1. Introduction
As a pillar industry of the national economy, the rapid development of the iron and steel industry is accompanied by the storage of a large amount of steel slag waste generated by steelmaking, which has become an urgent environmental problem to be solved. A large amount of steel slag not only encroaches on land resources, but also causes increasingly serious environmental hazards such as air pollution and water pollution [
1]. According to statistics, the cumulative stock of steel slag has continued to rise in the past decade, but its comprehensive utilization rate is still less than 30%. In this context, it is very important to promote the efficient resource utilization of steel slag [
2] for sustainable development and environmental protection.
The chemical composition of steel slag is complex, mainly including CaO, Fe
2O
3, MnO, SiO
2, MgO, P
2O
5, Al
2O
3, etc., and the composition fluctuates greatly. Its mineral composition is mainly composed of tricalcium silicate (C
3S), dicalcium silicate (C
2S), free calcium oxide (f-CaO), free magnesium oxide (f-MgO), and RO phase (solid solution of MgO, FeO, MnO, etc.). Among them, f-CaO and f-MgO are hydrated to form Ca(OH)
2 and Mg (OH)
2, respectively, with significant volume expansion (expansion rates of about 98% and 148%, respectively) [
3]. This expansion characteristic leads to poor volume stability of steel slag-based materials, which seriously restricts the large-scale application of steel slag fine aggregate in building cementitious materials and road engineering. Therefore, the effective digestion of f-CaO and f-MgO can solve the problem of volume stability, which is the core goal of realizing its high-value-added resource utilization. At present, many scholars are focusing on improving the volume stability of steel slag fine aggregate [
4,
5,
6].
The early methods to improve the stability of steel slag mainly include traditional treatment processes (such as hot stuffing method, drum method, granulation method, etc.) and physical/chemical modification technologies (such as grinding, chemical excitation [
7]). The traditional process generally has the disadvantages of high energy consumption, heavy pollution, uneven particle size, and low activity. Among them, only the heat stew method performs relatively better in terms of stability. Although physical or chemical modification can partially improve volume stability, it often faces challenges such as high cost and unstable effects. It is of great practical significance to develop green, efficient, and low-cost steel slag stability control technology, especially for the application requirements of fine aggregate. Seifritz proposed that mineral carbonation [
8] provides a new way to improve the stability of steel slag [
9]. As a solid waste with strong alkalinity and rich calcium, the carbonation process of steel slag (the reaction formula is shown by Equations (1) and (2) can not only realize the permanent storage of CO
2, but also effectively eliminate the expansion of f-CaO and f-MgO [
10,
11]. Carbonization technology is mainly divided into direct carbonization (dry/wet) and indirect carbonization [
12]. The dry carbonization process is simple, but the carbonization efficiency is low [
13]; by virtue of efficient mass transfer in the liquid phase environment, wet carbonization improves the reaction rate and CO
2 fixation [
14], but the process is complex, and energy consumption is high. Although the reaction rate and product quality of indirect carbonization are better [
15], the process is more complex and costly.
