1. Introduction
Controlling surface wettability is crucial for many scientific and industrial applications [
1,
2]. Wettability, defined by the hydrophilic or hydrophobic nature of a surface, influences processes such as liquid adhesion [
3], corrosion resistance [
4], heat transfer [
5], and self-cleaning effects [
6]. Inspired by natural phenomena such as the lotus leaf effect [
7] and fish scales [
8], researchers have sought to engineer surfaces that exhibit extreme water-repelling or water-attracting characteristics through precise micro- and nano-scale structuring [
9,
10,
11]. These advancements have led to the development of superhydrophobic and superhydrophilic surfaces, which are highly sought after in fields ranging from biomedical engineering [
12] and microfluidics [
13] to anti-icing coatings [
14] and energy-efficient systems [
15].
Among various surface modification techniques (e.g., chemical etching [
16], anodization [
17], plasma treatments [
18], physical vapor deposition [
19], and electrodeposition [
20]), laser-based processing has emerged as a highly versatile and precise method for tailoring surface properties [
21,
22,
23,
24,
25]. In contrast to traditional chemical treatments, which often require the use of specialized reagents [
26] and involve the need for proper disposal [
27], laser-based methods enable localized and repeatable modifications without generating harmful byproducts [
28,
29,
30]. Additionally, beyond modifying surface topography, laser processing can significantly influence surface chemistry through oxidation, which further affects wettability. The formation of oxide layers during or after laser ablation plays a critical role in determining long-term hydrophobic or hydrophilic behavior. By simultaneously controlling both the structural and chemical aspects of the surface, laser texturing provides an effective strategy for achieving durable and tunable wettability characteristics.
Among the various laser processing techniques, different pulse durations—ranging from femtosecond to nanosecond regimes—play a crucial role in determining the final surface characteristics [
31,
32]. Ultrafast femtosecond lasers offer high precision with minimal thermal effects [
33,
34], while longer pulse durations, such as nanosecond lasers, introduce controlled heat input, allowing for efficient and scalable surface texturing [
35,
36]. The selection of an appropriate laser system depends on the intended application, required processing speed, and cost-effectiveness [
37]. In this context, nanosecond laser ablation has emerged as a promising technique, balancing processing efficiency and surface modification capabilities [
38,
39,
40]. By leveraging the ability of nanosecond laser pulses to induce localized melting, re-solidification, and controlled oxidation, it is possible to engineer surface morphologies that influence wettability [
41]. These effects are particularly relevant for metallic surfaces, where laser processing not only alters topography but also modifies surface chemistry through oxidation and alloying effects [
42].
A key advantage of nanosecond laser processing is its ability to induce controlled surface texturing through rapid material ablation [
43], directly influencing wettability [
44]. By adjusting parameters such as energy density, pulse duration, and scanning speed, it is possible to achieve a broad spectrum of surface characteristics, from enhanced hydrophilicity to pronounced hydrophobicity [
45]. The interaction between the laser beam and the material surface leads to the formation of hierarchical micro- and nanoscale structures, which govern liquid adhesion through capillary effects and contact angle hysteresis [
46]. Additionally, chemical modifications induced by high-temperature reactions, such as oxide formation, further contribute to wettability changes [
47].
Unlike ultrafast femtosecond lasers and nanosecond systems offer a balance between cost-efficiency and scalability [
48], making them suitable for large-area processing in applications like corrosion-resistant coatings, tribological enhancements, and thermal management. Beyond initial structuring, post-laser treatments—including controlled oxidation; atmospheric exposure; or functional coatings—can further fine-tune surface properties; ensuring long-term stability and tailored performance for specific industrial and biomedical applications [
48].
Copper, especially high-purity Cu-ETP (Electrolytic Tough Pitch Copper), which consists of at least 99.9% Cu and a controlled oxygen content of around 0.02–0.04%, is a promising material for laser surface modification due to its excellent thermal and electrical conductivity, corrosion resistance, and antimicrobial properties. Hydrophobic copper surfaces are used in anti-corrosion coatings, heat exchangers, and oil-water separation systems, while hydrophilic surfaces are applied in water purification, biomedical implants, and microfluidic devices. Achieving controlled and reproducible hydrophobicity and hydrophilicity remains challenging due to the need for precise morphological and chemical modifications. Laser processing, particularly nanosecond-pulsed fiber laser ablation, enables the tuning of these properties through appropriate laser parameter selection, eliminating the drawbacks of chemical coatings.
Lim et al. [
49] used FEM simulations to model nanosecond pulsed laser ablation of copper, considering absorption, reflectivity, pulse energy, and beam profile. Their two-dimensional heat source model improved accuracy over one-dimensional approaches and was validated against Nd:YAG laser experiments (266 nm, 532 nm). Results showed that ablation efficiency saturates with increasing shots and depends more on reflectivity than absorption, consistent with Semerok et al. [
50]. The optimal pulse width for copper was 0.75 ns, highlighting the role of pulse duration in nanosecond laser processing. In contrast, experimental studies have focused on the surface modifications induced by nanosecond laser processing and their impact on wettability. Chun et al. [
51] used a Q-switched Nd:YAG laser (355 nm, 20 ns, 0.02 kHz) to texture copper, forming grids with burrs and debris. Low-temperature annealing (200 °C) with ethanol accelerated the CuO-to-Cu
2O transition, achieving a contact angle CA = 165°, similar to He et al. [
52], who also confirmed ethanol’s role in stabilizing superhydrophobicity. Expanding on this, Long et al. [
53] applied a fiber laser (1064 nm, 20 kHz) with pulse widths from 20 to 200 ns to create hierarchical micro/nanostructures. Fluorosilane treatment further enhanced hydrophobicity, yielding CA~170°. Shorter pulses produced taller microstructures with higher adhesion, while longer pulses formed denser nanoparticle layers, reducing adhesion—consistent with Chun et al.’s findings on roughness-enhanced wettability. He et al. [
52] refined this approach using a fiber laser (1060 nm, 25 ns) to create micro/nanopillar structures. Ethanol-assisted annealing (110 °C, 2.5 h) restored superhydrophobicity by increasing the contact angle from an initial 2° (superhydrophilic state after laser ablation) to 161° (superhydrophobic state). After wear (ultrasonic cleaning), the CA decreased to 121°, but subsequent ethanol-assisted annealing recovered it to 151°, demonstrating surface renewability. By varying groove spacing (50–300 μm), they controlled droplet retention, confirming that laser texturing and oxide transformation provide a scalable method for durable superhydrophobic copper surfaces. While previous studies focused on the role of laser-induced hierarchical microstructures in achieving superhydrophobicity, other research has expanded toward understanding wettability transitions and the stability of hydrophobic states under different conditions. Ta et al. [
54] demonstrated that nanosecond laser texturing (1064 nm, 220 ns, 75–93 J/cm
2) can induce superhydrophobicity on copper and brass surfaces, achieving a steady-state contact angle of ~152° after natural oxidation over 11–30 days. The transition from hydrophilicity to superhydrophobicity was attributed to the partial deoxidation of laser-induced oxides. This self-cleaning surface was further applied to chemical sensing, where methanol concentrations significantly influenced contact angle values, confirming the role of laser-induced structures in wetting-sensitive applications. Similarly, Long et al. [
55] investigated the formation mechanism of hierarchical micro/nanostructures on copper using a low-cost nanosecond fiber laser (1064 nm, 20 ns, 100 kHz). They identified that the resolidification of laser-melted material and redeposition of ablation plume were critical in forming stable hierarchical textures, providing an alternative explanation to oxide-driven wettability transitions observed by Ta et al. Their results showed that finer nanostructures significantly reduced water adhesion, aligning with earlier findings on Cassie-state stability. Chang et al. [
56] provided further insight into wettability transitions by studying CuO nanowire films, which naturally evolved from superhydrophilic to superhydrophobic states over time or after low-temperature annealing. XPS analysis confirmed that this transition was due to partial deoxidation of CuO to Cu
2O, a process also observed in laser-textured copper surfaces. Notably, they demonstrated that superhydrophilicity could be reversibly restored through high-temperature annealing, suggesting a pathway for tunable wettability. Pike et al. [
57] reinforced this understanding by showing that CuO nanoparticles, when reduced under controlled conditions, transition to a stable Cu
2O phase instead of metallic copper. This highlights a key aspect of oxide stability, which directly influences long-term wettability evolution in laser-textured copper surfaces. Wang et al. [
58] expanded on this concept, demonstrating that copper surfaces could undergo wettability cycling between superhydrophobicity (CA = 165°) and superhydrophilicity (CA~0°) through adsorption/desorption of a fluorocarbon monolayer. This cycling was thermally induced at 200 °C, offering a robust method for reversibly controlling wetting behavior without altering the microstructure. In contrast, Long et al. [
59] explored picosecond laser processing to fabricate periodic nanostructures on copper, achieving WDCA = 153.9° ± 3.2° with low sliding angles. Unlike oxide-driven transitions, their study emphasized the role of structural periodicity in controlling both superhydrophobicity and optical properties, producing colorful, biomimetic surfaces similar to Morpho butterfly wings. Further refining these principles, Long et al. [
60] systematically analyzed Cassie-state stability on metallic superhydrophobic surfaces using femtosecond laser texturing. Their results confirmed that densely packed nanoscale roughness is essential for maintaining a stable Cassie state under condensation and evaporation conditions, addressing a key limitation observed in oxide-based transitions. Collectively, these studies illustrate a progression from oxide-driven wettability transitions to structured-induced Cassie-state stability, highlighting how laser processing parameters, oxide chemistry, and hierarchical morphology collectively govern superhydrophobic copper surfaces.
