Effect of Enzymatic Glycosylation on Film-Processing Properties and Biological Activities of Black Soybean Protein
Abstract
1. Introduction
2. Materials and Sample Preparation
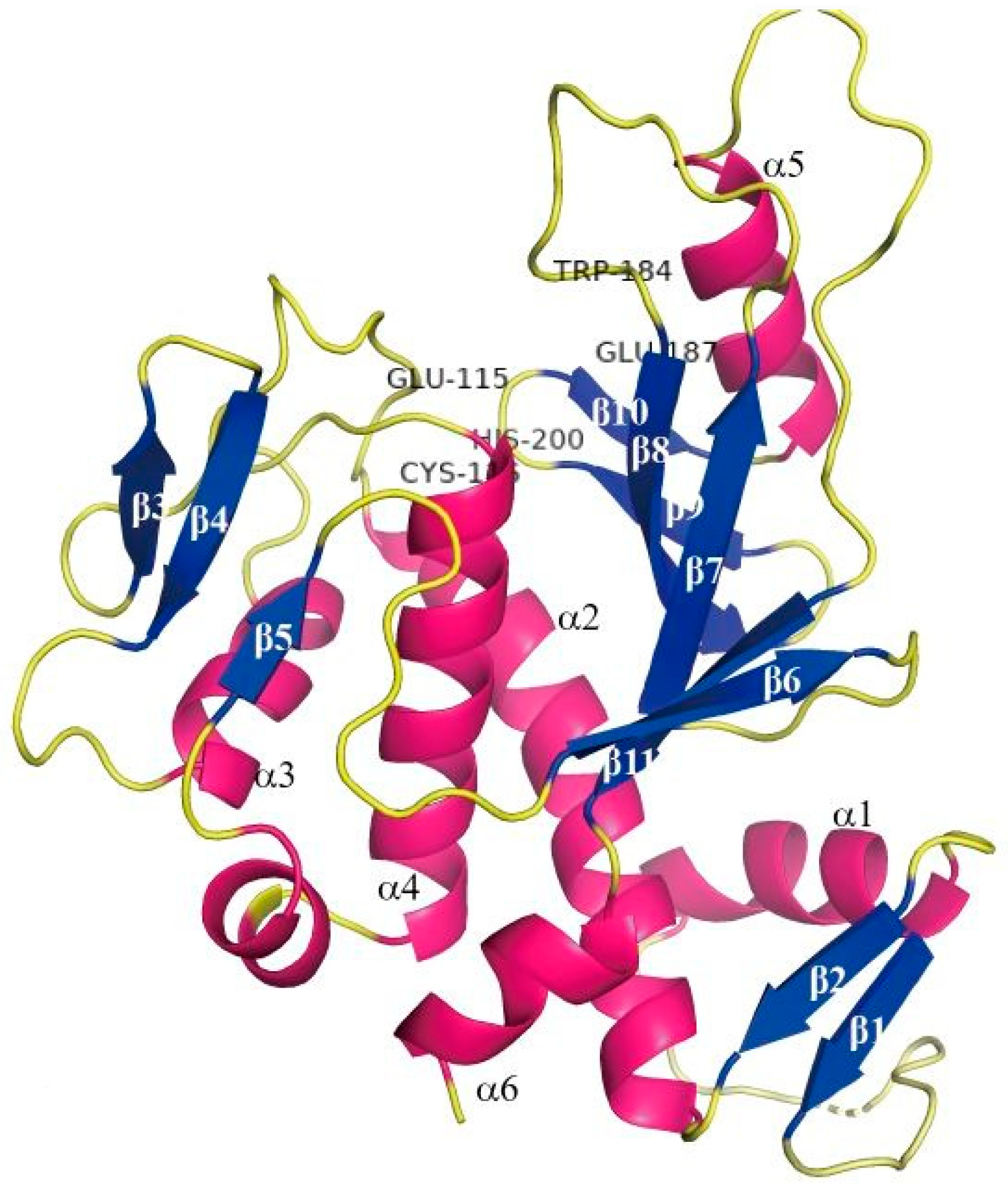
2.1. Preparation of Black Soybean Protein Isolated (BSP)
2.2. Preparation of Enzymatically Glycosylated Black Soybean Protein (EGBSP)
2.3. Preparation of Enzymatic Crosslinked Black Soybean Protein Isolated (ECBSP)
2.4. Experimental Animals and Tumor Strain
2.5. Instruments
3. Experimental Methods
3.1. Effect of Enzymatic Glycosylation on Film Processing Functions of BSP
3.1.1. Determination of Zeta Potential of BSP and Its Modified Products
3.1.2. Surface Hydrophobicity Analysis of BSP and Its Modified Products
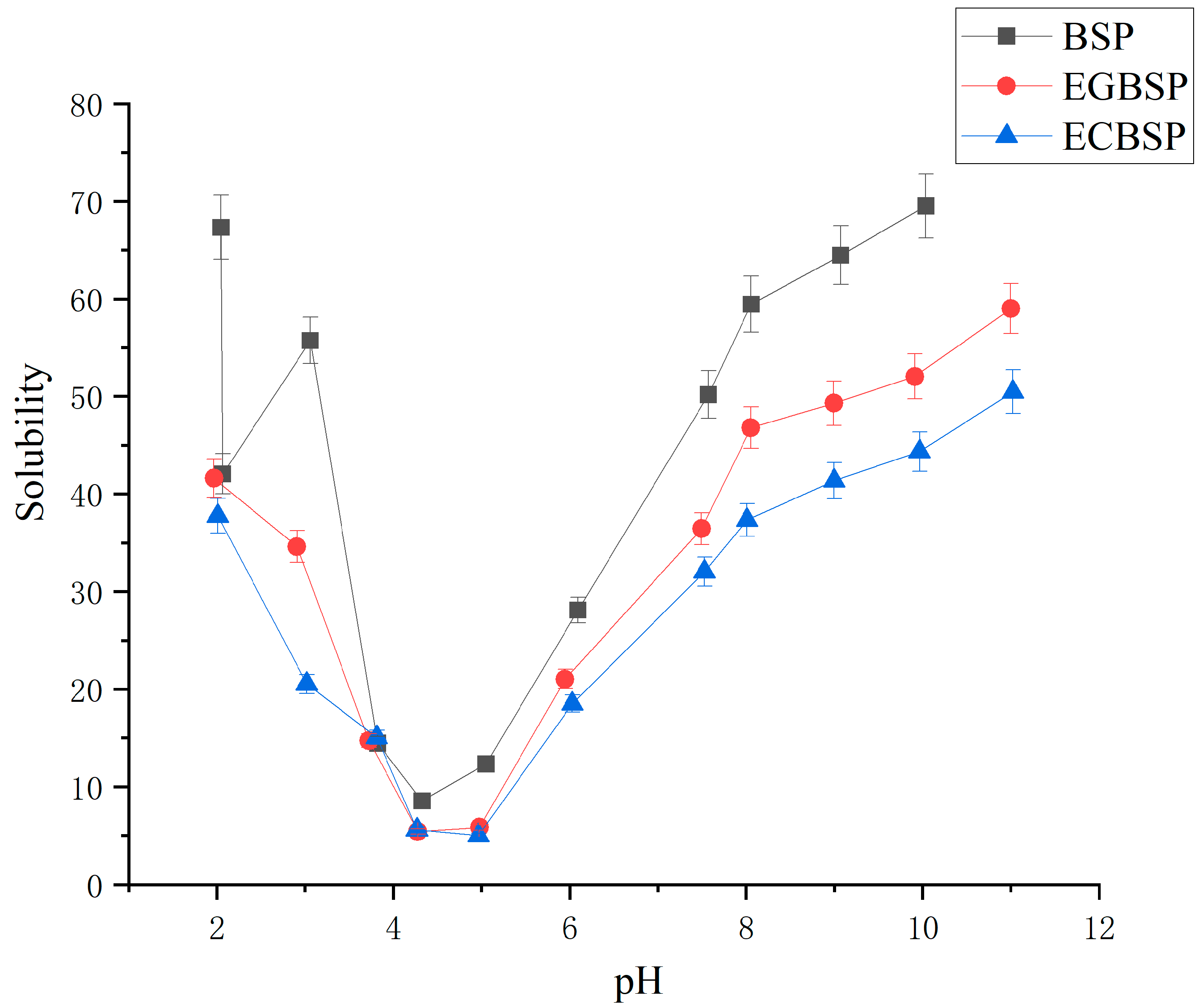
3.1.3. Determination of Solubility of BSP and Its Modified Products
3.1.4. Rheology of Acid-Induced Gel Formation of BSP and Its Modified Products
3.1.5. Film Preparation of BSP and Its Modified Products
3.1.6. Mechanical Properties of Films Made of BSP and Its Modified Products
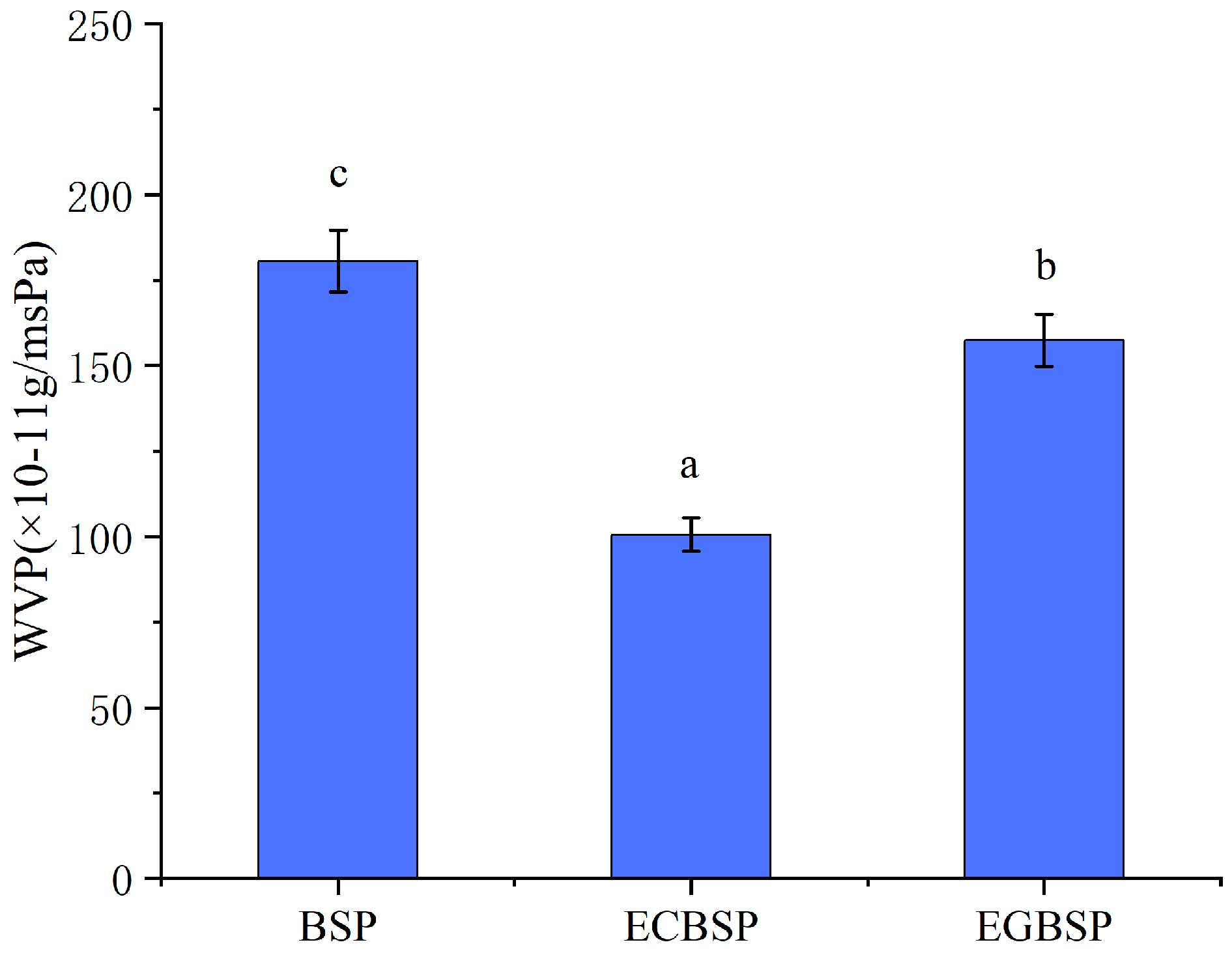
3.1.7. Permeability of Films Made of BSP and Its Modified Products
3.2. Effect of Enzymatic Glycosylation on Biological Activities of BSP
3.2.1. The Grouping and Administration of Mice
3.2.2. Morphological Observation of Mice Ascites Tumor
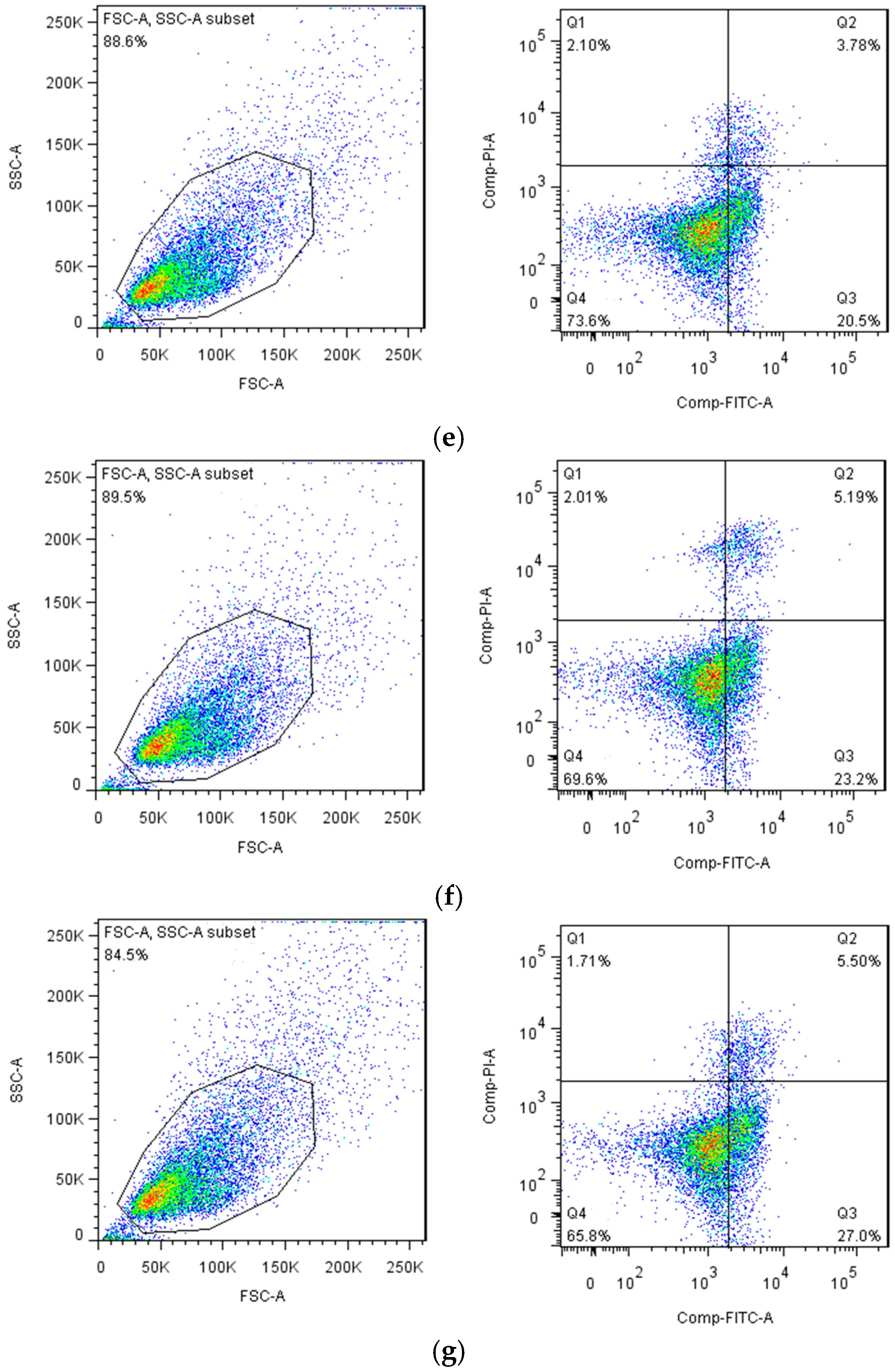
3.2.3. Flow Cytometry of Apoptosis of H22 Hepatocellular Carcinoma Cells in Tumor-Bearing Mice
3.2.4. Research on Lymphocyte Proliferation Activity
3.2.5. Research on Killing Activity of Mouse NK Cells
3.2.6. Research on Immune Factors
3.3. Statistical Analysis
4. Results and Discussion
4.1. Results of Effect of Enzymatic Glycosylation on Film Processing Functions of BSP
4.1.1. Results of Zeta Potential of BSP and Its Modified Products
4.1.2. Results of Surface Hydrophobicity Analysis of BSP and Its Modified Products
4.1.3. Results of Solubility of BSP and Its Modified Products
4.1.4. Results of Rheology of Acid-Induced Gel Formation of BSP and Its Modified Products