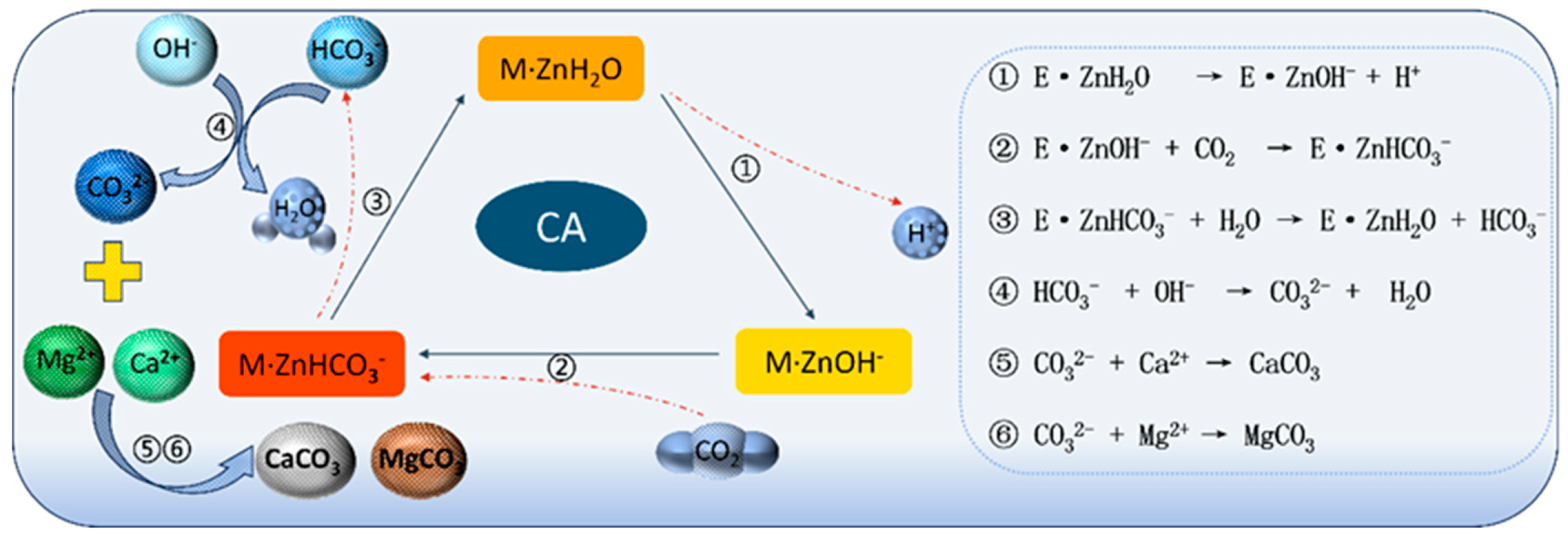
In recent years, microbially induced carbonate precipitation (MICP) refers to biologically assisted formation of CaCO
3/MgCO
3 via enhanced CO
2 hydration and carbonate generation, followed by nucleation on mineral surfaces. In practice, MICP commonly employs ureolytic strains or carbonic-anhydrase (CA)–producing
Bacillus to accelerate the conversion of dissolved CO
2/HCO
3−/CO
32− and to promote heterogeneous nucleation on porous substrates [
16,
17,
18]. Compared with purely physicochemical carbonation, MICP offers a greener route with mild conditions and self-selective nucleation on defects and rough surfaces, which helps fill pores and bind particles; however, its efficiency can be limited by the availability and transport of Ca
2+/Mg
2+ and by mass-transfer barriers in dense matrices [
16,
17,
18,
19,
20,
21]. This technology uses the metabolic activities of specific microorganisms (such as urease bacteria and carbonic anhydrase bacteria) to promote CO
2 hydration and precipitation of carbonate minerals. Among them, carbonic anhydrase (CA), as a widely existing metal enzyme, can efficiently catalyze the hydration reaction of CO
2, and its mineralization mechanism (as shown in
Figure 1) provides a new path for the modification of steel slag fine aggregate. Relevant studies have shown that microbial mineralization can accelerate the dissolution of Ca
2+/Mg
2+ ions in steel slag and their combination with CO
32− to form carbonate precipitates (CaCO
3/MgCO
3), thereby effectively reducing the expansion f-CaO/f-MgO content and filling and compacting the pore structure of the material. For example, Qian Chunxiang [
18,
19], etc., through the action of microorganisms, the fixation rate of CO
2 in steel slag fine aggregate is significantly improved, and the fixation rate of CO
2 reaches about 10%. It promotes the formation of carbonate precipitation. The linear expansion rate of the steel slag cementitious material is reduced to less than 0.05%, the compressive strength is increased to more than 40 MPa, and the engineering application shows good volume stability.
