The Design and Preparation of New Fe(21-x)CoNiCuAlTix High-Entropy-Alloy Wear- and Corrosion-Resistant Coatings and an Investigation of Their Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Test Methods and Characterization
3. Results and Discussion
3.1. Morphology of Laser-Clad HEA Specimens
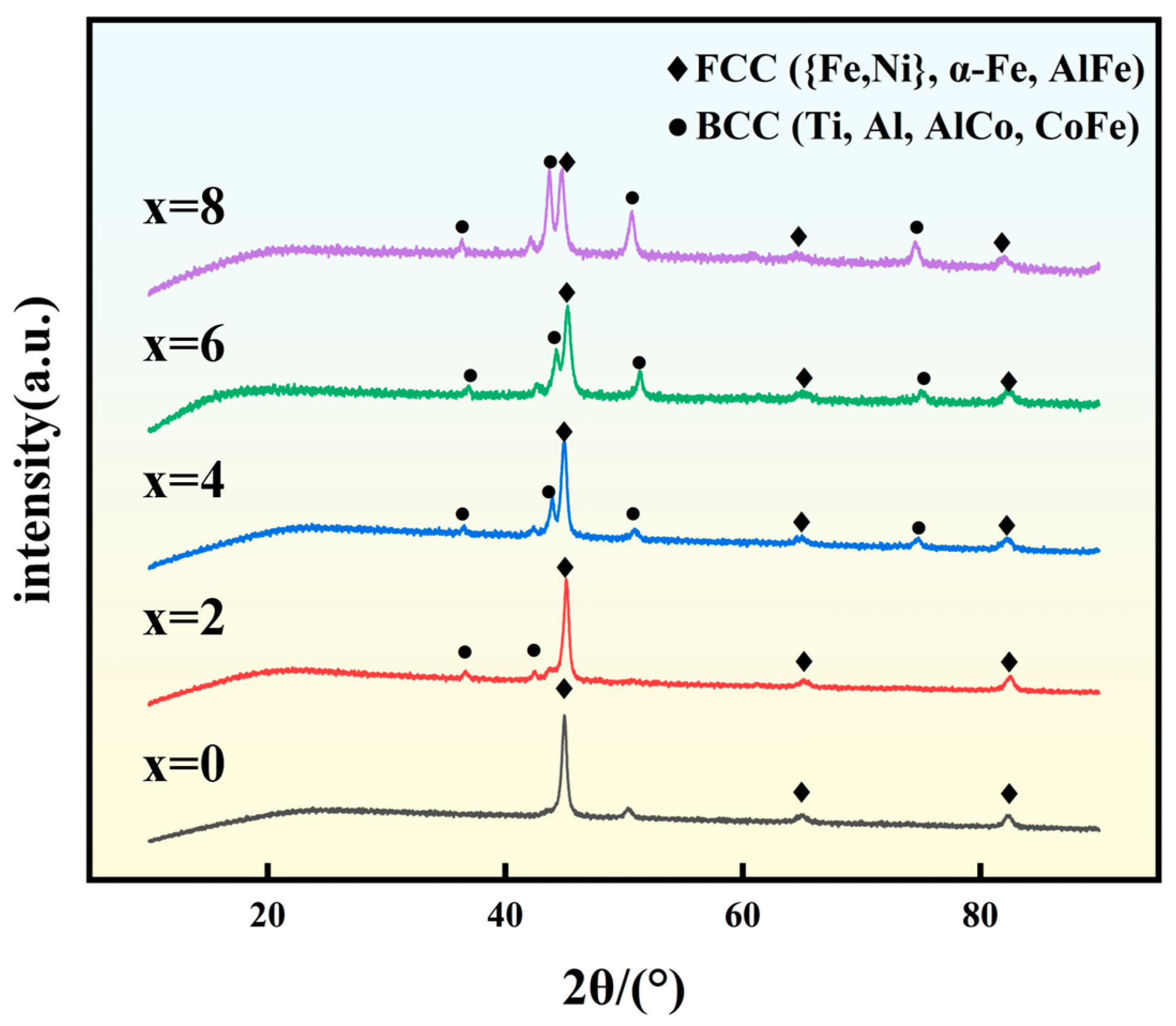
3.2. Physical Phase Analysis of Coatings
3.3. Microstructural Analysis of Coating
3.4. Microhardness of Coatings
