1. Introduction
Aluminum alloys are widely used in aerospace, electronic devices, transportation, and precision structural components owing to their excellent specific strength, formability, and inherent corrosion resistance [
1,
2,
3,
4]. However, in practical applications, the alloy surface is frequently exposed to aggressive service environments such as humidity and heat [
5], as well as salt spray [
6,
7], where its corrosion resistance remains a critical concern. These conditions impose stringent requirements on surface protection [
8,
9,
10], directly affecting service lifetime, structural safety, and the reliability of subsequent coating treatments [
11,
12].
Among numerous surface treatment techniques for aluminum alloys, conversion coatings have been extensively employed due to their advantages of simple processing, low cost, and environmental compatibility [
13]. Nevertheless, traditional chromate conversion coatings are increasingly restricted owing to the toxicity and environmental hazards associated with hexavalent chromium [
14], highlighting the urgent demand for environmentally benign alternatives. Vanadium-based [
15,
16] and zirconium-based [
17] conversion coatings have emerged as promising substitutes, offering eco-friendliness and excellent protective performance. Vanadium-based coatings are particularly valued for their self-healing capability, while zirconium-based coatings exhibit high density and chemical stability. However, single-component systems often fail to simultaneously meet all performance requirements. Their combination can yield synergistic effects [
18,
19,
20], integrating both density and defect-healing functions, thereby significantly enhancing overall corrosion protection. For instance, P. Ahmadi et al. investigated the corrosion behavior of hexafluorozirconic acid conversion coatings (ZrCC) and sodium metavanadate-modified coatings (ZrVCC) on AA 2024 aluminum alloy [
21], and reported that the incorporation of sodium metavanadate promoted the formation of a compact coating, markedly improving corrosion resistance.
To further refine the microstructure and composition of conversion coatings and enhance their protective performance, recent research has increasingly focused on the use of functional additives [
22,
23,
24,
25]. For example, Liu et al. [
26] demonstrated that 5-carboxybenzotriazole (BTC) incorporated into Ti–Zr conversion coatings effectively suppressed filiform corrosion (FFC) through complexation with metal ions, while simultaneously improving coating density and adhesion. These findings highlight that functional additives not only strengthen the protective effect of conversion coatings but also provide new strategies for addressing complex corrosion mechanisms.
Although preliminary studies have explored the role of individual salts and additives, research on the synergistic mechanisms, compositional regulation, and structural optimization strategies of functional additives within vanadium–zirconium composite conversion coatings remains limited. Therefore, systematically investigating the influence of different functional additives on coating structure and properties—particularly in V–Zr composite systems—is of significant academic value and practical relevance.
The present work focuses on the fabrication and performance optimization of vanadium–zirconium composite conversion coatings on 6061 aluminum alloy. The main objectives include (i) establishing the Optimized Processing conditions for V–Zr composite coatings on 6061 aluminum alloy by tuning parameters such as temperature, pH, and immersion time to obtain stable, corrosion-resistant films; (ii) systematically elucidating the effects of various functional additives on coating composition, microstructure, morphology, and defect distribution; and (iii) constructing the correlation between additives–coating structure–performance to reveal the intrinsic relationships between additive regulation, coating structure, and key performance metrics such as corrosion resistance and adhesion.
2. Materials and Methods
2.1. Materials and Substrate Preparation
The substrate material employed in this study was 6061 aluminum alloy (main elemental composition, wt.%: 0.5 Si, 0.7 Fe, 0.3 Cu, 0.15 Mn, 1.0 Mg, 0.3 Cr, 0.25 Zn, 0.15 Ti, and balance Al) (the material was supplied by Southwest Aluminum (Group) Co., Ltd., Chongqing, China).
Aluminum specimens with dimensions of 40 mm × 30 mm × 1 mm were subjected to a sequential pretreatment process. Initially, the surfaces were mechanically ground using SiC abrasive papers of 240#, 400#, 600#, 800#, and 1000# grit in sequence, followed by rinsing with deionized water. Subsequently, chemical pretreatments were performed in the following order: (i) degreasing in a solution containing 5 g/L OP emulsifier, 23 g/L Na3PO4·12H2O, and 8 g/L Na2CO3 at 50 °C, pH = 6 for 2 min, where oil contaminants were removed via the combined effects of solubility and saponification; (ii) alkali etching in 50 g/L NaOH solution at 50 °C for 40 s to remove the native oxide film and homogenize the surface; (iii) acid pickling in a solution containing 5 g/L thiourea and 10 mL/L HNO3 at 25 °C for 20 s to dissolve residual alumina and activate the surface; and (iv) activation in a solution containing 4 g/L Na2CO3, 1 g/L NaF, and 8 g/L sodium pyrophosphate decahydrate at 50 °C, pH = 7 for 1 min, to eliminate passive films and facilitate uniform nucleation and compact growth of the subsequent conversion coating.
Each step was followed by thorough rinsing with deionized water to prevent contamination and ensure the reliability of the pretreatment process.
2.2. Preparation of Vanadium–Zirconium Conversion Coatings
Pretreated aluminum alloy specimens were used as substrates for the preparation of vanadium–zirconium (V–Zr) conversion coatings. To optimize the processing parameters, the conversion bath was prepared with 2.5 g/L NaVO3 and 2.0 g/L K2ZrF6 as the baseline composition. A thermostatic magnetic stirrer (HJ-6A) was employed to maintain constant temperature and ensure homogeneous mixing of the solution, while a pH meter (PHS-3CB) was used for precise adjustment of the solution pH. Under these conditions, the effects of reaction temperature, immersion time, and pH were systematically investigated to establish the Optimized Processing parameters that yield coatings with superior structural integrity and corrosion resistance.
On the basis of the optimized conditions, additional functional additives were introduced into the conversion bath, including 0.1 g/L Ce(NO3)3·6H2O, 0.5 g/L sodium hexametaphosphate (SHMP), 3.5 g/L chitosan (CS), and 3.5 g/L polyvinyl alcohol (PVA), with the aim of further enhancing corrosion resistance and surface uniformity of the coatings.
2.3. Characterization
A multi-technique approach was adopted to comprehensively characterize the structure and chemical state of the V–Zr conversion coatings formed on 6061 aluminum alloy. Scanning electron microscopy (SEM, Sigma 300, Carl Zeiss Microscopy Limited, Oberkochen, Germany) was employed to examine high-resolution surface morphologies and cross-sectional structures, providing insights into coating density and thickness. Energy-dispersive X-ray spectroscopy (EDS) was used to analyze the elemental composition and spatial distribution of the coatings, confirming the enrichment and uniform incorporation of Zr, V, and Al. X-ray diffraction (XRD, D2 PHASER), based on Bragg diffraction principles, was conducted to identify crystalline and amorphous phases within the coatings, such as ZrO2 and V2O5. Atomic force microscopy (AFM, Bruker Dimension Icon) provided nanoscale surface topography, enabling quantitative analysis of surface roughness, grain size distribution, and microstructural features. These data established the correlations between coating compactness, uniformity, and enhanced corrosion resistance and adhesion. Overall, the multi-scale characterization techniques provided a comprehensive understanding of the structure–composition–performance relationships of the V–Zr conversion coatings.
2.4. Performance Evaluation
The protective performance and adhesion strength of the V–Zr conversion coatings on 6061 aluminum alloy were systematically evaluated by multiple testing methods.
First, the copper sulfate drop test was conducted to assess corrosion resistance (GB/T 6807-2001). The titration solution was prepared with 41 g/L CuSO4·5H2O, 35 g/L NaCl, and 13 mL/L HCl. Each specimen was tested at no fewer than three locations, and the time required for the surface to turn completely red was recorded. The average value was taken as the resistance time.
Second, weight-loss corrosion tests were performed to evaluate the overall protective performance in a neutral chloride environment. Specimens were immersed in 3.5 wt.% NaCl solution for specific time intervals, then removed, descaled, cleaned, and dried. The mass change before and after exposure was measured, and the corrosion rate was calculated as the mass loss per unit area. Each measurement was repeated at least three times, and the average corrosion rate was used for analysis to ensure experimental rigor. The results, combined with surface morphology observations, provided a comprehensive evaluation of corrosion resistance. To further validate long-term performance under accelerated conditions, neutral salt spray (NSS) tests were carried out according to ASTM B117. Specimens were exposed in a salt spray chamber (LS-UT-90) using a 5 wt.% NaCl solution under continuous spraying. Surface corrosion morphologies were monitored periodically to assess coating stability on a macroscopic scale.
Additionally, electrochemical measurements were conducted to obtain quantitative corrosion parameters. Potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS) tests were performed in 3.5 wt.% NaCl solution using an electrochemical workstation (CHI660E). A conventional three-electrode configuration was employed, with the specimen as the working electrode (exposed area: 1 cm2, sealed), a graphite electrode as the counter electrode, and Ag/AgCl as the reference electrode. The open-circuit potential (OCP) was monitored for 400 s, and when fluctuations were less than 20 mV, EIS measurements were carried out over the frequency range 10−2–105 Hz with a 5 mV perturbation at OCP, followed by polarization scans at a rate of 5 mV·s−1 over a potential range of OCP ± 0.5 V. The corrosion potential (Ecorr) and corrosion current density (icorr) were determined from the Tafel extrapolation of the polarization curves obtained by CHI660E. The EIS data were analyzed and fitted using the ZsimpWin software (version 3.30) to extract equivalent circuit parameters, providing insight into the corrosion resistance and interfacial electrochemical behavior of the coatings.
Finally, adhesion strength of the coatings was evaluated using the cross-cut test with a lattice cutter (QFH) in accordance with the ISO 2409:2013 standard [
27] (Paints and varnishes—Cross-cut test), and the results were graded accordingly.