However, the existing research on the improvement of steel slag performance by microbial mineralization mostly focuses on the cementitious material or powder level, and the research on the optimization of process conditions to improve volume stability at the fine aggregate scale of steel slag is still insufficient. There are differences between fine aggregate and cementitious material in particle size, reaction environment, and performance evaluation criteria. There may be deviations in directly extrapolating the conclusions of the cementitious material level to fine aggregate. In order to solve the long-standing volume stability problem of steel slag fine aggregate (SSFA), several competitive carbonation routes have been reported in the literature. Alkali-promoted accelerated carbonization (such as NaOH) can enhance the dissolution of Ca2+/Mg2+ and shorten the carbonization time, but it usually relies on strong alkali reagents and requires post-treatment of highly alkaline waste liquid, and is more effective at the powder scale rather than the aggregate scale. In contrast, hydrothermal/autoclaved carbonization can improve the reaction kinetics and carbonate crystallization by heating (steam) and pressurization, and can obtain a good stabilization effect, but it has high energy consumption and requires special equipment. In order to solve the long-standing volume stability problem of steel slag fine aggregate (SSFA), a modified route of boiling pretreatment combined with microbial mineralization (CA-MICP) was studied in this paper, and the process window with engineering feasibility was determined. We compared direct carbonization, MICP alone, and their synergistic effect with boiling pretreatment, and investigated the conversion of expansive f-CaO/f-MgO, CO2 absorption/carbonization rate, and mortar linear expansion rate. Eight groups (SS, K1–K8) were set at 0.3 MPa: containing/not containing 100 h of boiling, followed by carbonization or CA-mediated MICP for 3 h or 12 h. It was quantified by f-CaO/f-MgO titration, linear expansion test, XRD, SEM, FTIR, TG-DTG, BET, and other characterizations to compare different treatments and determine the optimal working conditions. The research framework also provides the mechanism basis and practical parameters for stabilizing SSFA using ‘boiling enhanced CA-MICP’. Promote the high-value utilization of steel slag in the field of concrete aggregate and road engineering aggregate.
2. Materials and Methods
2.1. Material
2.1.1. Steel Slag Fine Aggregates
The steel slag used in this study is from the Fuda Mine Iron and Steel Plant in Lingshou County, Shijiazhuang City, Hebei Province. The basicity is a key index to characterize the chemical properties of steel slag. It is usually expressed by the ratio of the mass fraction of alkaline oxide to acidic oxide, as shown in Equation (3). The basicity (R, calculated by Equation (3) of steel slag is calculated to be about 3.3. In order to evaluate the variability of components and the applicability of the method to other types of steel slag, three kinds of steel slag fine aggregates were selected in addition to the above steel slag fine aggregates.
Table 1 shows that the main oxides of these four steel slags have a wide range (CaO 23.90%–37.20%, Fe
2O
3 14.00%–37.06%, SiO
2 9.84%–16.10%, MgO 2.99%–5.49%), covering the representative medium–high alkalinity chemical composition in engineering practice. The ‘boiling + CA-MICP’ route is dominated by the reserves and mobility of Ca
2+/Mg
2+ and the digestion of expansive f-CaO/f-MgO; hence, it is effective in the above different component systems. The boiling step can enhance ion release and pore accessibility regardless of iron content and matrix difference. CA-mediated mineralization provides a rapid carbonate supply and heterogeneous nucleation, which supports the process to be extended to a variety of steel slag types without being limited by a single source. This experiment is not limited to a certain factory or a certain type of steel slag. The CaO/MgO content of the steel slag selected in the experiment is high, and the stability is poor, which is the most representative.
Table 1 shows the main oxide content in the chemical composition of steel slag determined by X-ray fluorescence spectrometry (XRF). In the experiment, steel slag with a particle size of 2.36 mm–4.75 mm was selected; its water absorption rate was 2.67%, and its apparent density was 3480 kg/m
3.