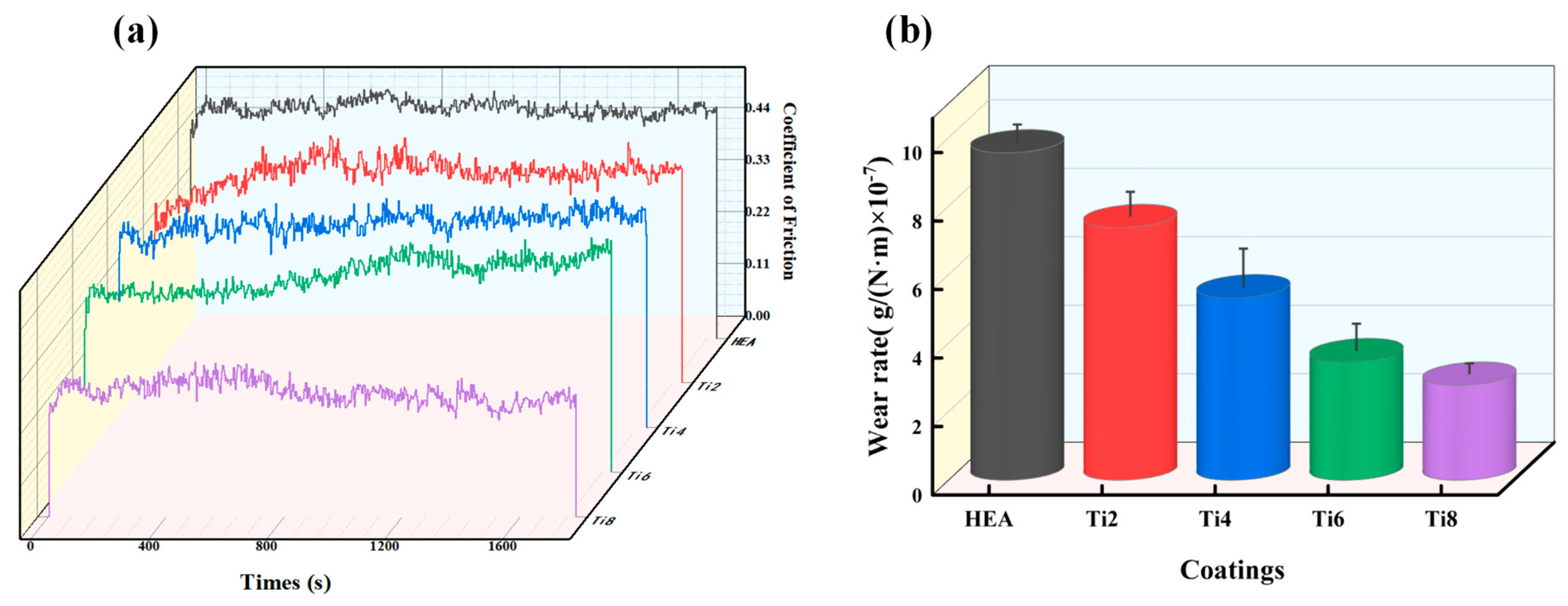
3.5. Frictional Wear Behavior of Coatings
3.5.1. Average Coefficient of Friction and Wear Rate
3.5.2. Coating Surface Wear Morphology and Wear Mechanism
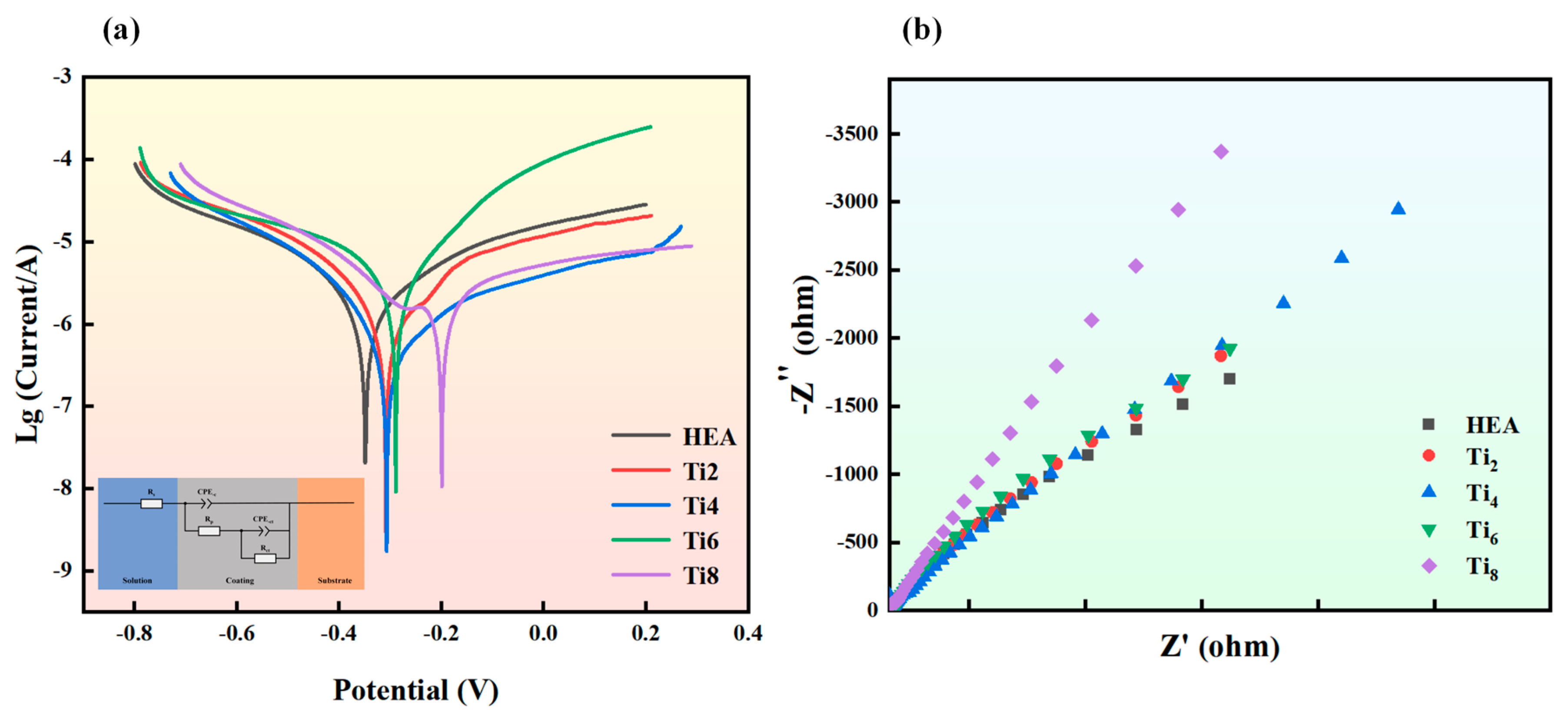
3.6. Corrosion Behavior of Coatings
4. Conclusions
- The Fe(21-x)CoNiCuAlTix coating initially exhibits a single FCC phase at x = 0, where the FCC phase corresponds to Fe and Ni solid solutions. Upon the introduction of Ti, a BCC phase begins to emerge, attributed to the Ti solid solution. As the Ti content increases, the BCC diffraction peaks intensify, reaching their maximum at x = 8. This indicates that the addition of Ti alters the alloy’s crystal structure, promoting the formation of the BCC phase. Furthermore, the corresponding FCC diffraction peaks shift to the left with increasing Ti content, suggesting that the incorporation of Ti induces lattice distortion.