3. Results
3.1. Optimization of Processing Parameters for Vanadium–Zirconium Conversion Coatings
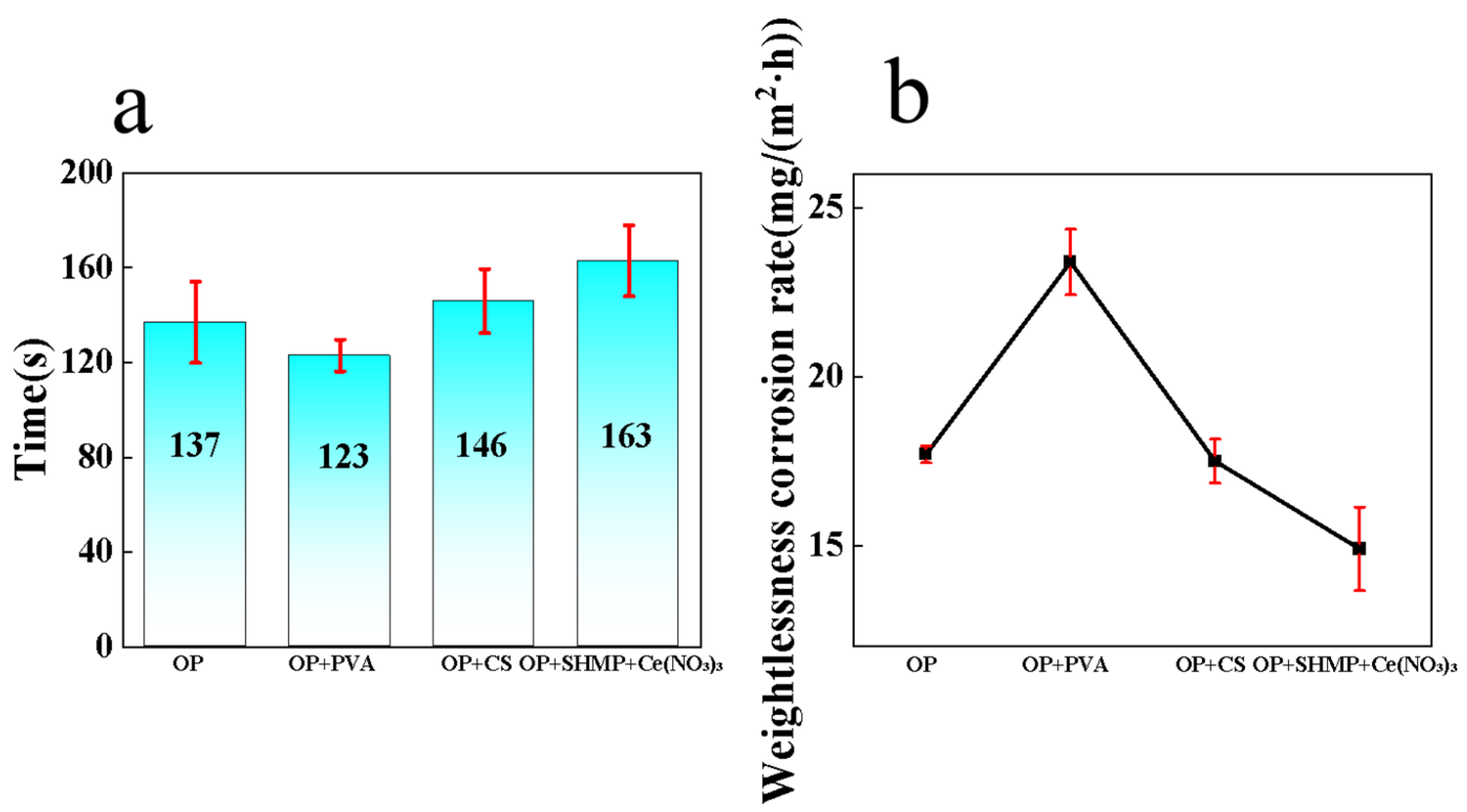
In this study, a vanadium–zirconium composite conversion system was constructed using sodium metavanadate and potassium hexafluorozirconate as precursors. By systematically adjusting three critical processing parameters—solution pH, reaction temperature, and immersion time—the coating performance was optimized. The results of the copper sulfate drop test (
Figure 1a) and weight-loss corrosion test (
Figure 1b) indicate that the optimal conditions for preparing the vanadium–zirconium mixed-salt conversion coating are pH = 3, reaction temperature 55 °C, and immersion time 25 min. Under these conditions, the coating exhibited excellent corrosion resistance.
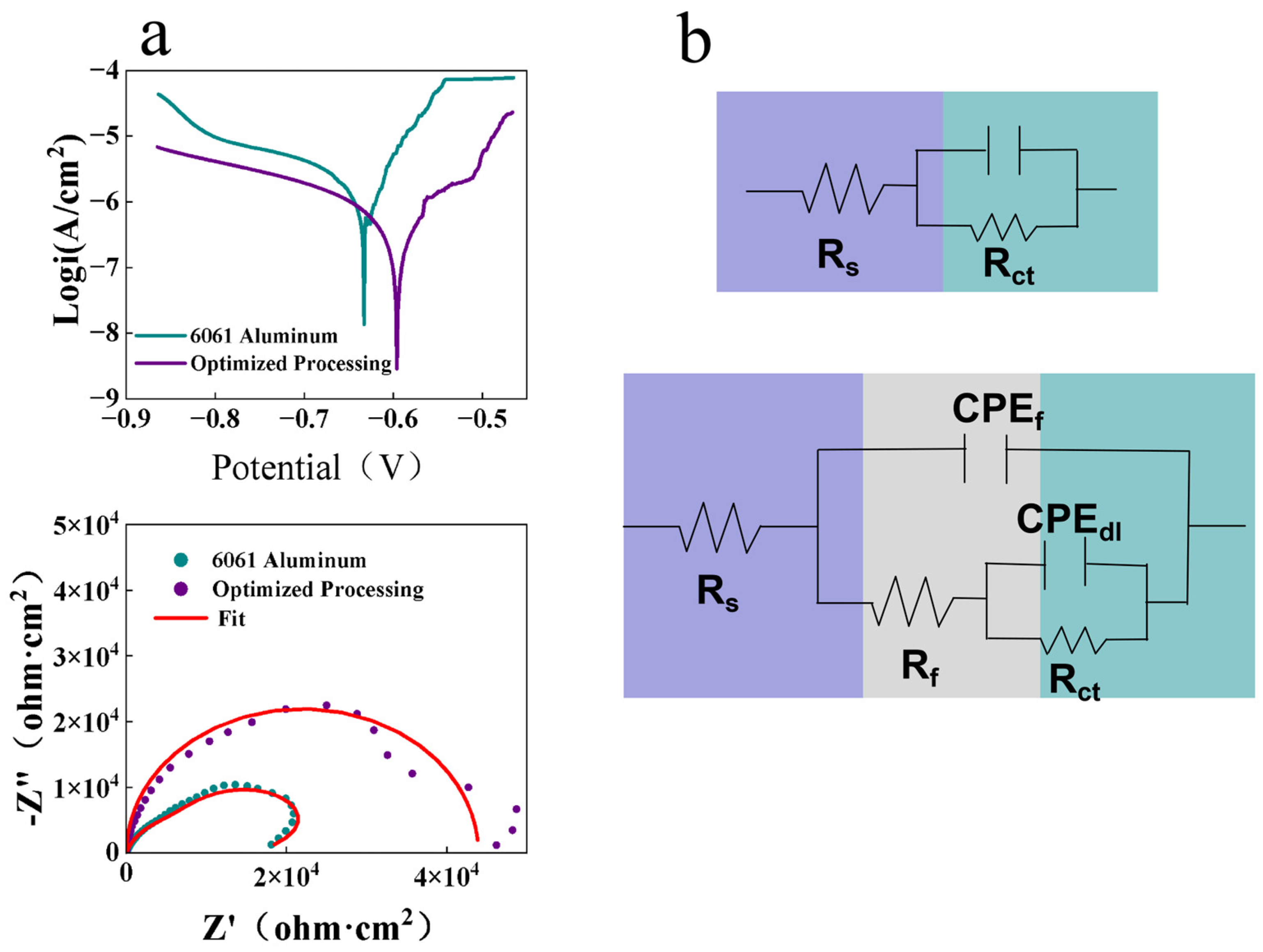
As shown in the polarization curves (
Figure 2a), the bare 6061 aluminum alloy substrate displayed a corrosion potential (E
corr) of −0.633 V with a corresponding corrosion current density (i
corr) of 2.871 μA·cm
−2, whereas under the optimized conditions, E
corr shifted positively to −0.596 V and i
corr decreased markedly to 0.335 μA·cm
−2. The detailed electrochemical parameters corresponding to these measurements are summarized in
Table 1. Furthermore, electrochemical impedance spectroscopy (EIS) results revealed a substantial increase in the diameter of the Nyquist plot, with |Z| rising from 2.1 kΩ·cm
2 to 48.7 kΩ·cm
2. The enlargement of the capacitive arc in the high-to-medium frequency region signifies stronger suppression of interfacial charge transfer, indicating that the corrosion reaction kinetics were effectively retarded, thereby markedly enhancing the corrosion resistance of the conversion coating. The equivalent circuit model used for EIS fitting is illustrated in
Figure 2b, providing further evidence for the improved electrochemical behavior. Similar findings were reported by H. et al., supporting the reliability of the present results [
28].
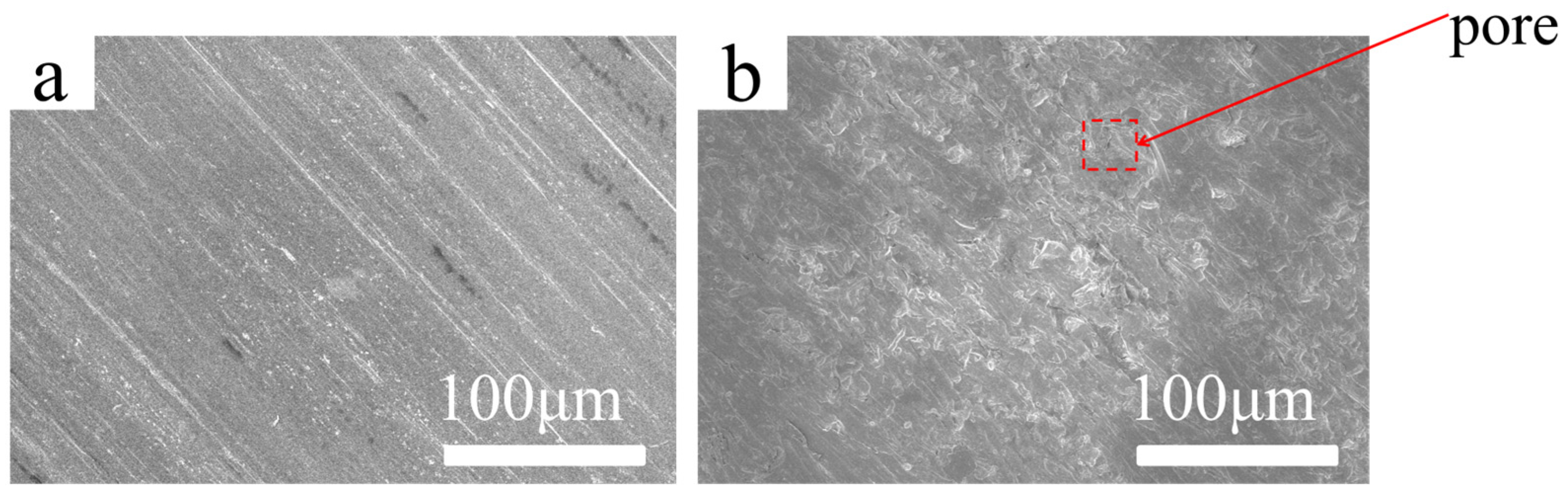
Scanning electron microscopy (SEM) observation provides intuitive insight into the surface uniformity and macrostructural quality of the conversion coating, serving as a critical foundation for performance evaluation and parameter optimization. As shown in
Figure 3a, the untreated 6061 aluminum alloy substrate displays a relatively smooth surface with prominent linear mechanical polishing scratches and localized surface roughness, indicative of bare metal exposure susceptible to environmental degradation. In contrast,
Figure 3b reveals that after the optimized conversion process, the surface is uniformly covered by a continuous coating with a significant reduction in visible defects. Only a few fine granular agglomerates and scattered shallow pits are observed. This transformation indicates that the optimized vanadium-based conversion treatment effectively enhances coating integrity and coverage. Combined with the electrochemical performance results (
Figure 2), the improved surface morphology directly correlates with enhanced corrosion resistance, as the uniform microstructure minimizes ion transport pathways and impedes electrolyte penetration.