The objective of this study is to examine how nanosecond laser ablation affects the wettability and microstructure of Cu-ETP copper sheets by varying fluence, pulse duration, and repetition rate. By exceeding conventional ablation thresholds, this research explores the interplay between surface morphology, oxidation, and wettability transitions. In addition to direct laser-induced modifications, this study also investigates the role of natural oxidation in ambient air and accelerated oxidation via low-temperature annealing in controlling wettability. Post-ablation oxidation processes significantly influence surface chemistry, leading to time-dependent wettability transitions. Using SEM, EDS, and contact angle measurements, the study analyzes hierarchical micro/nanostructures, their stability, and their role in hydrophilic and hydrophobic states. Findings will provide insights into laser-induced wettability control and oxidation-driven modifications, supporting scalable, chemical-free surface engineering for applications such as anti-corrosion coatings, heat exchangers, and functional surfaces requiring tunable wetting properties.
2. Materials and Methods
2.1. Materials
Commercial high-purity Cu-ETP copper was selected for this study, with its chemical composition (in wt.%) and UNS (Unified Numbering System) code shown in
Table 1. Samples with dimensions of 0.5 mm × 55 mm × 55 mm were cut from metal sheets. To remove the corrosion inhibitor from their surfaces, the samples underwent mechanical polishing followed by chemical polishing in a mixture of concentrated acids: orthophosphoric (V) (H
3PO
4), nitric (V) (HNO
3), and acetic (CH
3COOH) in a 3:1:1 volume ratio at 25 °C. The material was then cleaned with acetone in an ultrasonic bath and rinsed with distilled water.
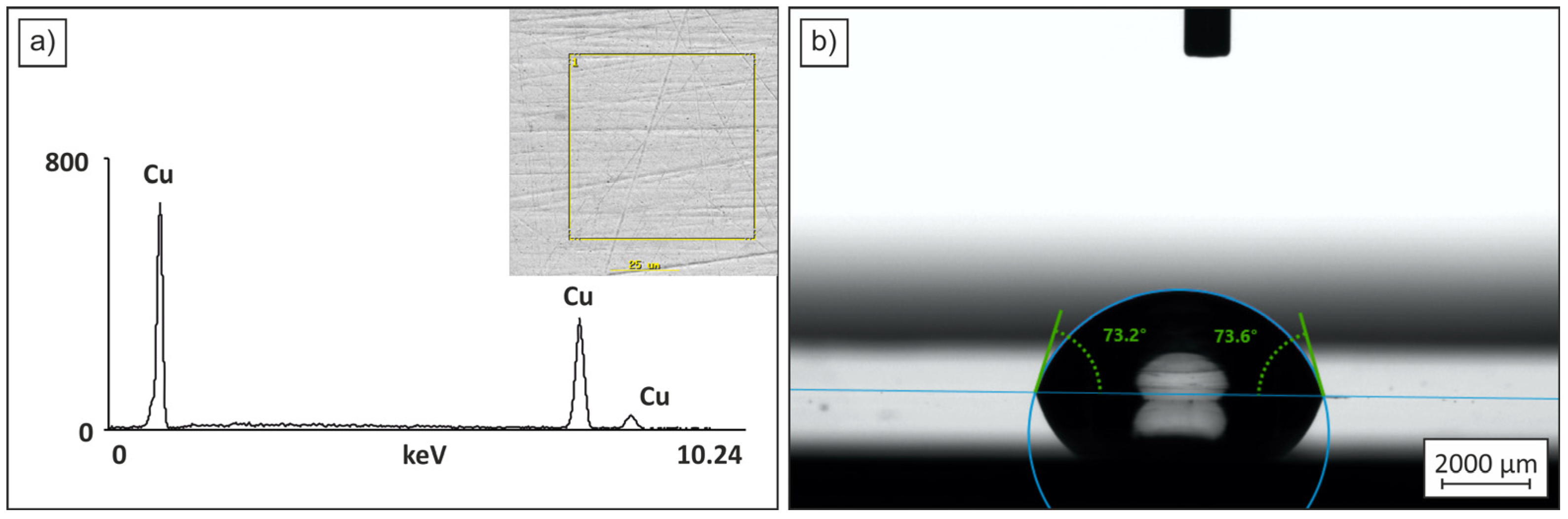
Figure 1 shows the surface morphology of a polycrystalline material obtained under casting and rolling conditions, with a grain size of approximately 20–50 μm. The figure presents an EDS plot of one analyzed point, confirming the elemental composition, and an image of a 2 μL water droplet on the surface, used for wettability assessment. The hardness of the tested sheet is 85 HV3, the roughness coefficient Ra is 0.1 μm, and the contact angle is 73.4°, indicating that the surface exhibits hydrophilic (wettable) properties.