- For the coating with the addition of Ti elements, the microstructure of the grain is significantly refined due to the high melting point of Ti and the high temperature of the laser cladding process precipitation, resulting in an increase in the solid solution, while Ti elements in the solidification process form stable TiC, TiB2, and other compounds; these compounds play a role in heterogeneous nucleation, which promotes the refinement of the coating grains. The distribution of Ti elements forms TiC and other high-hardness compounds, which can significantly improve the microhardness of the coating.
- The comprehensive coating physical phase analysis, tissue structure analysis, and microhardness analysis show that due to the addition of Ti elements to promote the formation of a BCC phase, compared to an FCC phase, the coating’s slip coefficient is smaller, it cannot slip as easily, and it has higher strength, and the presence of Ti will generate TiC and other high-hardness compounds at the same time, which greatly improves the abrasion resistance of the coatings; the addition of Ti elements to the coatings enhances both solid-solution strengthening and fine-grain strengthening. The significant lattice distortion caused by the atomic radius difference in the Ti atoms contributes to this effect. This distortion leads to an increase in the microhardness of the coatings, resulting in improved wear resistance as the Ti content is increased.
- As the Ti content increases, the corrosion potential of the Fe(21-x)CoNiCuAlTix coating gradually shifts to a more positive value, and the corrosion current density decreases. At x = 8, the corrosion potential reaches −0.199 V, representing a 42.98% increase compared to the coating without Ti addition. The corrosion current density at this composition is 3.513 × 10−7 A/cm2, an improvement of 387.8%. This enhancement is attributed to the formation of Al2O3 and TiO2 oxide films by the Al and Ti elements, respectively. The introduction of Ti significantly refines the microstructure, reducing the corrosion rate through grain size refinement. Additionally, Ti and other elements stabilize intermetallic compounds, further reducing the corrosion rate at grain boundaries and enhancing the overall corrosion resistance of the coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Sun, W.; Huang, J.; Wu, Y.; Lu, Y.; Wang, S. Effect of Annealing Temperature on Residual Stress and Microstructure of Ni60A Laser Cladding Repaired Gear. Coatings 2025, 15, 212. [Google Scholar] [CrossRef]