3.2. Effect of Additives on Coating Regulation
During the conversion coating process, the incorporation of functional additives into the treatment solution can effectively regulate the microstructure and protective performance of the coating. For instance, polyvinyl alcohol (PVA) enhances coating compactness and corrosion resistance through substrate inhibition and complexation-assisted deposition [
28]; chitosan (CS) strengthens interfacial adhesion by forming hydrogen bonds, thereby reducing cracks and improving corrosion resistance [
29,
30]; sodium hexametaphosphate (SHMP), as a stabilizer and dispersant, improves coating uniformity [
31]; while cerium nitrate (Ce(NO
3)
3) promotes nucleation and growth, leading to enhanced compactness [
32]. Although these additives have demonstrated significant modification effects in other conversion systems, their synergistic mechanisms in vanadium–zirconium composite coatings remain insufficiently understood. Therefore, in this study, on the basis of the Optimized Processing, 3.5 g/L PVA, 3.5 g/L CS, and a combined 0.1 g/L Ce(NO
3)
3 + 0.5 g/L SHMP were introduced to systematically investigate their influence on the structure and corrosion resistance of vanadium–zirconium conversion coatings.
3.2.1. Morphology Analysis
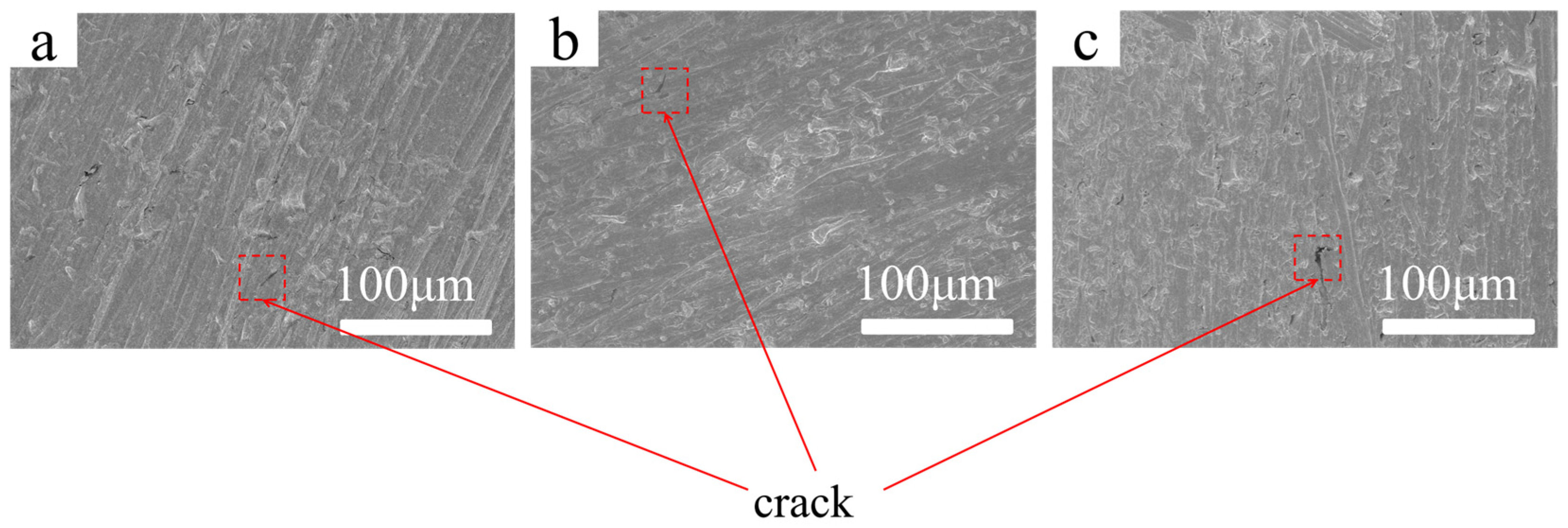
The surface morphology of the coatings prepared under different treatment conditions was further examined using SEM at 200× magnification. As shown in
Figure 4a, when PVA was introduced based on the Optimized Processing (OP), the coating surface displayed noticeable inhomogeneity. The micrograph reveals blurred streak-like regions and uneven surface contrast, indicating irregular deposition and local thinning of the coating. The presence of faint grooves and discontinuous features suggests that PVA may disturb the deposition process by forming viscous aggregates, leading to an insufficiently compact coating accompanied by microdefects that weaken structural uniformity. In
Figure 4b, after incorporating CS, the overall surface uniformity improved compared with the PVA-containing sample. However, the coating still exhibited scattered roughened areas and localized textural variations. These spot-like undulations imply that the macromolecular chains of CS partially hinder homogeneous nucleation during film growth, resulting in mild nonuniform deposition and regions where coating integrity remains suboptimal. In contrast,
Figure 4c shows that the introduction of SHMP combined with Ce(NO
3)
3 produced a markedly smoother and more uniform surface. The SEM image exhibits minimal surface irregularities, and the coating morphology appears continuous with fewer visible defects. This improvement can be attributed to the chelating capability of SHMP, which stabilizes metal ions and promotes uniform deposition, while Ce(NO
3)
3 facilitates dense nucleation and defect sealing. Together, these effects contribute to forming a compact and homogeneous coating with enhanced protective potential.
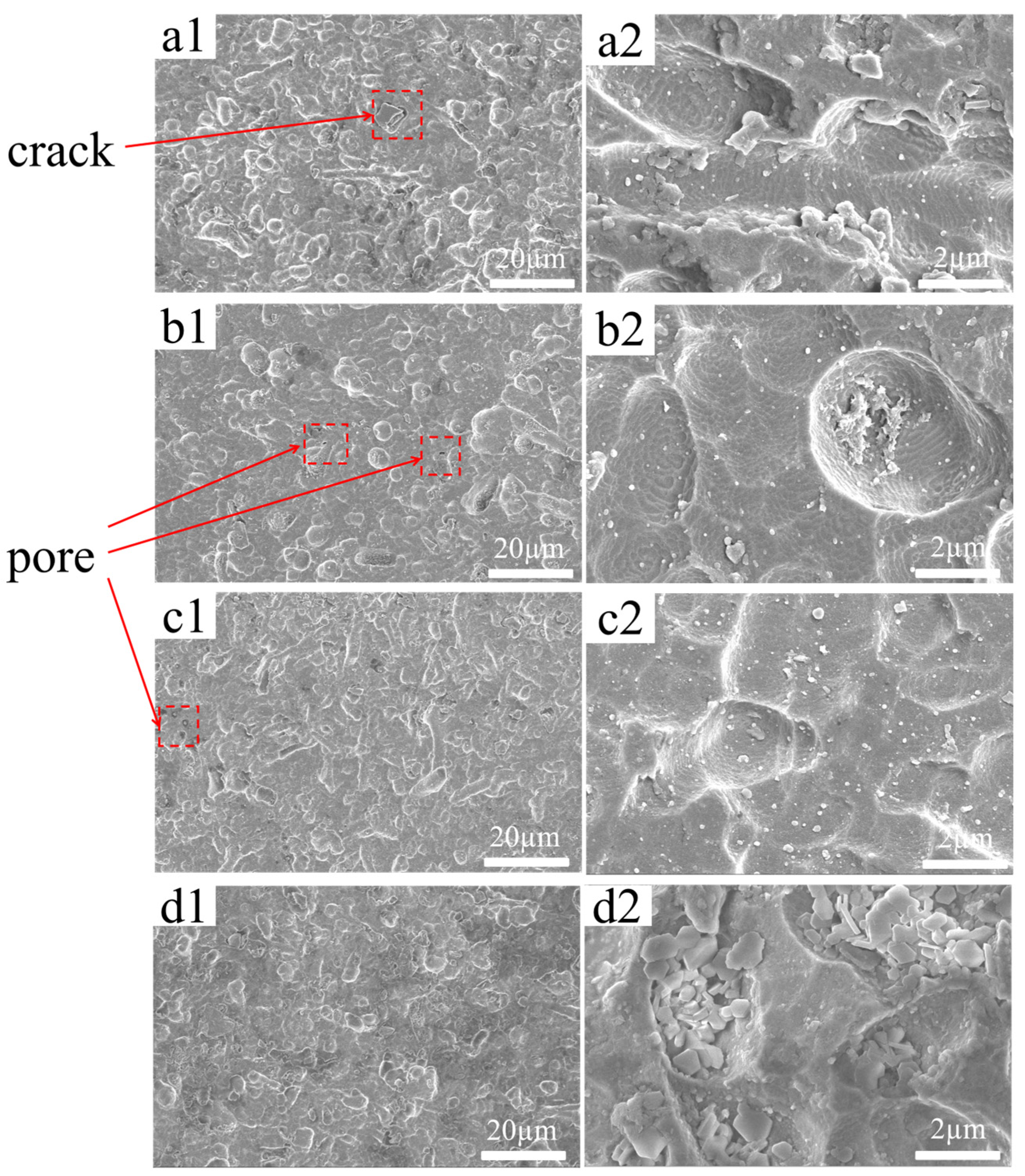
Scanning electron microscopy (SEM) was employed to further investigate the influence of different additives on the surface microstructure of the coatings. By analyzing the microscopic features, the uniformity, compactness, and distribution of defects could be evaluated, providing a theoretical basis for performance optimization. As shown in
Figure 5a, the OP coating exhibited a relatively uniform and continuous surface with overall smoothness, though some pores and cracks were present. In
Figure 5b, the addition of PVA led to the appearance of larger pits and irregularly deposited regions, with nonuniform particle distribution and localized agglomeration, resulting in increased surface roughness. This indicates that PVA may induce the formation of uneven gel-like structures during deposition, generating porosity, weakening interfacial adhesion, and thereby reducing overall protective performance. As shown in
Figure 5c, the addition of CS produced a coating with improved continuity compared with PVA, but local spot-like defects and nonuniform deposition persisted, with some areas showing sparse particle distribution. This suggests that the bulky molecular structure of CS may hinder homogeneous deposition, giving rise to localized defects that compromise coating uniformity and protection. In
Figure 5d, the incorporation of SHMP + Ce(NO
3)
3 resulted in a remarkably compact and well-ordered surface, with almost no visible pores or cracks, indicating excellent uniformity and continuity. The consistency with the results of Eslami, M et al. further confirms the reliability of this study [
33]. This improvement can be attributed to SHMP suppressing heterogeneous deposition through chelation, while Ce(NO
3)
3 facilitated dense nucleation and defect sealing.
