Abstract
Unsaturated polyester (UP) resin is widely utilized in the construction and automotive industries. The flammable nature of UP must be constrained when its products are manufactured. A novel organic flame retardant has been synthesized from 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) and triallyl isocyanate (TAIC). This DOPO-TAIC additive has been used to reduce the flammability of a matrix. Additionally, this flame retardant was then combined with dopamine-modified silicon carbide (M-SiC) to further diminish the flammability of UP. The limiting oxygen index (LOI) value combustion of a UP/DOPO-TAIC/M-SiC blend was 30.8% when the filler contents of DOPO-TAIC and M-SiC was 15 wt.% and 30 wt.%, respectively. These materials exhibited a UL 94 V-0 rating for combustion. Compared to the values for combustion of the neat UP, the peak heat release rate (Pk-HRR) and total heat release rate (THR) for this blend were reduced by 51% and 35%, respectively. The mode of action for flame retardant of UP blends containing DOPO-TAIC and M-SiC has been composed. The presence of a flame retardant containing P-Si elements can significantly reduce flammability compared to that of unmodified resin.
1. Introduction
Unsaturated polyester (UP) resin is a common thermosetting resin. It exhibits chemical resistance [1,2], excellent mechanical properties, and good processability. UP is typically synthesized through the polycondensation of dicarboxylic acids and diols [3,4]. The main chain of UP resin is made up of simple linear macromolecules consisting of C, H, and O elements [5]. After the polycondensation process, a small amount of styrene is added as a crosslinking diluent [6]. The pyrolysis products of the incorporated styrene segments are volatile styrene and methyl styrene [7]. These volatile, flammability component evolve when UP materials undergo thermal degradation upon being exposed to heat. This resulted in the highly flammable nature of UP-based materials [8] and significantly hinders the industrial application of these materials. Therefore, reducing the flammability UP and developing flame-retardant UP materials with excellent comprehensive properties are of great significance for expanding the scope of application for UP [9].
The introduction of additive flame retardants has become the facile and most direct method for reducing the flammability of polymeric materials [10,11]. Among them, DOPO and its derivatives have exhibited remarkable flame-retardant properties and low toxicity [12,13,14,15]. The active radicals in the flame zone of DOPO are quenched, which will inhibit the chain reaction of combustion in the matrix during the process of combustion. DOPO can also generate a phosphorus-containing char layer in the condensed phase, which will suppress the release of flammable volatiles [16,17,18,19,20,21]. In the present, DOPO and its derivatives have attracted widespread attention due to their low smoke emission, low toxicity, absence of corrosive gases during combustion, and anti-drip characteristics. They have been considered to be a kind of highly efficient and safe flame retardants [22,23]. Therefore, they are widely used to enhance the flame retardant properties of polylactic acid (PLA) [24,25], epoxy resin (EP) [26,27], and others. However, the poor compatibility between DOPO and several polymer matrixes has limited its efficiency as a flame-retardant. Chemical modifications have been generally employed to enhance compatibility. For example, 4,4′-diaminodiphenylmethane (DDM) and DOPO have been used as raw materials for the preparation of a flame retardant, HP-DOPO, via a one-step method [28]. The EP blend with this material exhibited excellent flame retardancy with a high limiting oxygen index (LOI) for combustion of 35.6% and a vertical burning rating UL-94 of V-0 when the loading of HP-DOPO was 5 wt.%. A novel phosphorus-nitrogen flame retardant has been synthesized from DOPO and DABP (4,4′-diaminobenzophenone). This DOPO-DABP flame retardant was combined with ammonium polyphosphate (APP) to generate a mixture for the preparation flame-retardant PLA foam materials via a hot-pressing method. The obtained PLA achieved a UL-94 V-0 rating, and an LOI of 34.4% was exhibited for a blend containing APP/DOPO-DABP in a ratio of 10:10 [29,30].
Recently, the combination of phosphorus-containing flame retardants with other flame-retardant compounds has been explored [31,32]. This strategy can lead to the enhancement of overall flame retardancy. Generally, organic flame retardants, particularly biological ones, have the advantages of being environmentally friendly and having minimal impact on polymeric materials [33]. While the flame-retardant mode of action for inorganic flame retardants is the promotion of crosslinking during thermal decomposition of the polymer matrix [34,35], which will promote the formation of a dense char layer on the surface [36]. Consequently, the heat transfer from the combustion will be inhibited, and the thermal release of decomposition products will be constrained. Vinyltriethoxysilane has been used to modify DOPO. This material was then mixed with montmorillonite to simultaneously promote both solid-phase and gas-phase activity in polycarbonate.
A new reactive flame retardant containing phosphaphenanthrene and triazine-trione groups has previously been synthesized and applied to improve the flame retardancy of unsaturated polyester resin [37]. The flammability of UP has now been reduced by using a combination of a DOPO derivatives and silicon carbide (SiC). A novel flame retardant was synthesized by using DOPO and triallyl isocyanurate (TAIC). The resultant DOPO-TAIC was combined with dopamine hydrochloride modified silicon carbide (M-SiC) to provide a composition for use as a flame retardant in a UP matrix. The chemical structure of DOPO-TAIC and the flame-retardant properties of UP blends containing different amounts of DOPO-TAIC or M-SiC have been described [38,39,40].
2. Materials and Methods
2.1. Materials
Unsaturated Polyester (UP7938) was provided by Xinyang Technology Co., Ltd., Xinyang, China, Curing Agent (OT 306-82-4) was purchased from Guangzhou Changshenghe Chemical Co., Ltd. (Guangzhou, China) 9,10-Dihydro-9-oxa-10-phosphaphenanthrene -10-oxide (DOPO), triallyl isocyanurate (TAIC), benzoyl peroxide (BPO), ethyl acetate (EA), dopamine hydrochloride, dichloromethane (DCM), and Tris reagent of analytical grade were all obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). N,N-Dimethylformamide (DMF) of analytical grade was provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Silicon Carbide (SiC) with 4000 mesh was purchased from Hubei Digem Technology Co., Ltd. (Yichang, China). All other chemical reagents were used without other purification.
2.2. Synthesis of DOPO-TAIC
Firstly, TAIC (23 g, 92.3 mmol) was added into a three-necked flask containing 120 mL of DMF solvent. The solution was heated to 125 °C and stirred until TAIC was completely dissolved. After cooling to 120 °C, DOPO (26 g, 120 mmol) and BPO (2.23 g, 9.2 mmol) were added in batches to ensure their complete dissolution in DMF solvent. The mixture was stirred at 120 °C under nitrogen protection for 24 h. After the reaction was complete, the DMF solvent was evaporated under vacuum. The product was purified by silica gel column chromatography using a mobile phase of DCM to EA (30:1), yielding a transparent light yellow oily compound. The synthesis route of DOPO-TAIC is shown in Figure 1a.