- Das, A.K. Effect of rare earth oxide additive in coating deposited by laser cladding: A review. Mater. Today Proc. 2022, 52, 1558–1564. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Q.; Hu, J.; Zhao, S.; Zheng, X.; Wang, H.; Liu, H. Effect of Recovery Treatment on the Microstructure and Tribological Properties of Ultrasonic Impacted Al2FeCoNiCrW0.5 High-Entropy Alloy Coatings. Coatings 2025, 15, 83. [Google Scholar] [CrossRef]
- Findik, F. Laser cladding and applications. Sustain. Eng. Innov. 2023, 5, 1–14. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Han, B.; Li, M.; Hu, C.; Wang, J. Comparative studies on microstructure and properties of CoCrFeMnNi high entropy alloy coatings fabricated by high-speed laser cladding and normal laser cladding. J. Alloys Compd. 2023, 947, 169517. [Google Scholar] [CrossRef]
- John, M.; Kuruveri, U.B.; Menezes, P.L. Laser cladding-based surface modification of carbon steel and high-alloy steel for extreme condition applications. Coatings 2022, 12, 1444. [Google Scholar] [CrossRef]
- Gong, N.; Meng, T.L.; Cao, J.; Wang, Y.; Karyappa, R.; Tan, C.; Suwardi, A.; Zhu, Q.; Ngo, A.; Misra, K.; et al. Laser-cladding of high entropy alloy coatings: An overview. Mater. Technol. 2023, 38, 2151696. [Google Scholar] [CrossRef]
- Straumal, B.B.; Klinger, L.; Kuzmin, A.; Lopez, G.A.; Korneva, A.; Straumal, A.B.; Vershinin, N.; Gornakova, A.S. High Entropy Alloys Coatings Deposited by Laser Cladding: A Review of Grain Boundary Wetting Phenomena. Coatings 2022, 12, 343. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, Y.; Wan, Y.; Jeyaprakash, N.; Wang, Y.; Ma, K.; Yang, J. Influence of scanning speed on microstructure and corrosion resistance of Fe-based amorphous coatings by high-speed laser cladding. Surf. Coat. Technol. 2024, 479, 130449. [Google Scholar] [CrossRef]
- Yuan, W.; Li, R.; Chen, Z.; Gu, J.; Tian, Y. A comparative study on microstructure and properties of traditional laser cladding and high-speed laser cladding of Ni45 alloy coatings. Surf. Coat. Technol. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Naizabekov, A.; Samodurova, M.; Bodrov, E.; Lezhnev, S.; Samoilova, O.; Trofimov, E.; Mikhailov, D.; Litvinyuk, K.; Trofimova, S.; Latfulina, Y.; et al. Use of laser cladding for the synthesis of coatings from high-entropy alloys reinforced with ceramic particles. Case Stud. Constr. Mater. 2023, 19, e02541. [Google Scholar] [CrossRef]
- Zhao, P.; Shi, Z.; Wang, X.; Li, Y.; Cao, Z.; Zhao, M.; Liang, J. A Review of the Laser Cladding of Metal-Based Alloys, Ceramic-Reinforced Composites, Amorphous Alloys, and High-Entropy Alloys on Aluminum Alloys. Lubricants 2023, 11, 482. [Google Scholar] [CrossRef]