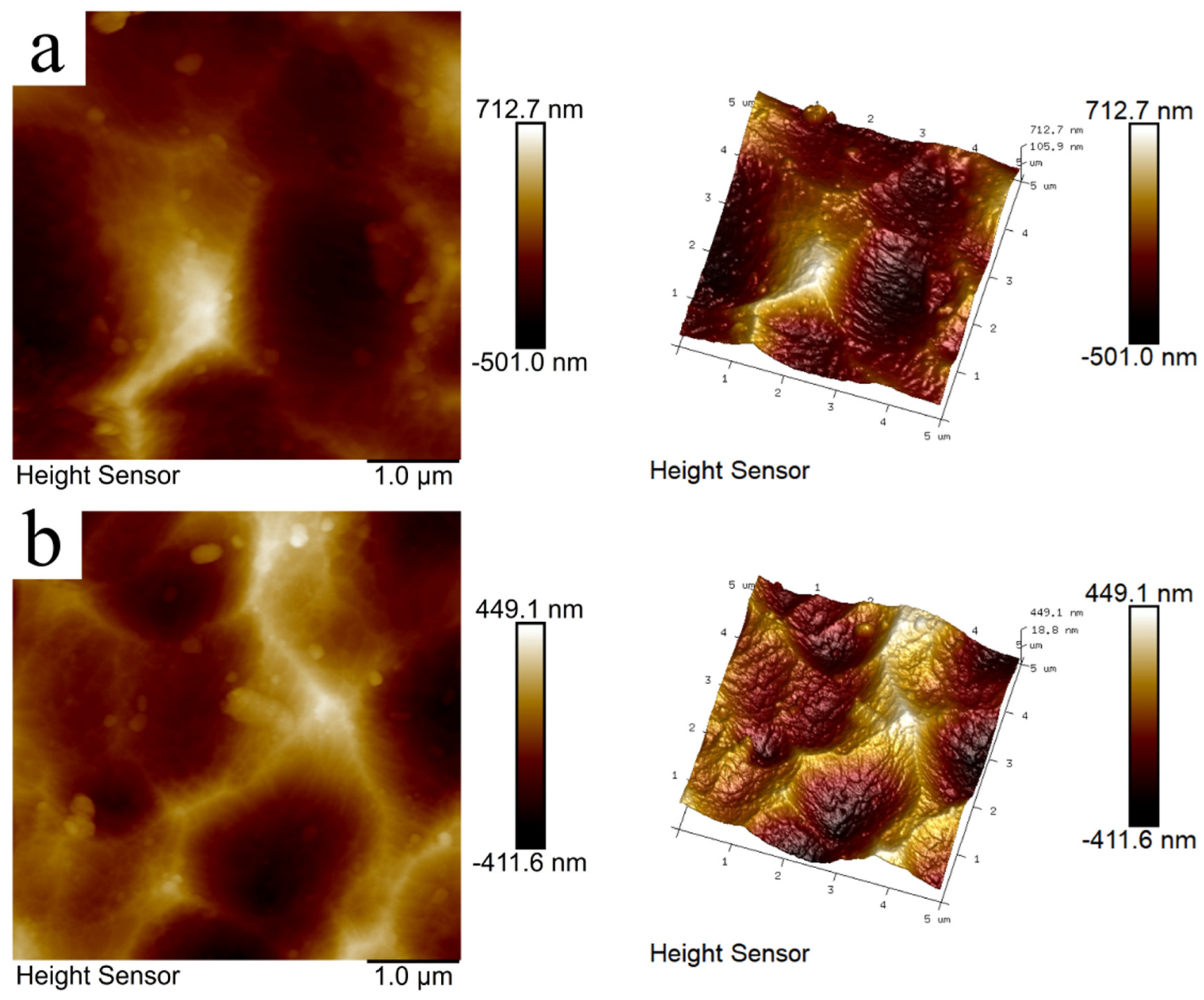
Atomic force microscopy (AFM) was used to characterize the nanoscale surface morphology and evaluate roughness and structural variation. AFM was conducted because nanoscale roughness directly reflects coating compactness and defect distribution, which are closely related to corrosion initiation and electrolyte penetration. These measurements provide quantitative evidence linking microstructural uniformity to corrosion-resistance performance. As shown in
Figure 6a, the OP sample exhibited a roughness of Rq = 174 nm and Ra = 136 nm. The surface displayed a granular accumulation and uneven local deposition with evident micropores and fine pits, suggesting the presence of structural defects that may undermine protective performance. Peng, D. et al. obtained comparable results, which reinforces the credibility of our observations [
34]. In contrast,
Figure 6b shows that with the introduction of SHMP + Ce(NO
3)
3, surface roughness significantly decreased to Rq = 131 nm and Ra = 107 nm. The coating became denser and smoother, with markedly fewer defects. The reduced nanoscale roughness corroborates the improved coating integrity, consistent with its higher impedance and lower corrosion current. These results demonstrate that SHMP + Ce(NO
3)
3 effectively improved the surface structure, enhancing coating continuity and compactness. The reduced roughness and defect density indicate an optimized microstructure, where fewer ion transport channels and diminished surface porosity hinder electrolyte penetration, thereby enhancing the corrosion resistance of the coating. Thus, AFM results directly support the discussion on structural uniformity and corrosion-resistance enhancement.
3.2.2. Composition and Structural Analysis
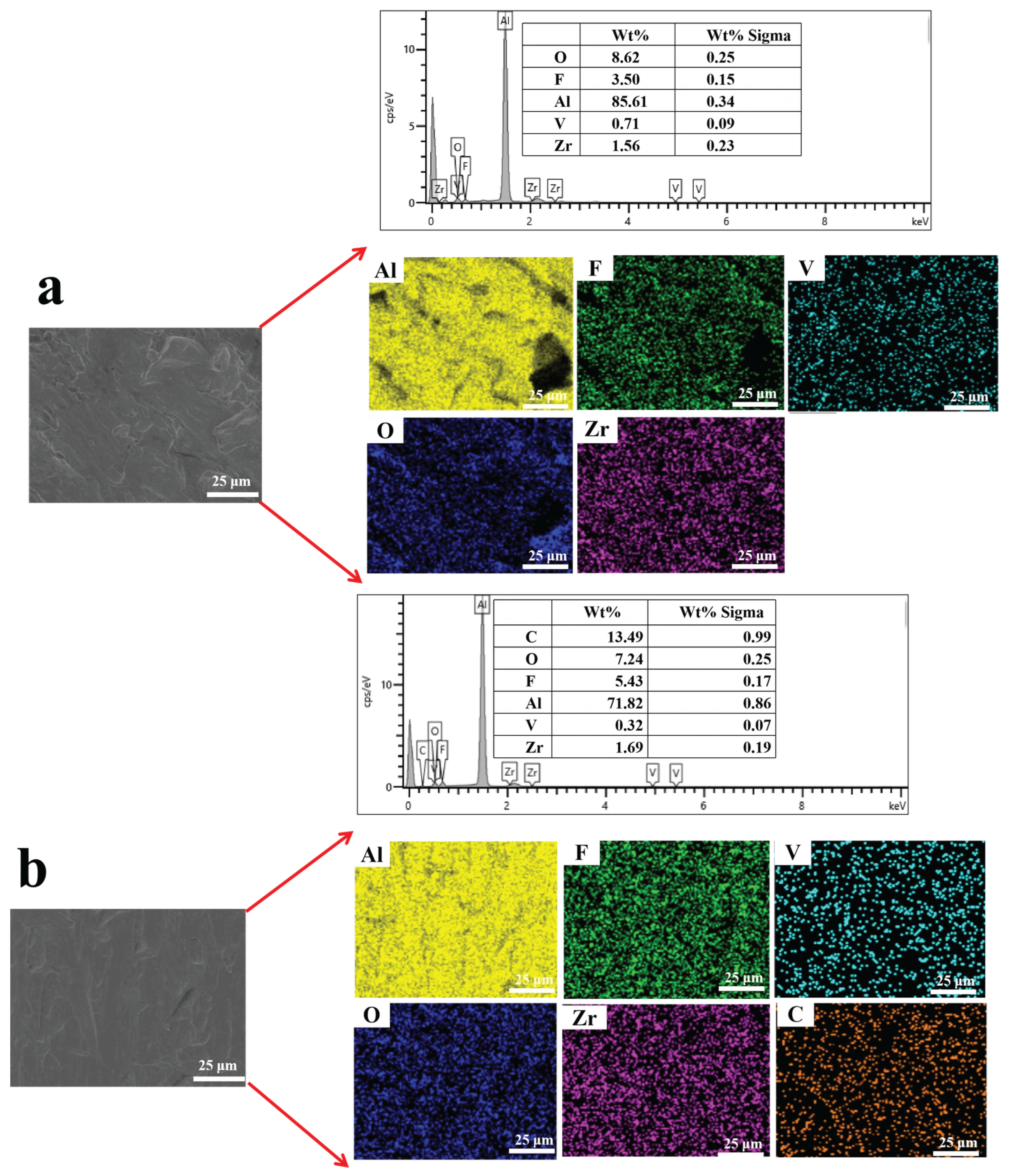
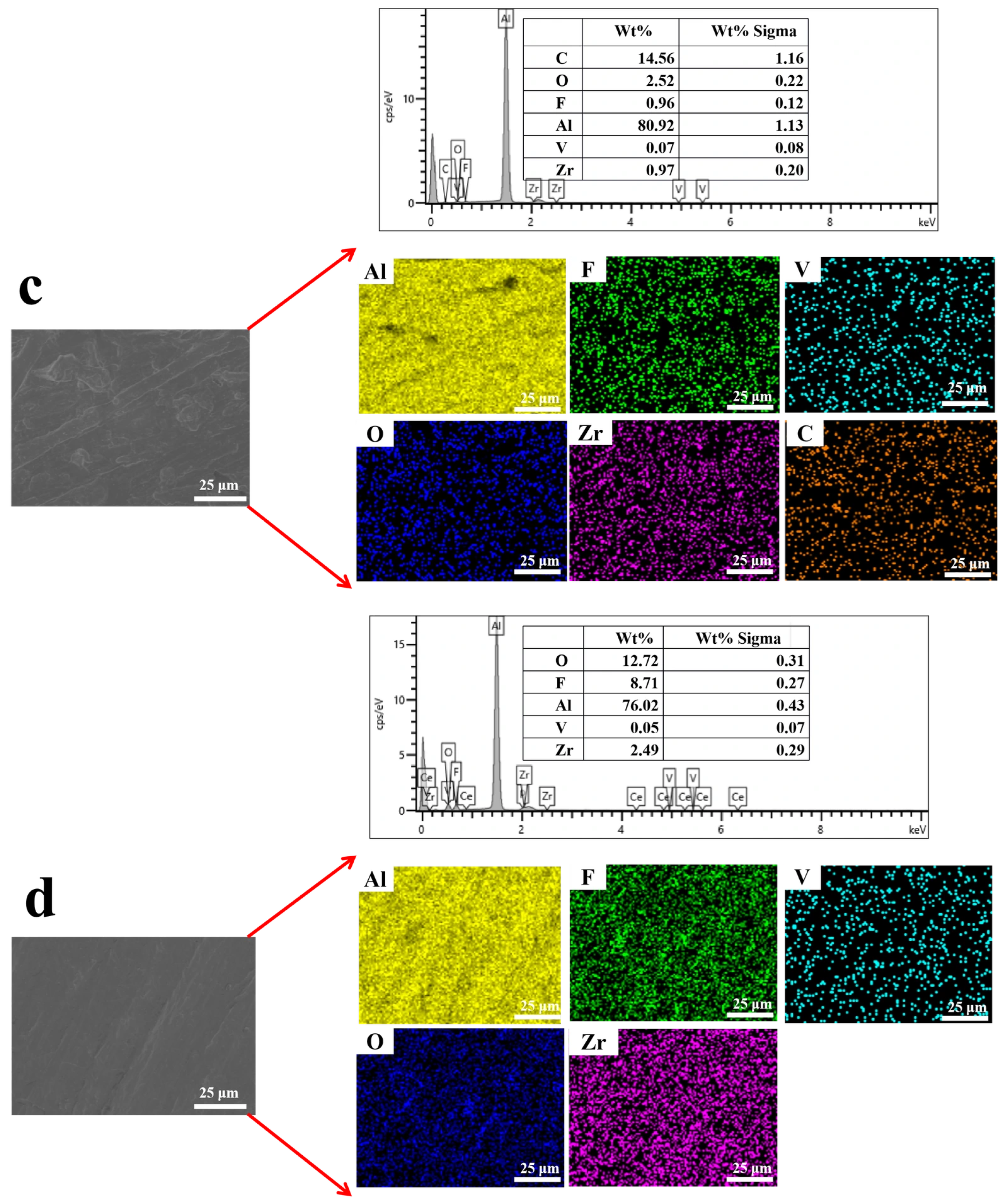
Figure 7 presents the EDS results of samples prepared with different additives. In
Figure 7b, after the introduction of PVA, the contents of F, V, and Zr decreased to 5.43%, 0.32%, and 1.69%, respectively, while Al content remained high (71.82%). This suggests that PVA interferes with the film-formation process, reducing coating thickness and compactness. By contrast, in
Figure 7c, with CS addition, the proportions of O and Zr increased to 2.52% and 0.97%, respectively, while V remained at 0.07%, indicating that CS promotes the deposition of inorganic elements, improving coating uniformity. In
Figure 7d, the incorporation of SHMP + Ce(NO
3)
3 maintained relatively high levels of Zr, suggesting that this combined treatment promotes uniform distribution of these elements in the coating and strengthens its protective properties.
