Effect of Different Surface Treatments and Toothbrushing Durations on Surface Roughness and Color Stability of CAD/CAM Interim Crown Material †
Abstract
1. Introduction
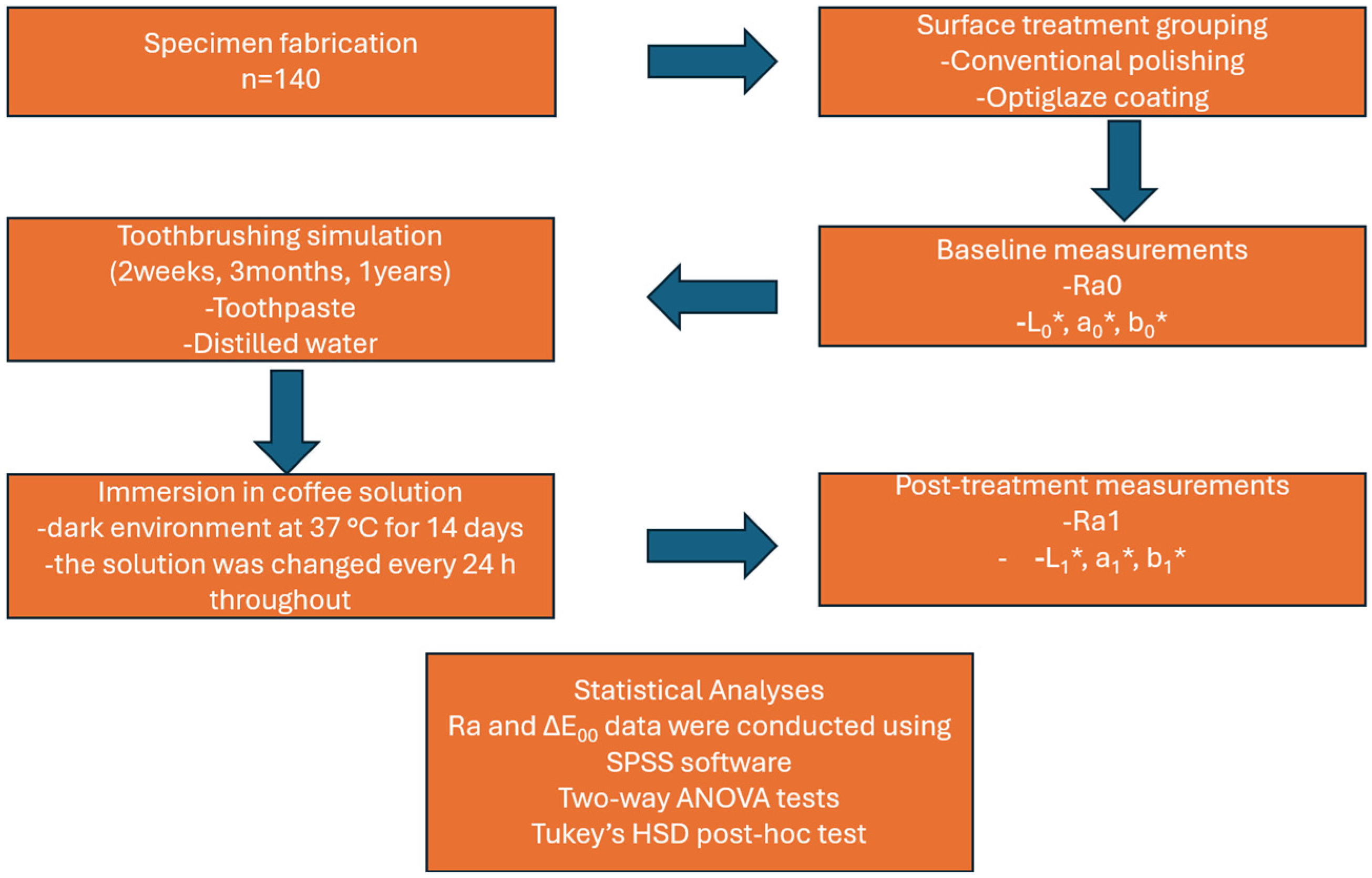
2. Materials and Methods
2.1. Specimen Fabrication
2.2. Surface Treatment and Finishing Procedures
2.3. Surface Roughness Measurement
2.4. Scanning Electron Microscopy (SEM)
2.5. Color Measurements
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAD-CAM | Computer-aided design and computer-aided manufacturing |
| Ra | Surface roughness |
| PMMA | Polymethyl methacrylate |
| ΔE00 | Color changes |
| Bis-GMA | Bisphenol A-glycidyl methacrylate |
| TEGDMA | Triethylene glycol dimethacrylate |
| THFMA | Tetrahydrofurfuryl methacrylate |
| UDMA | Urethane dimethacrylate |
References
- Sadek, H.A.; Wahsh, M.; Saeed, M. Surface Roughness and Color stability of Provisional Restorative Materials Fabricated Using Different Methods After Immersion in Various Storage Media. Egypt. Dent. J. 2023, 69, 683–695. [Google Scholar] [CrossRef]
- Song, S.Y.; Shin, Y.H.; Lee, J.Y.; Shin, S.W. Color stability of provisional restorative materials with different fabrication methods. J. Adv. Prosthodont. 2020, 12, 259. [Google Scholar] [CrossRef]
- Fox, C.W.; Abrams, B.L.; Doukoudakis, A. Provisional restorations for altered occlusions. J. Prosthet. Dent. 1984, 52, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Almohareb, T.; Alkatheeri, M.S.; Vohra, F.; Alrahlah, A. Influence of experimental staining on the color stability of indirect computer-aided design/computer-aided manufacturing dental provisional materials. Eur. J. Dent. 2018, 12, 269–274. [Google Scholar] [CrossRef]
- Mohsen AlMutairi, R. In-Vitro Marginal Fit of 3D-Printed vs Milled Provisional Crowns. J. Pioneer. Med. Sci. 2025, 14 (Suppl. S2), 176–180. [Google Scholar] [CrossRef]
- Igreț, A.; Rotar, R.N.; Ille, C.; Topală, F.; Jivănescu, A. Marginal fit of milled versus different 3D-printed materials for provisional fixed dental prostheses: An in vitro comparative study. Med. Pharm. Rep. 2023, 96, 298–304. [Google Scholar] [CrossRef]