- Li, Z.; Taheri, M.; Torkamany, P.; Heidarpour, I.; Torkamany, M.J. Laser cladding of NiCrCoFeNbMoX high-entropy alloy to increase resistance to corrosion of gas turbine blades. Vacuum 2024, 219, 112749. [Google Scholar] [CrossRef]
- Ma, M.; Nie, S.; Yu, H.; Huang, G.; Wang, X.; Zhang, N. Effect of Heat Treatment on Microstructure and Wear Behavior of Laser Clad FeCoCrMoNi High-Entropy Alloy Coatings. J. Mater. Eng. Perform. 2024, 1–11. [Google Scholar] [CrossRef]
- Han, Y.; Fu, H. Improvement of high temperature wear resistance of laser-cladding high-entropy alloy coatings: A review. Metals 2024, 14, 1065. [Google Scholar] [CrossRef]
- Wei, X.; Dai, F.; Ban, A.; Liu, L.; Li, N.; Zhang, C. Effect of Ni-Coated-Al2O3 Addition on Slurry Erosion Behavior of AlCoCrFeNi High-Entropy Alloy Coatings Prepared by Laser Cladding. J. Therm. Spray Technol. 2023, 32, 2123–2132. [Google Scholar] [CrossRef]
- Yuan, S.; Li, H.; Han, C.; Li, W.; Xu, X.; Chen, C.; Wei, R.; Wang, T.; Wu, S.; Li, F. FeCoNiCrAl0.6 high-entropy alloy coating on Q235 steel fabricated by laser cladding. Mater. Sci. Technol. 2023, 39, 705–713. [Google Scholar] [CrossRef]
- Yan, D.; Shi, C.; Wang, J.; Zhang, Y.; Sun, J.; Wang, Y.; Liu, P. Microstructure and properties of laser cladding AlxFeCoCrNiMn high entropy alloy of Q345 steel. Mater. Res. 2023, 26, e20220154. [Google Scholar] [CrossRef]
- Wangy, N. Corrosion Resistance of Laser Cladded FeCoCrxNiAly High Entropy Alloy Repair Layer on Surface of Q345 Welded Structure; Nanjing University of Aeronautics and Astronautics: Nanjing, China, 2022. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, G.; Song, Q.; Lu, X.; Li, Z.; Zhao, Q.; Chen, Y. Microstructure, mechanical properties, and corrosion resistance of SiC reinforced AlxCoCrFeNiTi1-x high-entropy alloy coatings prepared by laser cladding. Surf. Coat. Technol. 2022, 437, 128349. [Google Scholar] [CrossRef]
- Shen, Q.; Xue, J.; Yu, X.; Zheng, Z.; Ou, N. Triple-wire plasma arc cladding of Cr-Fe-Ni-Tix high-entropy alloy coatings. Surf. Coat. Technol. 2022, 443, 128638. [Google Scholar] [CrossRef]
- Gu, Z.; Xi, S.; Sun, C. Microstructure and properties of laser cladding and CoCr2.5FeNi2Tix high-entropy alloy composite coatings. J. Alloys Compd. 2020, 819, 152986. [Google Scholar] [CrossRef]
- Cai, Y.; Shan, M.; Manladan, S.M.; Zhu, L.; Gao, F.; Sun, D.; Han, J. Effect of high temperature heat treatment on microstructure and properties of FeCoCrNiAl high-entropy alloy laser cladding layer. Mater. Charact. 2022, 191, 112137. [Google Scholar] [CrossRef]