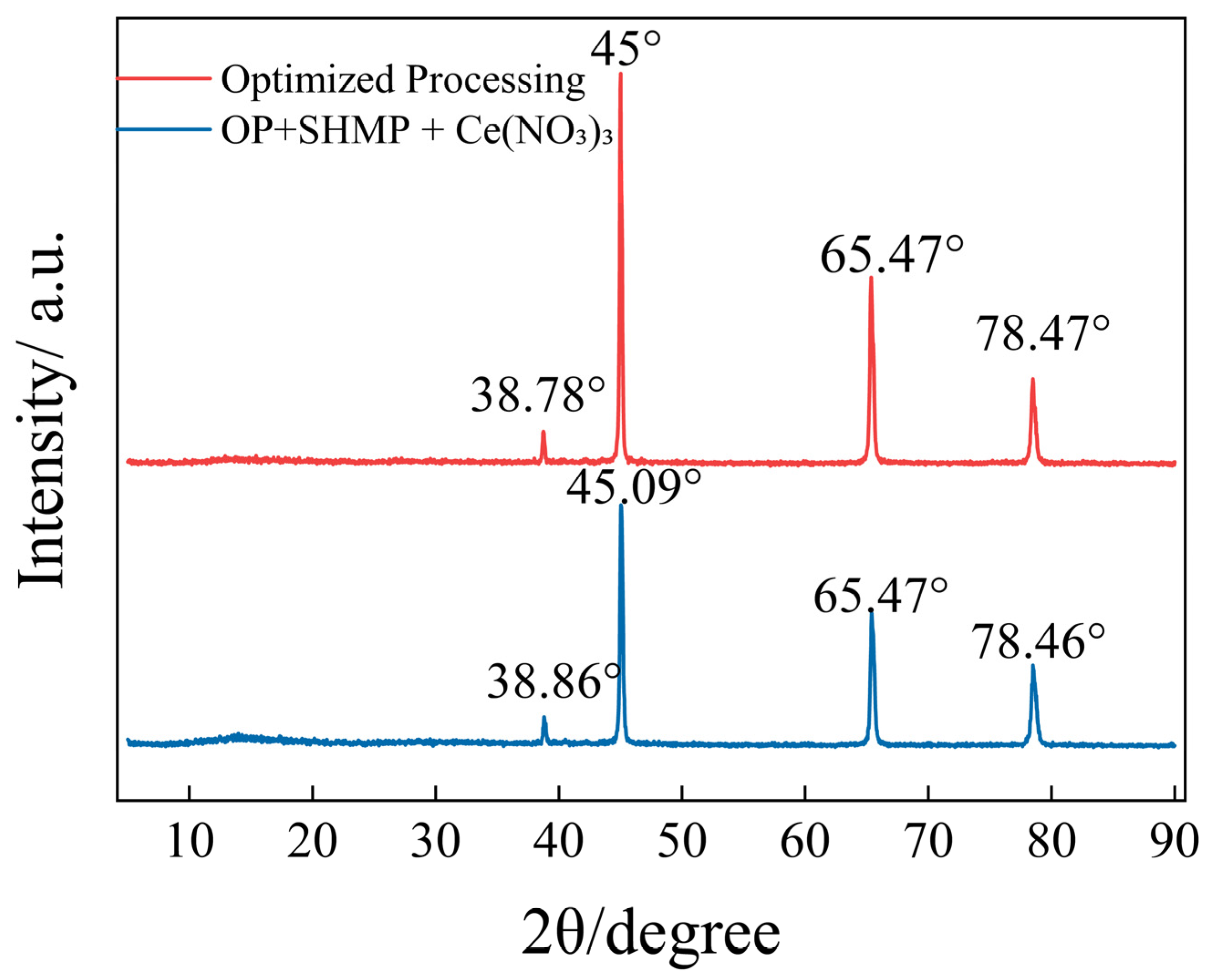
The XRD results in
Figure 8 compare the diffraction patterns of the OP sample and the OP + SHMP + Ce(NO
3)
3 sample. Both groups showed distinct diffraction peaks at 38.7°, 45.0°, 65.5°, and 78.5°, corresponding to the (111), (200), (220), and (311) planes of the aluminum substrate. No additional crystalline phases were detected. Notably, after the introduction of SHMP + Ce(NO
3)
3, the peak intensities decreased, indicating enhanced surface coverage of the aluminum substrate and suggesting that the conversion coating is predominantly amorphous in nature. The agreement with the findings of Wang, L. et al. provides additional evidence for the reliability of these results [
35]. This result is consistent with SEM observations of improved coating compactness.
3.3. Influence of Additives on Coating Performance
3.3.1. Corrosion Resistance
To further evaluate the influence of different additives on the corrosion resistance of the coatings, weight-loss corrosion tests and CuSO
4 droplet tests were conducted, complemented by electrochemical analyses. As shown in
Figure 9a,b, the specimens prepared with 0.1 g/L Ce(NO
3)
3 and 0.5 g/L SHMP exhibited the best corrosion resistance, with the lowest weight-loss rate (0.0149 g/(m
2·h)) and the longest CuSO
4 droplet resistance time (163 s). In contrast, coatings prepared with CS showed only moderate improvements in both corrosion rate and droplet resistance time, while those prepared with PVA not only failed to enhance protection but even decreased corrosion resistance. Comparable outcomes documented by Guo, H. et al. lend strong support to the reliability of our conclusions [
36].
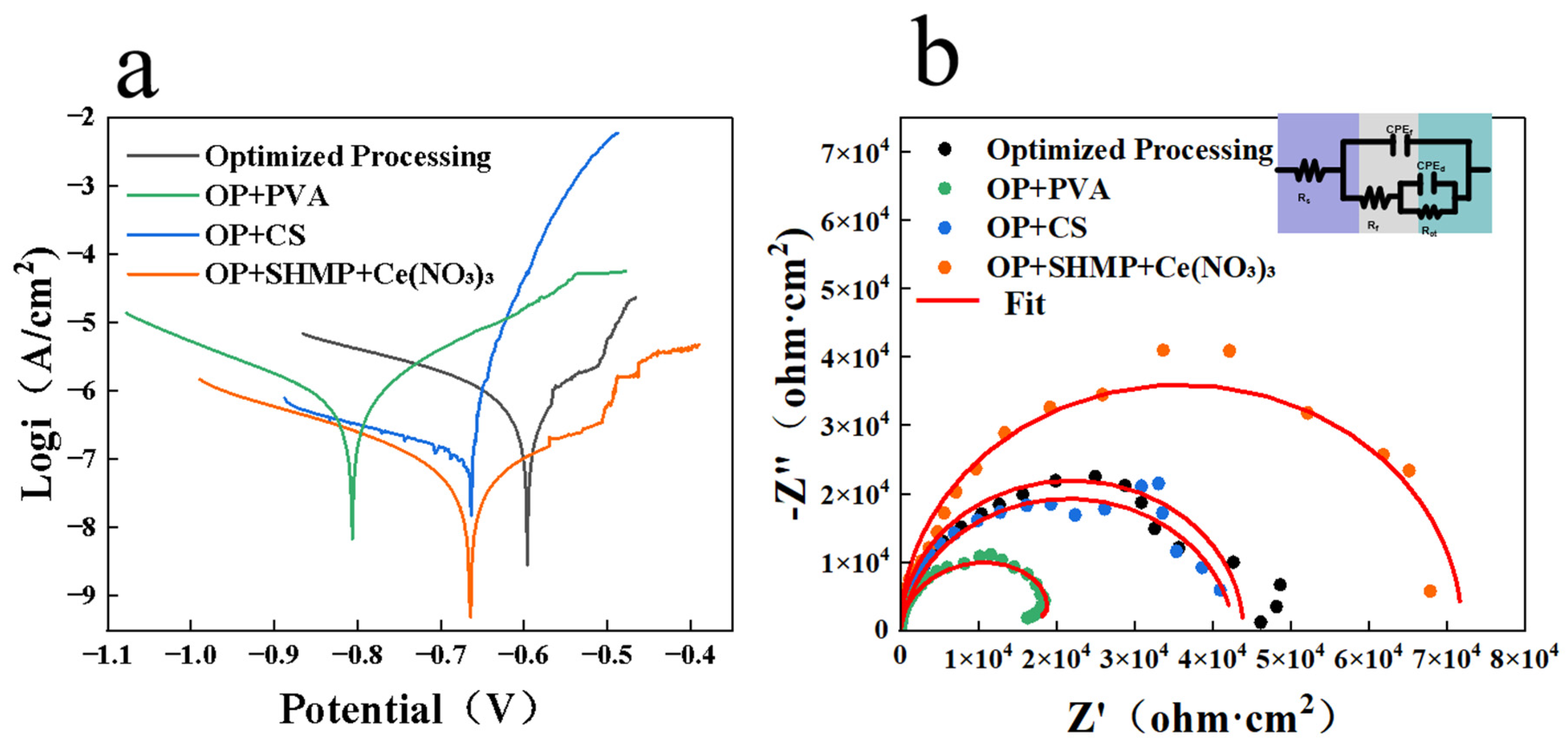
Further electrochemical results (
Figure 10a,b) corroborated these findings: SHMP + Ce(NO
3)
3 coatings exhibited the best performance, with i
corr reduced to 0.055 μA·cm
−2 and |Z| significantly increased to 67.9 kΩ·cm
2, indicating enhanced compactness and protective capacity. The corresponding electrochemical parameters are summarized in
Table 2 for clarity and comparison. It is worth noting that this corrosion current density was not only significantly lower than that of the optimized baseline coating, but also superior to values reported in the literature. For example, Zhan et al. [
31] reported that a Ti–Zr–Ce coating prepared on 6061 aluminum alloy exhibited an i
corr of 0.114 μA·cm
−2, while Wang et al. [
32] found that a Ti–Zr conversion coating modified with salicylic acid and Ce(NO
3)
3·6H
2O on 6061 aluminum alloy showed an even higher i
corr of 0.207 μA·cm
−2. These comparisons clearly highlight the superior corrosion resistance achieved in the present study by employing the SHMP + Ce(NO
3)
3 additive system. By comparison, the electrochemical behavior of the PVA coating was inferior to that of the OP sample, demonstrating weaker corrosion resistance. The CS-based coating provided slight improvement over OP, but its corrosion current density remained higher than that of the optimized OP coating. The results align well with those reported by Nam, D. et al., indicating the robustness and reliability of the findings [
37]. Taken together, the corrosion and electrochemical results confirm that among the three additives, SHMP + Ce(NO
3)
3 provided the most pronounced enhancement in corrosion resistance, CS had a moderate effect, and PVA was the least effective, even impairing overall protective performance.