4.1.5. Results of Mechanical Properties of Films Made of BSP and Its Modified Products
4.1.6. Results of Permeability of Films Made of BSP and Its Modified Products
4.2. Results of Effect of Enzymatic Glycosylation on Biological Activities of BSP
4.2.1. Results of Tumor Weight of Mice with Hepatoma H22
4.2.2. Results of Morphological Observation of Mice Ascites Tumor
4.2.3. Results of Flow Cytometry of Mice Ascites Tumor
4.2.4. Results of Lymphocyte Proliferation Activity
4.2.5. Results of Effect of BSP and EGBSP on Immune Cells of Mice with H22
4.2.6. Results of Effect of BSP and EGBSP on Serum IL-2 and IL-12 of Mice with Tumor H22
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitharwal, S.; Saini, A.; Chauhan, K.; Taneja, N.K.; Oberoi, H.S. Unveiling the nutrient-wealth of black soybean: A holistic review of its bioactive compounds and health implications. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70001. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Hao, X.; Ji, X.; Zhu, Y.; Chen, X.; Yao, Y. A systematic review of black soybean (Glycine max (L.) Merr.): Nutritional composition, bioactive compounds, health benefits, and processing to application. Food Front. 2024, 5, 1188–1211. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Guo, Y.R.; Hou, T.Y.; Ning, Q.R.; Han, W.Y.; Zhao, X.Y.; Cui, F.; Li, H. Formation of advanced glycation end products in glucose–amino acid models of Maillard reaction under dry- and wet-heating conditions. J. Sci. Food Agric. 2024, 105, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Mondaca-Navarro, B.A.; Torres-Arreola, W.; Ávila-Villa, L.A.; Villa-Lerma, A.G.; Hernández-Mendoza, A.; Wall-Medrano, A.; Ramírez, R.R. Obtaining glycoconjugates of marine origin via Maillard reaction and their cytotoxic effect: An alternative for the use of animal byproducts. J. Sci. Food Agric. 2024, 100, 3228–3235. [Google Scholar] [CrossRef] [PubMed]
