The Examination of the Effect of Water-Soluble Hydrophobic Agents on Physical–Mechanical Parameters and Resistance to Aggressive Environment of Concrete
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrophobic Impregnations
2.2. Concrete Substrates
2.3. Nanocellulose
2.4. Depth of Penetration of Hydrophobic Impregnation
2.5. Chemical Resistance
2.6. Compressive Strength
2.7. Water Tightness Test
2.8. Concrete Absorption
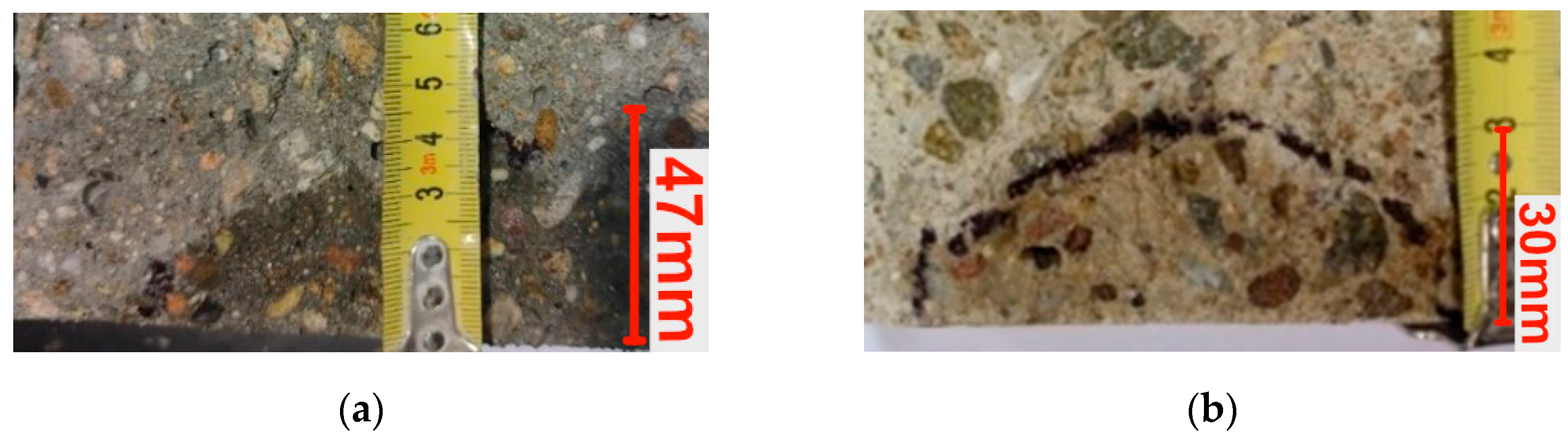
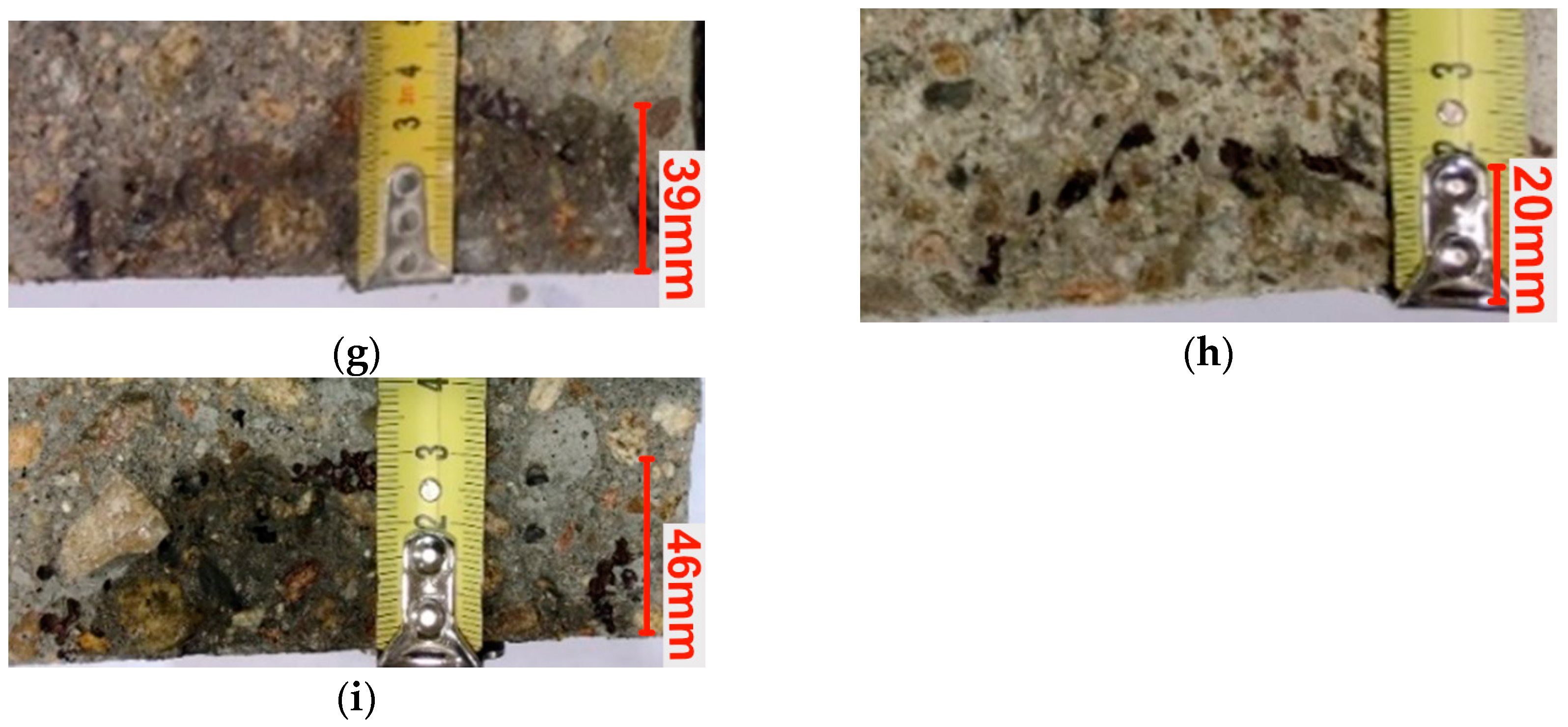
2.9. Depth of Penetration of Water Under Pressure
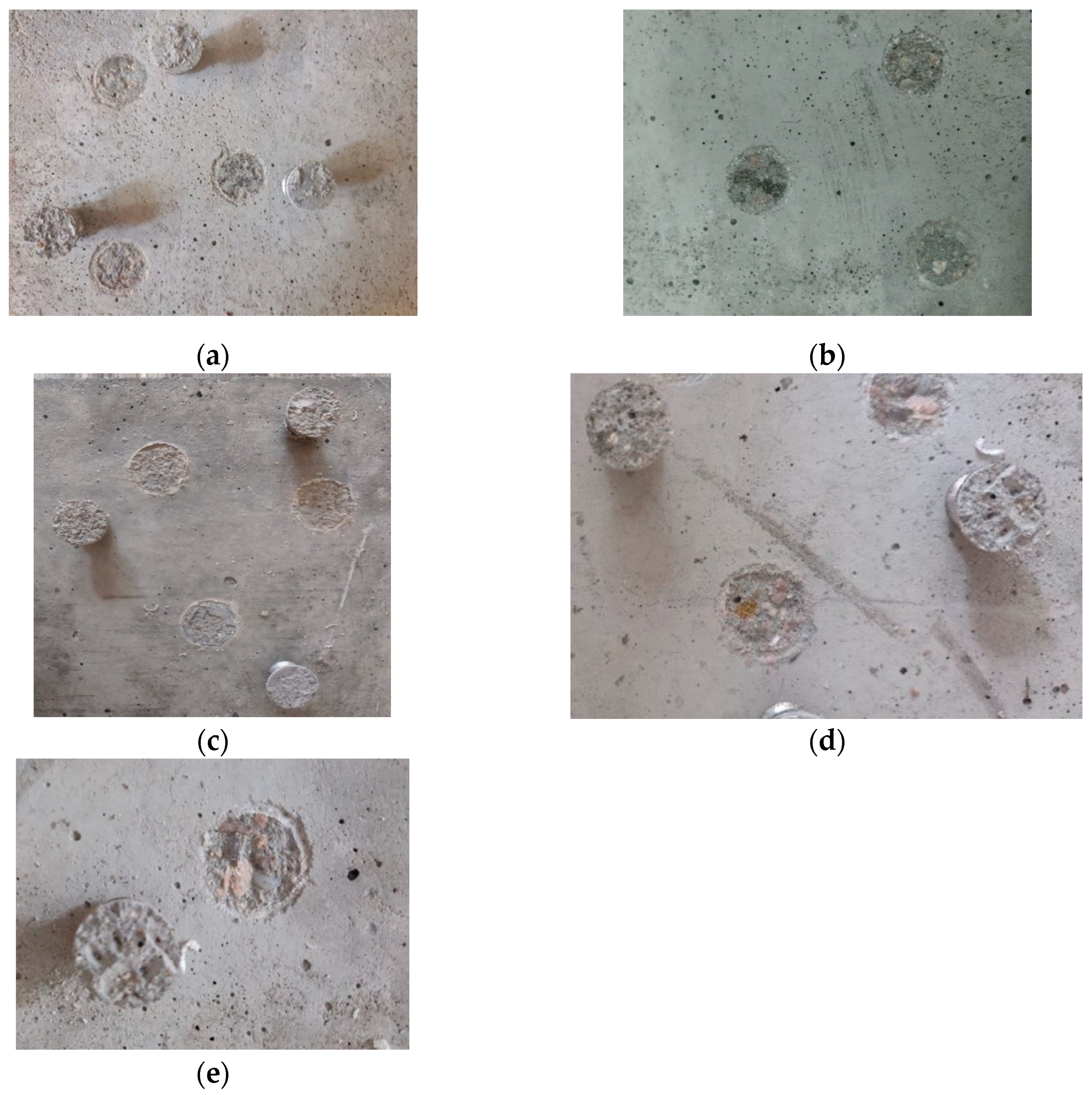
2.10. Adhesion of Hydrophobic Agents to Concrete
2.11. Resistance of Concrete Against the Action of Water and Chemical Deicers
2.12. Frost Resistance
2.13. Resistance to Aggressive CO2 and SO2 Gasses
2.14. Microstructure
2.15. Rapid Chloride Penetration Test (RCPT)
2.16. Vacuum Saturation Porosity
2.17. Concrete Sorptivity
2.18. Effect of Nanocellulose Addition on Hydrophobic Agents
3. Results and Discussion
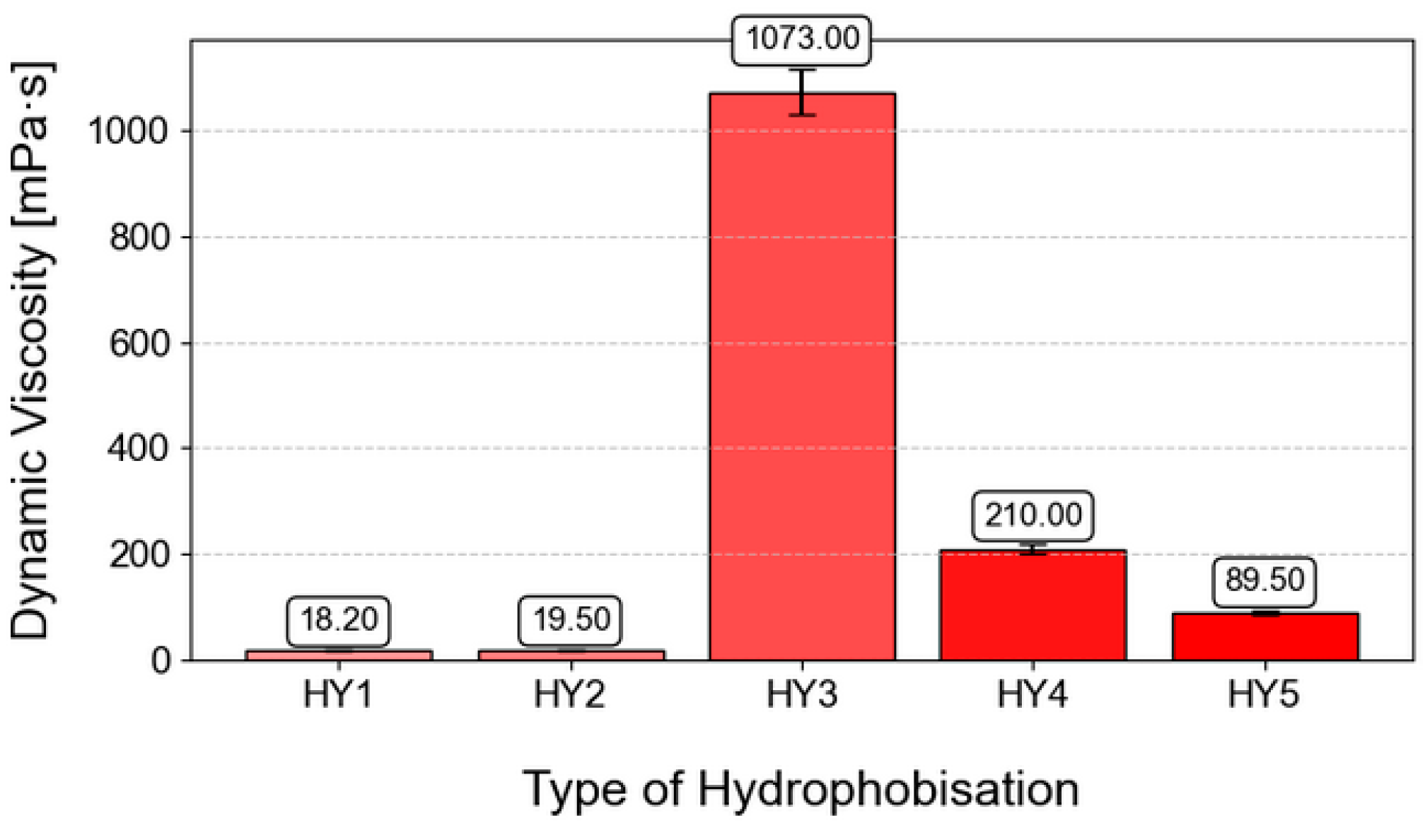
3.1. Dynamic Viscosity
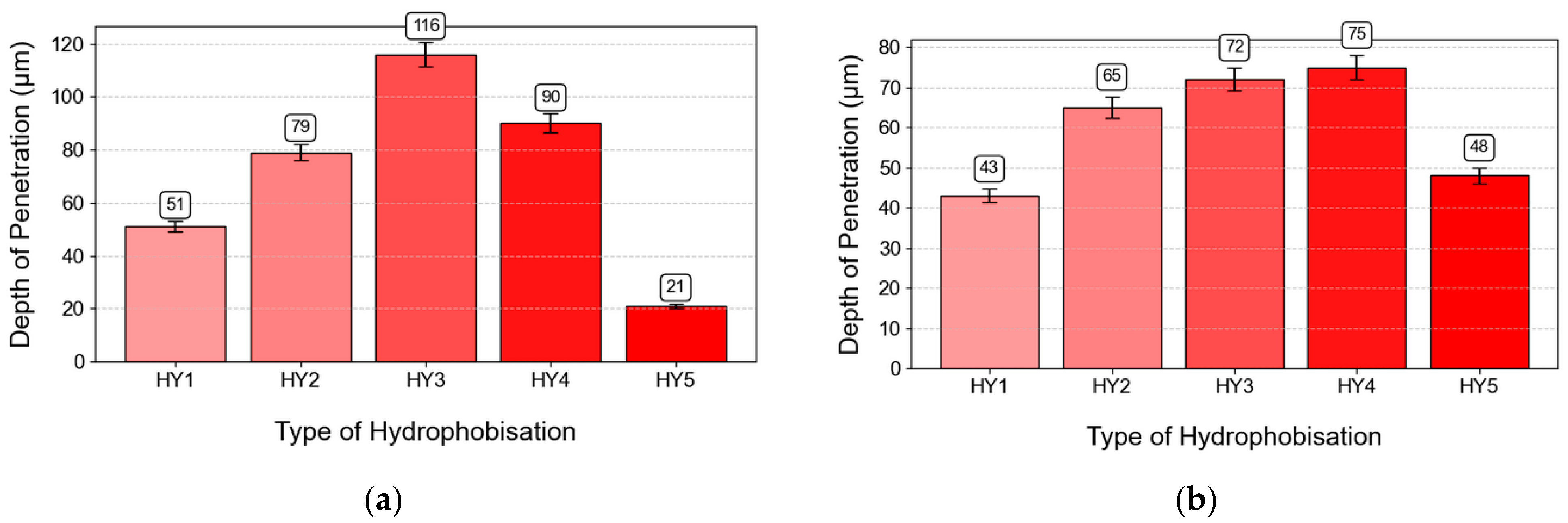
3.2. Depth of Penetration of Hydrophobic Impregnation
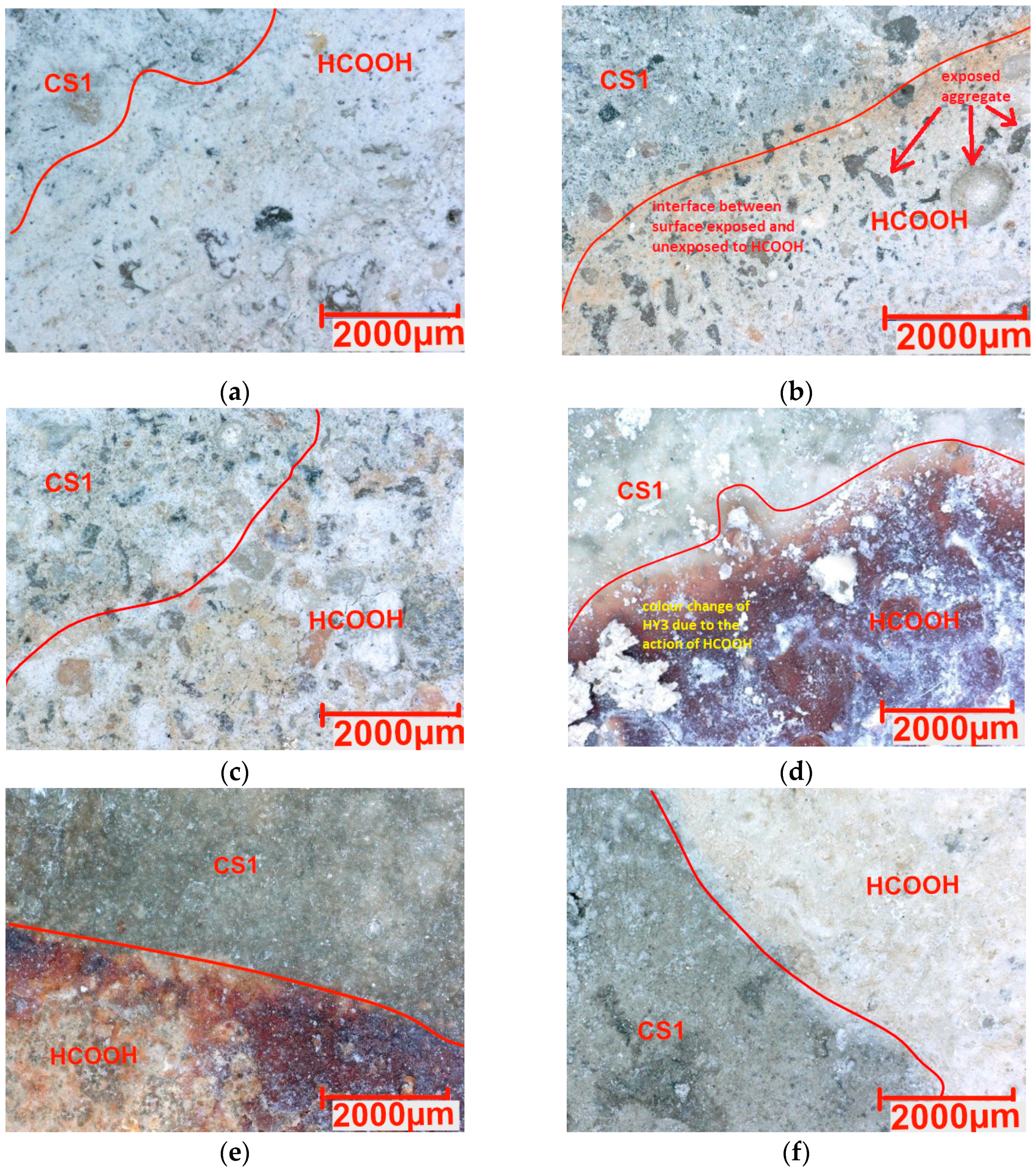
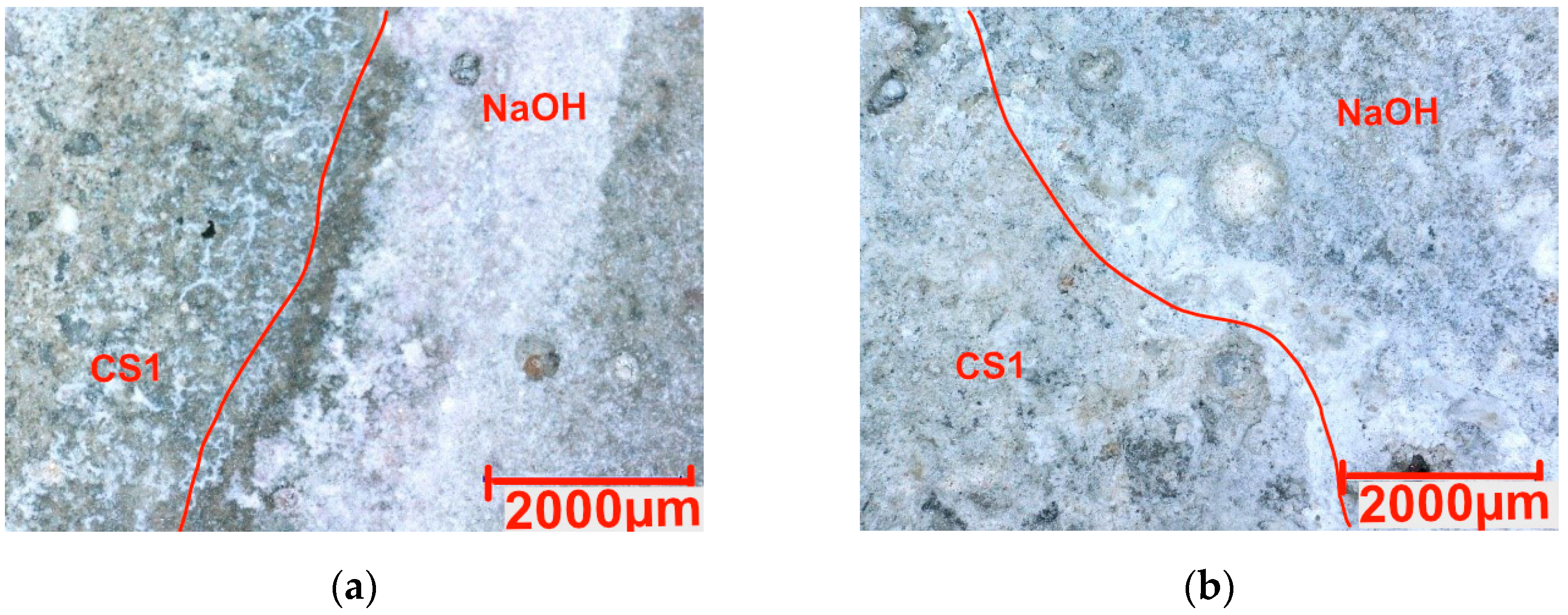
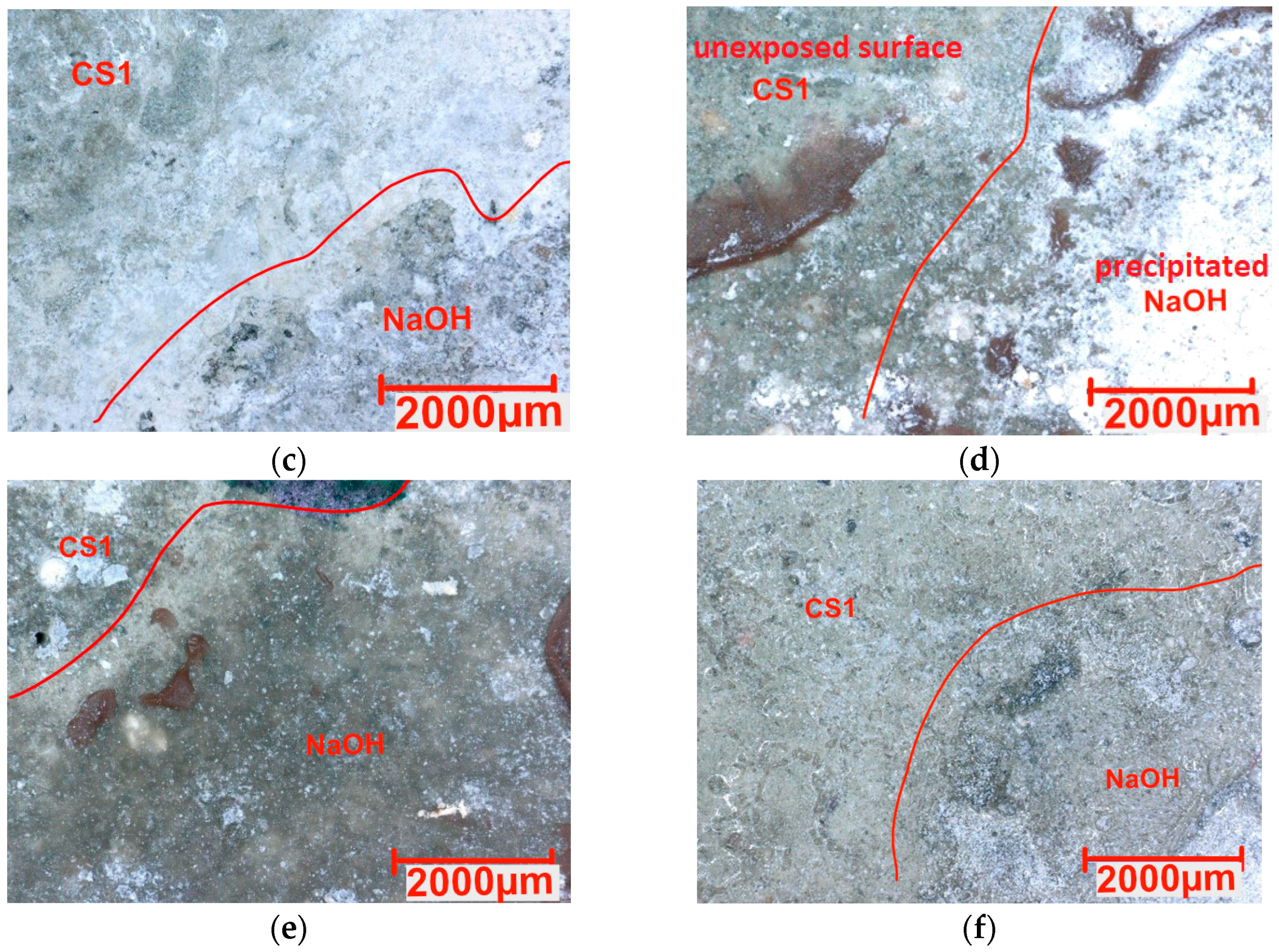
3.3. Chemical Resistance
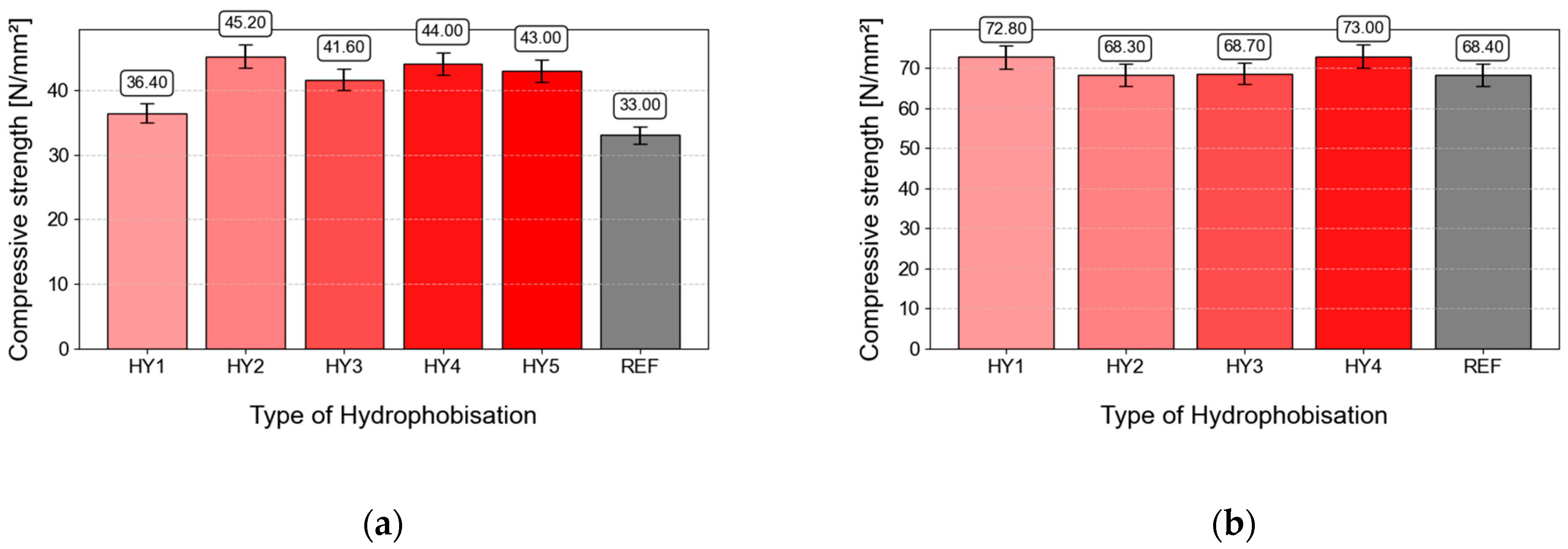
3.4. Compressive Strength
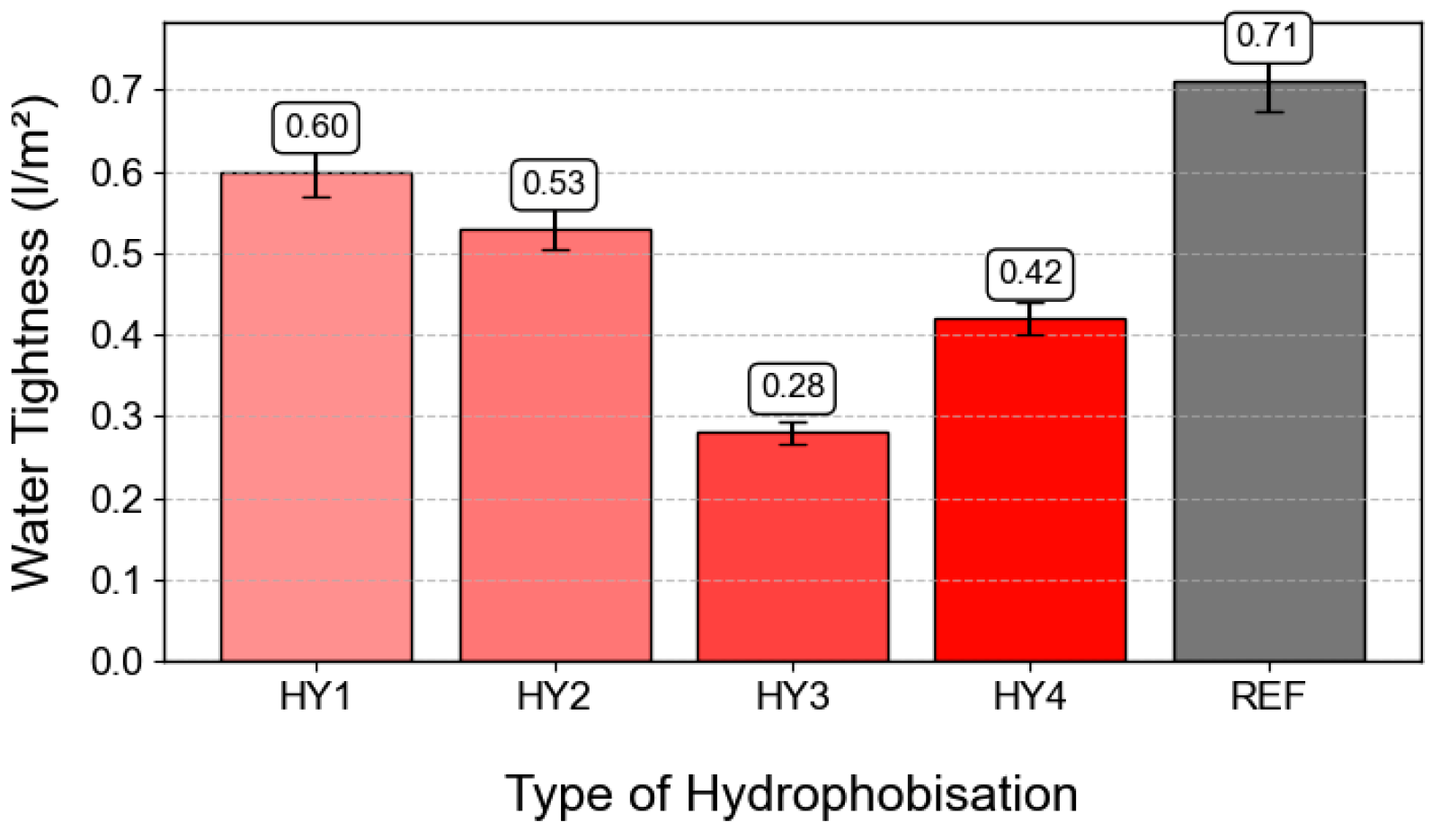
3.5. Water Tightness Test
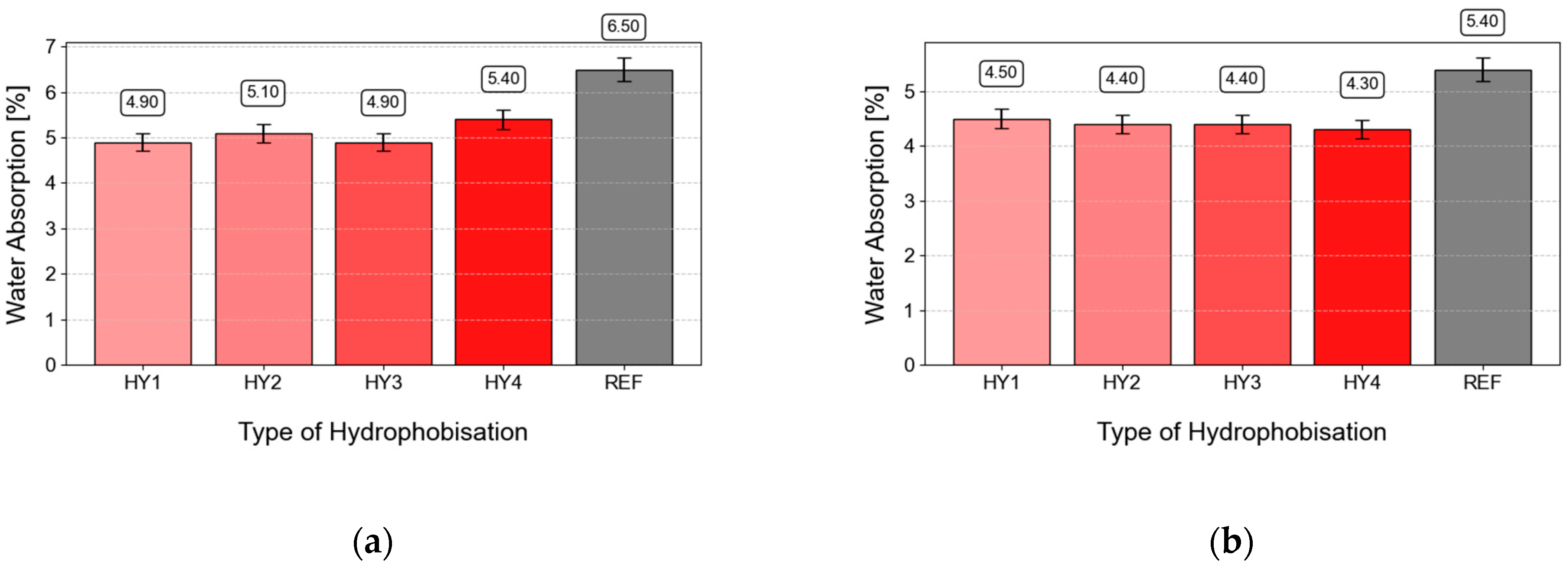
3.6. Concrete Absorption
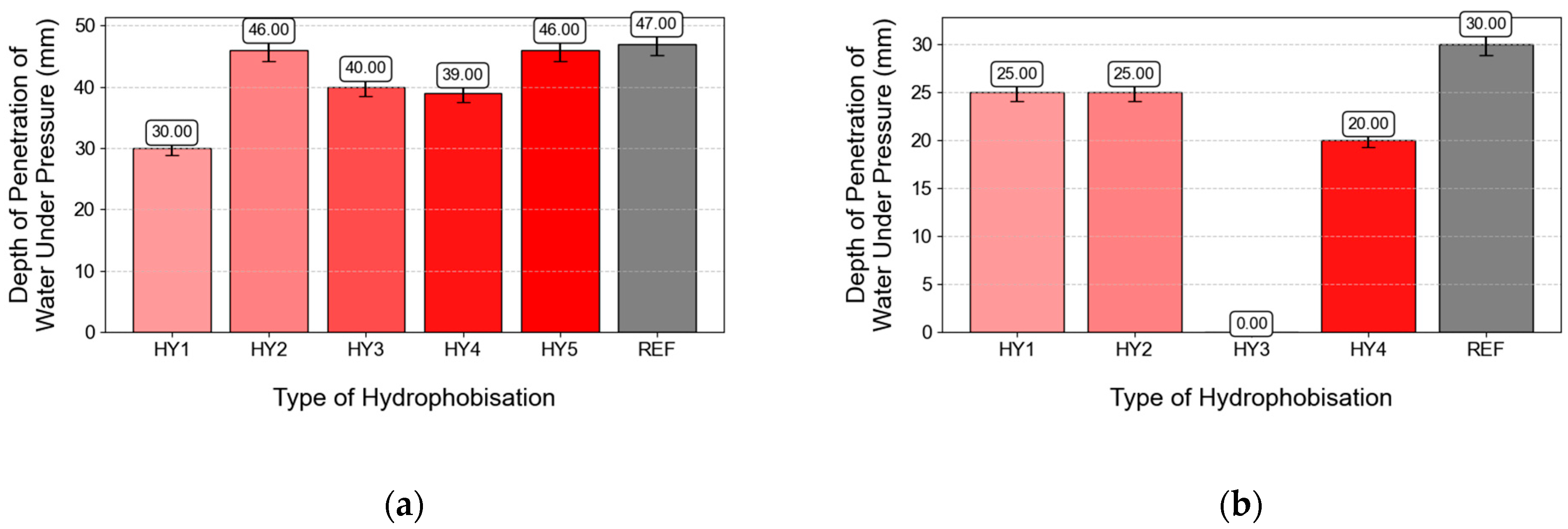
3.7. Depth of Penetration of Water Under Pressure
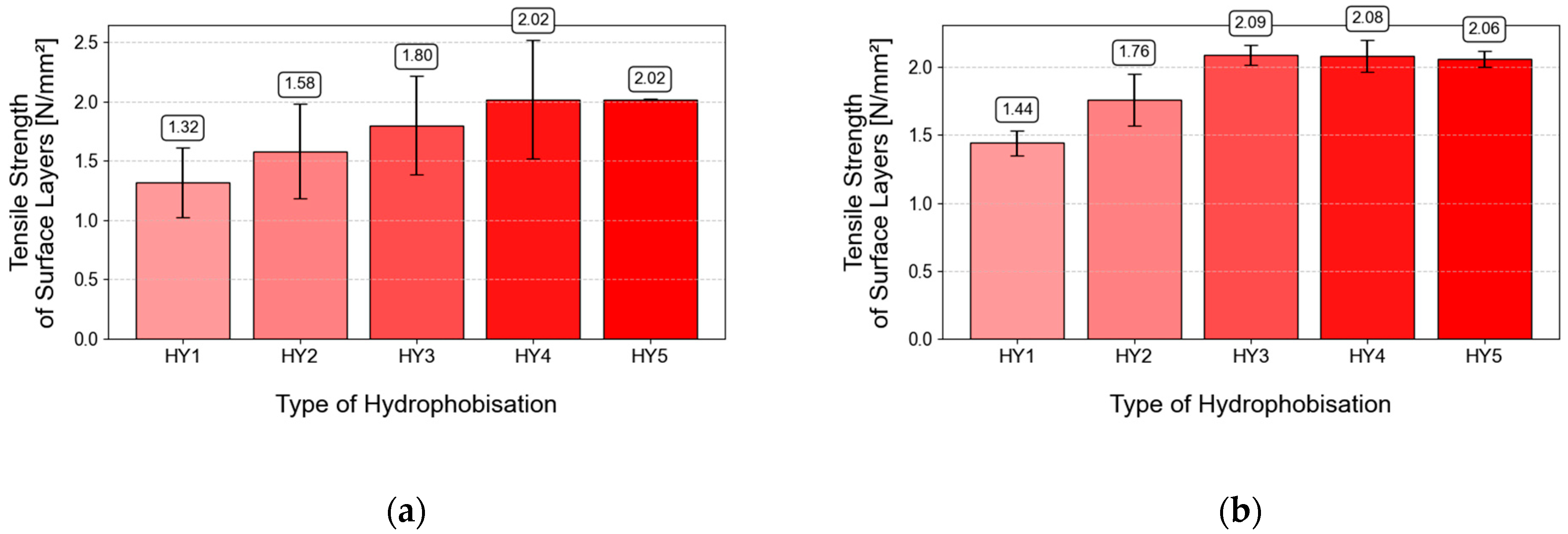
3.8. Adhesion of Hydrophobic Agents to Concrete
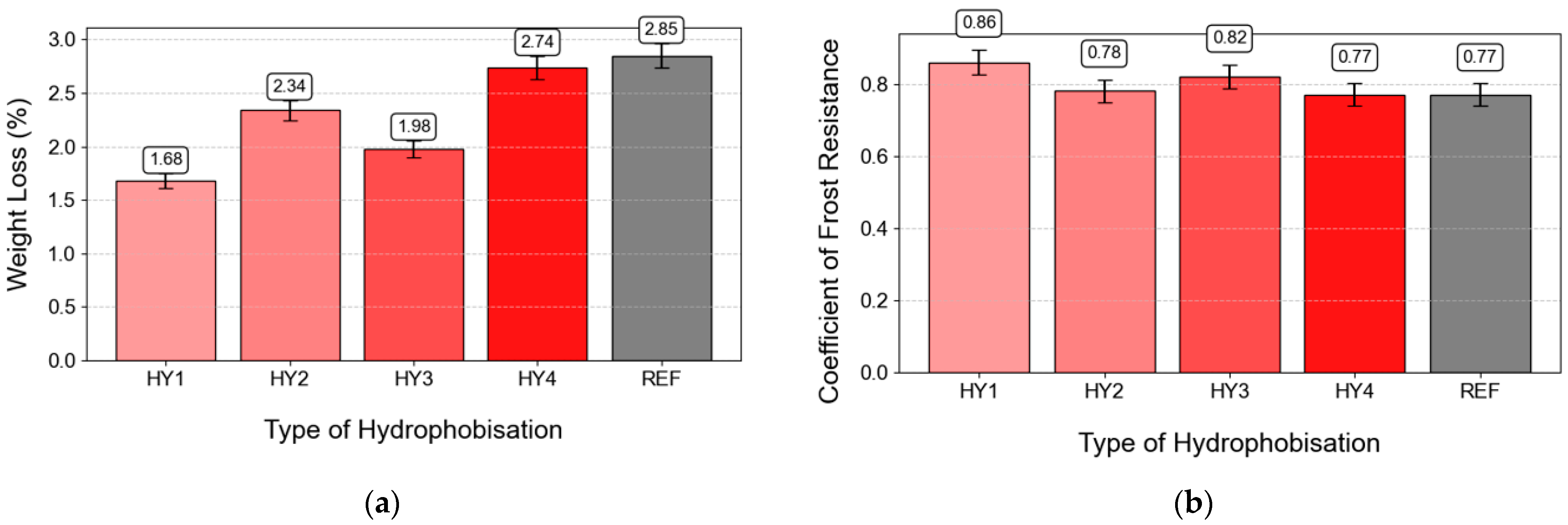
3.9. Frost Resistance
3.10. Resistance to Aggressive CO2 and SO2 Gasses
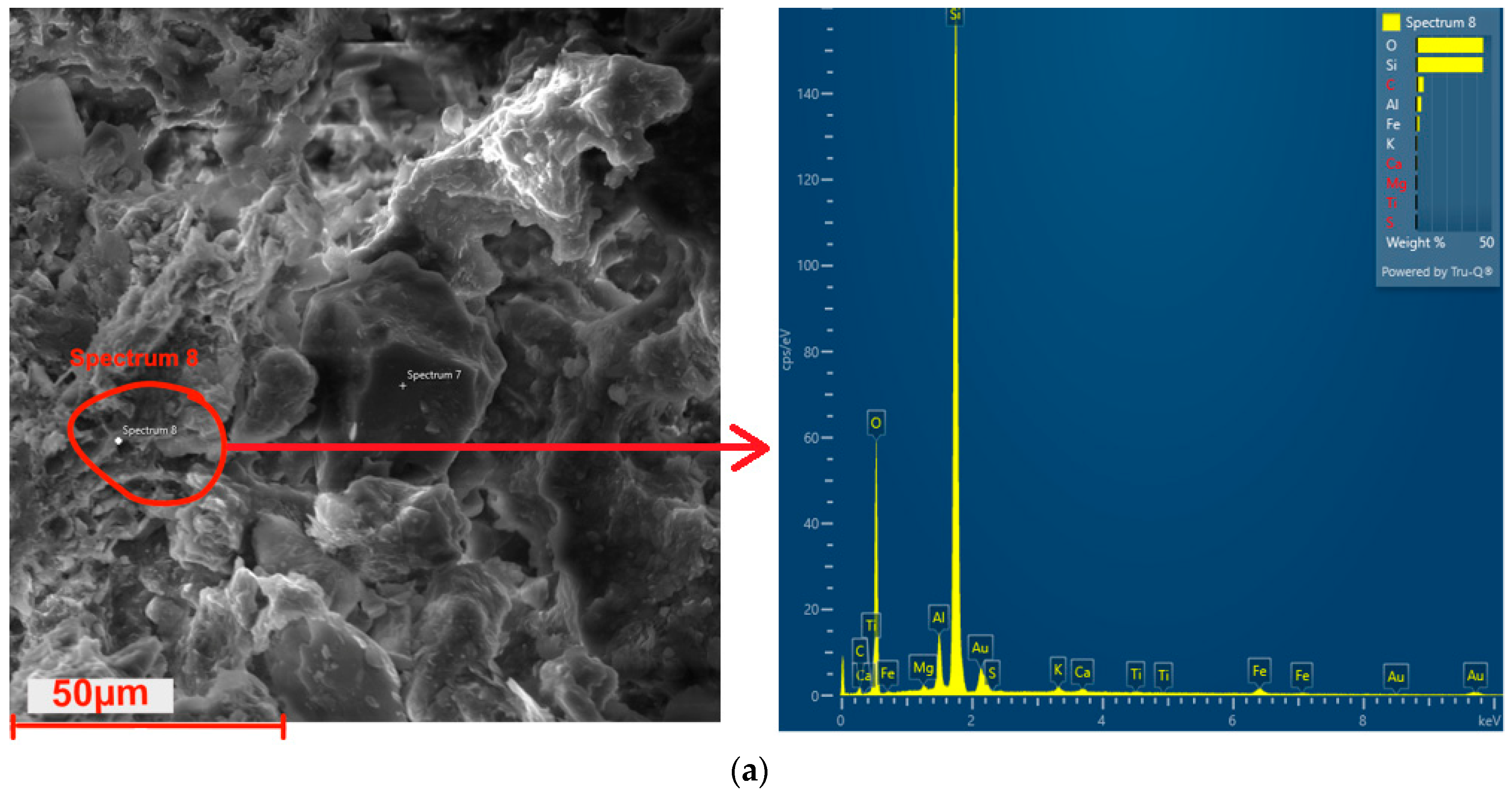
3.11. Microstructure
3.12. Vacuum Saturation Porosity
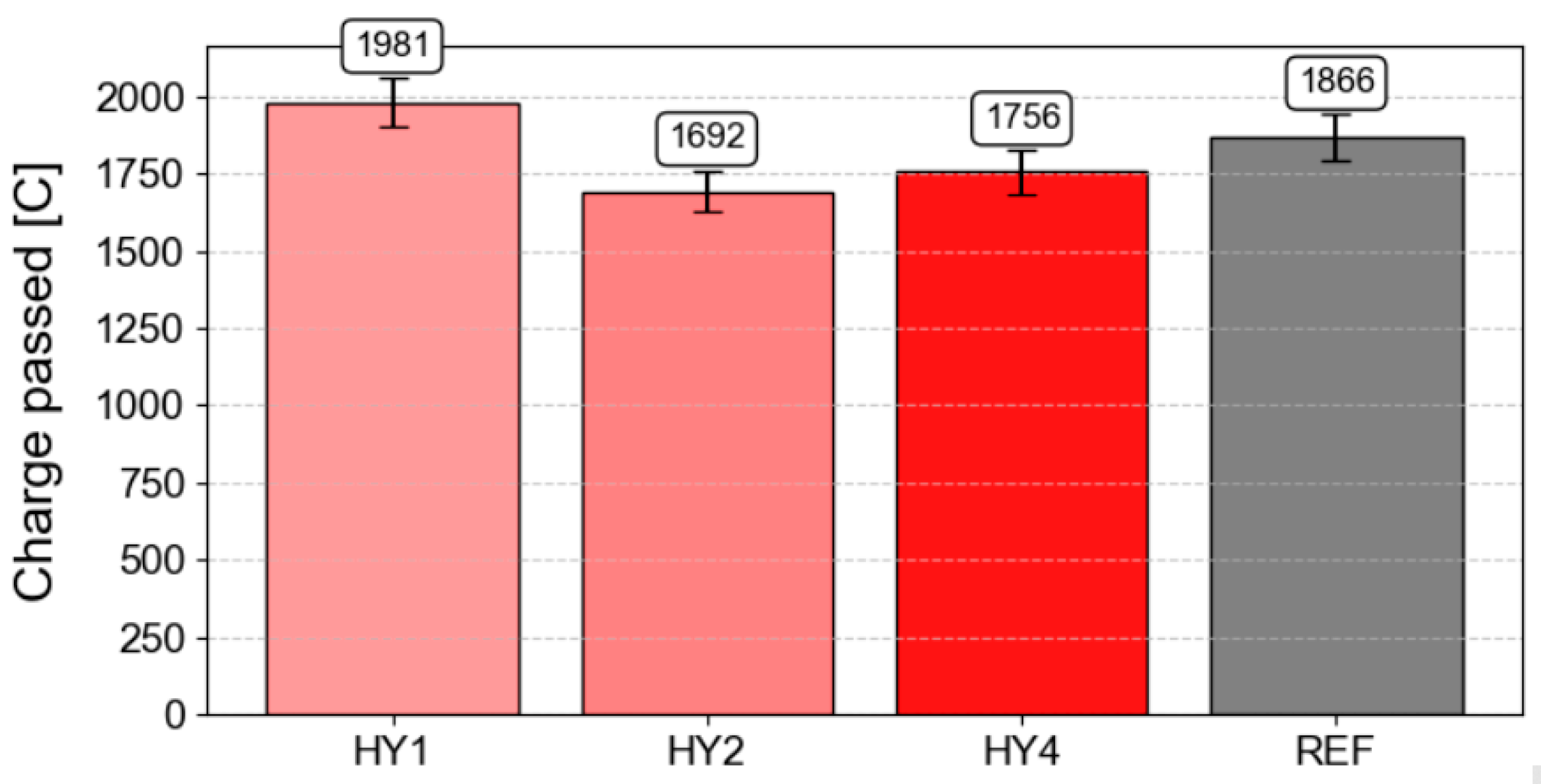
3.13. Rapid Chloride Permeability Test (RCPT)
3.14. Concrete Sorptivity
3.15. Effect of Nanocellulose Addition on Hydrophobization
4. Conclusions
- The hydrophobized samples exhibited low resistance to acid attack, limiting their use in acidic environments. However, in an alkaline environment with NaOH, they showed better stability than reference samples.
- The application of hydrophobic impregnations had a positive effect on the mechanical parameters of the concrete—with an increase after 28 days in the compressive strength of CS1 by up to 30% to 45.2 MPa or of the hydrophobically treated concrete HY2. This positive effect was caused mainly by the reduction in water evaporation during concrete hydration. The same effect was recorded with CS2, when the concrete hydrophobized by HY1 showed an increase in compressive strength of 4 MPa with respect to the reference concrete.