2.2. Laser System
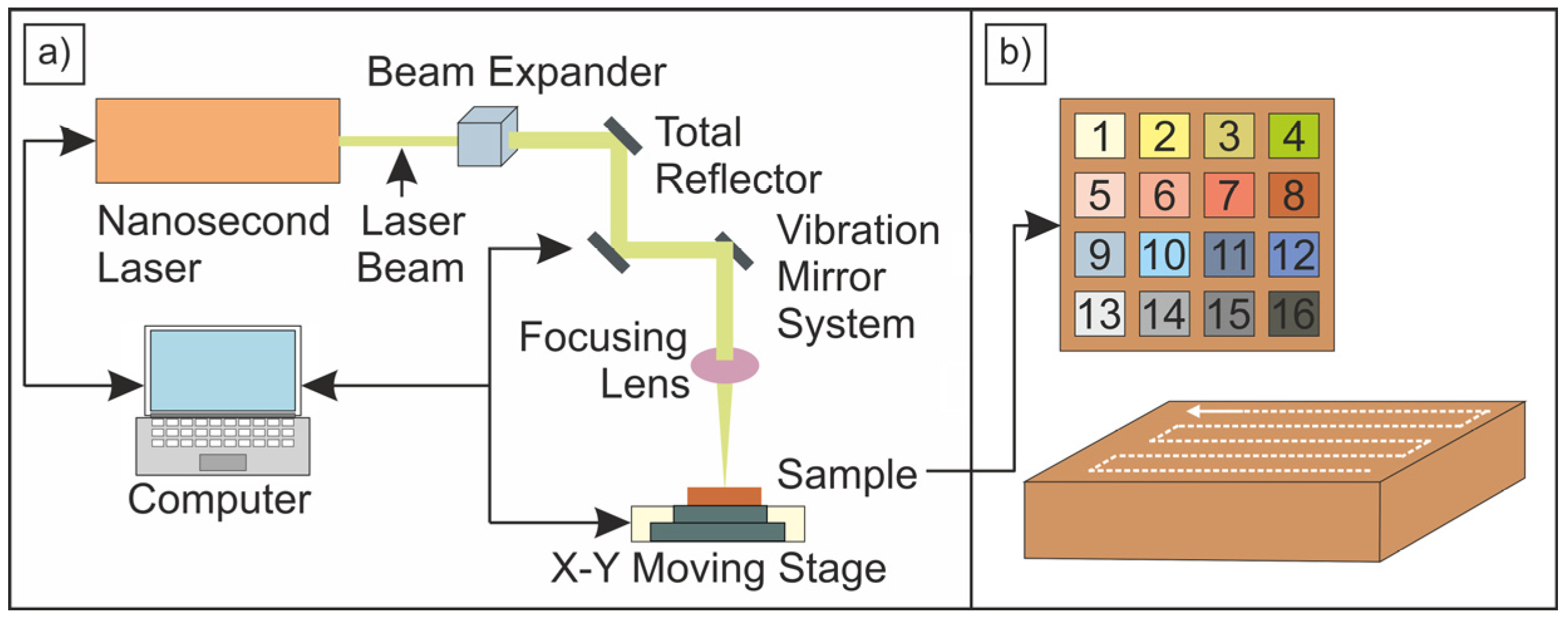
A MOPA 20 W nanosecond laser (wavelength 1064 nm, DK Lasertechnik, Krakow, Poland), commonly used in industrial applications, was employed for laser ablation, enabling reproducible results in laboratory settings that are scalable to industrial conditions on flat copper products. This type of laser is widely utilized in industry and allows for the fabrication of diverse surface patterns—such as grids; grooves; or spots—that can either support water droplets by minimizing their contact with the metal substrate or facilitate fluid spreading within the trenches; thereby increasing wettability.
Many previous studies, including those cited in the Introduction, have focused on a narrow range of nanosecond laser parameters (e.g., low fluence, frequency, or wavelength) and their impact on ablating engineering materials such as copper, typically evaluated through contact angle (CA) and surface morphology. To broaden this scope, we investigated nanosecond laser ablation at three repetition rates (25 kHz, 100 kHz, and 1000 kHz), sixteen pulse durations (range 2–500 ns), and a moderate scanning speed of 500 mm/s. The laser fluences were set to 25.46 J/cm
2, 254.65 J/cm
2, and 1018.59 J/cm
2, deliberately exceeding common values to assess the effect of high fluence on surface structural changes and the resultant CA, measured using the sessile drop method. This comprehensive approach offers a more extensive view of how key parameters—repetition rate; pulse duration; fluence; and scanning speed—influence copper ablation by a nanosecond laser; thereby enabling more effective tuning of its functional properties.
Figure 2 shows the schematic of the laser processing procedure and the Cu-ETP sample (0.5 mm × 55 mm × 55 mm) prepared for testing, featuring a 16-square grid pattern (10 mm × 10 mm each) created using dkGalvo software (v. 1.2.3.2.). Each square was processed with different ablation parameters by systematically varying the pulse duration and frequency while maintaining a step size of 30 µm, a constant scanning speed, and full laser power (100%). The fluence values reported in this study refer to the single-pulse fluence (J/cm
2) rather than the total accumulated fluence on the surface. The effective fluence depends on the pulse overlap and scanning parameters, which influence the total energy delivered to a given area.
2.3. Surface Properties
The surface wettability (contact angle) of the Cu-ETP samples after laser ablation and subsequent oxidation was measured using a drop shape analyzer (DSA25, Krüss GmbH, Hamburg, Germany). A 2 µL droplet of demineralized water was placed on each sample under controlled ambient conditions (20 °C ± 0.5 °C, 50% ± 2% relative humidity) to minimize external environmental influences. The system was calibrated using a reference standard before each measurement session to ensure accuracy. For each sample, six independent contact angle measurements were taken at different locations, and the results were averaged. The standard deviation of the measurements did not exceed ±1.5°. Wettability measurements were conducted at different oxidation stages to evaluate time-dependent changes induced by natural and accelerated oxidation. To ensure measurement consistency, the contact angle was recorded within 5 s after placing the water droplet on the sample surface to minimize time-dependent variations in wettability. The droplet volume used was 2 µL, resulting in an approximate droplet diameter of 1.3–1.5 mm. Compared to the micro- and nanoscale surface features (ranging from 5 to 50 µm for microstructures and 100–500 nm for nanostructures), the droplet size was significantly larger, interacting with multiple texture features simultaneously. Additionally, for samples exhibiting periodic groove textures (such as those processed at 100 kHz with 4–100 ns pulse durations), directional wettability effects were observed. Specifically, the droplet exhibited preferential spreading along the groove direction, with a reduced spreading tendency in the perpendicular direction. This anisotropic wetting behavior was most pronounced for shorter pulse durations (≤50 ns), where the groove aspect ratio was highest.
In addition to wettability measurements, surface roughness was evaluated using a profilometer (Hommel T1000, Jenoptik, Villingen-Schwenningen, Germany), following the PN-EN ISO 4288:2011 standard. The measurements were performed with a range of 80 µm, a total measurement length (Lt) of 4.8 mm, and a cut-off length (Lc) of 0.8 mm, maintaining an Lc/Ls ratio of 300. For grooved textures, roughness measurements were conducted both parallel and perpendicular to the groove direction. However, the roughness values presented in this study correspond to the perpendicular measurements, as this direction exhibited greater height variations due to the presence of ridges and valleys. In contrast, parallel measurements showed lower roughness values due to the relatively smoother profile along the groove orientation. For each sample, the roughness parameter Ra was determined based on measurements taken at six different locations, and the average values were reported. These roughness measurements provided insights into the correlation between surface topography and wettability changes occurring both after laser treatment and during the oxidation process.
Microstructural analysis was performed using a scanning electron microscope (Inspect S50, FEI Company, Hillsboro, OR, USA) equipped with a secondary electron (SE) detector. Observations were conducted at magnifications ranging from ×100 to ×4000, with an accelerating voltage of 20 kV, to ensure optimal resolution of surface features. To minimize charging effects, samples were not coated with a conductive layer. Energy-dispersive X-ray spectroscopy (EDS) analysis was carried out at an accelerating voltage of 15 kV, using a spot size of 2 µm, ensuring reliable elemental composition measurements. SEM and EDS analysis were conducted at different stages of oxidation to observe the evolution of surface morphology and chemical composition over time.
To evaluate the statistical significance of the effects of the processing parameters on the surface properties, an analysis of variance (ANOVA) was conducted. The ANOVA test was performed at a significance level of p < 0.05 to determine which factors had a statistically significant impact on the measured results. Statistical analysis was carried out using OriginPro 2023 (OriginLab, Northampton, MA, USA). Each set of parameters was tested with three repetitions, and the results are presented as mean ± standard deviation. The analysis also included an assessment of interactions between factors to determine possible combined effects.
2.4. Post-Oxidation Surface Process in the Final Modification of Copper Sheets
Following laser ablation, the samples underwent oxidation under two different conditions: natural oxidation in ambient air and accelerated oxidation in a controlled furnace environment. The samples selected for oxidation were chosen based on wettability measurements and microstructural analysis, ensuring a representative assessment of surface modifications.
Natural oxidation was performed at room temperature under laboratory conditions over a period ranging from 1 to 90 days, allowing an assessment of time-dependent wettability changes induced by atmospheric exposure. This process accounts for the natural evolution of the surface state due to gradual interaction with oxygen in the air, which can alter surface chemistry over time, potentially leading to hydrophobic behavior. The environmental conditions were controlled at 20 °C ± 0.5 °C with a relative humidity of 50% ± 2%, ensuring reproducibility of the oxidation process.