- Akharume, F.; Aluko, R.; Adedeji, A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Pourmohammadi, K. Chemical modifications and their effects on gluten protein: An extensive review. Int. J. Food Chem. 2021, 343, 128398. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Fu, Y.; Ma, L.; Dai, H.; Wang, H.; Chen, H.; Zhu, H.; Yu, Y.; Zhang, Y. Collagen glycopeptides from transglutaminase-induced glycosylation exhibit a significant salt taste-enhancing effect. J. Agric. Food Chem. 2023, 71, 8558–8568. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, Y.; Lu, S.; Yao, X.; Zheng, X.; Zhao, R.; Li, Z.; Shen, H.; Zhang, S. Effects of Modified Processing Methods on Structural Changes of Black Soybean Protein Isolate. Molecules 2018, 23, 2127. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Jo, Y.H.; Han, Y.S.; Jung, W.J. Production of chitin- and chitosan-oligosaccharide using the edible insect, Tenebrio molitor. Entomol. Res. 2022, 52, 207–213. [Google Scholar] [CrossRef]
- Sun, X.; Cui, Q.; Li, R.; Hao, L.; Liu, H.; Wang, X.; Xu, N.; Zhao, X. Structural and emulsifying properties of soybean protein isolate glycated with glucose based on pH treatment. J. Sci. Food Agric. 2022, 102, 4462–4472. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, J.; Zhao, L.; He, W.; Liu, T.; Liu, B. Analysis of the gel properties, microstructural characteristics, and intermolecular forces of soybean protein isolate gel induced by transglutaminase. Food Sci. Nutr. 2022, 10, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Cui, Q.; Yu, Z.; Wang, X.; Zhao, X.H. Effects of transglutaminase glycosylated soy protein isolate on its structure and interfacial properties. J. Sci. Food Agric. 2021, 101, 5097–5105. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Cao, Y.; Chang, S.K.C. Characteristics of soy films as affected by transglutaminase cross-linking and inclusions of pectin and protein enhancers. J. Food Sci. 2024, 89, 4389–4402. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, N.; Wang, Z.; Wang, X. Wheat bran dietary fibre-induced changes in gluten aggregation and conformation in a dough system. Int. J. Food Sci. 2021, 56, 86–92. [Google Scholar] [CrossRef]