- The depth of penetration of water under pressure, absorption, and watertightness were significantly decreased when water-soluble hydrophobic agents were used. CS2 impregnated by HY3 showed zero absorbency and watertightness of only 0.28 L/m2.
- The effectiveness of HY was also confirmed with respect to the frost resistance of the concrete, when the weight loss of the CS1 hydrophobized using HY1 was only 1.68% in comparison to untreated CS1, which showed a weight loss of 2.85%.
- Samples treated with HY3 and HY4 showed higher resistance to the gaseous aggressive environment, whilst higher resistance to carbonation was confirmed through the FF test, when the depth of carbonation of the concrete impregnated by HY3 was 9 mm, which is 13 mm lesser than the untreated concrete.
- The best results for resistance to chloride penetration were achieved with samples treated with hydrophobic agents HY2 and HY4, which showed the lowest penetration of chloride ions.
- The presence of nanocellulose in HY2 and HY4 reduced the depth of penetration of water under pressure in CS1 (with no water penetration with concrete hydrophobized using HY4 + nano). There was even an increase in the compressive strength after 28 days in the case of CS1 to 47.5 MPa for HY4 + nano.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, S.W.; Yao, Y.; Andrade, C.; Li, Z.J. Recent Durability Studies on Concrete Structure. Cem. Concr. Res. 2015, 78, 143–154. [Google Scholar] [CrossRef]
- Desmettre, C.; Charron, J.-P. Water Permeability of Reinforced Concrete With and Without Fiber Subjected to Static and Constant Tensile Loading. Cem. Concr. Res. 2012, 42, 945–952. [Google Scholar] [CrossRef]
- Chenxi, Z.; Jian, L.; Lingdong, C.; Weiqiang, L.; Jing, Z.; Jintao, Y.; Jie, F. Dark, Heat-Reflective, Anti-Ice Rain and Superhydrophobic Cement Concrete Surfaces. Constr. Build. Mater. 2019, 220, 21–28. [Google Scholar]
- Liao, J.; Wang, Y.; Sun, X.; Wang, Y. Chloride Penetration of Surface-Coated Concrete: Review and Outlook. Materials 2024, 17, 4121. [Google Scholar] [CrossRef]
- Matziaris, K.; Stefanidou, M.; Karagiannis, G. Impregnation and Superhydrophobicity of Coated Porous Low-Fired Clay Building Materials. Prog. Org. Coat. 2011, 72, 181–192. [Google Scholar] [CrossRef]