For accelerated oxidation, samples were subjected to low-temperature annealing in a muffle furnace (Czylok, Jastrzębie-Zdrój, Poland) at 200 °C for durations ranging from 1 to 12 h. Low-temperature annealing is known to accelerate oxidation processes, promoting the formation of a stable oxide layer that influences surface chemistry and wettability. During laser ablation, molten copper reacts with atmospheric oxygen, forming initial oxide layers that may not be chemically stable. Over time, these oxides can undergo phase transformations, leading to wettability transitions from hydrophilic to superhydrophobic states. By applying controlled thermal oxidation, we aim to stabilize the oxide layer composition and study its impact on surface-wetting properties.
3. Results and Discussion
3.1. Effects of Nanosecond Laser Ablation on the Surface Wettability
Figure 3 presents water droplet contact angle measurements on Cu-ETP copper sheet samples after laser ablation at 25 kHz, 100 kHz, and 1000 kHz, with pulse durations from 2 to 500 ns in ambient air. The results demonstrate a significant influence of frequency and pulse duration on surface wettability. At 1000 kHz, the surface becomes hydrophobic (θ > 90°) for pulse durations between 4 and 500 ns, which correlates with the low fluence of 25.46 J/cm
2. Limited oxidation and minimal surface modification likely contribute to this effect. However, at 2 ns, the surface is hydrophilic (θ = 81°), suggesting that the short pulse duration does not induce sufficient oxidation or structural changes to enhance hydrophobicity. At 25 kHz, a different trend is observed. For 2–9 ns, the surface behaves similarly to the untreated material, with a contact angle ≥ 90°. From 13 ns to 500 ns, the surface transitions to hydrophilic, with CA decreasing from 23° to 0°. This shift is attributed to the high fluence and low frequency, which promote intense material removal and oxidation, leading to the formation of a hydrophilic CuO layer. For the intermediate frequency of 100 kHz, hydrophilic properties are observed for pulse durations between 4 and 250 ns (θ = 0–75°). However, at 350 ns and 500 ns, the contact angle increases, likely due to excessive heating and surface structure formation, reducing wettability. Laser ablation results in the formation of a CuO layer, which is typically hydrophilic, but its effect depends on processing parameters. Low fluence and high-frequency limit oxidation, preserving hydrophobic properties, while high fluence and low frequency enhance oxidation and promote hydrophilicity.
Summarizing this part of the study, the wettability of Cu-ETP copper sheets after nanosecond laser processing is directly governed by the interplay between frequency, fluence, and pulse duration, which dictate both the oxidation state and the microstructural characteristics of the surface layer. The transition from hydrophilic to hydrophobic behavior is strongly correlated with the extent of oxidation and surface restructuring. At low frequencies (25 kHz) with high fluence, enhanced oxidation and material removal lead to the formation of a hydrophilic CuO layer. In contrast, at high frequencies (1000 kHz) with low fluence, oxidation is limited, and the resulting surface remains hydrophobic. The intermediate frequency (100 kHz) exhibits a mixed response, with hydrophilic properties observed for short and moderate pulse durations, while longer pulses promote partial hydrophobicity. These findings demonstrate that by precisely controlling laser parameters, it is possible to tailor the wettability of copper surfaces, which may have implications for applications requiring surface-functionalized metallic materials.
An alternative graphical representation of the contact angle values shown in
Figure 3 is provided in
Figure 4, illustrating the evolution of demineralized water droplet shape on Cu-ETP copper sheets after nanosecond laser ablation at different repetition rates, fluence, and pulse durations. The droplet morphology correlates with the contact angle (CA), reflecting surface modifications induced by laser processing. For hydrophilic surfaces (θ < 90°), observed at 25 kHz with high fluence (1018.59 J/cm
2), the droplet spreads significantly, forming a flattened profile. At very low CA values (~0°) for pulse durations of 250–500 ns, complete wetting occurs, indicating strong adhesion due to increased roughness and oxidation. A gradual decrease in CA from 23° to 0° is evident as pulse duration increases from 13 ns to 500 ns (
Figure 4a,d), confirming that extended laser-material interaction enhances wettability. Conversely, for hydrophobic surfaces (θc > 90°), particularly at 1000 kHz with low fluence (25.46 J/cm
2), the droplet remains more spherical, minimizing surface contact. This effect is most pronounced at longer pulse durations (100–500 ns) (
Figure 4f), where reduced oxidation and smoother surface features decrease wettability. Interestingly, for very short pulses (2 ns), the surface remains hydrophilic (θc = 81°), suggesting that oxidation and roughness are insufficient at this duration to induce hydrophobic behavior. Intermediate cases at 100 kHz (254.65 J/cm
2) (
Figure 4b,e) exhibit a gradual transition, with droplet spreading significantly at short to moderate pulse durations (4–250 ns, CA = 0–75°), whereas longer pulses (350–500 ns) show a noticeable increase in CA, likely due to thermal effects leading to surface smoothening and reduced oxidation.
Based on the CA values from
Figure 3 to
Figure 4, representative samples were selected for further investigation of surface morphology via SEM imaging, as shown in
Figure 5,
Figure 6 and
Figure 7. Additionally, these selected samples were subjected to an oxidation process under both natural and controlled furnace conditions to assess the impact of oxidation on surface properties. The selection includes samples processed at 25 kHz (9 ns, 13 ns, 200 ns, 500 ns), 100 kHz (4 ns, 45 ns, 100 ns), and 1000 kHz (2 ns, 500 ns).
The wettability alteration of engineering materials occurring during laser surface texturing results from the modification of the surface layer under various processing parameters, including laser fluence, wavelength, repetition rate, and pulse duration. This modification leads to the formation of different surface patterns characterized by varying roughness coefficients, which can take the form of spots, grids, or grooves, as reported by researchers in previous studies [
40,
53,
55]. In this study, a similar surface morphology was observed after the nanosecond laser ablation of copper sheets at repetition rates of 25 kHz (
Figure 5), 100 kHz (
Figure 6), and 1000 kHz (
Figure 7), as evidenced by SEM images.
The statistical analysis confirmed that the tested factors of nanosecond laser processing, such as repetition rate and pulse duration, had a statistically significant impact on the measured surface properties (p < 0.05). The interaction effects between parameters were analyzed, revealing that specific parameter combinations led to optimal surface modifications in terms of wettability.