- Deshpande, M.; Sathe, S.K. Interactions with 8-Anilinonaphthalene-1-sulfonic Acid (ANS) and Surface Hydrophobicity of Black Gram (Vigna mungo) Phaseolin. J. Food Sci. 2018, 83, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, F.; Lai, S.; Wang, H.; Yang, H. Impact of soybean protein isolate-chitosan edible coating on the softening of apricot fruit during storage. LWT 2018, 96, 604–611. [Google Scholar] [CrossRef]
- González, A.; Gastelú, G.; Barrera, G.N.; Ribotta, P.D.; Igarzabal, C.I.Á. Preparation and characterization of soy protein films reinforced with cellulose nanofibers obtained from soybean by-products. Food Hydrocoll. 2019, 89, 758–764. [Google Scholar] [CrossRef]
- Waldmann, T.A. The IL-2/IL-2 receptor system: A target for rational immune intervention. Trends Pharmacol. Sci. 1993, 14, 159–164. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, J.; Wang, F.; Li, Q.; Fan, Y.; Zhao, X.; Hao, L.; Hou, H. Stability of oil-in-water emulsion and immunomodulating activity in S180 tumor-bearing mice. J. Food Sci. 2024, 89, 5884–5899. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, Y.; Jing, X.; Liu, Y.; Liu, A. Structural Characterization of an Alkali-Soluble Polysaccharide from Angelica sinensis and Its Antitumor Activity in Vivo. Chem. Biodivers. 2021, 18, e2100089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Ma, T.; Zhang, X.; Zhao, Q.; Zhu, K.; Cao, J.; Liu, Z.; Shen, X.; Li, C. Holothuria leucospilota Polysaccharides Improve Immunity and the Gut Microbiota in CyclophosphamideTreated Immunosuppressed Mice. Nutr. Food Res. 2023, 67, 2200317. [Google Scholar] [CrossRef] [PubMed]
- San, Y.; Xing, Y.; Li, B.; Zheng, L. Effect of transglutaminase cross-linking on the structure and emulsification performance of heated black bean protein isolate. J. Sci. Food Agric. 2025, 105, 2382–2389. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, F.; Ji, W.; Yokoyama, W.; Shoemaker, C.F.; Zhu, S.; Xia, W. Functional properties of Maillard reaction products of rice protein hydrolysates with mono-, oligo- and polysaccharides. Food Hydrocoll. 2013, 30, 53–60. [Google Scholar] [CrossRef]