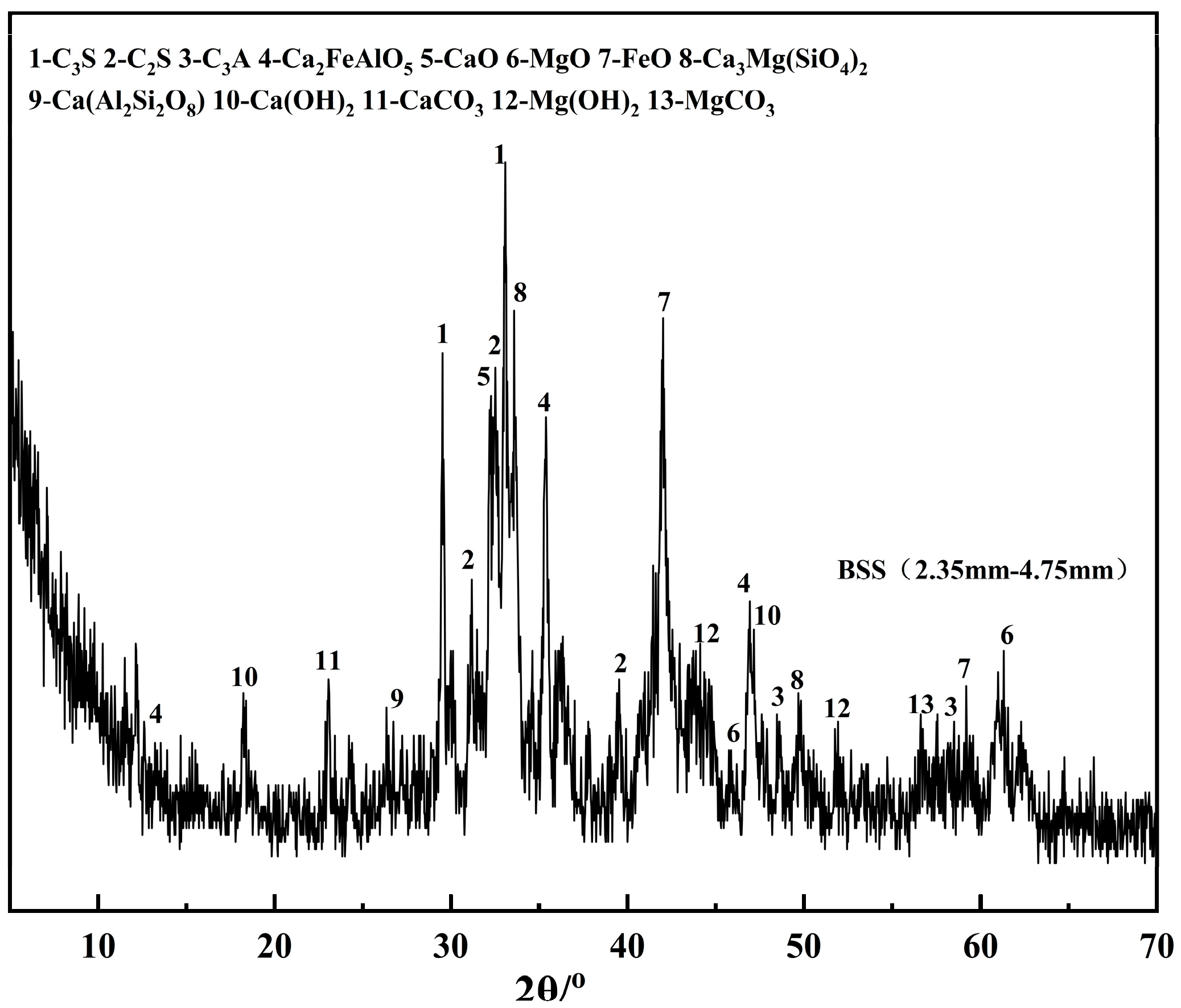
Figure 2 shows that the surface of steel slag fine aggregate is more porous and rough. The steel slag fine aggregate was analyzed by X-ray diffraction (XRD), and the results are shown in
Figure 3.