- González-Coneo, J.; Zarzuela, R.; Elhaddad, F.; Carrascosa, L.M.; Gil, M.L.A.; Mosquera, M.J. Alkylsiloxane/Alkoxysilane Sols as Hydrophobic Treatments for Concrete: A Comparative Study of Bulk vs. Surface Application. J. Build. Eng. 2022, 46, 103729. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.-C.; Li, N. A Review on Concrete Surface Treatment Part I: Types and Mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Szymańska, A.; Dutkiewicz, M.; Maciejewski, H.; Palacz, M. Simple and effective hydrophobic impregnation of concrete with functionalized polybutadienes. Constr. Build. Mater. 2022, 315, 125624. [Google Scholar] [CrossRef]
- Di Mundo, R.; Labianca, C.; Carbone, G.; Notarnicola, M. Recent Advances in Hydrophobic and Icephobic Surface Treatments of Concrete. Coatings 2020, 10, 449. [Google Scholar] [CrossRef]
- Schueremans, L.; Van Gemert, D.; Giessler, S. Chloride Penetration in RC-Structures in Marine Environment—Long Term Assessment of a Preventive Hydrophobic Treatment. Constr. Build. Mater. 2007, 21, 1238–1249. [Google Scholar] [CrossRef]
- Medeiros, M.; Helene, P. Efficacy of Surface Hydrophobic Agents in Reducing Water and Chloride Ion Penetration in Concrete. Mater. Struct. 2007, 41, 59–71. [Google Scholar] [CrossRef]
- Zhao, Z.; Qi, S.; Suo, Z.; Hu, T.; Hu, J.; Liu, T.; Gong, M. Development of a Superhydrophobic Protection Mechanism and Coating Materials for Cement Concrete Surfaces. Materials 2024, 17, 4390. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhang, L.; Feng, Y. A Review on Silane and Siloxane Materials: Enhancing Durability of Cementitious Materials Through Surface Treatments. J. Mater. Sci. 2024, 59, 10119–10139. [Google Scholar] [CrossRef]
- De Vries, J.; Polder, R.B. Hydrophobic Treatment of Concrete. Constr. Build. Mater. 1997, 11, 259–265. [Google Scholar] [CrossRef]
- Gnanaraj, J.S.; Vasugi, K. A Comprehensive Review of Hydrophobic Concrete: Surface and Bulk Modifications for Enhancing Corrosion Resistance. Eng. Res. Express. 2024, 6, 032101. [Google Scholar] [CrossRef]
- Wang, D.; Wu, X.; Yuan, L.; Wu, D.; Zhao, Q.; Pan, H.; Qi, W. Oil Absorption and Plant Symbiosis Capacity of Hydrophobic Modified Concrete: Preparation and Performance Analysis. Constr. Build. Mater. 2024, 413, 134897. [Google Scholar] [CrossRef]
- Rabczuk, T.; Zi, G.; Bordas, S.; Nguyen-Xuan, H. A Simple and Robust Three-Dimensional Cracking-Particle Method Without Enrichment. Comput. Methods Appl. Mech. Eng. 2010, 199, 2437–2455. [Google Scholar] [CrossRef]
- Tittarelli, F.; Moriconi, G. The Effect of Silane-Based Hydrophobic Admixture on Corrosion of Reinforcing Steel in Concrete. Cem. Concr. Res. 2008, 38, 1354–1357. [Google Scholar] [CrossRef]
- Barnat-Hunek, D.; Góra, J.; Widomski, M.K. Durability of Hydrophobic/Icephobic Coatings in Protection of Lightweight Concrete with Waste Aggregate. Materials 2021, 14, 101. [Google Scholar] [CrossRef]