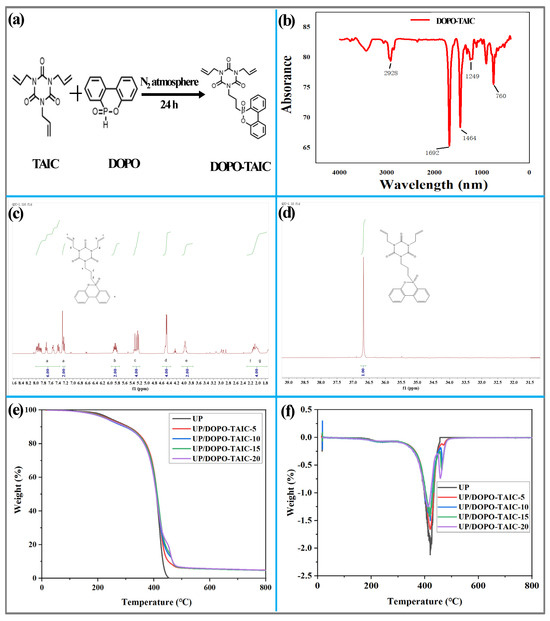
Figure 1.
DOPO-TAIC (a) synthesis route, (b) infrared spectrum, (c) 1H NMR spectrum, and (d) 32P NMR spectrum; (e) TG and (f) DTG curves of UP and UP/DOPO-TAIC.
2.3. Preparation of UP/DOPO-TAIC
Firstly, 50 g UP and a certain amount of DOPO-TAIC were mixed. An appropriate amount of methanol was added to the mixture, which was then stirred at 40 °C for 30 min until the UP and the DOPO-TAIC flame retardant were thoroughly mixed. The mixture was subsequently stirred at 70 °C for 3 h to evaporate the methanol solvent. Next, 0.5 g of OT curing agent was added to the mixture and stirred for 10 min until the OT curing agent was completely dissolved. The blend was then poured into a standard polytetrafluoroethylene (PTFE) mold and degassed in a vacuum drying chamber until all bubbles were completely removed. The mold was then placed in an oven and cured at 100 °C for 2 h, followed by an increase in temperature to 120 °C for an additional 1 h curing period. Consequently, a light yellow specimen with dimensions of 1300 × 150 × 3 mm3 was obtained. The specific formulation for the flame-retardant UP/DOPO-TAIC specimen is shown in Table 1.

Table 1.
Formulations of flame retardant-UP/DOPO-TAIC.
2.4. Preparation of Modified SiC(M-SiC)
A total of 1 g of dopamine hydrochloride was dispersed in 500 mL of deionized water, and the solution pH was adjusted to 8.5 using Tris reagent. Subsequently, 22.5 g SiC powder was added to the aforementioned mixture, and the solution was continuously stirred at 60 °C for 8 h. After that, the mixture was dried in a vacuum oven at 60 °C for 24 h and then ground into a powder using a mortar to obtain the gray M-SiC powder material.
2.5. Preparation of UP/DOPO-TAIC/M-SiC Composites
Firstly, 50 g UP matrix and 15% of the organic DOPO-TAIC flame retardant were mixed. An appropriate amount of methanol was added to the mixture, which was then stirred at 40 °C for 30 min until the UP matrix and DOPO-TAIC were thoroughly mixed. Subsequently, different proportions of M-SiC powder were added and stirred for 30 min until the powder was uniformly dispersed in the mixture. The mixture was then stirred at 70 °C for 3 h to evaporate the methanol. After that, 0.5 g of OT curing agent was added into this mixture.
Secondly, the mixture was poured into a standard polytetrafluoroethylene (PTFE) mold and placed in a vacuum drying chamber to remove internal bubbles until the surface was smooth and bubble-free. The mold was then subjected to a two-step thermal curing process in an oven. First, the temperature was raised to 80 °C, and the mixture was held at this temperature for 2 h. Subsequently, the temperature was increased to 120 °C and the mixture was cured for an additional 1 h to ensure complete thermal curing of the composite material. After the curing reaction was complete, the specimen was naturally cooled in air to obtain a gray, opaque specimen with dimensions of 1300 × 150 × 3 mm3. These samples were UP composites modified with M-SiC and DOPO-TAIC (UP/DOPO-TAIC/M-SiC). The specific formulation for the flame-retardant UP/DOPO-TAIC/M-SiC specimen is shown in Table 2.

Table 2.
Formulations of flame retardant-UP/DOPO-TAIC/M-SiC.
2.6. Characterization
Fourier-transform infrared spectroscopy (FTIR) analysis of samples was performed using a Nicolet 6700 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) with the potassium bromide (KBr) pellet method over a wave number range of 4000–400 cm−1. The 1H and 31P NMR spectra of DOPO-TAIC were obtained using a Bruker AV400 NMR spectrometer (Bruker Corporation, Berlin, Germany). Thermogravimetric analysis (TGA) was conducted using an SDT-Q600 instrument (TA Instruments, New Castle, DE, USA) under a nitrogen atmosphere with a heating rate of 10 °C/min and a temperature range of 0–800 °C.
The limiting oxygen index (LOI) was measured at room temperature according to the ASTM D2863 standard using a JF-3 oxygen index meter (Jiangning Analytical Instrument Company, Nanjing, China). The dimensions of all samples were 100 × 6.5 × 3 mm3. Vertical burning tests were performed on samples with dimensions of 130 × 13 × 3 mm3 according to the UL-94 standard using a NK8017A instrument (Nklsky Instrument Co., Dongguan, China). Cone calorimetry analysis was conducted using a Vouch 6810 instrument (Servomex Analytical Instruments, Crowborough, UK) according to the ISO 5660 standard. The test heat flux was 35 kW/m2, and the sample dimensions were 100 × 100 × 3 mm3.
The microstructure of SiC fillers before and after modification and the residual carbon after cone calorimetry testing was observed using a scanning electron microscope (SEM, JSM-5500, JEOL, Tokyo, Japan). The samples were coated with gold in a vacuum, and the test voltage was set at 15 kV. Elemental analysis of the residual carbon after combustion was performed using an energy-dispersive X-ray spectrometer (EDX, Ultim Max 100, Oxford Instruments, Oxford, UK).
3. Results and Discussion
3.1. Structural Analysis of DOPO-TAIC
The infrared spectrum of DOPO-TAIC is shown in Figure 1b. The characteristic absorption peak at 760 cm−1 corresponded to the 1,2-disubstitution of the benzene ring, which was attributed to the P-O-Ph group. The presence of this peak indicated the existence of a triazine trione group in the chemical structure of DOPO-TAIC. The absorption peak near 1249 cm−1 is due to the stretching vibration of P=O [41]. The peak near 1464 cm−1 was associated with the stretching vibration of Ph-P after the reaction of the benzene ring. The peak at 1692 cm−1 was a characteristic absorption peak caused by the stretching and bending vibrations of P-O. The absorption peak at 2928 cm−1 was attributed to the stretching vibration of the -CH2 group. The characteristic absorption peak of P-H stretching vibration in DOPO was located in the wave number range of 2425–2325 cm−1 [42]. This medium intensive peak is relatively stable in position and is less influenced by the rest of the molecular structure. The absence of a distinct peak at this wave number in Figure 1b suggests that the P-H in DOPO had reacted with TAIC.