- Hao, X.; Liu, H.; Zhang, X.; Tao, J.C.; Wang, Y.; Yang, C.; Liu, Y. Microstructure and wear resistance of in-situ TiN/(Nb, Ti) 5Si3 reinforced MoNbTaWTi-based refractory high entropy alloy composite coatings by laser cladding. Appl. Surf. Sci. 2023, 626, 157240. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, Y.; Wan, Y.; Chen, H.; Wang, Y.; Yang, J. Laser clad CoCrFeNiTixNby hypoeutectic high-entropy alloy coating: Effects of Ti and Nb content on mechanical, wear and corrosion properties. Surf. Coat. Technol. 2024, 494, 131346. [Google Scholar] [CrossRef]
- Lian, G.; Yang, J.; Que, L.; Lan, R.; Tang, X. Effects of Ti content on the microstructure and properties of AlCoCrFeNiTix high-entropy alloys prepared by laser cladding. J. Mater. Sci. 2024, 59, 1027–1043. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, N.; Yu, H.; Hao, D.; Ma, Y. AlxCoCrFeNiSi high entropy alloy coatings with high microhardness and improved wear resistance. Surf. Coat. Technol. 2020, 402, 126328. [Google Scholar] [CrossRef]
- Hao, C.; Meng, J.; Ding, H.; Li, Q.; Chen, M. Microstructure and Wear Resistance of AlxCoCrFeCuNi High Entropy Alloy Coating by Argon Arc Cladding. Surf. Technol. 2023, 52, 360–368. [Google Scholar] [CrossRef]
- Wang, X.; Hu, S.; Li, W.; Hu, Y. Corrosion monitoring for prestressed concrete cylinder pipe spigot with combined use of Tafel extrapolation and surface acoustic wave methods. Constr. Build. Mater. 2022, 337, 127572. [Google Scholar] [CrossRef]
- Ye, B.; Pan, G.; Yang, X.; Qi, Y.; Fang, Q.; She, L.; Di, Y. Electrochemical corrosion behavior and theoretical simulation of cobalt in chemical mechanical polishing process. Electrochim. Acta 2023, 468, 143184. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, P.; Li, L.; Liu, Q.; Guo, S. Enhancing biocompatibility, mechanical properties, and corrosion resistance of laser cladding β-TiNb coatings. J. Vac. Sci. Technol. A 2023, 41, 063103. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Chen, C. Microstructure and properties of Ni-Ti based gradient laser cladding layer of Ti6Al4V alloy by laser powder bed fusion. Addit. Manuf. 2024, 79, 103906. [Google Scholar] [CrossRef]

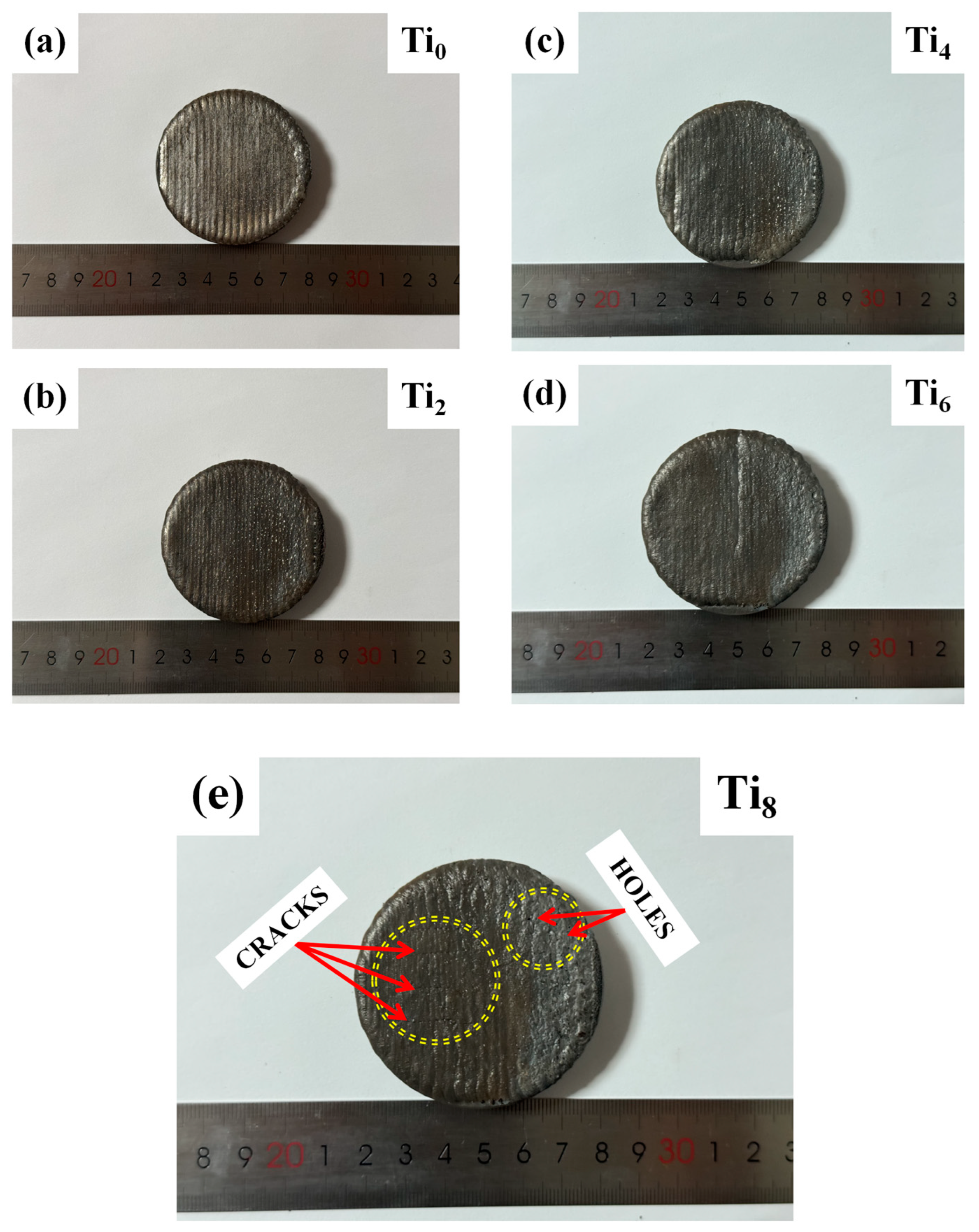



| Ti | Fe | Cu | Ni | Al | Co | |
|---|---|---|---|---|---|---|
| 0 | 0 | 21 | 24.12 | 22.47 | 10.49 | Bal. |
| Ti2 | 2 | 19 | 24.12 | 22.47 | 10.49 | Bal. |
| Ti4 | 4 | 17 | 24.12 | 22.47 | 10.49 | Bal. |
| Ti6 | 6 | 15 | 24.12 | 22.47 | 10.49 | Bal. |
| Ti8 | 8 | 13 | 24.12 | 22.47 | 10.49 | Bal. |
| Coatings | Coefficient of Friction | Average Coefficient of Friction | Dispersion Errors (Standard Deviation) |
|---|---|---|---|
| HEA | 0.391 | 0.49 | 0.07 |
| 0.517 | |||
| 0.550 | |||
| Ti2 | 0.415 | 0.44 | 0.04 |