- Rabczuk, T.; Belytschko, T. Cracking Particles: A Simplified Meshfree Method for Arbitrary Evolving Cracks. Int. J. Numer. Methods Eng. 2004, 61, 2316–2343. [Google Scholar] [CrossRef]
- Materak, K.; Wieczorek, A.; Bednarska, D.; Koniorczyk, M. Internal Hydrophobization of Cement-Based Materials by Means of Silanes. J. Phys. Conf. Ser. 2023, 2521, 012009. [Google Scholar] [CrossRef]
- Avrămescu, R.-E.; Ghica, M.V.; Dinu-Pîrvu, C.; Prisada, R.; Popa, L. Superhydrophobic Natural and Artificial Surfaces—A Structural Approach. Materials 2018, 11, 866. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Xie, Z.; Huang, C.; Yuan, Q.; Yu, Z. Recent Progress of Hydrophobic Cement-Based Materials: Preparation, Characterization and Properties. Constr. Build. Mater. 2021, 299, 124255. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Luongo, N.; Massari, S.; Spagnolo, S.L.; Daniotti, B.; Pedeferri, M.P. Durability of Self-Cleaning Cement-Based Materials. Constr. Build. Mater. 2021, 280, 122442. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Qian, B.; Xiong, Y. Effect of Nano-Modified Permeable Silicone Emulsion on the Durability of Concrete Curbstone. Constr. Build. Mater. 2022, 324, 126620. [Google Scholar] [CrossRef]
- Li, S.; Jiang, J.; Geng, Y.; Hu, J.; Sui, S.; Liu, A.; Hu, M.; Shan, Y.; Liu, Z. Application of Silane Protective Materials in the Concrete Durability Improvement in Recent Years: A Review. Eng. Fail. Anal. 2024, 160, 108140. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Characterization and Distribution of Water-repellent, Self-cleaning Plant Surfaces. Ann. Bot. 1997, 76, 667–677. [Google Scholar] [CrossRef]
- Sørli, J.B.; Hansen, J.S.; Nørgaard, A.W.; Levin, M.; Larsen, S.T. An In Vitro Method for Predicting Inhalation Toxicity of Impregnation Spray Products. ALTE X Altern. Anim. Exp. 2015, 32, 101–111. [Google Scholar]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent Advances in Vegetable Oils Based Environment Friendly Coatings: A Review. Ind. Crops Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, L.; Shu, X.; Yang, Y.; She, W.; Ran, Q. A Review on Recent Advances in the Fabrication and Evaluation of Superhydrophobic Concrete. Compos. Part B Eng. 2022, 237, 109867. [Google Scholar] [CrossRef]
- Uğur, M.; Şimşek, B.; Uygunoğlu, T.; Kocakerim, M.M. Comparison of Effectiveness of Blending and Impregnation Applications of Dispersed Nanoparticles on Performance of Cementitious Composites. Constr. Build. Mater. 2023, 392, 132009. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated Cellulose—Its Barrier Properties and Applications in Cellulosic Materials: A Review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yue, J.; Guo, C.; Ji, Y. Influences of Modified Nanoparticles on Hydrophobicity of Concrete with Organic Film Coating. Constr. Build. Mater. 2018, 169, 1–7. [Google Scholar] [CrossRef]