Nuclear Magnetic Resonance (NMR) spectroscopy was further employed to analyze the chemical structure of DOPO-TAIC. Figure 1c,d shows the 1H and 31P NMR spectra of DOPO-TAIC, respectively. It can be found from Figure 1d that the 31P NMR spectrum of the flame-retardant DOPO-TAIC exhibited a characteristic absorption peak of the P atom in the DOPO residue at 39.9 ppm, appearing as a singlet. The NMR results further demonstrated the successful synthesis of DOPO-TAIC.
3.2. Thermal Stability and Flame Retardancy of UP/DOPO-TAIC
The TG and DTG curves of UP and UP/DOPO-TAIC under a nitrogen atmosphere are shown in Figure 1e,f, while the main thermal degradation data are listed in Table 3. The UP matrix had a T5% of 243 °C and a Tmax of 422 °C, and it was completely decomposed at 800 °C. The thermal degradation process of the UP/DOPO-TAIC blends was similar to that of the UP matrix. However, compared with neat UP, the T5% and Tmax of the UP/DOPO-TAIC blends were both reduced, while the residual content at 800 °C increased significantly. The T5% decreased from 243 °C to 227 °C for neat UP to UP/DOPO-TAIC-20, and the Tmax also decreased from 422 °C to 413 °C for neat UP to UP/DOPO-TAIC-20. The residual content at 800 °C slightly increased by 4.89%. This indicated that the UP/DOPO-TAIC blends exhibited better thermal stability and char-forming performance. The decrease in T5% and Tmax, along with the increase in residual content at 800 °C for UP/DOPO-TAIC blends may be attributed to the early decomposition of the phosphorus-containing groups in DOPO within the DOPO-TAIC flame retardant, generating phosphoric acid and polyphosphoric acid compounds [14]. These decomposition products can promote the degradation of the UP matrix at lower temperatures and facilitate the charring of the UP matrix at higher temperatures. The TG and DTG curve data demonstrated that the DOPO-TAIC flame retardant can be used in the UP matrix and can enhance the flame retardancy of UP matrix.

Table 3.
TG and DTG data for UP and UP/DOPO-TAIC.
The Limiting Oxygen Index (LOI) and UL-94 vertical burning test are important standards for evaluating the flame retardancy of materials, and the test results are shown in Table 4. The neat UP thermoset had a Limiting Oxygen Index (LOI) of only 18.8% and fails the UL-94 vertical burning test. However, the flame retardancy of the UP/DOPO-TAIC blends was significantly improved. The LOI value of the UP gradually increased with the content of DOPO-TAIC. The LOI value significantly increased to 28%, and the UL-94 test achieved a V-0 rating when the content of the flame retardant-DOPO-TAIC in UP/DOPO-TAIC reached 20% and the phosphorus content in the system reached 1.34 wt.%. Additionally, the UP/DOPO-TAIC thermoset resin containing 10% DOPO-TAIC reached a V-2 rating in the UL-94 vertical burning test, while the UP/DTAC-15 thermoset resin containing 15% DOPO-TAIC achieved a V-0 rating.

Table 4.
UL-94 and limiting oxygen index (LOI) for UP and UP/DOPO-TAIC.
The combustion behavior of neat UP and UP/DOPO-TAIC materials was studied using a cone calorimeter to further investigate the effect of the flame retardant-DOPO-TAIC on the flame retardancy of UP matrix materials. Total Heat Release (THR), Effective Heat of Combustion (EHC), Peak Heat Release Rate (PK-HRR), and Time to Ignition (TTI) are important parameters and indicators for evaluating the flame retardancy of materials. Figure 2 shows the curves of THR, HRR, and Residual Mass for UP/DOPO-TAIC materials. The main data from the cone calorimeter are listed in Table 5. It can be seen that the PK-HRR and average HRR (av-HRR) of UP/DOPO-TAIC materials are significantly lower than those of neat UP. The peak heat release rate (PK-HRR) decreased remarkably from 1012 kW/m2 for neat UP material to 498 kW/m2 for UP/DOPO-TAIC-20 blends. The addition of the flame retardant led to a significant reduction in HRR, thereby indicating improved flame retardancy. The EHC and THR data can explain the combustion of volatile organic compounds in the gas phase. It can be seen that the gas-phase quenching effect of the DOPO-TAIC flame retardant is evident by analyzing the corresponding data of THR, av-EHC, av-COY, and av-CO2Y. The data for THR, av-EHC, and av-CO2Y of UP all decreased with the addition of DOPO-TAIC, while the data for av-COY, representing the incomplete combustion product CO, increased. This suggests that DOPO-TAIC decomposes during combustion to produce quenching radicals such as PO•, PO2• [35], and phenoxyl radicals, which will inhibit chain radical reactions and reduce the release of flammable gases. Consequently, the decomposition products play a flame-retardant role in the gas phase, thereby preventing further combustion of the UP matrix. The increase in char yield indicated that DOPO-TAIC also played an important role in the condensed phase. Compared with neat UP, the char yields of UP/DOPO-TAIC samples with 5%, 10%, 15%, and 20% DOPO-TAIC in Table 5 increased by 5.66%, 256%, 754%, and 470%, respectively. Therefore, the addition of DOPO-TAIC effectively promoted the charring of the UP matrix. The char layer can isolate flammable substances and prevent further combustion of the material, thereby exerting a flame-retardant effect in the condensed phase.

Figure 2.
Total heat release (THR) (a), heat release rate (HRR) (b), and residual mass (c) curves for UP and UP/DOPO-TAIC.

Table 5.
Cone calorimetry data for UP and UP/DOPO-TAIC.
The key indicators av-HRR, THR, and EHC of the UP/DOPO-TAIC-15 material in the cone calorimeter test decreased more significantly compared to that of UP/DOPO-TAIC-20. This indicated that the addition of 15% flame retardant had produced the optimal flame-retardant effect for the UP matrix. The possible reason was that the high loading of DOPO-TAIC would lead to the increase in viscosity of UP, which subsequently would lead to its difficult dispersion in UP matrix. Overall, the addition of the flame retardant-DOPO-TAIC can effectively promote the charring of UP and exert flame-retardant effects in both the condensed and gas phases.
3.3. Morphologies and Structure of M-SiC
The dimensions of the SiC filler before and after modification were analyzed by using SEM observation in Figure 3. It can be found that the size distribution of M-SiC was more uniform than that of SiC. In addition, thermogravimetric analysis (TGA) was performed on SiC and M-SiC in Figure 3e. The SiC powder exhibited no change in weight at 800 °C, demonstrating the inherent stability of SiC powder. In contrast, the modified M-SiC powder underwent a slight decomposition process as the temperature increased. The residual mass of M-SiC was 96.8% at 800 °C, which was attributed to the decomposition of polydopamine (PDA) attached to the surface of SiC.