- Gu, X.; Campbell, L.J.; Euston, S.R. Influence of sugars on the characteristics of glucono-δ-lactone-induced soy protein isolate gels. Food Hydrocoll. 2009, 23, 314–326. [Google Scholar] [CrossRef]
- Hiller, B.; Lorenzen, P.C. Surface Hydrophobicity of Physicochemically and Enzymatically Treated Milk Proteins in Relation to Techno-functional Properties. J. Agric. Food Chem. 2008, 56, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Lv, L.; Li, Z.; Mi, N.; Chen, H.; Lin, H. Effect of transglutaminase-catalyzed glycosylation on the allergenicity and conformational structure of shrimp (Metapenaeus ensis) tropomyosin. Food Chem. 2017, 219, 215–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, C.; Zhang, X.; Xia, S.; Jia, C.; Karangwa, E.; Shabbar, A.; Feng, B.; Zhong, F. Effects of maltodextrin glycosylation following limited enzymatic hydrolysis on the functional and conformational properties of soybean protein isolate. Eur. Food Res. Technol. 2014, 238, 957–968. [Google Scholar] [CrossRef]
- Chang, Y.; Su, H.; Shiau, S. Rheological and textural characteristics of black soybean touhua (soft soybean curd) prepared with glucono-δ-lactone. Food Chem. 2009, 115, 585–591. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Physical and mechanical properties of compression molded and solution casting soybean protein concentrate based films. Food Hydrocoll. 2014, 38, 193–204. [Google Scholar] [CrossRef]
- Xu, Y.P.; Wang, Y.; Zhang, T.; Mu, G.Q.; Jiang, S.J.; Zhu, X.M.; Tuo, Y.F.; Qian, F. Evaluation of the properties of whey protein films with modifications. J. Food Sci. 2021, 86, 923–931. [Google Scholar] [CrossRef]