- Keyence. Elemental Analysis with Digital Microscopes Using LIBS (Laser Induced Breakdown Spectroscopy). Available online: https://www.keyence.eu/ss/products/microscope/vhx-casestudy/other/libs.jsp (accessed on 15 January 2025).
- EN 12390-3; Testing Hardened Concrete—Part 3: Compressive Strength of Test Specimens. European Committee for Standardization (CEN): Brussels, Belgium, 2019.
- ČSN 73 2578; Watertightness Test of Surface Treatment of Building Structures. Czech Standardization Institute (CSI): Prague, Czech Republic, 1982.
- ČSN 73 1316; Determination of Moisture, Absorptivity and Capillarity of Concrete. Czech Standardization Institute (CSI): Prague, Czech Republic, 1969.
- EN 12390-8; Testing Hardened Concrete—Part 8: Testing hardened concrete—Depth of Penetration of Water Under Pressure. European Committee for Standardization (CEN): Brussels, Belgium, 2019.
- EN ISO 4624; Paints and Varnishes—Pull-Off Test for Adhesion. European Committee for Standardization (CEN): Brussels, Belgium, 2023.
- ČSN 73 1326; Determination of the Resistance of Cement Concrete Surface to Water and Chemical De-Icing Agents. Czech Standardization Institute (CSI): Prague, Czech Republic, 1985.
- ČSN 73 1322; Determination of Frost Resistance of Concrete, Change Z1. Czech Standardization Institute (CSI): Prague, Czech Republic, 2003.
- EN ISO 3231; Paints and Varnishes—Determination of Resistance to Humid Atmospheres Containing Sulfur Dioxide. European Committee for Standardization (CEN): Brussels, Belgium, 1993.
- ASTM C1202-19; Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. ASTM International: West Conshohocken, PA, USA, 2022.
- EN 13057; Products and Systems for the Protection and Repair of Concrete Structures—Test Methods—Determination of Resistance of Capillary Absorption. European Committee for Standardization (CEN): Brussels, Belgium, 2022.
- Xu, E.; Zhang, Y.; Lin, L. Improvement of Mechanical, Hydrophobicity and Thermal Properties of Chinese Fir Wood by Impregnation of Nano Silica Sol. Polymers 2020, 12, 1632. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, Y.; Liu, N.; Yin, H.; Li, B.; Sun, D.; Ran, Q. Epoxy Adhesive with High Underwater Adhesion and Stability Based on Low Viscosity Modified Mannich Bases. J. Appl. Polym. Sci. 2017, 135, 45688. [Google Scholar] [CrossRef]