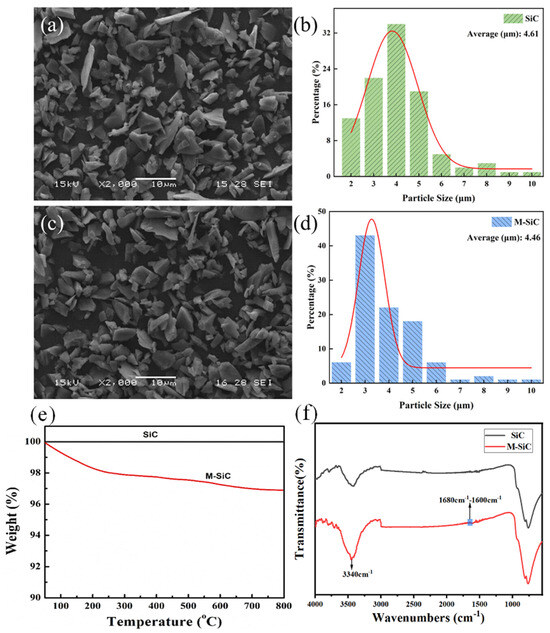
Figure 3.
Morphologies, infrared spectrum, and thermal analysis of M-SiC and SiC. (a–d) SEM observation, (e) TG, and (f) infrared spectrum.
In addition, infrared spectroscopy analysis was conducted on SiC and M-SiC. As shown in Figure 3f, new absorption peaks appeared at 1600–1680 cm−1 and 3340 cm−1 for M-SiC. The absorption peak at 3340 cm−1 is attributed to the O-H and N-H stretching vibrations of polydopamine (PDA) attached to the surface of the modified M-SiC material [43]. The absorption peak intensity in the 3000–3500 cm−1 region of M-SiC is significantly stronger than that of SiC material. This is because the peak intensity in the 3000–3500 cm−1 region is enhanced by the functional groups of PDA [44,45], which has introduced -OH and -NH bonds on the surface of M-SiC due to the deposition of PDA.
The distinct absorption peaks in the 1600–1680 cm−1 region are attributed to the overlapping resonance vibrations of the aromatic C-C bonds in PDA and the bending vibrations of the N-H bonds in polydopamine. The infrared spectra of M-SiC showed clear differences from those of SiC, indicating that the structure of M-SiC had changed after modification with dopamine hydrochloride. Both the thermogravimetric and infrared spectroscopy data confirmed that PDA had been successfully attached to the SiC surface, indicating the successful synthesis of M-SiC material.
3.4. Microstructural Analysis of UP/DOPO-TAIC/M-SiC Composites
Based on the above cone calorimeter test results, the content of DOPO-TAIC in UP/DOPO-TAIC/M-SiC composites was fixed at 15% to keep the optimal flame-retardant effect. To further investigate the effects of the cooperative flame retardants M-SiC and DOPO-TAIC on the microstructural properties of UP matrix, SEM analysis was conducted on UP and UP/DOPO-TAIC/M-SiC composites. The SEM images are shown in Figure 4. It was evident that as the incorporation of M-SiC powder increased the surface roughness of UP matrix. However, the addition of the cooperative flame retardants did not result in significant cracks or pores on the fracture surface of the UP/DOPO-TAIC/M-SiC composite. This is because the PDA attached to the surface of SiC after modification with dopamine hydrochloride, which enhanced the compatibility between M-SiC and the UP matrix.
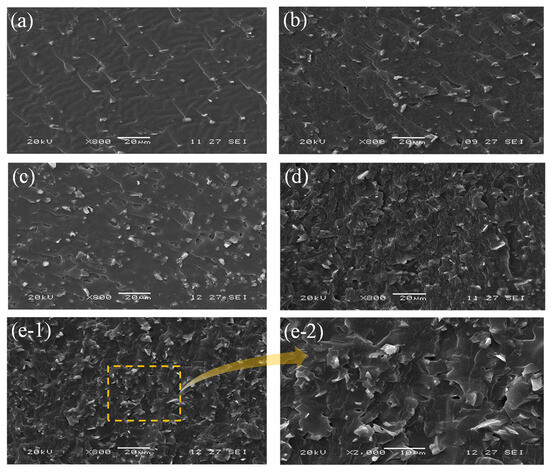
Figure 4.
SEM images of UP/DOPO-TAIC/M-SiC. UP/DOPO-TAIC/M-SiC-5 (a), UP/DOPO-TAIC/M-SiC-10 (b), UP/DOPO-TAIC/M-SiC-15 (c), UP/DOPO-TAIC/M-SiC-20 (d), and UP/DOPO-TAIC/M-SiC-5 (e-1,e-2).
Thermogravimetric analysis (TGA) was conducted on UP and UP/DOPO-TAIC/M-SiC composites to further investigate the thermal stability of flame-retardant composite UP/DOPO-TAIC/M-SiC. The main thermal degradation parameters are listed in Table 6. As shown in Figure 5a, neat UP material began to decompose progressively with the increase in temperature. The thermal degradation behavior of the UP/DOPO-TAIC/M-SiC composite was similar to that of the UP matrix. However, the T5% of UP/DOPO-TAIC/M-SiC-30 decreased by 13.5% compared to neat UP. This phenomenon can be attributed to the initial cleavage of some weaker bonds in the organic flame retardant-DOPO-TAIC, such as P-O-C and P-C bonds. Additionally, the polydopamine (PDA) in the M-SiC powder also decomposed with increasing temperature, which would lead to the decrease in T5% of UP/DOPO-TAIC/M-SiC. Similarly, the maximum decomposition temperature of UP/DOPO-TAIC/M-SiC-30 reached 393 °C, which was a 6.9% reduction in Tmax compared to neat UP.

Table 6.
TG and DTG data for UP and UP/DOPO-TAIC/M-SiC composites with different M-SiC contents.
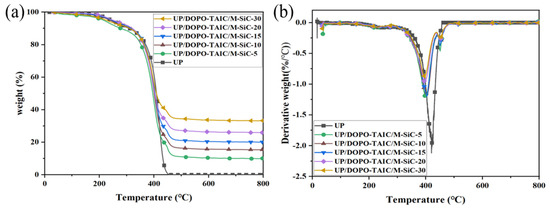
Figure 5.
TG (a) and DTG (b) curves of UP and UP/DOPO-TAIC/M-SiC.
In addition, compared to the UP/DOPO-TAIC blends discussed in Section 2.2, the Tmax of the UP/DOPO-TAIC/M-SiC-30 composite was 393 °C. This represented a decrease of 5.52% compared to the 416 °C of UP/DOPO-TAIC-15. Additionally, the T5% of UP/DOPO-TAIC/M-SiC-30 was also decreased by 9.10% compared to UP/DOPO-TAIC-15. The decrease of T5% and Tmax for UP/DOPO-TAIC/M-SiC composite is attributed to the decomposition of polydopamine (PDA) in the M-SiC powder at a high temperature. However, the residual mass of UP/DOPO-TAIC/M-SiC at 800 °C significantly increased compared to that of UP/DOPO-TAIC. Specifically, it was raised from 6.11% for UP/DOPO-TAIC-10 to 33.3% for UP/DOPO-TAIC/M-SiC-30, an increase of 445%. This indicated that although the early thermal decomposition of PDA in M-SiC would lead to a decrease in T5% and Tmax, it could significantly promote the formation of residual mass in the UP matrix and further enhance the thermal stability of UP matrix.