3.2. Effects of Nanosecond Laser Ablation on the Microstructure of Sample
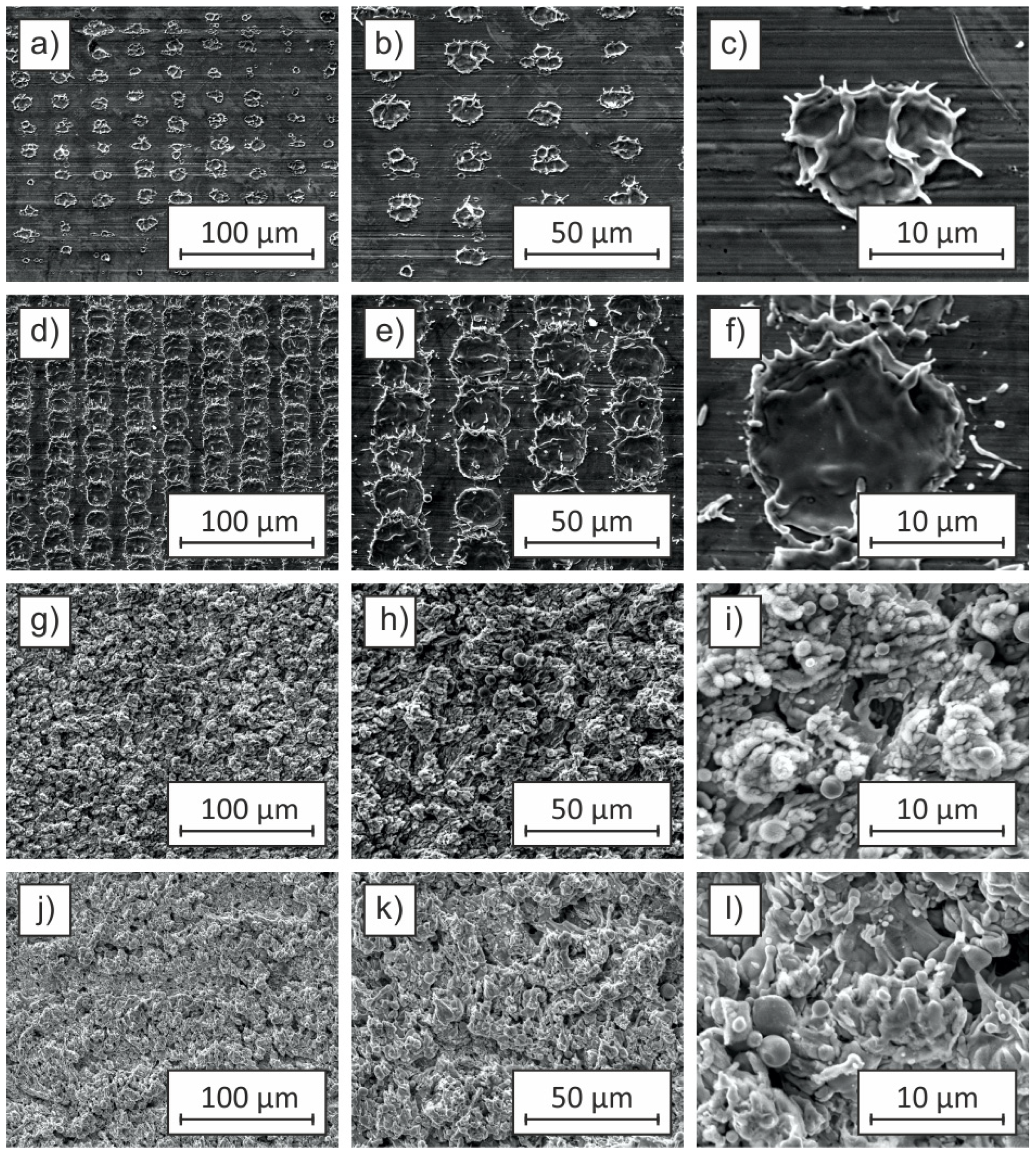
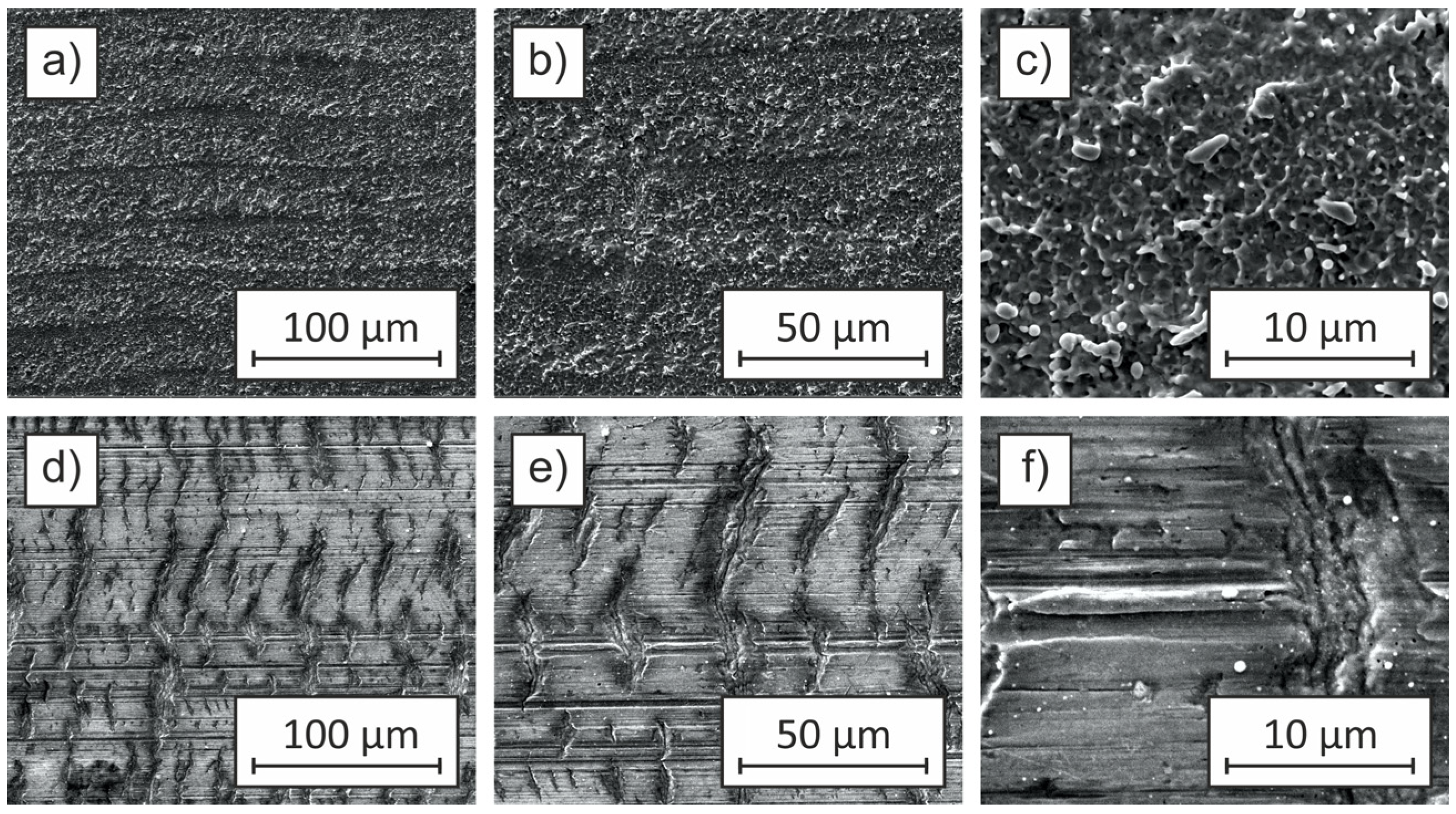
Figure 5 presents SEM images of Cu-ETP copper sheet samples subjected to laser ablation at a pulse repetition frequency of 25 kHz and a fluence of 1018.59 J/cm
2. The effect of varying pulse durations (9 ns, 13 ns, 200 ns, and 500 ns) on the surface morphology is depicted, with images at different magnifications illustrating the microstructural modifications induced by the laser treatment.
Sample (a), ablated with a pulse duration of 9 ns, exhibits a periodic arrangement of discrete microstructures resembling a spot pattern. The features are evenly distributed across the surface, with individual structures measuring approximately 15 × 15 µm. Higher magnification images (b, c) reveal that the ablated regions have well-defined edges with minimal material re-deposition, indicating a clean ablation process with limited thermal effects. The surface roughness (Ra) remains relatively low at 0.2 µm, comparable to the initial untreated surface (Ra ≈ 0.1 µm). The contact angle of 91° suggests that the wettability remains similar to that of unmodified Cu-ETP, indicating that the applied laser parameters were insufficient to induce substantial changes in surface topography and wetting behavior.
Sample (d), processed with a pulse duration of 13 ns, demonstrates a distinct surface morphology characterized by a grid pattern, consisting of an interconnected matrix of ablation sites. The size of individual structures increases to 20 × 20 µm, with a denser distribution compared to sample (a). Enlarged images (e, f) show the presence of well-defined features with slightly more pronounced material redeposition at the ablation sites. The roughness parameter increases to Ra = 0.4 µm, suggesting a more significant surface texturing effect. The wettability characteristics are markedly altered, with the contact angle decreasing to 23°, indicative of an increased hydrophilic response due to enhanced microstructural complexity.