- Sham, A.S.K.; Chu, F.C.S.; Chai, J.; Chow, T.W. Color stability of provisional prosthodontic materials. J. Prosthet. Dent. 2004, 91, 447–452. [Google Scholar] [CrossRef]
- Knobloch, L.A.; Kerby, R.E.; Pulido, T.; Johnston, W.M. Relative fracture toughness of bis-acryl interim resin materials. J. Prosthet. Dent. 2011, 106, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Lieu, C.; Nguyen, T.M.; Payant, L. In vitro comparison of peak polymerization temperatures of 5 provisional restoration resins. J. Can. Dent. Assoc. 2001, 67, 36–39. [Google Scholar] [PubMed]
- Köroğlu, A.; Şahin, O.; Küçükekenci, A.S.; Dede, D.Ö.; Yıldırım, H.; Yilmaz, B. Influences of Toothbrushing and Different Toothpastes on the Surface Roughness and Color Stability of Interim Prosthodontic Materials. Materials 2022, 15, 5831. [Google Scholar] [CrossRef]
- Jehan, A.; Chidambaranathan, A.S.; Balasubramanium, M. Application and trends in provisional dental restorative materials for fixed partial denture. J. Oral. Res. Rev. 2023, 15, 65–71. [Google Scholar] [CrossRef]
- Rayyan, M.M.; Aboushelib, M.; Sayed, N.M.; Ibrahim, A.; Jimbo, R. Comparison of interim restorations fabricated by CAD/CAM with those fabricated manually. J. Prosthet. Dent. 2015, 114, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, A.; Rajaraman, V.; Maiti, S. Comparative analysis of various temporary computer-aided design/computer-aided manufacturing polymethyl methacrylate crown materials based on color stability, flexural strength, and surface roughness. J. Adv. Pharm. Technol. Res. 2022, 13 (Suppl. S1), S130–S135. [Google Scholar] [CrossRef]
- Nam, N.E.; Shin, S.H.; Lim, J.H.; Shim, J.S.; Kim, J.E. Effects of Artificial Tooth Brushing and Hydrothermal Aging on the Mechanical Properties and Color Stability of Dental 3D Printed and CAD/CAM Materials. Materials 2021, 14, 6207. [Google Scholar] [CrossRef] [PubMed]