3.5. Flame Retardancy Properties of UP/DOPO-TAIC/M-SiC Composites
The UP/DOPO-TAIC/M-SiC composite was also subjected to Limiting Oxygen Index (LOI) and UL-94 vertical burning tests to further evaluate the flame-retardant effect of the cooperative flame retardants DOPO-TAIC and M-SiC on the UP matrix. The LOI and UL-94 vertical burning test data for UP/DOPO-TAIC/M-SiC are presented in Table 7. The data clearly illustrated that the LOI value of UP composites was greatly increased to 30.8 with the addition of the cooperative flame retardants DOPO-TAIC and M-SiC, representing a significant enhancement of 59.6% compared to the neat UP. The UL-94 rating of the UP matrix was NR (Not Rated), while that of UP/DOPO-TAIC/M-SiC-30 achieved the highest V-0 rating. Furthermore, the LOI value of UP/DOPO-TAIC/M-SiC composites was higher than that of UP/DOPO-TAIC systems when the content of M-SiC in UP/DOPO-TAIC/M-SiC composites exceeded 10 wt.%. This indicated that the addition of the cooperative flame retardants can result in a further enhancement of the flame-retardant properties for UP matrix. It demonstrated that the incorporation of M-SiC can further increase the LOI value of the UP matrix, thereby significantly improving its flame-retardant performance.

Table 7.
UL-94 and limiting oxygen index (LOI) for UP and UP/DOPO-TAIC/M-SiC composites with different M-SiC contents.
Subsequently, the UP/DOPO-TAIC/M-SiC composite was also subjected to cone calorimetry testing. Table 8 shows the curves of Total Heat Release (THR), Heat Release Rate (HRR), and Residual Mass results for UP/DOPO-TAIC/M-SiC with different contents of M-SiC. The main data from the cone calorimetry test are presented in Table 8. The peak heat release rate (Pk-HRR) decreased from 1012 kW/m2 of the UP matrix to 488 kW/m2 of the UP/DOPO-TAIC/M-SiC-30 composite, a reduction of 51.8%. Compared to UP/DOPO-TAIC-15, it also decreased by 24.2%, indicating that the addition of M-SiC can cooperatively work with DOPO-TAIC to significantly enhance the flame-retardant performance of the UP matrix.

Table 8.
Cone calorimetry data for UP, UP/DOPO-TAIC-15 blends, and UP/DOPO-TAIC/M-SiC composites with different M-SiC contents.
Total Heat Release (THR) is one of the important parameters for assessing the intensity of a fire. According to the THR data in Table 8, UP matrix already had a relatively high total heat release at 600 s. The THR of UP/DOPO-TAIC-15 was significantly reduced. However, the THR of UP/DOPO-TAIC/M-SiC-5 and UP/DOPO-TAIC/M-SiC-30 was slightly increased. This may be attributed to the addition of the inorganic flame retardant M-SiC, which can form a stable char layer more quickly, providing thermal insulation and oxygen isolation [34,35]. This extends the combustion time of the flame-retardant UP matrix, leading to an increase in the THR value.
During combustion behavior testing, the release of CO is caused by the incomplete combustion of gaseous decomposition products. As shown in Table 8, the CO generation in samples with flame retardants was higher than that of neat UP, especially after the addition of cooperative flame retardants. The content of incomplete combustion products (CO) increased significantly, which can dilute the concentration of combustible gases and, thereby, suppressed the combustion of UP matrix in the gas phase. Figure 6c showed that the introduction of M-SiC obviously increased the char yield of the UP/DOPO-TAIC/M-SiC-5 and UP/DOPO-TAIC/M-SiC-30 composites compared to UP matrix and UP/DOPO-TAIC-15 blends. This is closely related to the intumescent flame-retardant mode of action for the cooperative flame retardants M-SiC and DOPO-TAIC. The introduction of Si and P elements not only can enhance the flame-retardant effect in the gas phase but also can promote the flame-retardant action in the condensed phase. This helps to form a denser and more complete char layer, which can more effectively insulate heat and oxygen, thereby slowing down the combustion of the UP matrix. In addition, the THR value of UP/DOPO-TAIC/M-SiC-30 composites was significantly decreased compared to that of UP by using phosphophenanthrene and triazinone as flame-retardant in the previous literature [37].

Figure 6.
Total heat release (THR) (a), heat release rate (HRR) (b), and residual mass (c) curves for UP, UP/DOPO-TAIC-15, UP/DOPO-TAIC/M-SiC-5, and UP/DOPO-TAIC/M-SiC-30.
3.6. Characterization of Residual Carbon in UP/DOPO-TAIC/M-SiC Composite After Combustion
The residual carbon data of UP/DOPO-TAIC/M-SiC composites after cone calorimetry testing were analyzed using Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), and Energy Dispersive X-ray Spectroscopy (EDX). The FTIR spectra can reflect changes in the material structure. As shown in Figure 7, obvious absorption peaks appeared at 3048 cm−1, 1070 cm−1, and 810 cm−1 for UP/DOPO-TAIC-15, UP/DOPO-TAIC/M-SiC-5, and UP/DOPO-TAIC/M-SiC-30. Additionally, a relatively strong new peak was observed in the range of 3400–3600 cm−1, specifically at 3480 cm−1, which corresponded to the O-H stretching vibrations from the M-SiC material and the O-H bonds in the DOPO-TAIC flame retardant. The absorption peak at 1070 cm−1 was attributed to the P-O-C bond in DOPO-TAIC within the cooperative flame retardant. The peak at 810 cm−1 was attributed to the P-O-P bond in DOPO-TAIC. A strong peak appeared in the range of 800–960 cm−1 for UP/DOPO-TAIC/M-SiC-5 and UP/DOPO-TAIC/M-SiC-30 composites, which was associated with the vibration of Si-C bonds [46].

Figure 7.
Infrared spectra of residual carbon for UP, UP/DOPO-TAIC-15, UP/DOPO-TAIC/M-SiC-5, UP/DOPO-TAIC/M-SiC-30 composites after cone calorimetry tests.
The FTIR results indicated that the residues of UP/DOPO-TAIC/M-SiC-5 and UP/DOPO-TAIC/M-SiC-30 after combustion were significantly different from those of UP and UP/DOPO-TAIC-15. The residue of neat UP matrix material after combustion mainly consisted of compounds containing unsaturated C-H bonds. The residue of UP/DOPO-TAIC-15 blends after combustion was primarily composed of organic phosphorus compounds. However, the residues of UP/DOPO-TAIC/M-SiC-5 and UP/DOPO-TAIC/M-SiC-30 composites after combustion mainly included organic phosphorus compounds and carbon-silicon-containing residues. All of these combustion products are low toxicity by taking consideration of non-generation of halogen element [12].
The SEM images of the residual carbon after cone calorimetry testing were shown in Figure 8. For UP matrix material, the sample was almost completely combusted after burning, as shown in Figure 8a. The residue was loose and incomplete and had many pores and cracks on the surface of the residual carbon. In addition, no dense carbon layer was formed. While the carbon layer became denser after the addition of the organic flame retardant, as shown in Figure 8b, This is because that the organic flame retardant-DOPO-TAIC will decompose to form phosphorus-oxygen radicals [14], which can inhibit the radical chain combustion reaction [15] and also promote the formation of the carbon layer.

Figure 8.