2.1.2. Microorganism and Microbial Solution Culture
The microorganism used in this study is
Bacillus mucilaginosus bio-51767 (internal collection number). It is an alkali-resistant, Gram-positive, spore-forming bacterium that is known to secrete carbonic anhydrase (CA). The nearly full-length 16S rRNA gene sequence (~1.5 kb) was compared with reference sequences in the NCBI GenBank database using BLASTn (
https://blast.ncbi.nlm.nih.gov/, accessed on 16 September 2025), which supported its identification as
Bacillus mucilaginosus [
22]. The cells were resuscitated from frozen strains and cultured in alkaline medium (initial pH 9.5–10.5) at 30 °C and 180 rpm. Using the bacterial powder with well-configured nutrients as the raw material, the bacterial powder is activated by a shaker, and the carbonic anhydrase secreted by the bacterial powder can catalyze the hydration reaction of carbon dioxide [
23]. The preparation process of the microbial solution is shown in
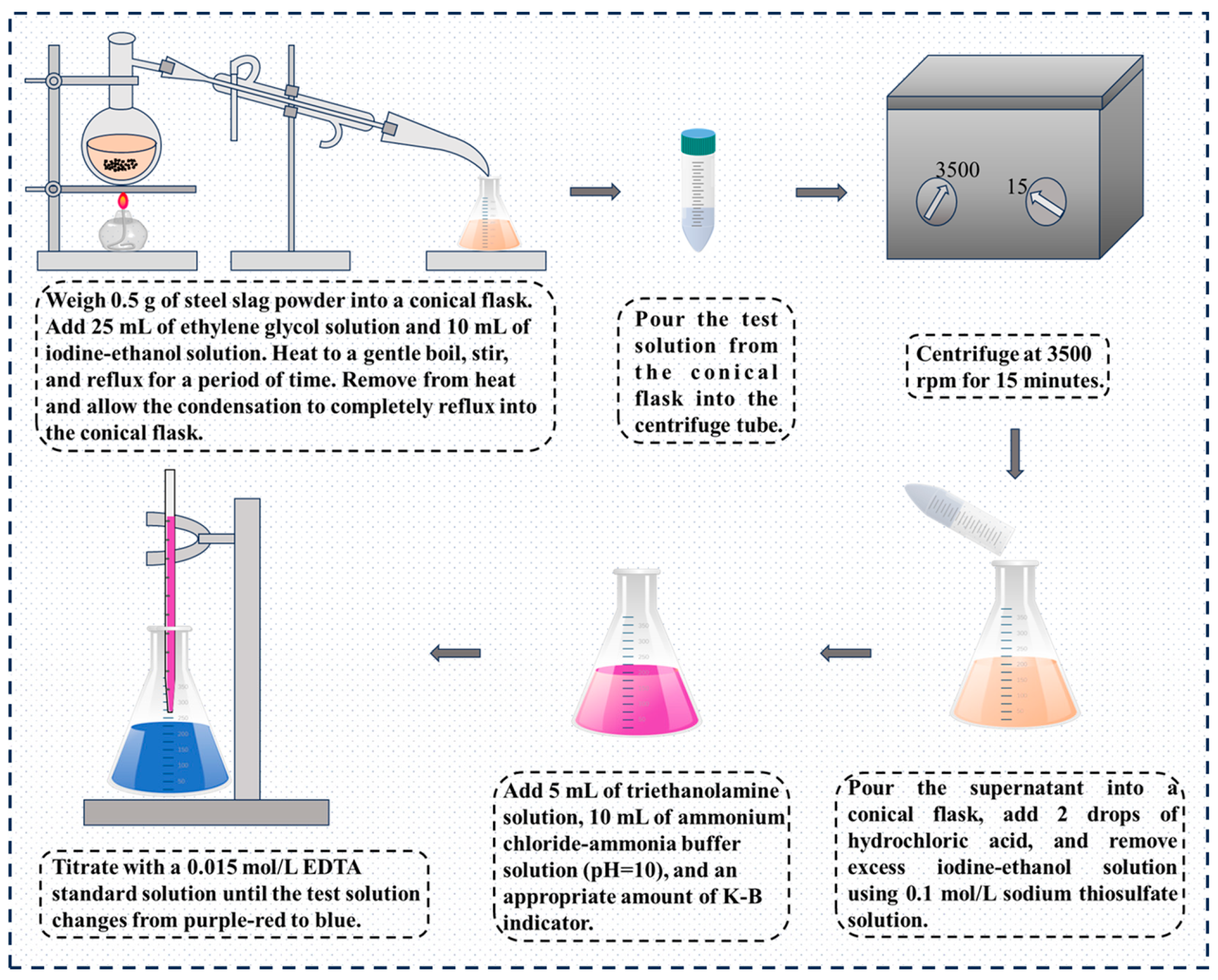
Figure 4. The specific steps are as follows: 15 g of bacterial powder and 500 mL of distilled water are added to the conical flask, resurrected in a shaker at 30 °C and 180 r/min for 24 h, and the inorganic carrier is filtered to obtain the bacterial solution, that is, the required bacterial solution is obtained.
2.1.3. Water
The water used in the microbial culture process is deionized water, and the water used in other processes is tap water.
2.2. Modification Techniques
Previous exploration experiments showed that the effect of carbonization treatment at normal temperature and pressure was not significant [
17,
24,
25]. In order to improve the reaction efficiency, this study used the method of boiling pretreatment combined with microbial mineralization under 0.3 MPa pressure to explore the carbonization, microbial mineralization, and boiling pretreatment. The individual and synergistic effects of factors on carbonization efficiency and volume stability, this study designed eight groups of control experiments (
Table 2). CO