The neutral salt spray test (NSST) was performed to evaluate the long-term stability and protective ability of the conversion coatings under salt-fog conditions [
38]. After 72 h of salt spray exposure, the substrate surface exhibited severe corrosion, with uneven coloration and localized blackening (
Figure 11a). In contrast, the OP sample (
Figure 11b) showed only a few black spots, and the overall corrosion extent was reduced, indicating a certain protective effect. However, local corrosion remained, likely due to nonuniformity and insufficient compactness of the coating. As shown in
Figure 11c, the PVA-modified coating suffered significant degradation, with pitting and black spots, suggesting that the uneven and loose structure weakened corrosion protection by creating local weak areas. In
Figure 11d, the CS coating exhibited somewhat improved surface protection compared with OP, but localized pitting was still present, indicating that although CS contributed to improved compactness, the overall uniformity and stability were not optimal. As shown in
Figure 11e, the surface condition after SHMP + Ce(NO
3)
3 composite treatment was optimal, with no evident signs of corrosion. This confirms that the synergistic effect of SHMP + Ce(NO
3)
3 markedly improved coating compactness and integrity, thereby providing stable corrosion protection in salt-fog environments. The NSST results were consistent with electrochemical findings, further validating the superior protective performance of the SHMP + Ce(NO
3)
3-modified coatings.
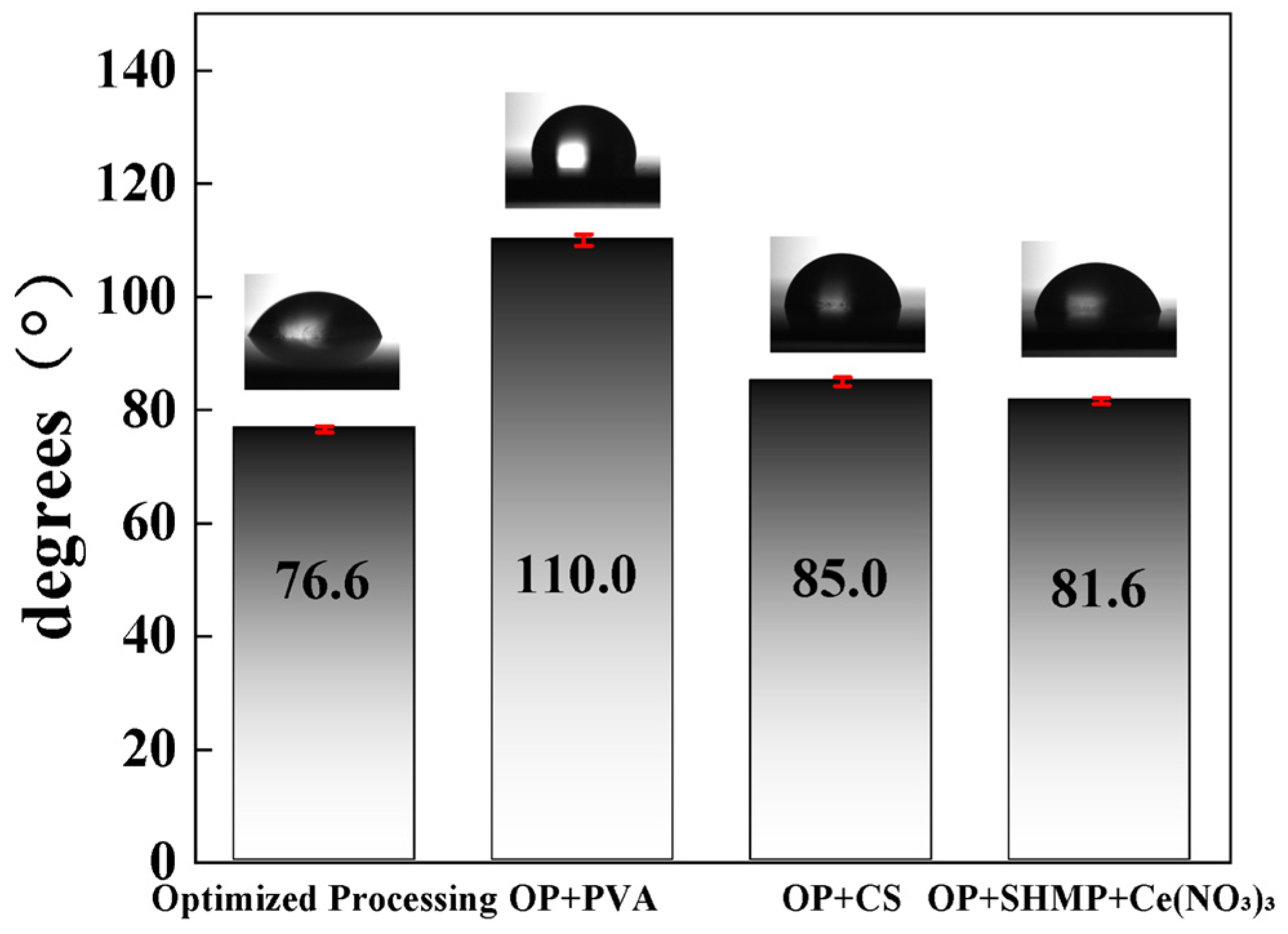
3.3.2. Surface Wettability
Contact angle measurements were conducted to evaluate the correlation between surface wettability and resistance to electrolyte penetration, reflecting the influence of microstructural characteristics on protective performance [
39]. As shown in
Figure 12, all additive-containing groups exhibited higher contact angles than the OP group. Interestingly, although the PVA group achieved the highest hydrophobicity (109°), its uneven and porous coating structure (
Figure 4a and
Figure 5b) still allowed electrolyte penetration, leading to the poorest corrosion resistance, as confirmed by electrochemical results. This highlights that hydrophobicity alone does not guarantee corrosion protection. In contrast, the SHMP + Ce(NO
3)
3 group showed only moderate hydrophobicity (82°), but in combination with its compact structure (
Figure 5d), a synergistic protective mechanism was established: reducing water adhesion while simultaneously blocking transport pathways, thereby yielding excellent corrosion resistance.
3.3.3. Interfacial Adhesion
The adhesion performance of the coatings is shown in
Figure 13. The OP coating exhibited slight peeling along the cross-cut lines, with the affected area below 5%, corresponding to an adhesion rating of 4B. In contrast, all additive-containing coatings showed intact cut edges without peeling or powdering, achieving the highest adhesion rating of 5B. Although the macroscopic appearances in
Figure 13 seem similar, AFM and EDS analyses provide supporting microstructural evidence. AFM results indicate that the additive-modified coatings present reduced nanoscale roughness and improved surface continuity compared with the OP coating, suggesting a denser and more coherent interface conducive to enhanced adhesion. EDS data (
Figure 7) further confirm this trend without requiring extensive elemental changes. The PVA-containing coating shows reduced key inorganic signals, indicating inhibited film growth. CS slightly improves the deposition of inorganic species, while the SHMP + Ce(NO
3)
3 system produces a more uniform elemental distribution, consistent with the formation of a compact protective layer. These microstructural improvements align with the superior 5B adhesion ratings observed in the additive-containing coatings. The concurrence with the work of Proença, C.S. et al. strengthens the reliability and validity of the present results [
40].
4. Discussion
Micromorphological observations (stereomicroscopy, SEM, AFM) indicate that coating uniformity and compactness are key factors influencing protective performance. Under the Optimized Processing (OP), the coating exhibited relatively smooth coverage but still contained local particle agglomerations and micropore defects. With the introduction of PVA, the surface displayed uneven deposition, pits, and agglomerations, which disrupted coating compactness, created weak regions, and ultimately reduced protection. CS improved coating continuity to some extent, yet its macromolecular structure may have hindered uniform deposition, leading to localized spots and defects that compromised integrity. By contrast, the synergistic effect of SHMP and Ce(NO3)3 significantly improved coating morphology: SHMP suppressed non-uniform deposition through chelation, while Ce(NO3)3 promoted dense nucleation and pore sealing, yielding a well-organized, fine, and continuous structure with reduced roughness and defect density, thereby enhancing interfacial integrity. Elemental and structural analyses (EDS, XRD) further confirmed these findings. In the PVA-modified coatings, the content of inorganic elements decreased, reflecting inferior coating quality. The CS coating showed only limited improvement, whereas the SHMP + Ce(NO3)3 system effectively increased the proportion of Zr, V, and O, contributing to greater compactness. These results aligned with SEM and AFM observations.
Electrochemical and corrosion performance tests (Tafel polarization, EIS, weight-loss, CuSO4 droplet, and salt spray tests) established the intrinsic link between structural evolution and protective behavior. Although PVA enhanced hydrophobicity, its non-uniform and porous structure caused a decline in corrosion resistance. CS imparted moderate improvements but was still restricted by local defects. The SHMP + Ce(NO3)3 process demonstrated the most comprehensive corrosion resistance, with a significant reduction in corrosion current, a substantial increase in |Z|, and no apparent corrosion observed in the salt spray test. These results were markedly superior to those of other processes, underscoring the effectiveness of a dense and continuous coating in impeding electrolyte penetration and retarding localized corrosion. Finally, the interfacial adhesion tests indicated that additives generally enhance the bonding strength between the coating and the substrate, with the SHMP + Ce(NO3)3 process providing the most pronounced improvement, thereby further consolidating the stability and practical applicability of the conversion coating.