SEM images of residues after cone calorimeter tests: (a) UP, (b) UP/DOPO-TAIC/M-SiC-5, (c) UP/DOPO-TAIC/M-SiC-5, and (d) UP/DOPO-TAIC/M-SiC-30.
The SEM images of the residual carbon of UP/DOPO-TAIC/M-SiC-5 and UP/DOPO-TAIC/M-SiC-30 composites after combustion were shown in Figure 8c,d. A more compact and highly carbonized surface morphology was observed. M-SiC particles were clearly found on the surface of residual carbon image. The possible reason can be explained in the following. The M-SiC powder accumulates on the sample surface, forming silicon carbide and silicon oxide [37], which will effectively block the transfer of oxygen and combustible gases, thereby preventing further combustion of the UP matrix. Therefore, UP/DOPO-TAIC/M-SiC-30 composites exhibited better flame-retardant performance than that of UP/DOPO-TAIC/M-SiC-5. The relatively larger volume of the carbon layer is typically associated with lower thermal conductivity. The P-Si elements in the hybrid flame retardants [47] can work cooperatively to form a dense carbon layer, which can suppress the combustion performance of UP matrix and provide better thermal insulation and smoke suppression. The SEM results indicated that the hybrid flame-retardant effect of M-SiC and DOPO-TAIC can significantly enhance the flame-retardant properties of UP materials.
The residual carbon of UP, UP/DOPO-TAIC-15, UP/DOPO-TAIC/M-SiC-5, and UP/DOPO-TAIC/M-SiC-30 materials was analyzed using EDX (Energy Dispersive X-ray Spectroscopy) to further explore the flame-retardant mode of action of the effect between M-SiC and the organic flame-retardant DOPO-TAIC. According to the data in Table 9, the addition of M-SiC significantly increased the Si content in the residual carbon of UP/DOPO-TAIC/M-SiC-30, while the O and C contents decreased. This phenomenon is likely caused by the cooperative action of P and Si elements in the flame retardants, which will generate large amounts of non-combustible CO and CO2 gases in the gas phase. These gases can isolate combustible gases, reducing the contact between UP and combustible gases and preventing further combustion of UP matrix. Meanwhile, The SEM and EDX results indicated that the combination of M-SiC and DOPO-TAIC exerted a cooperative flame-retardant effect, which can significantly improve the flame-retardant properties of UP materials.

Table 9.
EDX elemental analysis results of residual carbon after combustion of UP, UP/DOPO-TAIC/M-SiC-5, and UP/DOPO-TAIC/M-SiC-30.
The possible mode of action by using DOPO derivatives and SiC to enhance the flame retardant effect of UP matrix can be explained in the following. DOPO derivatives can undergo decomposition to form PO· radicals during the degrading process of polymer matrix. Some of these survive and react with components of the UP matrix, which will escape to the gas phase where they efficiently scavenge combustion propagating radicals. DOPO derivatives display little solid-phase activity. PO· radicals are consumed by reaction with matrix components, and this may contribute in a small way, to the formation of a char layer. However, the formation of phosphoric acids with sufficient lifetime to promote cationic crosslinking is not observed [48]. It is likely that the presence of Si-C facilitates the formation of a char layer at the surface of the degrading polymer matrix. This dense and stable carbon layer will effectively block oxygen, combustible gases, and heat transfer, acting in the condensed phase to slow down the further combustion of UP matrix and enhance the safety performance of the UP material. This char layer acts as an insulation barrier to inhibit heat feedback from the combustion zone to decrease the rate of polymer degradation and consequent formation of volatile fuel fragments. Thus. The presence of both a DOPO and Si-C act in a manner to enhance the overall flame-retardant effect [49,50].
3.7. Mechanical Properties of UP/DOPO-TAIC/M-SiC Composite After Combustion
The effect of M-SiC on the mechanical properties of UP/DOPO-TAIC/M-SiC composites was given in Figure 9. The incorporation of DOPO-TAIC can increase the tensile strength of UP. The tensile strength increased from 36.2 MPa for neat UP to 41 MPa for UP/DOPO-TAIC-15 blends. The possible reason is that DOPO-TAIC might participate in the crosslinking reaction of UP during the curing process. In addition, the optimum M-SiC content for achieving the highest tensile strength of UP/DOPO-TAIC/M-SiC composites is 15 wt.%. A slight increase in tensile strength was obtained for UP/DOPO-TAIC blends after incorporating M-SIC. The tensile strength of UP/DOPO-TAIC/M-SiC composites began to decrease after the weight fraction of M-SIC exceeded 15 wt.%. The exceeding M-SiC content will lead to an increase in viscosity of UP during the fabricating process of its composites, which will consequently result in the difficult dispersion of M-SiC.

Figure 9.
Effect of M-SiC content on the mechanical properties of UP/DOPO-TAIC/M-SiC composites.
4. Conclusions
The novel organic flame-retardant DOPO-TAIC was successfully synthesized using DOPO and TAIC as raw materials, and the M-SiC material was successfully prepared from SiC. These materials were combined to enhance the flame-retardant effect of UP resin. Compared to UP/DOPO-TAIC-15 blends, UP/DOPO-TAIC/M-SiC composites showed significant improvements in flame-retardant properties when the content of DOPO-TAIC and M-SiC was 15 wt.% and 30%. The LOI value of the UP/DOPO-TAIC/M-SiC-30 composite increased to 30.8%. Meanwhile, the HRR value of this composite decreased by 14.4%, and the char yield increased by 251%.
These results indicated that the organic flame retardant-DOPO-TAIC decomposes upon heating to release PO• radicals in the condensed phase, which can inhibit the chain combustion reactions of the UP material and promote the charring of the UP matrix. M-SiC will cover the surfaces of polymer material during the process of combustion, forming a more dense and continuous char layer. In the gas phase, the combustion of DOPO-TAIC releases substances such as PO2 and HPO2, which isolate combustible gases and exert a flame-retardant effect. The cooperative incorporation of inorganic M-SiC and organic flame-retardant DOPO-TAIC releases non-combustible gases in the gas phase to isolate oxygen and other combustible gases. While it can promote the formation of a char layer to prevent further combustion of UP matrix in the condensed phase. This strategy of simultaneously using organic DOPO-TAIC and inorganic M-SiC flame retardants can provide excellent flame-retardant performance for UP resin, which also provide a facile method for the flame retardant of other polymer materials.