2 (99% purity) was supplied from a commercial compressed-gas vendor in cylinders and used as delivered to maintain 0.3 MPa during carbonation/mineralization tests. Due to the limited carbonation conversion under environmental conditions, in order to obtain sufficient gas–liquid–solid mass transfer under mild and scalable conditions, we fixed the CO
2 pressure at 0.3 MPa and maintained the temperature/relative humidity at about 20 °C/ ≈ 70%. The pretreatment of 100 h boiling was selected for sufficient water to combine and consume the expansive f-CaO/f-MgO and reconstruct the pore network, thereby providing a higher specific surface area and ion accessibility for subsequent carbonic anhydrase (CA)-mediated biomineralization. Keeping the pressure constant helps us to focus on the synergistic effect of ‘boiling + CA-MICP’, and quantify the performance response in two dimensions of treatment (with/without boiling) and biomineralization time (3 h vs. 12 h), avoiding the confounding effects of pressure variables. The modification process of steel slag fine aggregate is shown in
Figure 5, and experiments are carried out under different modification process conditions. The boiling pretreatment time is short, and the expansive material content of the steel slag fine aggregate is partially digested. Therefore, the pretreatment time is selected as 100 h to ensure the full hydration of free oxides and create the best conditions for subsequent microbial mineralization. After boiling pretreatment, the sample is naturally cooled and washed to neutral, and then subjected to microbial mineralization treatment to ensure the stability of bacterial activity. The macroscopic properties and microstructure of the modified samples were characterized, and the performance changes of steel slag fine aggregate under different treatment conditions were clarified.
2.3. Mix Proportion Design of Steel Slag Fine Aggregate Mortar
The design results of the natural mortar mix ratio are shown in
Table 3. Due to the different density between steel slag and natural sand, ρ
steel slag = 3.48 g/cm
3, ρ
sand = 2.63 g/cm
3, according to Equation (4).