- Bozoğulları, H.N.; Köroğlu, A.; Şahin, O.; İzzetağa, B. The Effect of the Toothbrushing and Surface Treatments on the Surface Roughness of Interim Crown Materials Used for Conventional, Subtractive, and Additive Manufacturing Techniques: An in Vitro Study. Selcuk. Dent. J. 2023, 10, 554–559. [Google Scholar] [CrossRef]
- Şen, D.; Göller, G.; İşsever, H. The effect of two polishing pastes on the surface roughness of bis-acryl composite and methacrylate-based resins. J. Prosthet. Dent. 2002, 88, 527–532. [Google Scholar] [CrossRef]
- Yildiz, P.; Güneş Ünlü, D. Farkli Yöntemler İle Kullanilan Geçici Materyallerinin Renk Stabilitesine Polisaj Patlarinin Etkisi. Selcuk. Dent. J. 2021, 8, 420–426. [Google Scholar] [CrossRef]
- Sattawatthamrong, S.; Kamonkhantikul, K.; Homsiang, W.; Arksornnukit, M. Effect of toothbrushing on surface roughness and gloss of CAD-CAM versus conventional interim materials with different surface treatments. J. Prosthet. Dent. 2025, 133, 282.e1–282.e12. [Google Scholar] [CrossRef] [PubMed]
- Gantz, L.; Fauxpoint, G.; Arntz, Y.; Pelletier, H.; Etienne, O. In vitro comparison of the surface roughness of polymethyl methacrylate and bis-acrylic resins for interim restorations before and after polishing. J. Prosthet. Dent. 2021, 125, 833.e1–833.e10. [Google Scholar] [CrossRef] [PubMed]
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J. A Comparison of the Flexural and Impact Strengths and Flexural Modulus of CAD/CAM and Conventional Heat-Cured Polymethyl Methacrylate (PMMA). J. Prosthodont. 2020, 29, 341–349. [Google Scholar] [CrossRef]
- Al-Thobity, A.M. The Impact of Polymerization Technique and Glass-Fiber Reinforcement on the Flexural Properties of Denture Base Resin Material. Eur. J. Dent. 2020, 14, 092–099. [Google Scholar] [CrossRef] [PubMed]
- Aysan, İ.; Uçar, Y.; Üşümez, A. Üç Farkli Kaide Materyalinin Farkli Solüsyonlardaki Renk Stabilitesinin Karşilaştirilmasi. Atatürk Üniv. Diş Hek. Fak. Derg. 2011, 21, 219–225. [Google Scholar]