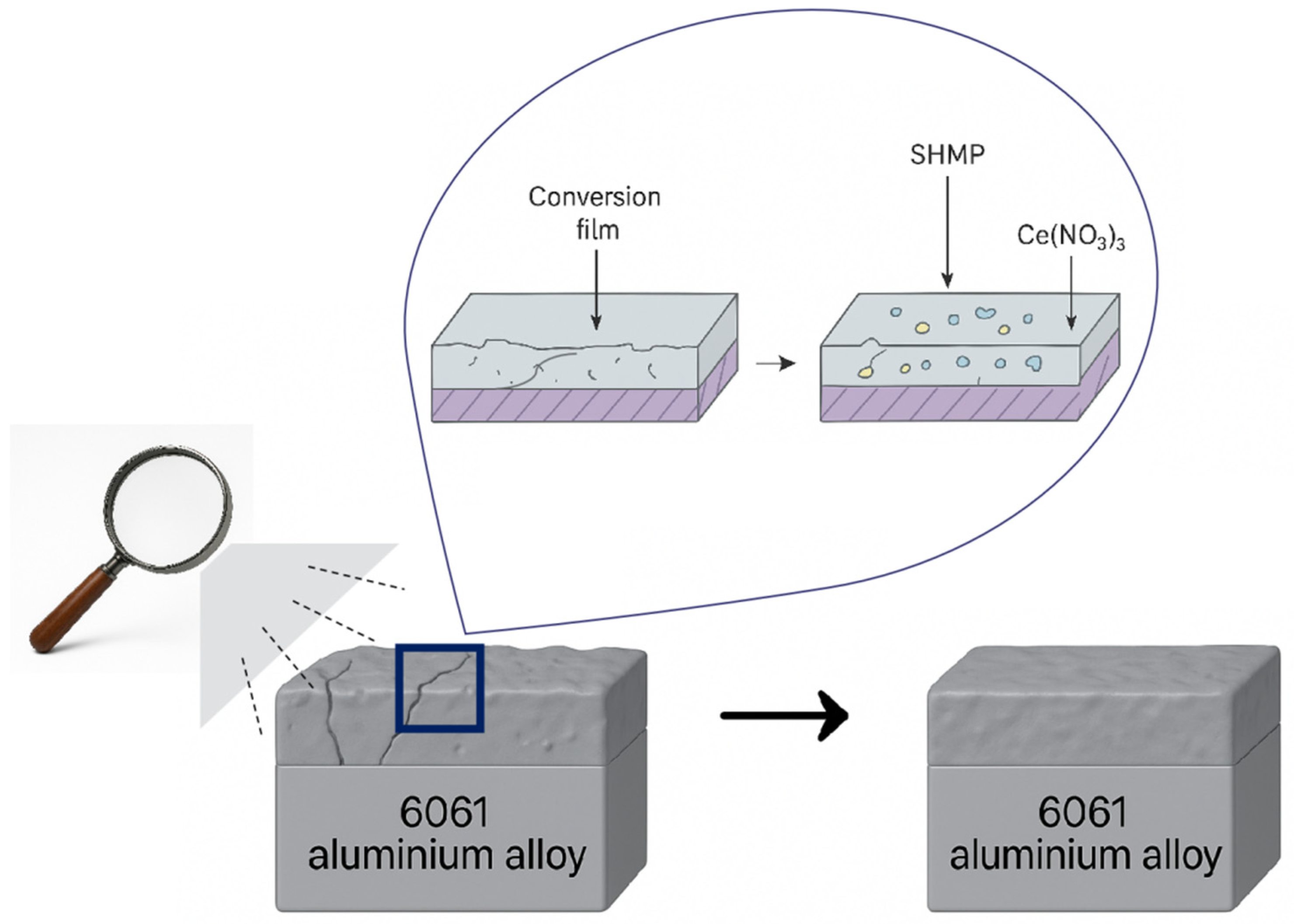
In summary, the three additives exhibited different mechanisms of action: PVA primarily enhanced hydrophobicity but reduced coating uniformity, resulting in poor overall protection; CS slightly improved compactness but with limited effect; while the synergistic action of SHMP and Ce(NO
3)
3 significantly enhanced coating uniformity, compactness, and interfacial integrity through the combined pathways of chelation-induced uniform deposition and nucleation-driven pore sealing (as illustrated in
Figure 14). This led to comprehensive optimization of corrosion resistance, wettability, and adhesion. Therefore, SHMP + Ce(NO
3)
3 represents the most promising additive system for chromate-free V–Zr conversion coatings.
5. Conclusions
Vanadium–zirconium conversion coatings were successfully fabricated on 6061 aluminum alloy via in-situ chemical conversion. The characteristics, formation mechanism, and performance of the coatings were systematically investigated, with emphasis on the effects of solution pH, reaction temperature, and immersion time.
The Optimized Processing parameters were identified as NaVO3 2.5 g/L, K2ZrF6 2.0 g/L, pH = 3, 55 °C, and 25 min. Under these conditions, the V–Zr conversion coating exhibited excellent corrosion resistance: the weight-loss corrosion rate decreased to 0.0188 g/(m2·h), the CuSO4 droplet time extended to 130 s, and electrochemical testing showed a positive shift of Ecorr to −0.596 V, a significant reduction of icorr to 0.335 μA·cm−2, and an increase in impedance modulus from 2.1 to 48.7 kΩ·cm2. Further optimization with additives revealed that the SHMP + Ce(NO3)3 system provided the most remarkable enhancement: the weight-loss rate decreased to 0.38 mg/cm2, icorr further dropped to 0.055 μA/cm2, |Z| increased to 67.9 kΩ·cm2, and no significant pitting was observed even after 72 h salt spray exposure. By contrast, the CS-modified coatings showed only moderate improvement, whereas PVA led to decreased corrosion resistance.
These enhancements originated from structural optimization of the coatings. SEM and AFM results demonstrated that SHMP + Ce(NO3)3 produced a denser and more uniform coating with fewer pores, reducing roughness from Rq = 174 nm, Ra = 136 nm to Rq = 131 nm, Ra = 107 nm. Such compact structures effectively hinder electrolyte penetration and diffusion, thereby improving long-term protection. Contact angle and adhesion tests further confirmed that additives could regulate wettability and interfacial bonding. Although PVA increased hydrophobicity, it was accompanied by decreased corrosion resistance, whereas SHMP + Ce(NO3)3 achieved optimal corrosion protection while maintaining strong adhesion.
Author Contributions
Conceptualization, S.L.; methodology, S.L.; validation, J.F.; formal analysis, J.F.; investigation, J.F. and Z.S.; resources, L.Z.; data curation, J.F. and X.P.; writing—original draft preparation, J.F.; writing—review and editing, J.F.; visualization, J.F. and S.L.; supervision, S.L.; project administration, S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Sichuan Province Science and Technology Program (2024YFHZ0034).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PVA | polyvinyl alcohol |
| CS | chitosan |
| SHMP | sodium hexametaphosphate |
References
- Vargel, C. Corrosion of Aluminium, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-08-099925-8. [Google Scholar]
- Guillaumin, V.; Mankowski, G. Localized Corrosion of 1913 T240 Aluminium Alloy in Chloride Media. Corros. Sci. 1999, 41, 421–438. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, X.; Du, A.; Ma, R.; Zhang, X.; Shi, T.; Fan, Y.; Zhao, X. Investigation on Adhesion Strength and Corrosion Resistance of Ti-Zr Aminotrimethylene Phosphonic Acid Composite Conversion Coating on 7A52 Aluminum Alloy. Appl. Surf. Sci. 2018, 458, 350–359. [Google Scholar] [CrossRef]
- Niu, Y.; Song, Z.; Le, Q.; Hou, J.; Ning, F. Excellent Mechanical Properties Obtained by Low Temperature Extrusion Based on Mg-2Zn-1Al Alloy. J. Alloys Compd. 2019, 801, 415–427. [Google Scholar] [CrossRef]
- Mao, S.; Li, W.; Zeng, X.; Yi, A.; Liao, Z.; Zhu, W. Multiple Transitional Metal Oxides Conversion Coating on AA6063 toward Corrosion Protection and Electrical Conductivity. Surf. Coat. Technol. 2020, 397, 125819. [Google Scholar] [CrossRef]
- Banjo, N.; Sasaki, T.T.; Hono, K. Microstructural Origin of Adhesion and Corrosion Properties of Ti-Based Conversion Coatings on A6063 Alloy. Appl. Surf. Sci. 2022, 604, 154411. [Google Scholar] [CrossRef]
- Munson, C.A.; McFall-Boegeman, S.A.; Swain, G.M. Cross Comparison of TCP Conversion Coating Performance on Aluminum Alloys during Neutral Salt-Spray and Thin-Layer Mist Accelerated Degradation Testing. Electrochim. Acta 2018, 282, 171–184. [Google Scholar] [CrossRef]
- Kim, M.; Brewer, L.N.; Kubacki, G.W. Microstructure and Corrosion Resistance of Chromate Conversion Coating on Cold Sprayed Aluminum Alloy 2024. Surf. Coat. Technol. 2023, 460, 129423. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, Y.; Liu, L.; Yu, B.; Zhang, T.; Wang, F. Critical Role of Pretreatment on the Corrosion Resistance of Zr Conversion Coating on 6061 Aluminum Alloy: The Combined Effect of Surface Topography and Potential Difference between Different Phases. Surf. Coat. Technol. 2019, 377, 124904. [Google Scholar] [CrossRef]
- Zhang, H.; Zuo, Y. The Improvement of Corrosion Resistance of Ce Conversion Films on Aluminum Alloy by Phosphate Post-Treatment. Appl. Surf. Sci. 2008, 254, 4930–4935. [Google Scholar] [CrossRef]
- Boag, A.; Taylor, R.J.; Muster, T.H.; Goodman, N.; McCulloch, D.; Ryan, C.; Rout, B.; Jamieson, D.; Hughes, A.E. Stable Pit Formation on AA2024-T3 in a NaCl Environment. Corros. Sci. 2010, 52, 90–103. [Google Scholar] [CrossRef]
- Proença, C.S.; Pereira, A.M.; Cabral, A.M.; Pigliaru, L.; Rohr, T.; Araújo, M.E.M.; Correia, J. Trivalent Chromium Conversion Coating on AA2024-T3 Used in Aeronautical and Aerospace Industry. Surf. Interface Anal. 2019, 51, 1298–1311. [Google Scholar] [CrossRef]
- Hughes, A.E.; Mol, J.M.C.; Zheludkevich, M.L.; Buchheit, R.G. (Eds.) Active Protective Coatings: New-Generation Coatings for Metals; Springer Series in Materials Science; Springer: Dordrecht, The Netherlands, 2016; Volume 233, ISBN 978-94-017-7538-0. [Google Scholar]
- Gharbi, O.; Thomas, S.; Smith, C.; Birbilis, N. Chromate Replacement: What Does the Future Hold? npj Mater. Degrad. 2018, 2, 12. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, F.; Luo, Y.; Su, Z.; Li, W.; Yi, A.; Liao, Z.; Li, K.; Chen, K.; Hu, Y.; et al. Vanadium and Tannic Acid-Based Composite Conversion Coating for 6063 Aluminum Alloy. Front. Mater. 2021, 8, 802468. [Google Scholar] [CrossRef]
- Zhong, X.; Wu, X.; Jia, Y.; Liu, Y. Self-Repairing Vanadium–Zirconium Composite Conversion Coating for Aluminum Alloys. Appl. Surf. Sci. 2013, 280, 489–493. [Google Scholar] [CrossRef]
- Bin Mohamad Sultan, B.; Persson, D.; Thierry, D.; Han, J.; Ogle, K. On the Dissolution Kinetics during Acid Pickling and Zr-Based Conversion Coating of Aluminum Alloys Using Element-Resolved Electrochemistry. Electrochim. Acta 2024, 503, 144820. [Google Scholar] [CrossRef]
- Zhu, W.; Li, W.; Mu, S.; Yang, Y.; Zuo, X. The Adhesion Performance of Epoxy Coating on AA6063 Treated in Ti/Zr/V Based Solution. Appl. Surf. Sci. 2016, 384, 333–340. [Google Scholar] [CrossRef]
- Zhao, W.; Suhaily, S.S.; Huang, F.; Jingjing, P. Preparation and Anticorrosion Properties of Co-Ti-Zr Composite Conversion Coating on 6061 Aluminium Alloy of the Scooter Profile. Mater. Lett. 2024, 377, 137344. [Google Scholar] [CrossRef]
- Han, X.; Li, N.; Wu, B.; Liu, T.; Lyu, C.; Wang, R.; Li, D. Corrosion Resistance and Microstructural Characterization of Ti-Zr-V/Waterborne Resin Composite Conversion Coating on 1060 Aluminum Alloy. Colloids Surf. A Physicochem. Eng. Asp. 2023, 678, 132503. [Google Scholar] [CrossRef]
- Ahmadi, P.; Sarabi, A.A.; Eivaz Mohammadloo, H.; Behgam, R. Effect of Practical Parameters on the Structure and Corrosion Behavior of Vanadium/Zirconium Conversion Coating on AA 2024 Aluminum Alloy. J. Coat. Technol. Res. 2019, 16, 1503–1513. [Google Scholar] [CrossRef]