With further extension of the pulse duration to 200 ns, as seen in sample (g), the laser-induced modifications become more pronounced. Unlike the structured arrangements observed in samples (a) and (d), the surface morphology transitions into a more irregular texture, lacking discernible periodicity. This shift is likely due to the increased thermal effects and material re-solidification associated with longer pulse durations. The surface roughness increases significantly to Ra = 1.4 µm, with higher magnifications (h, i) revealing extensive material melting and resolidification. The contact angle of 4° indicates an almost complete transition to a hydrophilic state, likely due to the formation of micro- and nano-scale surface features enhancing capillary effects and liquid adhesion.
Sample (j), which underwent laser ablation with the longest pulse duration of 500 ns, exhibits an even more complex and heterogeneous surface morphology. The SEM images illustrate a surface characterized by extensive material melting, deep depressions, and irregular protrusions. The absence of periodic structures, as observed in samples (a) and (d), suggests that prolonged pulse durations result in excessive heat accumulation, leading to significant reflow and surface instability. The roughness reaches its highest value at Ra = 1.8 µm, accompanied by a contact angle of 0°, indicating near-total surface wetting. High-magnification images (k, l) reveal a highly porous structure, likely resulting from cumulative thermal effects, vaporization, and subsequent condensation of molten copper droplets, further contributing to increased roughness and hydrophilicity.
The results presented in
Figure 5 demonstrate that increasing pulse duration at a constant repetition frequency of 25 kHz and fluence of 1018.59 J/cm
2 progressively shifts the ablation mechanism from precision patterning (9 ns, 13 ns) to extensive surface restructuring dominated by thermal effects and re-solidification (200 ns, 500 ns). This transition results in a significant increase in surface roughness and enhanced wettability, with longer pulse durations promoting hydrophilic behavior through the formation of micro/nanostructures and porous features.
Further examination of the effects of laser processing at higher repetition frequencies (100 kHz and 1000 kHz) will be discussed in the following sections, with
Figure 6 and
Figure 7 providing insights into how fluence and pulse duration influence the microstructural evolution of Cu-ETP copper sheets under these conditions.
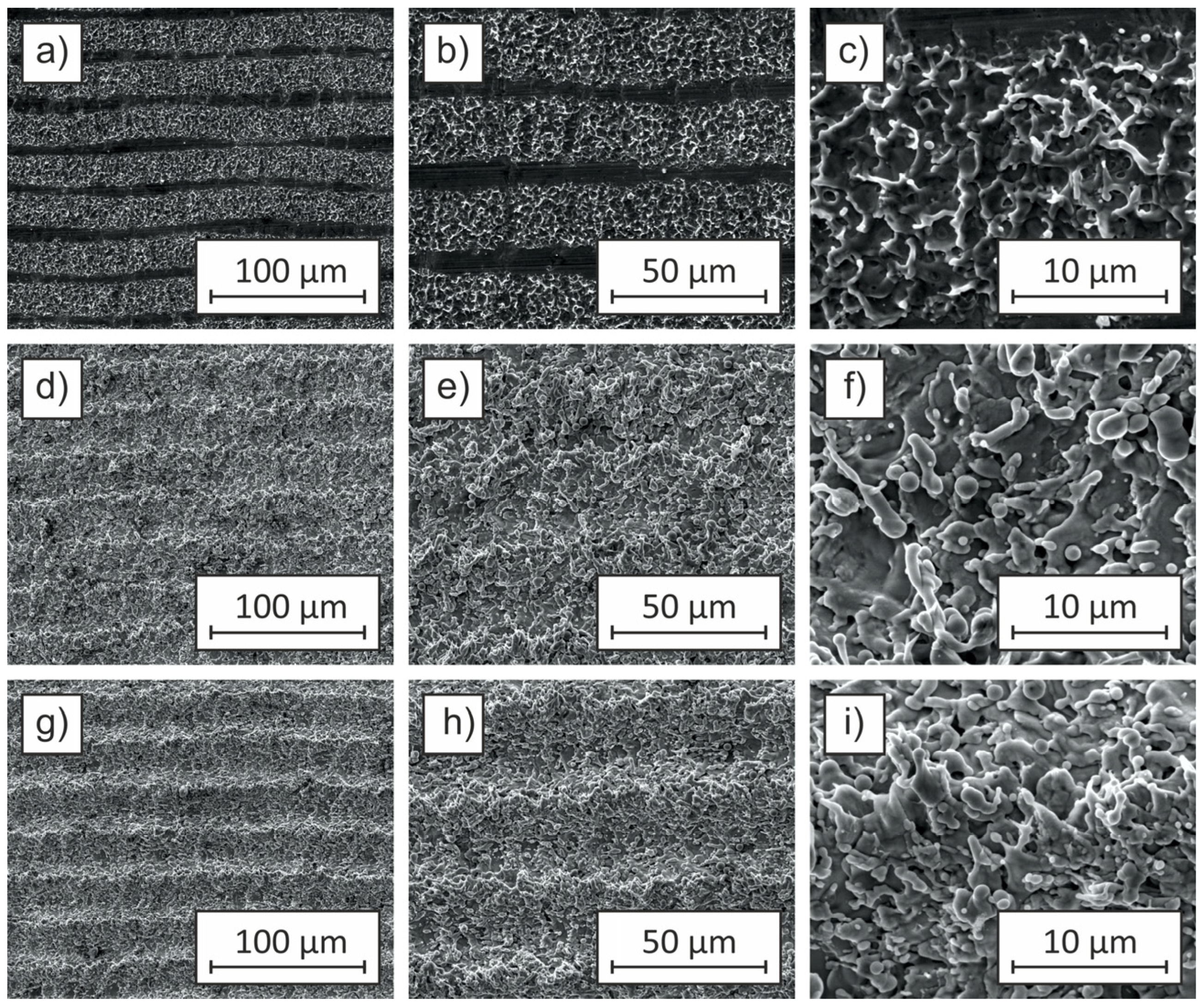
Figure 6 presents SEM images illustrating the surface morphology of Cu-ETP copper sheet samples after laser ablation at a pulse repetition frequency of 100 kHz and a fluence of 254.66 J/cm
2. The figure depicts the effect of varying pulse durations (4 ns, 45 ns, and 100 ns) on the surface topography, highlighting differences in groove formation and microstructural evolution.
The sample processed with a 4 ns pulse duration (
Figure 6a–c) exhibits a well-defined groove pattern, characterized by evenly spaced, parallel lines with a hatch spacing of approximately 30 µm. The grooves remain regular across the surface, with minimal material redeposition, suggesting a clean ablation process dominated by photomechanical effects with limited thermal diffusion. The measured surface roughness is Ra = 0.3 µm, indicating a slight increase compared to untreated copper. The contact angle of 75° suggests that the surface retains a degree of hydrophobicity, implying that the laser-induced modifications at this pulse duration were insufficient to significantly alter the wetting characteristics of the material.
For the sample treated with a 45 ns pulse duration (
Figure 6d–f), the surface morphology becomes increasingly irregular. While the groove pattern remains visible, higher magnifications reveal pronounced topographical variations along the groove edges. The surface roughness increases substantially to Ra = 1.1 µm, indicating stronger material modification due to increased thermal effects. The prolonged pulse duration leads to partial melting and localized material re-solidification, promoting a rougher surface with distinct microstructural irregularities. This effect is further reflected in the contact angle, which drops to 0°, confirming complete wettability and the transition to a superhydrophilic state. The increased surface roughness enhances capillary effects, which contribute to enhanced liquid adhesion, following the Wenzel model.
The sample processed with a 100 ns pulse duration (
Figure 6g–i) exhibits further evolution in surface morphology, retaining the groove pattern but displaying significantly enhanced microstructural complexity. High-magnification SEM images reveal irregularities within the grooves, indicative of intensified laser-material interactions and the onset of surface melting. The roughness parameter is Ra = 1.0 µm, comparable to the 45 ns sample, suggesting that heat accumulation and material redistribution stabilize beyond a certain pulse duration. The presence of surface re-solidification features suggests that heat diffusion extends beyond the initial ablation zone, promoting a more pronounced restructuring of the surface. The contact angle of 10° reinforces the strong hydrophilic nature of the surface, although it does not reach the extreme wetting behavior observed at 45 ns.