- Tatar, J.; Torrence, C.E.; Mecholskz, J.J., Jr.; Taylor, C.R.; Hamilton, H.R. Effects of Silane Surface Functionalization on Interfacial Fracture Energy and Durability of Adhesive Bond Between Cement Paste and Epoxy. Int. J. Adhes. Adhes. 2018, 84, 132–142. [Google Scholar] [CrossRef]
- Johansson, A.; Janz, M.; Silfwerbrand, J.; Trägardh, J. Penetration Depth for Water Repellent Agents in Concrete as a Function of Humidity, Porosity and Time. Restor. Build. Monum. 2007, 13, 3–16. [Google Scholar]
- Zhan, H.; Wittmann, F.H.; Zhao, T. Relation Between the Silicon Resin Profiles in Water Repellent Treated Concrete and the Effectiveness as a Chloride Barrier. Int. J. Restor. Build. Monum. 2005, 11, 35–46. [Google Scholar]
- Xue, X.; Li, Y.; Yang, Z.; He, Z.; Dai, J.-G.; Xu, L.; Zhang, W. A Systematic Investigation of the Waterproofing Performance and Chloride Resistance of a Self-Developed Waterborne Silane-Based Hydrophobic Agent for Mortar and Concrete. Constr. Build. Mater. 2017, 155, 939–946. [Google Scholar] [CrossRef]
- Dai, J.G.; Akira, Y.; Yokota, Y.; Wittmann, F.H. Various Surface Impregnation Treatments of Pre-Conditioned Concrete Subjected to Seawater Immersion Test. Restor. Build. Monum. 2007, 13, 229–240. [Google Scholar]
- Suleiman, A.R.; Soliman, A.M.; Nehdi, M.L. Effect of Surface Treatment on Durability of Concrete Exposed to Physical Sulfate Attack. Constr. Build. Mater. 2014, 73, 674–681. [Google Scholar] [CrossRef]
- Almusallam, A.A.; Khan, F.M.; Dulaijan, S.U.; Al-Amoudi, O.S.B. Effectiveness of Surface Coatings in Improving Concrete Durability. Cem. Concr. Compos. 2003, 25, 473–481. [Google Scholar] [CrossRef]
- Wegmann, A. Chemical Resistance of Waterborne Epoxy/Amine Coatings. Prog. Org. Coat. 1997, 32, 231–239. [Google Scholar] [CrossRef]
- Yuan, G.; Li, Q. The Use of Surface Coating in Enhancing the Mechanical Properties and Durability of Concrete Exposed to Elevated Temperature. Constr. Build. Mater. 2015, 95, 375–383. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.A. Application of Epoxy Resins in Building Materials: Progress and Prospects. Polym. Bull. 2022, 79, 1949–1975. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.-C.; Li, N. A Review on Surface Treatment for Concrete—Part 2: Performance. Constr. Build. Mater. 2017, 133, 81–90. [Google Scholar] [CrossRef]
- Zhang, L.V.; Nehdi, M.L.; Suleiman, A.R.; Allaf, M.M.; Gan, M.; Marani, A.; Tuyan, M. Crack Self-Healing in Bio-Green Concrete. Compos. Part B Eng. 2021, 227, 109397. [Google Scholar] [CrossRef]
- Husni, H.; Nazari, M.R.; Yee, H.M.; Rohim, R.; Yusuff, A.; Ariff, M.A.M.; Ahmad, N.N.R.; Leo, C.P.; Junaidi, M.U.M. Superhydrophobic Rice Husk Ash Coating on Concrete. Constr. Build. Mater. 2017, 144, 385–391. [Google Scholar] [CrossRef]