- Lepri, C.P.; Palma-Dibb, R.G. Surface roughness and color change of a composite: Influence of beverages and brushing. Dent. Mater. J. 2012, 31, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Peñate, L.; Mercade, M.; Arregui, M.; Roig, M.; Basilio, J.; Cedeño, R. Color Stability of CAD/CAM Interim Material for Long-Term Fixed Dental Prostheses vs. Conventional Materials after Immersion in Different Staining Solutions. J. Compos. Sci. 2021, 5, 106. [Google Scholar] [CrossRef]
- Yao, Q.; Morton, D.; Eckert, G.J.; Lin, W.S. The effect of surface treatments on the color stability of CAD-CAM interim fixed dental prostheses. J. Prosthet. Dent. 2021, 126, 248–253. [Google Scholar] [CrossRef]
- Haselton, D.R.; Diaz-Arnold, A.M.; Dawson, D.V. Effect of Storage Solution on Surface Roughness of Provisional Crown and Fixed Partial Denture Materials. J. Prosthodont. 2004, 13, 227–232. [Google Scholar] [CrossRef]
- Bollenl, C.M.L.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Santos, M.; Soo, S.; Petridis, H. The effect of Parylene coating on the surface roughness of PMMA after brushing. J. Dent. 2013, 41, 802–808. [Google Scholar] [CrossRef]
- Rao, D.; Kalavathy, N.; Mohammad, H.; Hariprasad, A.; Kumar, C.R. Evaluation of the surface roughness of three heat-cured acrylic denture base resins with different conventional lathe polishing techniques: A comparative study. J. Indian Prosthodont. Soc. 2015, 15, 374. [Google Scholar] [CrossRef]
- Potluri, N.; Savitha, P.N. Comparison of two surface sealants on surface roughness and color stability of a commercially available denture base material—An in vitro study. IP Ann. Prosthodont. Restor. Dent. 2023, 9, 22–26. [Google Scholar]
- Takeuchi, C.Y.G.; Orbegoso Flores, V.H.; Palma Dibb, R.G.; Panzeri, H.; Lara, E.H.G.; Dinelli, W. Assessing the surface roughness of a posterior resin composite: Effect of surface sealing. Oper. Dent. 2003, 28, 281–286. [Google Scholar] [PubMed]
- Korkut, B.; Bud, M.; Kukey, P.; Sancakli, H. Effect of surface sealants on color stability of different resin composites. Med. Pharm. Rep. 2022, 95, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.C.C.; Barão, V.A.R.; Wee, A.G.; Alfaro, M.F.; Afshari, F.S.; Sukotjo, C. Effect of brushing and thermocycling on the shade and surface roughness of CAD-CAM ceramic restorations. J. Prosthet. Dent. 2018, 119, 1000–1006. [Google Scholar] [CrossRef]