The results in
Figure 6 indicate that at 100 kHz, the higher repetition rate reduces energy per pulse compared to lower frequencies (e.g., 25 kHz in
Figure 5), leading to a more uniform groove formation. However, as pulse duration increases, cumulative heat effects become more pronounced, resulting in surface restructuring, increased roughness, and enhanced wettability. The transition from a well-ordered groove pattern at 4 ns to a more textured and highly hydrophilic surface at 45 ns and 100 ns highlights the direct influence of thermal accumulation on surface morphology. The observed increase in Ra values and reduction in contact angle confirm that prolonged pulse durations enhance surface wettability through a combination of increased roughness and surface energy modifications.
Further investigation into the laser-processed Cu-ETP sheets at 1000 kHz, where different pulse durations continue to influence surface characteristics, is presented in
Figure 7.
Figure 7 presents SEM images illustrating the surface morphology of Cu-ETP copper sheets after laser texturing at a pulse repetition frequency of 1000 kHz with a fluence of 25.46 J/cm
2 and different pulse durations (2 ns and 500 ns). Unlike the groove structures observed at 100 kHz (
Figure 6), the increase in repetition frequency to 1000 kHz leads to reduced thermal effects per pulse, resulting in surface morphologies that retain a high degree of regularity while influencing surface wettability.
The sample processed with a 2 ns pulse duration (
Figure 7a–c) exhibits a well-defined groove pattern, characterized by evenly spaced, parallel lines with a hatch spacing of approximately 30 µm. The grooves remain continuous and uniform, with minimal material redeposition, suggesting that at this pulse duration, photomechanical effects dominate over thermal diffusion. The measured surface roughness is Ra = 0.3 µm, confirming the smooth nature of the surface, while the contact angle of 81° indicates moderate hydrophobicity. The presence of slight irregularities at groove edges suggests localized variations in the ablation process, but these do not significantly affect the overall pattern uniformity.
For the sample treated with a 500 ns pulse duration (
Figure 7d–f), the surface maintains a groove pattern, but with subtle differences compared to the 2 ns case. While the grooves remain clearly visible, higher magnifications reveal minor morphological alterations likely due to the extended pulse duration. Despite the longer interaction time, the surface roughness remains relatively low at Ra = 0.3 µm, similar to the 2 ns case, indicating that heat accumulation does not induce significant melting or material redistribution. However, a noticeable increase in contact angle to 98° suggests enhanced hydrophobicity, potentially due to minor changes in surface chemistry or the preservation of an unoxidized metallic state.
At 1000 kHz, the higher repetition rate reduces the energy delivered per pulse, limiting material expulsion and minimizing thermal effects compared to lower frequencies. As a result, surface morphology remains largely consistent across different pulse durations, with groove patterns persisting even at 500 ns. The transition from moderate hydrophobicity (81° at 2 ns) to increased hydrophobicity (98° at 500 ns) suggests that subtle changes in surface roughness and chemistry play a dominant role in modifying wettability at this frequency.
Table 2 presents the EDS analysis of the Cu-ETP surface after laser ablation, corresponding to
Figure 5g,j and
Figure 7d, indicating a relationship between laser parameters and surface oxidation. At 25 kHz and longer pulse durations (200–500 ns), the oxygen content reaches 8.77% at 200 ns and 5.81% at 500 ns, which corresponds to the increased surface roughness. Lower repetition frequencies and prolonged laser-material interaction enhance ablation, leading to a more developed surface topography that facilitates oxygen adsorption and oxide formation. The slight decrease in oxygen content at 500 ns may be associated with thermal effects influencing oxide redistribution. At 1000 kHz and 500 ns, the oxygen content decreases to 2.82%, while Cu increases to 97.18%, suggesting reduced oxidation. As seen in
Figure 7d, higher frequencies and lower pulse energy result in a smoother surface, which may limit oxidation. These findings indicate that low-frequency, high-energy pulses promote oxidation and roughening, whereas high-frequency, low-energy pulses lead to a smoother, less oxidized surface. The ability to control these effects by adjusting laser parameters may be relevant for applications requiring specific surface properties, such as corrosion resistance or wettability modification.
Fluence values in
Table 2 correspond to the single-pulse fluence, calculated as F = E/A. The effective total fluence on the surface is influenced by the pulse overlap, repetition rate, and scanning speed.
3.3. Effects of Nanosecond Laser Ablation on the Surface Roughness Analysis
Figure 8 presents the surface roughness profiles of Cu-ETP copper sheets processed by nanosecond laser ablation at different frequencies and pulse durations. The data indicate that surface roughness is directly influenced by both the laser frequency and pulse duration, with notable variations across the tested parameters.
At 25 kHz, surface roughness progressively increases as pulse duration extends from 9 ns to 500 ns. This trend is attributed to enhanced thermal effects that promote localized melting and material re-solidification. Since fluence directly influences the depth of material removal and surface texturing, its interaction with pulse duration should be further examined to optimize roughness parameters for specific applications. Shorter pulse durations, such as 9 ns and 13 ns, produce well-defined structures with moderate roughness, while 200 ns and 500 ns lead to more irregular morphologies due to intensified heat accumulation and subsequent redistribution of molten material.
At 100 kHz, an increase in roughness is particularly evident for pulse durations of 45 ns and 100 ns, where significant surface restructuring occurs. The transition from 100 kHz to 1000 kHz marks a shift in laser-material interaction, where thermal effects become less dominant, leading to reduced surface roughness despite prolonged pulse durations. Unlike at lower frequencies, where the transition from well-defined patterns to irregular morphologies is gradual, at 100 kHz, roughness variations appear more abrupt due to the interplay between energy absorption and material redeposition. The observed microstructures within the grooves suggest that localized melting and resolidification contribute to the enhanced roughness observed at longer pulse durations.
At 1000 kHz, surface roughness remains relatively low, even with an extended pulse duration of 500 ns. At higher repetition rates, the heat input per pulse is lower, and the time between pulses is shorter, preventing excessive material vaporization and promoting smoother surface formation. This behavior can be attributed to the significantly higher repetition rate, which reduces per-pulse energy input, preventing excessive heat accumulation and suppressing extensive melting. The groove structures remain well-preserved, and the roughness values measured for 2 ns and 500 ns remain consistent, indicating that high-frequency laser processing mitigates excessive surface deformations and preserves the original surface integrity.
The observed trends suggest that laser frequency plays a crucial role in controlling surface roughness. Lower frequencies such as 25 kHz allow for greater heat diffusion and material reorganization, leading to increased roughness as pulse duration increases. In contrast, higher frequencies such as 1000 kHz suppress extensive melting, resulting in smoother and more uniform surfaces. The increase in roughness at lower frequencies with extended pulse durations is a direct result of higher energy absorption per pulse, leading to increased localized melting and re-solidification. This promotes microstructural irregularities and enhances surface roughness. As pulse duration increases, the transition from a well-defined groove pattern to more disordered microstructures is observed, primarily due to enhanced heat diffusion and the subsequent redistribution of molten material. The correlation between roughness and wettability suggests that microstructural evolution due to different pulse durations can significantly impact surface interactions with liquids, highlighting the importance of precise laser processing for wettability control.
Understanding the correlation between laser parameters and surface roughness is critical for applications in microelectronics, biomedical implants, and tribological coatings, where precise surface properties dictate performance. These findings highlight the significance of selecting appropriate laser parameters to achieve desired surface properties, particularly in applications where roughness and wettability are critical factors.
3.4. Effects of Oxidation Process on the Surface Properties of Copper Sheet
The wettability of Cu-ETP copper sheets after nanosecond laser ablation is influenced by multiple factors, including surface morphology, oxidation state, and adsorption of airborne contaminants. While previous studies suggested that natural oxidation alone could induce a transition from hydrophilic (θ < 90°) to hydrophobic (θ > 90°), this assumption has been revised based on well-established findings in surface science. Recent literature demonstrates that all metals and metal oxides are intrinsically hydrophilic due to their high surface free energy and that the observed hydrophobic transition is primarily driven by the adsorption of volatile organic compounds (VOCs) rather than oxidation alone [
61,
62,
63].