- Yu, Z.; Ni, H.; Zhang, G.; Wang, Y.; Dong, S. A Study of the Composhion and Structure of Chromate Conversion Coating on Aluminum. Appl. Surf. Sci. 1992, 62, 217–221. [Google Scholar] [CrossRef]
- Zhichun, C.; Jianzhong, L.; Zhiying, Z.; Wenhu, Y.; Donghai, Q. Effects of Addition of Polyvinyl Pyrrolidone (PVP) as a Corrosion Inhibitor to Zr/V-Based Conversion Coating on Aluminum Alloy. Chem. Pap. 2023, 77, 5847–5857. [Google Scholar] [CrossRef]
- Koryakin, A.S.; Kuzenkov, Y.A.; Oleinik, S.V. Chromateless Conversion Protective Coatings on 1441 Aluminum Alloy. Prot. Met. Phys. Chem. Surf. 2018, 54, 1320–1325. [Google Scholar] [CrossRef]
- Zhou, S.; Mao, J. Evaluation of Anticorrosive and Self-Healing Performances of TiO2 -Added Cerium Conversion Coatings Developed on 211Z Aluminium Alloy. Mater. Res. Express 2020, 7, 026556. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, H.; Jawad, M.; Fan, B.; Tabish, M.; Mubeen, M.; Mahmood, M.; Wang, J. Filiform Corrosion Inhibition of Zn-Al-Mg Coated Steel by Ti-Zr Conversion Coating: Crucial Role of Benzotriazole-5-Carboxylic Acid. Langmuir 2025, 41, 17475–17493. [Google Scholar]
- ISO 2409:2013; Paints and Varnishes—Cross-Cut Test. ISO: Geneva, Switzerland, 2013. (In German)
- Li, H.; Xue, C.; Cheng, W.; Gao, L.; Wang, X.; Wei, H.; Nan, H.; Wang, G.; Lin, H. The Synergistic Effect of Polyvinyl Alcohol and Graphene Oxide on the Corrosion Resistance of Waterborne Coatings. Prog. Org. Coat. 2021, 160, 106526. [Google Scholar] [CrossRef]
- Chen, W.; Su, W.; Li, X.; Cui, X.; Xie, E.; Wang, Y.; Jin, G. Effect of Chitosan on Microstructure and Corrosion Resistance of Ti-Zr-based Conversion Coating. Surf. Technol. 2018, 47, 189–194. [Google Scholar] [CrossRef]
- Salmas, C.E.; Leontiou, A.; Kollia, E.; Zaharioudakis, K.; Kopsacheili, A.; Avdylaj, L.; Georgopoulos, S.; Karabagias, V.K.; Karydis-Messinis, A.; Kehayias, G.; et al. Active Coatings Development Based on Chitosan/Polyvinyl Alcohol Polymeric Matrix Incorporated with Thymol Modified Activated Carbon Nanohybrids. Coatings 2023, 13, 1503. [Google Scholar] [CrossRef]
- Zhan, W.; Qian, X.; Gui, B.; Liu, L.; Liu, X.; Li, Z.; Hu, L. Preparation and Corrosion Resistance of Titanium–Zirconium–Cerium Based Conversion Coating on 6061 Aluminum Alloy. Mater. Corros. 2020, 71, 419–429. [Google Scholar] [CrossRef]
- Wang, X.; Zhan, W.; Gui, B. Effect of Cerium Nitrate and Salicylic Acid on the Titanium–Zirconium Chemical Conversion Coating of 6061 Aluminum Alloy. Anti-Corros. Methods Mater. 2020, 67, 205–213. [Google Scholar] [CrossRef]
- Eslami, M.; Fedel, M.; Speranza, G.; Deflorian, F.; Andersson, N.-E.; Zanella, C. Study of Selective Deposition Mechanism of Cerium-Based Conversion Coating on Rheo-HPDC Aluminium-Silicon Alloys. Electrochim. Acta 2017, 255, 449–462. [Google Scholar] [CrossRef]
- Peng, D.; Wu, J.; Yan, X.; Du, X.; Yan, Y.; Li, X. The Formation and Corrosion Behavior of a Zirconium-Based Conversion Coating on the Aluminum Alloy AA6061. J. Coat. Technol. Res. 2016, 13, 837–850. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; He, H.; Sun, W.; Zong, Q.; Liu, G. Enhanced Corrosion Resistance of MgAl Hydrotalcite Conversion Coating on Aluminum by Chemical Conversion Treatment. Surf. Coat. Technol. 2013, 235, 484–488. [Google Scholar] [CrossRef]
- Guo, H.; Wang, C.; Liu, H. Effect of Different Sealers on the Properties of Phosphate Conversion Coatings on 30CrMnSi Alloys. ACMM 2024, 71, 506–513. [Google Scholar] [CrossRef]
- Nam, D.; Lim, D.; Kim, S.-D.; Seo, D.; Shim, S.E.; Baeck, S.-H. The Fabrication of a Conversion Film on AZ31 Containing Carbonate Product and Evaluation of Its Corrosion Resistance. J. Alloys Compd. 2018, 737, 597–602. [Google Scholar] [CrossRef]
- Xia, M.; Wang, Y.; Xu, S. Study on Surface Characteristics and Stochastic Model of Corroded Steel in Neutral Salt Spray Environment. Constr. Build. Mater. 2021, 272, 121915. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, S.; Chen, S.; Xu, S.; Yin, L. Influence of Three Different Anions on Morphology and Property of Trivalent Chromium Based Conversion Coating on Aluminum Alloy. Int. J. Electrochem. Sci. 2016, 11, 843–854. [Google Scholar] [CrossRef]
- Proença, C.S.; Pereira, A.M.; Pigliaru, L.; Rohr, T.; Correia, J.; Cabral, A.M. Alternative Chemical Conversion Pre-Treatment for Aeronautical Aluminium Alloy: Characterisation and Anticorrosion Performance. CEAS Space J. 2023, 15, 265–280. [Google Scholar] [CrossRef]
Figure 1.
Corrosion resistance of coatings prepared under different processing parameters. (a) Copper sulfate drop test: time to surface discoloration; (b) Weight-loss corrosion test: corrosion rate.
Figure 1.
Corrosion resistance of coatings prepared under different processing parameters. (a) Copper sulfate drop test: time to surface discoloration; (b) Weight-loss corrosion test: corrosion rate.
Figure 2.
(a) Electrochemical measurements of the substrate and the Optimized Processing (OP); (b) Equivalent circuit.
Figure 2.
(a) Electrochemical measurements of the substrate and the Optimized Processing (OP); (b) Equivalent circuit.
Figure 3.
SEM images at 200× magnification): (a) Substrate; (b) Optimized Processing (OP).
Figure 3.
SEM images at 200× magnification): (a) Substrate; (b) Optimized Processing (OP).
Figure 4.
SEM images at 200× magnification: (a) OP + PVA, (b) OP + CS, (c) OP + SHMP + Ce(NO3)3.
Figure 4.
SEM images at 200× magnification: (a) OP + PVA, (b) OP + CS, (c) OP + SHMP + Ce(NO3)3.
Figure 5.
SEM images at 1k× and 6k× magnification: (a) OP, (b) OP + PVA, (c) OP + CS, (d) OP + SHMP + Ce(NO3)3.
Figure 5.
SEM images at 1k× and 6k× magnification: (a) OP, (b) OP + PVA, (c) OP + CS, (d) OP + SHMP + Ce(NO3)3.
Figure 6.
AFM images: (a) OP, (b) OP + SHMP + Ce(NO3)3.
Figure 6.
AFM images: (a) OP, (b) OP + SHMP + Ce(NO3)3.
Figure 7.
EDS spectra: (a) OP, (b) OP + PVA, (c) OP + CS, (d) OP + SHMP + Ce(NO3)3.
Figure 7.
EDS spectra: (a) OP, (b) OP + PVA, (c) OP + CS, (d) OP + SHMP + Ce(NO3)3.
Figure 8.
XRD patterns: OP and OP + SHMP + Ce(NO3)3.
Figure 8.
XRD patterns: OP and OP + SHMP + Ce(NO3)3.
Figure 9.
Corrosion resistance with different additives: (a) CuSO4 droplet test—time to color change, (b) weight-loss corrosion test—corrosion rate.
Figure 9.
Corrosion resistance with different additives: (a) CuSO4 droplet test—time to color change, (b) weight-loss corrosion test—corrosion rate.
Figure 10.
Corrosion resistance with different additives: (a) potentiodynamic polarization curves, (b) Nyquist plots.
Figure 10.
Corrosion resistance with different additives: (a) potentiodynamic polarization curves, (b) Nyquist plots.
Figure 11.
Neutral salt spray test: (a) Al 6061 substrate, (b) OP, (c) OP + PVA, (d) OP + CS, (e) OP + SHMP + Ce(NO3)3. “1” represents the sample before testing, “2” represents the sample after 72 h of exposure.
Figure 11.
Neutral salt spray test: (a) Al 6061 substrate, (b) OP, (c) OP + PVA, (d) OP + CS, (e) OP + SHMP + Ce(NO3)3. “1” represents the sample before testing, “2” represents the sample after 72 h of exposure.
Figure 12.
Contact angle measurements.
Figure 12.
Contact angle measurements.
Figure 13.
Adhesion test results: (a) OP, (b) OP + PVA, (c) OP + CS, (d) OP + SHMP + Ce(NO3)3.
Figure 13.
Adhesion test results: (a) OP, (b) OP + PVA, (c) OP + CS, (d) OP + SHMP + Ce(NO3)3.
Figure 14.
Schematic of the effects of SHMP and Ce(NO3)3.
Figure 14.
Schematic of the effects of SHMP and Ce(NO3)3.
Table 1.
Obtained electrochemical data from PDP and EIS measurements.
Table 1.
Obtained electrochemical data from PDP and EIS measurements.
| Sample | Ecorr (V) | icorr (μA·cm−2) | |Z| (kΩ·cm2) |
|---|
| 6061 Aluminum | −0.633 | 2.871 | 2.1 |
| Optimized Processing | −0.596 | 0.335 | 48.7 |
Table 2.
Obtained electrochemical data from PDP and EIS measurements.
Table 2.
Obtained electrochemical data from PDP and EIS measurements.
| Sample | Ecorr (V) | icorr (μA·cm−2) | |Z| (kΩ·cm2) |
|---|
| Optimized Processing | −0.596 | 0.335 | 48.7 |
| OP + PVA | −0.806 | 0.771 | 18.9 |
| OP + CS | −0.663 | 0.598 | 41.0 |
| OP + SHMP + Ce(NO3)3 | −0.664 | 0.055 | 67.9 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).