- Goldstein, G.R.; Lerner, T. The effect of toothbrushing on a hybrid composite resin. J. Prosthet. Dent. 1991, 66, 498–500. [Google Scholar] [CrossRef]
- Aydın, N.; Karaoglanoglu, S.; Oktay, E.A. Investigation the effects of whitening toothpastes on color change of resin-based CAD/CAM blocks. J. Esthet. Restor. Dent. 2021, 33, 884–890. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, Y.; Li, S.; Yang, F.; Liang, S.; Xing, W. Effect of staining solutions on color and translucency stability of resin-composite computer-aided design and computer-aided manufacturing blocks. J. Am. Dent. Assoc. 2024, 155, 1012–1021. [Google Scholar] [CrossRef]
- Ghinea, R.; Pérez, M.M.; Herrera, L.J.; Rivas, M.J.; Yebra, A.; Paravina, R.D. Color difference thresholds in dental ceramics. J. Dent. 2010, 38, e57–e64. [Google Scholar] [CrossRef]
- Paravina, R.D.; Pérez, M.M.; Ghinea, R. Acceptability and perceptibility thresholds in dentistry: A comprehensive review of clinical and research applications. J. Esthet. Restor. Dent. 2019, 31, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Stawarczyk, B.; Vogt, K.; Hickel, R.; Edelhoff, D.; Reymus, M. Influence of cleaning methods after 3D printing on two-body wear and fracture load of resin-based temporary crown and bridge material. Clin. Oral. Investig. 2021, 25, 5987–5996. [Google Scholar] [CrossRef]
- Wechkunanukul, N.; Klomjit, K.; Kumtun, T.; Jaikumpun, P.; Kengtanyakich, S.; Katheng, A. Comparison of Mechanical and Surface Properties between Conventional and CAD/CAM Provisional Restorations. Eur. J. Dent. 2025, 19, 697–703. [Google Scholar] [CrossRef]
- Alt, V.; Hannig, M.; Wöstmann, B.; Balkenhol, M. Fracture strength of temporary fixed partial dentures: CAD/CAM versus directly fabricated restorations. Dent. Mater. 2011, 27, 339–347. [Google Scholar] [CrossRef]