This section examines the evolution of wettability as a function of laser processing parameters (frequency, pulse duration, and fluence) and subsequent exposure to two different environmental conditions: natural air exposure (
Figure 9) and controlled oxidation at 200 °C (
Figure 10). The discussion considers the interplay between surface roughness, oxide layer formation, and the adsorption of VOCs.
Figure 9 presents the changes in water contact angle over time for laser-textured copper surfaces exposed to ambient air. For the tested pulse durations (2–500 ns) and frequencies (25 kHz, 100 kHz, and 1000 kHz), a gradual increase in contact angle is observed within the first 10–20 days, after which the values stabilize. However, this transition cannot be attributed solely to copper oxidation, as CuO and Cu
2O remain inherently hydrophilic. Instead, the adsorption of airborne organic species is the dominant factor governing the increase in contact angle.
At low frequencies (25 kHz), surfaces treated with longer pulse durations (200 ns and 500 ns) exhibit a faster increase in contact angle, exceeding 120° after approximately 60 days. This behavior correlates with higher surface roughness (Ra up to 1.8 µm), which facilitates the trapping of organic contaminants, enhancing hydrophobicity. Initially, the Wenzel model applies, with the roughened hydrophilic surface promoting water spreading. Over time, as VOCs accumulate within the micro/nanostructures, the surface transitions into the Cassie-Baxter regime, where air pockets reduce the solid–liquid contact area, increasing the apparent contact angle.
At higher frequencies (100 kHz and 1000 kHz), the transition to hydrophobicity is less pronounced. Surfaces processed at 100 kHz reach contact angles exceeding 120°, but the transition occurs more gradually. At 1000 kHz, wettability remains relatively stable, with contact angles around 110° regardless of pulse duration. This trend correlates with lower surface roughness (Ra~0.3 µm) and a reduced ability to retain organic contaminants, leading to a weaker hydrophobic effect.
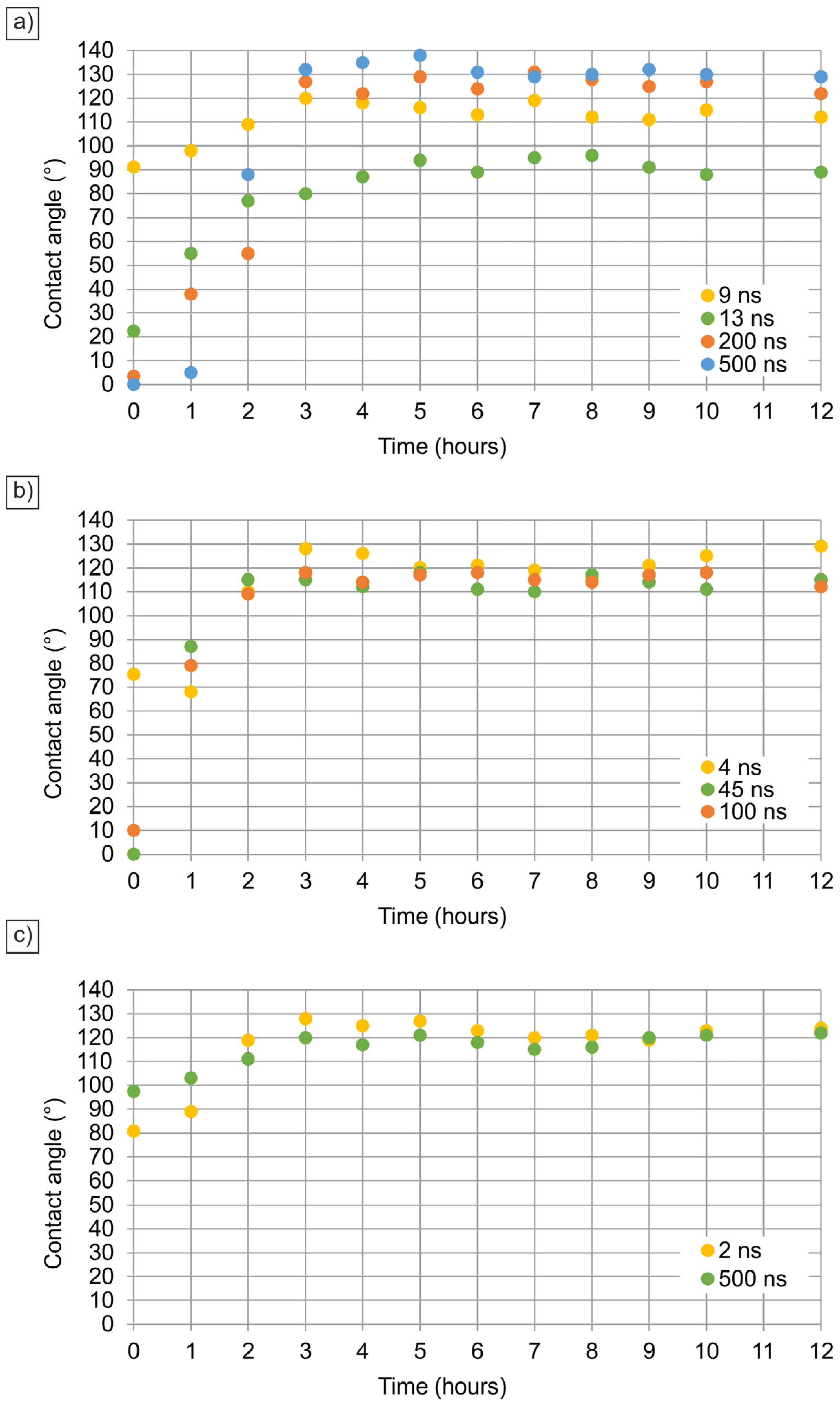
Figure 10 illustrates the impact of thermal oxidation at 200 °C on the wettability of laser-ablated copper surfaces. Compared to natural oxidation, heat treatment significantly accelerates the adsorption of organic molecules, leading to a faster transition to hydrophobicity. The elevated temperature enhances molecular mobility and adsorption kinetics, resulting in stable high contact angles within just a few hours.
At 25 kHz, surfaces treated with longer pulse durations (200 ns and 500 ns) rapidly reach superhydrophobic states (contact angles > 130°), stabilizing within 6 h. The combination of high roughness (Ra up to 1.8 µm) and rapid VOC adsorption promotes the Cassie-Baxter state. Conversely, shorter pulse durations (9 ns and 13 ns) result in lower roughness (~0.2–0.4 µm), leading to a slower and less significant increase in contact angle.
At 100 kHz, a similar trend is observed, although maximum contact angles are slightly lower due to moderate roughness values (Ra~1.1 µm). Initially, the Wenzel model dominates as the freshly processed surfaces are hydrophilic, but prolonged exposure to high temperature facilitates VOC adsorption, causing a transition to the Cassie-Baxter regime [
64,
65].
At 1000 kHz, the effect of thermal oxidation is the least pronounced. Samples processed at 2 ns and 500 ns stabilize at contact angles of approximately 125° after 8 h, with minimal further changes. The limited surface roughness (Ra~0.3 µm) and thinner oxide layers restrict the extent of wettability modification, supporting the conclusion that surface oxidation alone does not induce hydrophobicity.
Our findings confirm that while laser ablation induces structural modifications that influence wettability, the observed hydrophobic transition is predominantly driven by the adsorption of airborne organic compounds rather than oxidation alone. The presence of VOCs lowers the surface energy and stabilizes the Cassie-Baxter state on roughened surfaces. Additionally, heat treatment accelerates this adsorption process, highlighting the importance of controlled environmental exposure in tuning surface properties.
These insights are crucial for industrial applications such as anti-corrosion coatings, self-cleaning surfaces, and heat exchangers, where precise control over wettability is desired.