- Gao, C.; Wang, F.; Yuan, L.; Liu, J.; Sun, D.; Li, X. Physicochemical property, antioxidant activity, and cytoprotective effect of the germinated soybean proteins. Food Sci. Nutr. 2019, 7, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.; Quinta-Costa, M.; Leite, P.S.; Guimarães, J.E. Critical evaluation of techniques to detect and measure cell death--study in a model of uv radiation of the leukaemic cell line hl60. Anal. Cell. Pathol. Cell. Oncol. 1999, 19, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Hou, X.; Su, D.; He, Z.; Zhao, J.; Liu, T. Effect of PhenylEthanol Glycosides from Cistanche Tubulosa on Autophagy and Apoptosis in H22 Tumor-Bearing Mice. Evid. Based Complement. Altern. Med. 2022, 14, 3993445. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Zhu, M.; Chen, K.; Xie, H.; Bai, H.; Chen, Q. Z-Guggulsterone Induces Apoptosis in Gastric Cancer Cells through the Intrinsic Mitochondria-Dependent Pathway. Sci. World J. 2021, 6, 3152304. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Luo, S.; Luo, X.; Hu, M.; Ma, F.; Wang, Y.; Lai, X.; Zhou, L. Polysaccharides from Chinese Herbal Lycium barbarum Induced Systemic and Local Immune Responses in H22 Tumor-Bearing Mice. J. Immunol. Res. 2018, 12, 3431782. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, C.; Zhao, S.; Wang, M.; Shang, L.; Zhou, J.; Ma, Y. The role of tumor-associated macrophages in hepatocellular carc inoma progression: A narrative review. Cancer Med. 2023, 12, 22109–22129. [Google Scholar] [CrossRef]
- Halim, N.R.A.; Azlan, A.; Yusof, H.M.; Sarbon, N.M. Antioxidant and anticancer activities of enzymatic eel (monopterus sp.) protein hydrolysate as influenced by different molecular weight. Biocatal. Agric. Biotechnol. 2018, 16, 10–16. [Google Scholar] [CrossRef]
- Paglia, P.; Chiodoni, C.; Rodolfo, M.; Colombo, M.P. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic t lymphocytes against tumor antigen in vivo. Clin. Exp. Immunol. 1996, 183, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Liu, W.; Huang, L.; Wang, L.; Yu, J.; Wang, Y.; Wu, D.T.; Wang, S. Quality evaluation of immunomodulatory polysaccharides from Agaricus bisporus by an integrated fingerprint technique. Food Front. 2023, 4, 474–490. [Google Scholar] [CrossRef]