Author Contributions
Conceptualization, P.W.; Validation, C.L.; Formal analysis, F.Y.; Investigation, P.W. and J.H.; Resources, M.X.; Data curation, P.H.; Writing—original draft, P.W.; Writing—review & editing, H.Y.; Project administration, M.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vahabi, H.; Kandola, B.K.; Saeb, M.R. Flame Retardancy Index for Thermoplastic Composites. Polymers 2019, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Kicko-Walczak, E.; Rymarz, G. Recent developments in fire-retardant thermoset resins using inorganic-organic hybrid flame retardants. J. Polym. Eng. 2018, 38, 563–571. [Google Scholar]
- Kandola, B.K.; Krishnan, L.; Deli, D.; Ebdon, J.R. Blends of unsaturated polyester and phenolic resins for application as fire-resistant matrices in fibre-reinforced composites. Part 2: Effects of resin structure, compatibility and composition on fire performance. Polym. Degrad. Stab. 2015, 113, 154–167. [Google Scholar]
- Seraji, S.M.; Gan, H.; Swan, S.R.; Varley, R.J. Phosphazene as an effective flame retardant for rapid curing epoxy resins. React. Funct. Polym. 2021, 164, 104910. [Google Scholar]
- Weil, E.D.; Levchik, S.V. Commercial Flame Retardancy of Unsaturated Polyester and Vinyl Resins: Review. J. Fire Sci. 2004, 22, 293–303. [Google Scholar]
- Kandola, B.K.; Ebdon, J.R.; Chowdhury, K.P. Chowdhury. Flame Retardance and Physical Properties of Novel Cured Blends of Unsaturated Polyester and Furan Resins. Polymers 2015, 7, 298–315. [Google Scholar]
- Wazarkar, K.; Kathalewar, M.; Sabnis, A. Flammability behavior of unsaturated polyesters modified with novel phosphorous containing flame retardants. Polym. Compos. 2017, 38, 1483–1491. [Google Scholar]
- Zhang, Q.; Xu, B.; Zhou, H.; Qian, L. A facile strategy to simultaneously enhance the flame retardancy, toughness and ultraviolet shielding performance of unsaturated polyester resin: Adjusting the unsaturated degree of flame retardant. Polymer 2024, 301, 127035. [Google Scholar]
- Reuter, J.; Greiner, L.; Kukla, P.; Doring, M. Efficient flame retardant interplay of unsaturated polyester resin formulations based on ammonium polyphosphate. Polym. Degrad. Stab. 2020, 178, 109134. [Google Scholar]
- Kandola, B.K.; Ebdon, J.R.; Luangtriratana, P.; Krishnan, L. Novel flame retardant thermoset resin blends derived from a free-radically cured vinylbenzylated phenolic novolac and an unsaturated polyester for marine composites. Polym. Degrad. Stab. 2016, 127, 56–64. [Google Scholar]
- Reuter, J.; Greiner, L.; Schönberger, F.; Döring, M. Cooperative flame retardant interplay of phosphorus containing flame retardants with aluminum trihydrate depending on the specific surface area in unsaturated polyester resin. J. Appl. Polym. Sci. 2019, 136, 47270. [Google Scholar]
- Seraji, S.M.; Song, P.; Varley, R.J.; Bourbigot, S.; Voice, D.; Wang, H. Fire-retardant unsaturated polyester thermosets: The state-of-the-art, challenges and opportunities. Chem. Eng. J. 2022, 430, 132785. [Google Scholar]
- Li, P.; Wang, J.; Wang, C.; Xu, C.; Ni, A. The flame retardant and mechanical properties of the epoxy modified by an efficient DOPO-based flame retardant. Polymers 2024, 16, 631. [Google Scholar] [CrossRef]
- Jiao, D.; Zhao, H.; Sima, H.; Cheng, C.; Liu, B.; Zhang, C. Engineering flame retardant epoxy resins with strengthened mechanical property by using reactive catechol functionalized DOPO compounds. Chem. Eng. J. 2024, 485, 149910. [Google Scholar]
- Yang, Y.; Wang, D.Y.; Jian, R.K.; Liu, Z.; Huang, G. Chemical structure construction of DOPO-containing compounds for flame retardancy of epoxy resin: A review. Prog. Org. Coat. 2023, 175, 107316. [Google Scholar]
- Liang, S.; Hemberger, P.; Steglich, M.; Simonetti, P.; Levalois-Grützmacher, J.; Grützmacher, H.; Gaan, S. The underlying chemistry to the formation of PO2 radicals from organophosphorus compounds: A missing puzzle piece in flame chemistry. Chem. Eur. J. 2020, 26, 10795–10800. [Google Scholar]
- Chu, F.; Hu, W.; Song, L.; Hu, Y. State-of-the-Art Research in Flame-Retardant Unsaturated Polyester Resins: Progress, Challenges and Prospects. Fire Technol. 2022, 60, 1077–1118. [Google Scholar]
- Battig, A.; Müller, P.; Bertin, A.; Schartel, B. Hyperbranched rigid aromatic phosphorus-containing flame retardants for epoxy resins. Macromol. Mater. Eng. 2021, 306, 2000731. [Google Scholar]
- Chi, Z.; Guo, Z.; Xu, Z.; Zhang, M.; Li, M.; Shang, L.; Ao, Y. A DOPO-based phosphorus-nitrogen flame retardant bio-based epoxy resin from diphenolic acid: Synthesis, flame-retardant behavior and mechanism. Polym. Degrad. Stab. 2020, 176, 109151. [Google Scholar]
- Jin, S.; Liu, Z.; Qian, L.; Qiu, Y.; Chen, Y.; Xu, B. Epoxy thermoset with enhanced flame retardancy and physical-mechanical properties based on reactive phosphaphenanthrene compound. Polym. Degrad. Stab. 2020, 172, 109063. [Google Scholar]
- Wang, J.; Li, J.; Meng, X.; Gao, X.; Yan, H. Synthesis of a novel DOPO-substituted charring agent containing triazine for reducing the fire hazard of polypropylene. J. Polym. Res. 2022, 29, 380. [Google Scholar] [CrossRef]
- Wang, S.; Lou, S.; Fan, P.; Ma, L.; Liu, J.; Tang, T. A novel aromatic imine-containing DOPO-based reactive flame retardant towards enhanced flame-retardant and mechanical properties of epoxy resin. Polym. Degrad. Stab. 2023, 213, 110364. [Google Scholar]
- Chen, R.; Hu, K.; Tang, H.; Wang, J.; Zhu, F.; Zhou, H. A novel flame retardant derived from DOPO and piperazine and its application in epoxy resin: Flame retardance, thermal stability and pyrolysis behavior. Polym. Degrad. Stab. 2019, 166, 334–343. [Google Scholar] [CrossRef]
- Wang, X.; He, W.; Long, L.; Huang, S.; Qin, S.; Xu, G. A phosphorus-and nitrogen-containing DOPO derivative as flame retardant for polylactic acid (PLA). J. Therm. Anal. 2021, 145, 331–343. [Google Scholar] [CrossRef]