- Kumagai, S.; Sakuraba, H.; Miyata, A.; Sasaki, I.; Nishizaki, I. Improvement of the Adhesion Between Epoxy-Based Surface Coating and Hydrophobic Impregnated Concrete. Adv. Mater. Res. 2015, 1129, 256–262. [Google Scholar] [CrossRef]
- Liu, J.; Vipulanandan, C. Tensile Bonding Strength of Epoxy Coatings to Concrete Substrate. Cem. Concr. Res. 2005, 35, 1412–1419. [Google Scholar] [CrossRef]
- Brown, P.W.; Hooton, R.D.; Clark, B.A. The Co-Existence of Thaumasite and Ettringite in Concrete Exposed to Magnesium Sulfate at Room Temperature and the Influence of Blast-Furnace Slag Substitution on Sulfate Resistance. Cem. Concr. Compos. 2003, 25, 939–945. [Google Scholar] [CrossRef]
- Bu, C.; Liu, L.; Lu, X.; Zhu, D.; Sun, Y.; Yu, L.; OuYang, Y.; Cao, X.; Wei, Q. The Durability of Recycled Fine Aggregate Concrete: A Review. Materials 2022, 15, 1110. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-G.; Kou, S.-C.; Poon, C.-S.; Dai, J.-G.; Li, Q.-Y. Influence of Silane-Based Water Repellent on the Durability Properties of Recycled Aggregate Concrete. Cem. Concr. Compos. 2013, 35, 32–38. [Google Scholar] [CrossRef]
- Hewayde, E.; Nehdi, M.; Allouche, E.; Nakhla, G. Effect of Geopolymer Cement on Microstructure, Compressive Strength and Sulphuric Acid Resistance of Concrete. Mag. Concr. Res. 2006, 58, 321–331. [Google Scholar] [CrossRef]
- Zarzuela, R.; Luna, M.; Coneo, J.G.; Gemelli, G.; Andreouli, D.; Kaloidas, V.; Mosquera, M.J. Multifunctional Silane-Based Superhydrophobic/Impregnation Treatments for Concrete Producing C-S-H Gel: Validation on Mockup Specimens from European Heritage Structures. Constr. Build. Mater. 2023, 367, 130258. [Google Scholar] [CrossRef]
- Maes, M.; De Belie, M. Resistance of Concrete and Mortar Against Combined Attack of Chloride and Sodium Sulphate. Cem. Concr. Compos. 2014, 53, 59–72. [Google Scholar] [CrossRef]
- Brown, P.W.; Badger, S. The Distributions of Bound Sulfates and Chlorides in Concrete Subjected to Mixed NaCl, MgSO4, Na2SO4 Attack. Cem. Concr. Res. 2000, 30, 1535–1542. [Google Scholar] [CrossRef]
- Basheer, L.; Cleland, D.J. Durability and Water Absorption Properties of Surface Treated Concretes. Mater. Struct. 2011, 44, 957–967. [Google Scholar] [CrossRef]
- Wu, X.; Yang, F.; Lu, G.; Zhao, X.; Chen, Z.; Qian, S. A Breathable and Environmentally Friendly Superhydrophobic Coating for Anti-Condensation Applications. Chem. Eng. 2021, 412, 128725. [Google Scholar] [CrossRef]





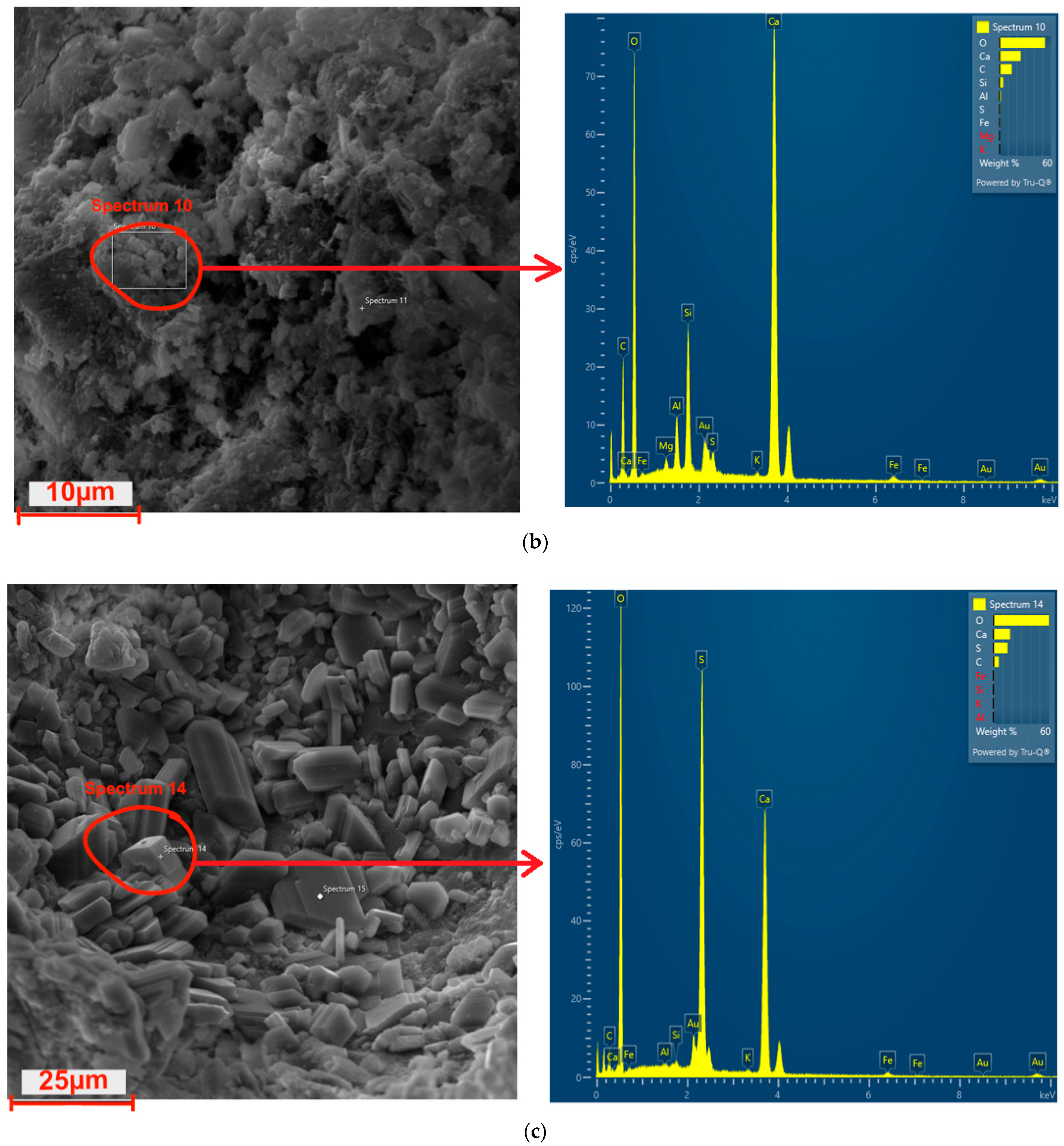


| Type of Hydrophobization | Chemical Base | Consumption (kg/m2) | Application Method | Color |