- Kan, Y.; Liu, Y.; Huang, Y.; Zhao, L.; Jiang, C.; Zhu, Y.; Pang, Z.; Hu, J.; Pang, W.; Lin, W. The regulatory effects of Pseudostellaria heterophylla polysaccharide on immune function and gut flora in immunosuppressed mice. Food Sci. Nutr. 2022, 10, 3828–3841. [Google Scholar] [CrossRef]
- Wang, C.; Tang, J.; Crowley-Nowick, P.A.; Wilson, C.M.; Kaslow, R.A.; Geisler, W.M. Interleukin (IL)-2 and IL-12 responses to Chlamydia trachomatis infection in adolescents. J. Exp. Med. 2005, 142, 548–554. [Google Scholar] [CrossRef]
- Thomas, G.R.; Chen, Z.; Enamorado, I.; Bancroft, C.; Van Waes, C. IL-12- and IL-2-induced tumor regression in a new murine model of oral squamous-cell carcinoma is promoted by expression of the CD80 co-stimulatory molecule and interferon-γ. Int. J. Cancer 2000, 86, 368–374. [Google Scholar] [CrossRef]
- Bagheri, Y.; Barati, A.; Aghebati-Maleki, A.; Aghebati-Maleki, L.; Yousefi, M. Current Progress in cancer Immunotherapy Based on Natural Killer Cells. Cell. Biol. Int. 2021, 45, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dou, L.; Sui, L.; Xue, Y.; Xu, S. Natural killer cells in cancer immunotherapy. MedComm 2024, 5, 626–656. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Lee, H.Y.; Shin, D.Y.; Jeong, H.N.; Hwang, H.M.; Park, H.Y.; Kim, S.H.; Kim, M.J.; Kang, H.J.; Kim, J.H.; et al. Immune-Enhancing Activity of Vitis coignetiae Extract via Increasing Cytokine and Natural Killer Cell Activity in Splenocytes and Cyclophosphamide-Induced Immunosuppressed Rats. J. Food Biochem. 2024, 12, 5010095. [Google Scholar] [CrossRef]









| Sample | BSP | ECBSP | EGBSP |
|---|---|---|---|
| surface hydrophobicity | 16.82 ± 0.03 c | 19.51 ± 0.06 b | 5.07 ± 0.07 a |
| Groups | Dosage/mg·kg−1 | Tumor Weight/g | Inhibitory Rate/% |
|---|---|---|---|
| Normal control | - | 4.21 ± 0.35 | - |
| 100 | 4.16 ± 0.26 | 0.71 | |
| BSP | 200 | 3.87 ± 0.21 * | 8.08 |
| 400 | 4.33 ± 0.33 | −2.85 | |
| EGBSP | 100 | 3.16 ± 0.25 ** | 24.94 |