- Souza, A.L.C.; de Oliveira Cruvinel Filho, J.L.; da Rocha, S.S. Flexural strength and Vickers hardness of milled and 3D-printed resins for provisional dental restorations. Braz. J. Oral. Sci. 2023, 22, e238439. [Google Scholar] [CrossRef]
- Farina, A.P.; Cecchin, D.; Soares, R.G.; Botelho, A.L.; Takahashi, J.M.F.K.; Mazzetto, M.O.; Mesquita, M.F. Evaluation of Vickers hardness of different types of acrylic denture base resins with and without glass fibre reinforcement. Gerodontology 2012, 29, e155–e160. [Google Scholar] [CrossRef]
- Rençber Kizilkaya, A.; Dursun, M.N. The Effect of Beverages and Polishing on Color Change of Different Temporary Prosthetic Materials: In Vitro Study. Selcuk. Dent. J. 2023, 10, 560–564. [Google Scholar] [CrossRef]
- Eslemez Topcu, E.; Şahin, O.; Köroğlu, A.; Cömert, F.; Yilmaz, B. Surface roughness and Streptococcus mutans adhesion on surface sealant agent coupled interim crown materials after dynamic loading. BMC Oral Health 2022, 22, 299. [Google Scholar] [CrossRef]
- Rizzante, F.A.; Bombonatti, J.S.; Vasconcelos, L.; Porto, T.S.; Teich, S.; Mondelli, R.F. Influence of resin-coating agents on the roughness and color of composite resins. J. Prosthet. Dent. 2019, 122, 332.e1–332.e5. [Google Scholar] [CrossRef]
- Mahrous, A.A.; Alhammad, A.; Alqahtani, F.; Aljar, Y.; Alkadi, A.; Taymour, N.; Alotaibi, A.; Akhtar, S.; Gad, M.M. The Toothbrushing Effects on Surface Properties and Color Stability of CAD/CAM and Pressable Ceramic Fixed Restorations—An In Vitro Study. Materials 2023, 16, 2950. [Google Scholar] [CrossRef] [PubMed]
- Radwan, H.; Elnaggar, G.; El Deen, I.S. Surface roughness and color stability of 3D printed temporary crown material in different oral media (In vitro study). Int. J. Appl. Dent. Sci. 2021, 7, 327–334. [Google Scholar] [CrossRef]
- Bezgin, T.; Özer, L.; Tulga Öz, F.; Özkan, P. Effect of Toothbrushing on Color Changes of Esthetic Restorative Materials. J. Esthet. Restor. Dent. 2015, 27 (Suppl. S1), S65–S73. [Google Scholar] [CrossRef] [PubMed]