- Yang, J.; Song, X.; Chen, X.; Wang, Y.; Shi, J.; Zheng, Z.; Xu, H.; Liu, L. A soluble Salen-DOPO flame retardant for efficiently improving PBAT/PLLA film. Chem. Eng. J. 2023, 476, 146669. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Z.; Ma, H.; Zhou, Y.; Tian, X.; Sheng, D.; Liu, X.; Yang, Y. Waterborne Polyurethane/Cellulose Composite Aerogel with Passive Cooling and Thermal Insulation Properties. J. Appl. Polym. Sci. 2024, 141, e56158. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, W.; Li, Z.; Huang, G.; Wu, G. Efficient flame retardancy, good thermal stability, mechanical enhancement, and transparency of DOPO-conjugated structure compound on epoxy resin. Chem. Eng. J. 2022, 450, 138424. [Google Scholar] [CrossRef]
- He, Y.; Cui, X.; Liu, Z.; Lan, F.; Sun, J.; Li, H.; Gu, X.; Zhang, S. A new approach to prepare flame retardant epoxy resin with excellent transmittance, mechanical properties, and anti-aging performance by the incorporation of DOPO derivative. Polym. Degrad. Stab. 2023, 218, 110579. [Google Scholar] [CrossRef]
- Cheng, R.; Chen, H.; Chen, Y.; Shen, C.; Gao, S. Effects of a phosphorus-nitrogen containing DOPO derivative on the flame retardancy and mechanical properties of polylactic acid foamed materials. Polym. Degrad. Stab. 2024, 225, 110790. [Google Scholar]
- Zhao, W.; Zhao, H.-B.; Cheng, J.-B.; Li, W.; Zhang, J.; Wang, Y.-Z. A green, durable and effective flame-retardant coating for expandable polystyrene foams. Chem. Eng. J. 2022, 440, 135807. [Google Scholar]
- Arfiana; Saputra, A.H.; Murti, S.D.S.; Saputra, H. Study on the Flammability and Thermal Stability of Non Halogen UPR-Based Fire Retardant Composite through Combination of Carbon Black and Hydroxide Additives. Macromol. Symp. 2020, 391, 1900176. [Google Scholar]
- Zhang, S.; Li, B.; Lin, M.; Li, Q.; Gao, S.; Yi, W. Effect of a novel phosphorus-conataining compound on the flame retardancy and thermal degradation of intumescent flame retardant polypropylene. J. Appl. Polym. Sci. 2011, 122, 3430–3439. [Google Scholar]
- Zhang, Y.; Yan, H.; Feng, G.; Liu, R.; Yang, K.; Feng, W.; Zhang, S.; He, C. Non-aromatic Si, P, N-containing hyperbranched flame retardant on reducing fire hazards of epoxy resin with desirable mechanical properties and lower curing temperature. Compos. Part B Eng. 2021, 222, 109043. [Google Scholar]
- Sun, J.; Zhang, D.; Wang, B.; Xia, Y.; Zhang, Y.; Guo, Z.; Fang, Z.; Li, J.; Chen, P. Flame retardancy and toughness of epoxy resin induced by a star-shaped flame retardant containing P/Si/B. React. Funct. Polym. 2023, 190, 105649. [Google Scholar]
- Hu, Z.; Chen, L.; Zhao, B.; Luo, Y.; Wang, D.-Y.; Wang, Y.-Z. A novel efficient halogen-free flame retardant system for polycarbonate. Polym. Degrad. Stab. 2011, 96, 320–327. [Google Scholar]
- Tibiletti, L.; Ferry, L.; Longuet, C.; Mas, A.; Robin, J.-J.; Lopez-Cuesta, J.-M. Thermal degradation and fire behavior of thermoset resins modified with phosphorus containing styrene. Polym. Degrad. Stab. 2012, 97, 2602–2610. [Google Scholar]
- Wu, P.; Peng, Y.; Zhang, X.; Zhang, G.; Ran, J.; Xu, M. Unsaturated polyester resin modified with a novel reactive flame retardant: Effects on thermal stability and flammability. J. Polym. Eng. 2022, 42, 818–826. [Google Scholar]
- Jin, X.; Xu, J.; Pan, Y.; Wang, H.; Ma, B.; Liu, F.; Yan, X.; Wu, C.; Huang, H.; Cheng, H.; et al. Light weight multiscale needle-punched quartz fiber felt reinforced silicon carbide modified phenolic aerogel nanocomposites with enhanced mechanical, insulating and flame retardant properties. Compos. Sci. Technol. 2021, 215, 109100. [Google Scholar]
- Zhang, L.; Wang, H.; Li, Y. Dopamine-modified ammonium polyphosphate as an efficient intumescent flame retardant for thermoplastic polyurethane elastomers. J. Appl. Polym. Sci. 2023, 130, 1234–1245. [Google Scholar]
- Shcherbakov, A.; Mostovoy, A.; Bekeshev, A.; Burmistrov, I.; Arzamastsev, S.; Lopukhova, M. Effect of Microwave Irradiation at Different Stages of Manufacturing Unsaturated Polyester Nanocomposite. Polymers 2022, 14, 4594. [Google Scholar] [CrossRef]
- Lei, D.; Ma, W.; Wang, L.; Zhang, D. Preparation of 2-ethyl-4- methylimidazole derivatives as latent curing agents and their application in curing epoxy resin. J. Appl. Polym. Sci. 2015, 132, 42563. [Google Scholar]
- Liu, L.; Li, M. Curing mechanisms and kinetic analysis of DGEBA cured with a novel imidazole derivative curing agent using DSC techniques. J. Appl. Polym. Sci. 2010, 117, 3220–3227. [Google Scholar]
- Shi, X.; Li, H.; Guan, H.; Li, D.; Wang, C.; Wang, Y.; Lu, X. Tribological behavior of polydopamine-modified boron nitride nanoplatelets-reinforced silicate ceramic coatings. Ceram. Int. 2025. [Google Scholar] [CrossRef]
- Wang, X.; Jia, X.; Ren, H.; Yang, J.; Song, H. Breathable, self-cleaning superhydrophobic DDA-PDA@ BNNS/silicon resin coating. Prog. Org. Coat. 2024, 192, 108515. [Google Scholar]
- Zhang, H.L.; Zuo, X.B.; Sun, Q.Q.; Liu, J.Y.; Zou, Y.X.; Zhang, T.T.; Tian, J.L. Preparation of h-BN @ ZnO composite epoxy coating for improve durability and antibacterial properties of concrete. Constr. Build. Mater. 2024, 438, 137082. [Google Scholar]
- Song, C.; Chen, Z.; Duan, C.; Li, C.; Kawi, S.; Li, Y. Polydopamine-boron nitride nanosheet composites with core-shell structures modified PMIA separator for enhanced performance of high-power lithium-ion batteries. J. Energy Storage 2024, 98, 113020. [Google Scholar]
- Jiang, Q.; Luo, Z.; Wang, B. A phosphorous/nitrogen/silicon containing diphenylphosphoramide silicon oil toward effective flame retardancy for polycarbonate with comparable mechanical properties. J. Appl. Polym. Sci. 2022, 139, 51755. [Google Scholar]
- Howell, B.A. Thermal degradation of organophosphorus flame retardants. Polymers 2022, 14, 4929. [Google Scholar] [CrossRef]
- Schäfer, A.; Seibold, S.; Lohstroh, W.; Walter, O.; Döring, M. Synthesis and properties of flame-retardant epoxy resins based on 9,10-dihydro-9-oxa-10-phosphaphe-nanthrene-10-oxide and one of its analogues. J. Appl. Polym. Sci. 2007, 105, 685–696. [Google Scholar]
- Liang, S.; Hemberger, P.; Neisius, N.M.; Bodi, A.; Grützmacher, H.; Levalois-Grützmacher, J.; Gaan, S. Elucidating the Thermal Decomposition of Dimethyl Methylphosphonate by Vacuum Ultraviolet (VUV) Photoionization: Pathways to the PO Radical, a Key Species in Flame-Retardant Mechanisms. Chem. A. Eur. J. 2015, 21, 1073–1080. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).