|---|---|---|---|---|
| HY1 | Oil | 0.23 | Spraying | White |
| HY2 | Silane–siloxane | 0.48 | Spraying | Colorless |
| HY3 | Epoxy resin | 0.23 | Painting | Faint white |
| HY4 | Epoxy resin | 0.23 | Spraying | Faint white |
| HY5 | Acrylic copolymers | 0.15 | Spraying | Colorless |
| Component | Unit | CS1 | CS2 |
|---|---|---|---|
| Portland composite cement CEM II/B-M (S-LL) 32.5 R | kg | 300 | - |
| White Portland cement CEM I 52.5 R SR5 | kg | - | 380 |
| High-temperature fly ash | kg | 60 | - |
| Water | kg | 140 | 152 |
| Fine quarried aggregates (0–4 mm) | Kg | 950 | 908 |
| Fine quarried aggregates (4–8 mm) | Kg | 380 | 363 |
| Coarse crushed aggregates (8–16 mm) | Kg | 570 | 545 |
| Superplasticizer | l | 3.0 | 4.18 |
| Aerating additive Micropon 0.3% of mc | l | - | 1.14 |
| Charge Passed [C] | Chloride Ion Penetrability (CIP) |
|---|---|
| >4000 | High |
| 2000–4000 | Moderate |
| 1000–2000 | Low |
| 100–1000 | Very low |
| <100 | Negligible |
| Type of Substrate | Type of Hydrophobization | Depth of Carbonation [mm] |
|---|---|---|
| CS1 | REF | 22 |
| HY1 | 12 | |
| HY2 | 22 | |
| HY3 | 9 | |
| HY4 | 10 | |
| HY5 | 18 |
| Type of Substrate | Type of Hydrophobization | Depth of Penetration of Water [mm] |
|---|---|---|
| CS1 | HY2 + nano | 25 |
| HY4 + nano | 0 |
| Type of Substrate | Type of Hydrophobization | Compressive Strength [N/mm2] |
|---|---|---|
| CS1 | HY2 + nano | 47.5 |
| HY4 + nano | 46.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodul, J.; Beníková, T.; Drochytka, R.; Borg, R.P. The Examination of the Effect of Water-Soluble Hydrophobic Agents on Physical–Mechanical Parameters and Resistance to Aggressive Environment of Concrete. Coatings 2025, 15, 175. https://doi.org/10.3390/coatings15020175
Hodul J, Beníková T, Drochytka R, Borg RP. The Examination of the Effect of Water-Soluble Hydrophobic Agents on Physical–Mechanical Parameters and Resistance to Aggressive Environment of Concrete. Coatings. 2025; 15(2):175. https://doi.org/10.3390/coatings15020175
Chicago/Turabian StyleHodul, Jakub, Tatiana Beníková, Rostislav Drochytka, and Ruben Paul Borg. 2025. "The Examination of the Effect of Water-Soluble Hydrophobic Agents on Physical–Mechanical Parameters and Resistance to Aggressive Environment of Concrete" Coatings 15, no. 2: 175. https://doi.org/10.3390/coatings15020175
APA StyleHodul, J., Beníková, T., Drochytka, R., & Borg, R. P. (2025). The Examination of the Effect of Water-Soluble Hydrophobic Agents on Physical–Mechanical Parameters and Resistance to Aggressive Environment of Concrete. Coatings, 15(2), 175. https://doi.org/10.3390/coatings15020175