- Şahin, O.; Köroğlu, A.; Dede, D.Ö.; Yıldırım, H.; Yağcı, Ü.; Erdal, S.G. Influence of Toothbrushing and Toothpaste on Surface Roughness and Color Stability of CAD/CAM Interim Crown Material. In Proceedings of the 48th EPA Annual Conference, Cappadocia, Türkiye, 11–13 September 2025; Abstract no. SS215. p. 102. [Google Scholar]


| Tests of Between-Subject Effects | ||||||
|---|---|---|---|---|---|---|
| Dependent Variable: Ra | ||||||
| Source | Type III Sum of Squares | df | Mean Square | F | p | Partial Eta Squared |
| Surface_treatment (A) | 1.479 | 1 | 1.479 | 443.273 | <0.001 | 0.779 |
| Brushing_agent (B) | 0.191 | 1 | 0.191 | 57.311 | <0.001 | 0.313 |
| Brushing_time (C) | 0.353 | 2 | 0.176 | 52.875 | <0.001 | 0.456 |
| A × B | 0.009 | 1 | 0.009 | 2.650 | 0.106 | 0.021 |
| A × C | 0.006 | 2 | 0.003 | 0.968 | 0.383 | 0.015 |
| B × C | 0.062 | 2 | 0.031 | 9.254 | <0.001 | 0.128 |
| A × B × C | 0.012 | 2 | 0.006 | 1.849 | 0.162 | 0.029 |
| Error | 0.420 | 126 | 0.003 | |||
| Total | 12.879 | 140 | ||||
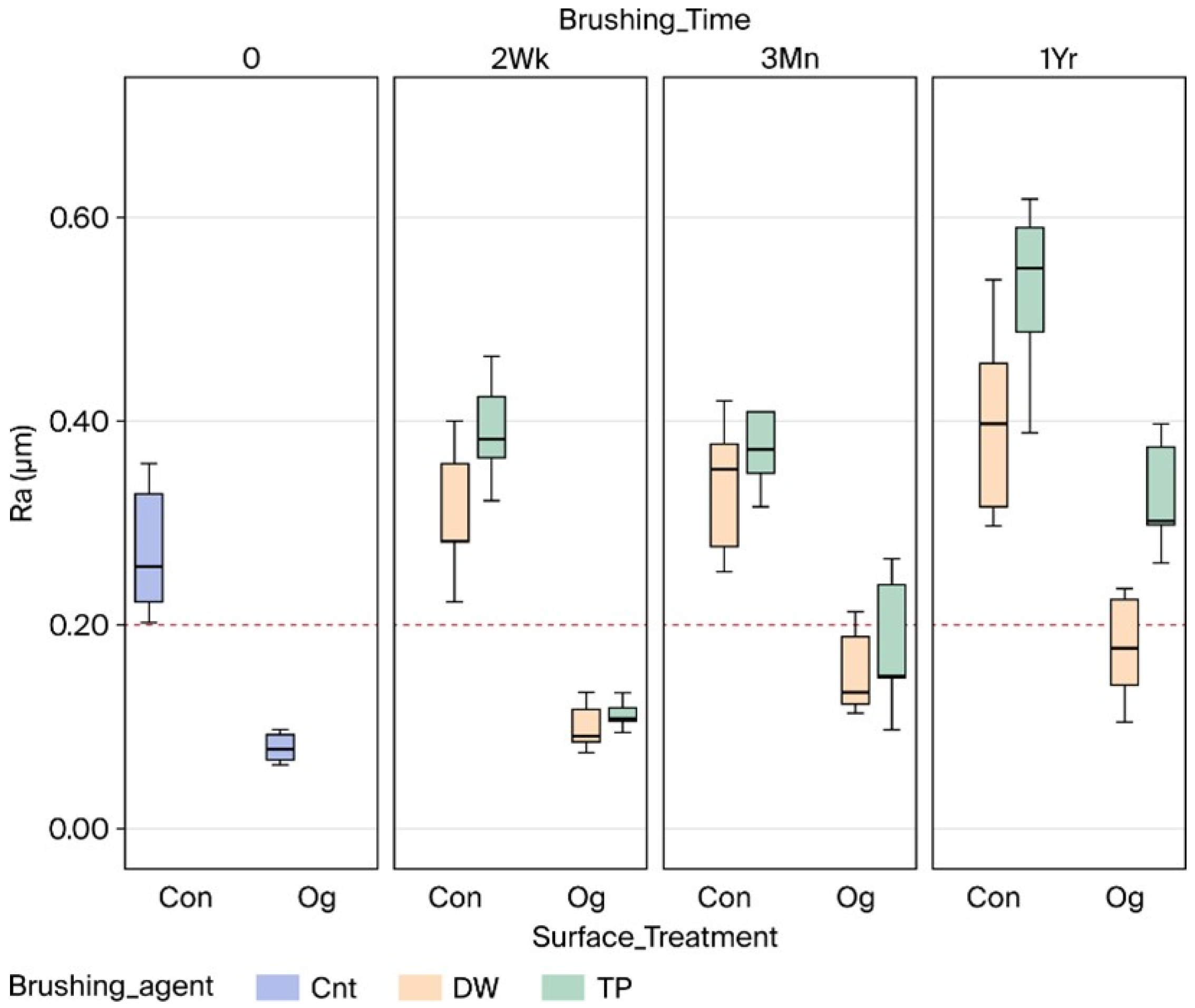
| Time | 0 (Ra0) | 2 Wk (Ra1) | 3 Mn (Ra1) | 1 Yr (Ra1) | Pooled | ||
|---|---|---|---|---|---|---|---|
| ST | BA | ||||||
| Con | Cnt | 0.28 ± 0.06 C | |||||
| Dw | 0.30 ± 0.06 C | 0.33 ± 0.06 CD | 0.40 ± 0.08 D | 0.34 ± 0.08 a | |||
| Tp | 0.39 ± 0.05 D | 0.40 ± 0.09 D | 0.53 ± 0.08 F | 0.44 ± 0.10 b | |||
| Pooled | 0.35 ± 0.07 # | 0.37 ± 0.08 # | 0.46 ± 0.1 ^ | ||||
| Og | Cnt | 0.08 ± 0.01 A | |||||
| Dw | 0.10 ± 0.02 A | 0.15 ± 0.04 AB | 0.17 ± 0.05 B | 0.14 ± 0.0 a | |||
| Tp | 0.11 ± 0.01 A | 0.17 ± 0.06 B | 0.33 ± 0.05 C | 0.20 ± 0.10 b | |||
| Pooled | 0.11 ± 0.02 # | 0.16 ± 0.05 # | 0.25 ± 0.09 ^ | ||||
| Tests of Between-Subject Effects | ||||||
|---|---|---|---|---|---|---|
| Dependent Variable: ΔE00 | ||||||
| Source | Type III Sum of Squares | df | Mean Square | F | p | Partial Eta Squared |
| Surface_treatment (A) | 0.902 | 1 | 0.902 | 42.547 | <0.001 | 0.252 |
| Brushing_agent (B) | 0.327 | 1 | 0.327 | 15.442 | <0.001 | 0.109 |
| Brushing_time (C) | 4.437 | 2 | 2.218 | 104.625 | <0.001 | 0.624 |
| A × B | 0.000 | 1 | 0.000 | 0.009 | 0.924 | 0.000 |
| A × C | 0.247 | 2 | 0.123 | 5.820 | 0.004 | 0.085 |