| 200 | 3.06 ± 0.20 ** | 27.32 | |
| 400 | 3.10 ± 0.27 ** | 26.37 |
| Groups | Dose/mg·kg−1 | Spleen Index /mg·g−1 | Thymus Index /mg·g−1 |
|---|---|---|---|
| Normal control | - | 7.67 ± 0.69 | 2.73 ± 0.21 |
| BSP | 100 | 7.78 ± 0.66 | 2.78 ± 0.25 |
| 200 | 8.15 ± 0.60 ** | 2.91 ± 0.24 * | |
| 400 | 8.51 ± 0.73 ** | 2.87 ± 0.24 * | |
| EGBSP | 100 | 9.26 ± 0.85 ** | 3.46 ± 0.24 ** |
| 200 | 9.55 ± 0.82 ** | 3.55 ± 0.24 ** | |
| 400 | 8.69 ± 0.87 ** | 3.89 ± 0.24 ** |
| Groups | Dose/mg·kg−1 | Killing Activity of Mice NK Cells (%) | Lymphocyte (%) |
|---|---|---|---|
| Normal control | - | 25.85 ± 2.55 | 18.13 ± 1.69 |
| BSP | 100 | 26.93 ± 3.92 | 19.98 ± 1.29 |
| 200 | 27.26 ± 2.67 * | 24.64 ± 1.72 ** | |
| 400 | 28.43 ± 2.26 * | 19.59 ± 2.11 | |
| EGBSP | 100 | 28.81 ± 2.10 * | 28.67 ± 2.58 ** |
| 200 | 32.14 ± 2.81 ** | 30.28 ± 2.36 ** | |
| 400 | 29.44 ± 2.07 * | 29.93 ± 1.63 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Gong, X.; Wang, J.; Dou, B.; Hou, L.; Xiao, W.; Chang, J.; Li, D. Effect of Enzymatic Glycosylation on Film-Processing Properties and Biological Activities of Black Soybean Protein. Coatings 2025, 15, 238. https://doi.org/10.3390/coatings15020238
Zhang Y, Gong X, Wang J, Dou B, Hou L, Xiao W, Chang J, Li D. Effect of Enzymatic Glycosylation on Film-Processing Properties and Biological Activities of Black Soybean Protein. Coatings. 2025; 15(2):238. https://doi.org/10.3390/coatings15020238
Chicago/Turabian StyleZhang, Yinglei, Xue Gong, Jing Wang, Boxin Dou, Lida Hou, Wei Xiao, Jiang Chang, and Danting Li. 2025. "Effect of Enzymatic Glycosylation on Film-Processing Properties and Biological Activities of Black Soybean Protein" Coatings 15, no. 2: 238. https://doi.org/10.3390/coatings15020238
APA StyleZhang, Y., Gong, X., Wang, J., Dou, B., Hou, L., Xiao, W., Chang, J., & Li, D. (2025). Effect of Enzymatic Glycosylation on Film-Processing Properties and Biological Activities of Black Soybean Protein. Coatings, 15(2), 238. https://doi.org/10.3390/coatings15020238







