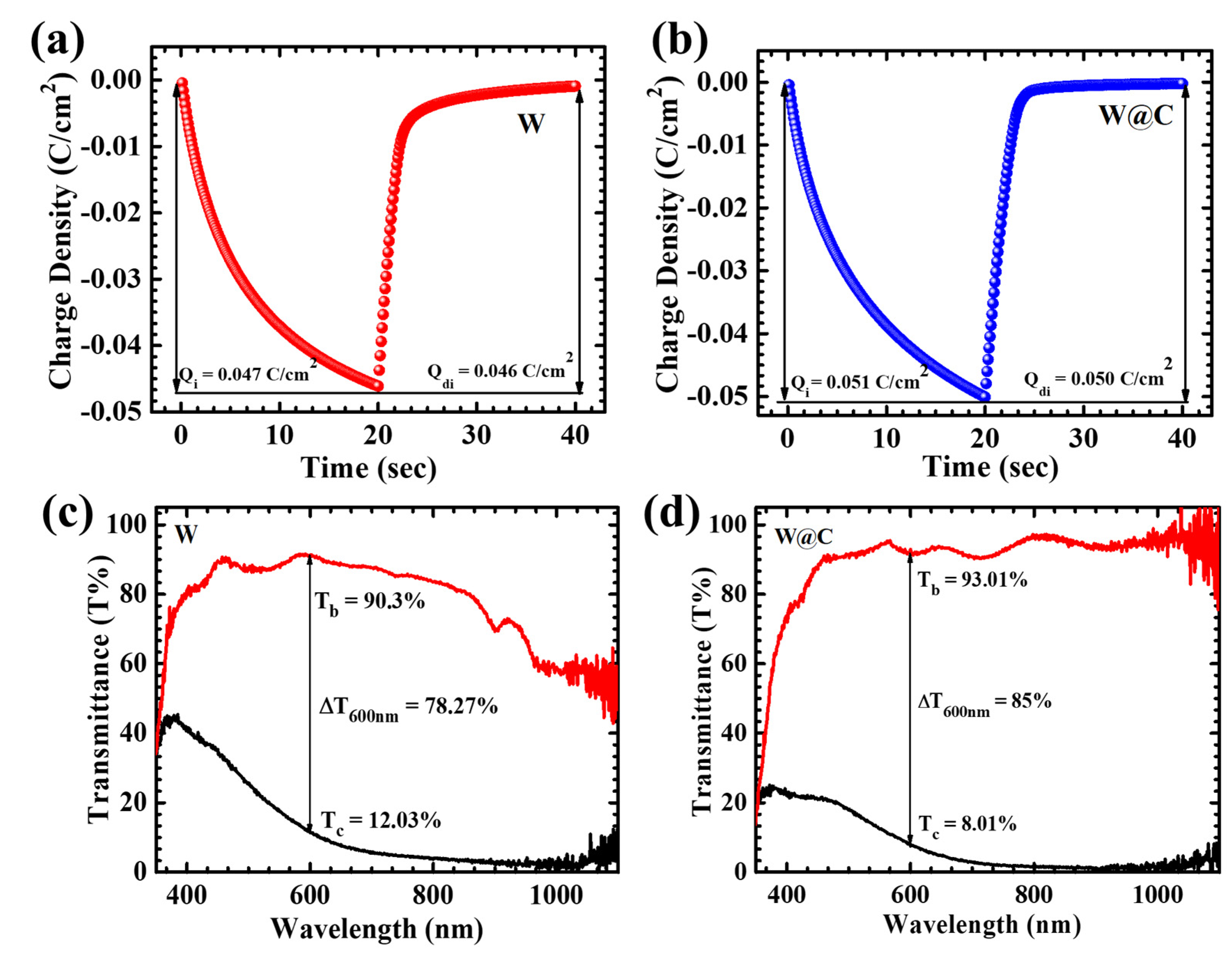


| Sample Name | Charge Intercalation ) (C/cm2) | Charge Deintercalation ) (C/cm2) | Reversibility (%) | Coloration Time (s) (TC) | Bleaching Time (s) (Tb) | Tb % | TC % | Optical Modulation (ΔT600nm%) | Optical Density(ΔOD) | Coloration Efficiency (cm2/C) |
|---|---|---|---|---|---|---|---|---|---|---|
| W | 0.047 | 0.046 | 97.87% | 11.2 | 6.02 | 90.3 | 12.03 | 78.27 | 2.01 | 85.53 |
| W@C | 0.051 | 0.050 | 98.03% | 12.6 | 5.4 | 93.01 | 8.01 | 85.0 | 2.48 | 96.07 |
| Se. No. | Material | Deposition Technique | Reversibility (%) | Optical Modulation (ΔT600nm%) | Coloration Efficiency (cm2/C) | Reference |
|---|---|---|---|---|---|---|
| 1. | WO3–Nb2O5 | Magnetron Sputtering | 82.40 | 33 | 30.9 | [7] |
| 2. | WO3 | Electrodeposition | 87 | 68.89 | 154.93 | [23] |
| 3. | MnO2/WO3 | Electrodeposition | - | 55.6 | 51.7 | [24] |
| 4. | MnO2/Ni (OH)2 | Electrodeposition | - | - | 34 | [25] |
| 5. | TiO2/WO3 | Hydrothermal | - | 79.5 | 443.4 | [26] |
| 6. | TiO2–Co3O4 | Hydrothermal | - | - | 91 | [27] |
| 7. | WO3/(CoO) | Electrodeposition | 98.03 | 85 | 96.07 | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morankar, P.J.; Amate, R.U.; Bhosale, M.K.; Ahir, N.A.; Jeon, C.-W. Advanced Electrochromic Functionality via Layered Cobalt Oxide Deposition on Tungsten Oxide Electrodes. Coatings 2025, 15, 403. https://doi.org/10.3390/coatings15040403
Morankar PJ, Amate RU, Bhosale MK, Ahir NA, Jeon C-W. Advanced Electrochromic Functionality via Layered Cobalt Oxide Deposition on Tungsten Oxide Electrodes. Coatings. 2025; 15(4):403. https://doi.org/10.3390/coatings15040403
Chicago/Turabian StyleMorankar, Pritam J., Rutuja U. Amate, Mrunal K. Bhosale, Namita A. Ahir, and Chan-Wook Jeon. 2025. "Advanced Electrochromic Functionality via Layered Cobalt Oxide Deposition on Tungsten Oxide Electrodes" Coatings 15, no. 4: 403. https://doi.org/10.3390/coatings15040403
APA StyleMorankar, P. J., Amate, R. U., Bhosale, M. K., Ahir, N. A., & Jeon, C.-W. (2025). Advanced Electrochromic Functionality via Layered Cobalt Oxide Deposition on Tungsten Oxide Electrodes. Coatings, 15(4), 403. https://doi.org/10.3390/coatings15040403