| 0.501 | |||
| 0.413 | |||
| Ti4 | 0.386 | 0.43 | 0.05 |
| 0.497 | |||
| 0.416 | |||
| Ti6 | 0.491 | 0.42 | 0.06 |
| 0.354 | |||
| 0.403 | |||
| Ti8 | 0.216 | 0.26 | 0.03 |
| 0.285 | |||
| 0.291 |
| Coatings | Amount of Wear/g | Average/g | Dispersion Errors (Standard Deviation) |
|---|---|---|---|
| HEA | 0.0218 | 0.022 | 0.003 |
| 0.0194 | |||
| 0.0260 | |||
| Ti2 | 0.0138 | 0.015 | 0.004 |
| 0.0112 | |||
| 0.0212 | |||
| Ti4 | 0.0097 | 0.010 | 0.0006 |
| 0.0101 | |||
| 0.0111 | |||
| Ti6 | 0.0048 | 0.007 | 0.0013 |
| 0.0077 | |||
| 0.0073 | |||
| Ti8 | 0.0039 | 0.006 | 0.002 |
| 0.0049 | |||
| 0.0086 |
| Coatings | Wear Rate g/(N·m) | Average g/(N·m) | Dispersion Errors (Standard Deviation) |
|---|---|---|---|
| HEA | 9.64 × 10−7 | 9.9 × 10−7 | 1.2 × 10−7 |
| 8.58 × 10−7 | |||
| 11.50 × 10−7 | |||
| Ti2 | 6.10 × 10−7 | 6.9 × 10−7 | 1.9 × 10−7 |
| 4.95 × 10−7 | |||
| 9.38 × 10−7 | |||
| Ti4 | 4.29 × 10−7 | 4.6 × 10−7 | 2.6 × 10−8 |
| 4.47 × 10−7 | |||
| 4.91 × 10−7 | |||
| Ti6 | 2.12 × 10−7 | 2.9 × 10−7 | 5.7 × 10−8 |
| 3.41 × 10−7 | |||
| 3.23 × 10−7 | |||
| Ti8 | 1.72 × 10−7 | 2.6 × 10−7 | 8.9 × 10−8 |
| 2.17 × 10−7 | |||
| 3.80 × 10−7 |
| Coatings | Ecorr/V | Icorr/A/cm2 |
|---|---|---|
| HEA | −0.349 | 1.714 × 10−6 |
| Ti2 | −0.309 | 1.094 × 10−6 |
| Ti4 | −0.307 | 5.966 × 10−7 |
| Ti6 | −0.289 | 4.761 × 10−7 |
| Ti8 | −0.199 | 3.513 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Huang, G.; Hu, R.; Lin, Q.; Zhang, X.; Li, W.; Chen, L. The Design and Preparation of New Fe(21-x)CoNiCuAlTix High-Entropy-Alloy Wear- and Corrosion-Resistant Coatings and an Investigation of Their Performance. Coatings 2025, 15, 396. https://doi.org/10.3390/coatings15040396
Guo C, Huang G, Hu R, Lin Q, Zhang X, Li W, Chen L. The Design and Preparation of New Fe(21-x)CoNiCuAlTix High-Entropy-Alloy Wear- and Corrosion-Resistant Coatings and an Investigation of Their Performance. Coatings. 2025; 15(4):396. https://doi.org/10.3390/coatings15040396
Chicago/Turabian StyleGuo, Chun, Guangcan Huang, Ruizhang Hu, Qingcheng Lin, Xinyu Zhang, Wenqing Li, and Linting Chen. 2025. "The Design and Preparation of New Fe(21-x)CoNiCuAlTix High-Entropy-Alloy Wear- and Corrosion-Resistant Coatings and an Investigation of Their Performance" Coatings 15, no. 4: 396. https://doi.org/10.3390/coatings15040396
APA StyleGuo, C., Huang, G., Hu, R., Lin, Q., Zhang, X., Li, W., & Chen, L. (2025). The Design and Preparation of New Fe(21-x)CoNiCuAlTix High-Entropy-Alloy Wear- and Corrosion-Resistant Coatings and an Investigation of Their Performance. Coatings, 15(4), 396. https://doi.org/10.3390/coatings15040396