| B × C | 0.151 | 2 | 0.076 | 3.563 | 0.031 | 0.054 |
| A × B × C | 0.033 | 2 | 0.017 | 0.787 | 0.457 | 0.012 |
| Error | 2.671 | 126 | 0.021 | |||
| Total | 137.326 | 140 | ||||
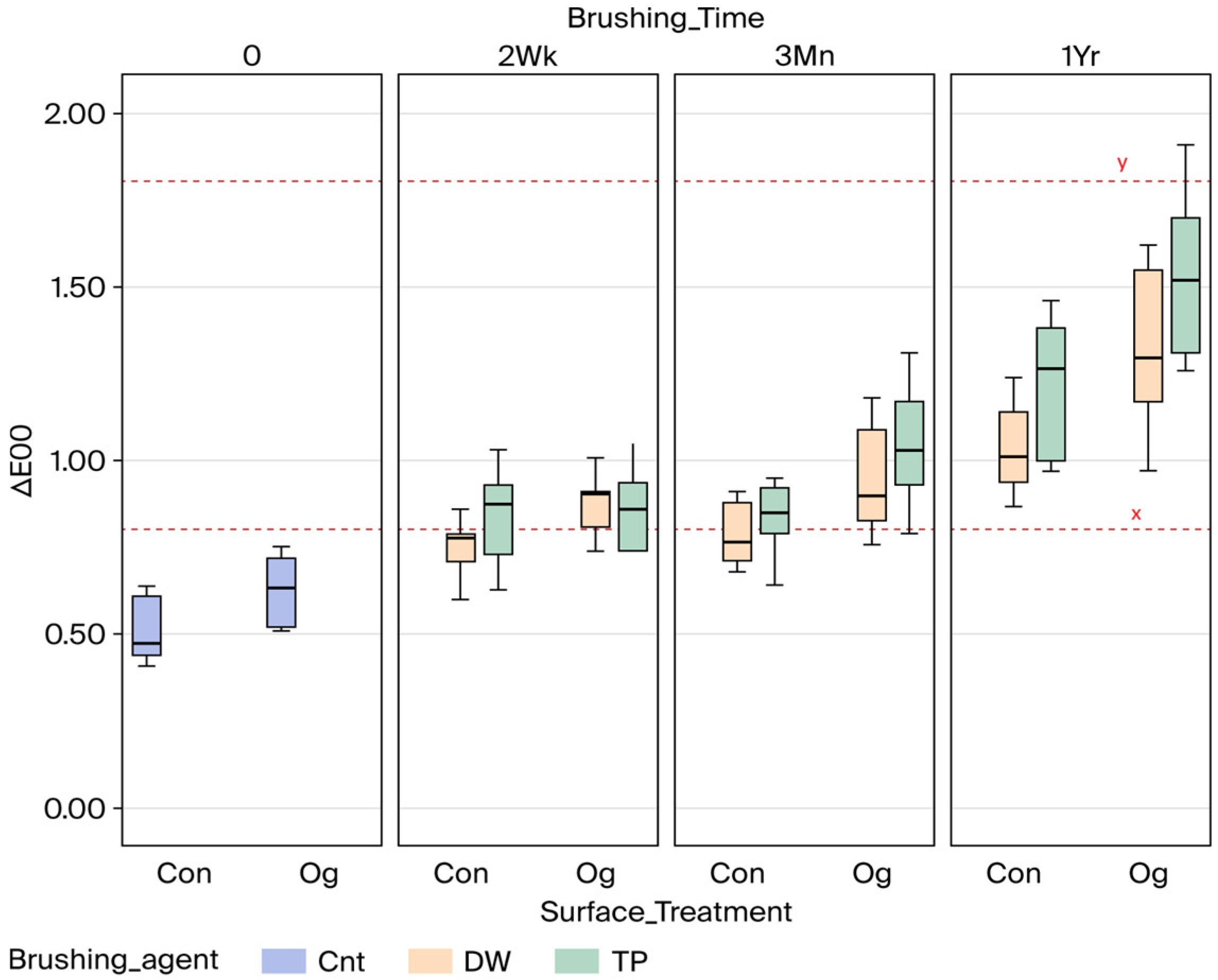
| Time | 0 | 2 Wk | 3 Mn | 1 Yr | Pooled | |
|---|---|---|---|---|---|---|
| ST | BA | |||||
| Con | Cnt | 0.51 ± 0.09 A | ||||
| Dw | 0.77 ± 0.15 B | 0.79 ± 0.09 B | 1.04 ± 0.12 C | 0.87 ± 0.17 a | ||
| Tp | 0.85 ± 0.13 B | 0.83 ± 0.11 B | 1.22 ± 0.18 C | 0.97 ± 0.23 b | ||
| Pooled | 0.81 ± 0.14 # | 0.81 ± 0.10 # | 1.13 ± 0.18 ^ | |||
| Og | Cnt | 0.63 ± 0.10 A | ||||
| Dw | 0.88 ± 0.08 B | 0.94 ± 0.15 BC | 1.31 ± 0.22 D | 1.05 ± 0.25 a | ||
| Tp | 0.87 ± 0.12 B | 1.05 ± 0.17 C | 1.53 ± 0.22 D | 1.15 ± 0.33 b | ||
| Pooled | 0.88 ± 0.10 # | 1.00 ± 0.16 # | 1.42 ± 0.24 ^ | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şahin, O.; Köroğlu, A.; Dede, D.Ö.; Yıldırım, H.; Yağcı, Ü.; Erdal, S.G. Effect of Different Surface Treatments and Toothbrushing Durations on Surface Roughness and Color Stability of CAD/CAM Interim Crown Material. Coatings 2025, 15, 1377. https://doi.org/10.3390/coatings15121377
Şahin O, Köroğlu A, Dede DÖ, Yıldırım H, Yağcı Ü, Erdal SG. Effect of Different Surface Treatments and Toothbrushing Durations on Surface Roughness and Color Stability of CAD/CAM Interim Crown Material. Coatings. 2025; 15(12):1377. https://doi.org/10.3390/coatings15121377
Chicago/Turabian StyleŞahin, Onur, Ayşegül Köroğlu, Doğu Ömür Dede, Hüsniye Yıldırım, Ünsun Yağcı, and Selda Gökçe Erdal. 2025. "Effect of Different Surface Treatments and Toothbrushing Durations on Surface Roughness and Color Stability of CAD/CAM Interim Crown Material" Coatings 15, no. 12: 1377. https://doi.org/10.3390/coatings15121377
APA StyleŞahin, O., Köroğlu, A., Dede, D. Ö., Yıldırım, H., Yağcı, Ü., & Erdal, S. G. (2025). Effect of Different Surface Treatments and Toothbrushing Durations on Surface Roughness and Color Stability of CAD/CAM Interim Crown Material. Coatings, 15(12), 1377. https://doi.org/10.3390/coatings15121377