It is concluded that m
steel slag = 1786 g, and the mix design results of steel slag mortar shown in the following table are shown in
Table 4.
Table 3.
Natural mortar mix ratio design results.
Table 3.
Natural mortar mix ratio design results.
| Water (g) | Cement (g) | Sand (g) |
|---|
| 225 | 450 | 1350 |
Table 4.
Mix proportion design results of steel slag fine aggregate mortar.
Table 4.
Mix proportion design results of steel slag fine aggregate mortar.
| Water (g) | Cement (g) | Steel Slag (g) |
|---|
| 225 | 450 | 1786 |
2.4. Linear Expansion Rate Test
For the steel slag fine aggregate treated by different modification processes, it was molded into a steel slag mortar specimen with a size of 25 mm × 25 mm × 280 mm. After curing for a certain period of time (7 days) in a standard curing environment, the initial length L0 was measured by a specific length meter. The specimen was then placed in a boiling tank for water bath heating, and the length L1 of the specimen was measured every 5 h until the specimen broke. The linear expansion rate LA of steel slag mortar is calculated according to Equation (5):
In the formula, the linear expansion rate LA unit is %, 280 is the original length unit of the specimen in mm. In order to improve the reliability of the experimental results, each group of samples was subjected to three independent tests, and three repeated measurements were performed in each test. The final results were taken as the average of all measured values. The test instrument of the length meter is shown in
Figure 6.
2.5. The Contents of f-CaO and f-MgO Were Determined by EDTA Titration
For the determination of f-CaO content in steel slag, according to GB/T 38216.3-2023 ‘determination of free f-CaO content in steel slag-EDTA titration method and thermogravimetric analysis method’ standard [
26], the EDTA titration method was used for titration. The specific operation process is shown in
Figure 7.
The total mass fraction of f-CaO and Ca(OH)
2 is calculated by Equation (6).
In the formula, w1 is the mass fraction (%) of the total f-CaO and Ca(OH)2; TCaO is equivalent to the mass of CaO per milliliter of EDTA standard solution, in units of (mg/mL); V is the volume of EDTA standard titration solution consumed during titration (mL); m is the mass of the sample (g); 1000 is the unit conversion coefficient.
The content of Ca(OH)
2 was determined by thermogravimetric analysis. The mass loss percentage of the weight loss steps observed in the temperature range of 400 °C to 500 °C is recorded as w
2. w
3 is the mass fraction % of Ca(OH)
2; 4.1111 is the ratio of Ca(OH)
2 to water molecular weight; 0.7567 is the molecular weight ratio of CaO to Ca(OH)
2; the content w
3 of Ca(OH)
2 in steel slag was calculated by Equation (7), and the content w of f-CaO was calculated according to Equation (8).
The determination process of f-MgO content is shown in
Figure 8, and its content is calculated according to Equation (9):
In the formula, WMgO denotes the mass fraction (%) of f-MgO; TMgO is equivalent to the mass of MgO per milliliter of EDTA standard solution, in units of (mg/mL); V2 and V are the volume of EDTA solution (mL) consumed when titrating the total amount of calcium and magnesium and titrating CaO alone, respectively; m is the sample mass (g); 1000 is the conversion coefficient of mass unit (mg and g).
Figure 8.
Flow chart for the determination of f-MgO content.
Figure 8.
Flow chart for the determination of f-MgO content.
2.6. Determination of CO2 Absorption Rate and Carbonation Rate
According to the mass loss of 600 °C–800 °C on the thermogravimetric curve, the CO
2 absorption rate and carbonization rate were calculated according to Equations (10) and (11), respectively.
In the formula, w1 is the mass loss of CO2 (%) of steel slag fine aggregate at 600 °C–800 °C; c is the carbonation rate (%); m1 is the molar mass of CO2 (44 kg/mol); m2 is the molar mass of CaO (56 kg/mol); w2 is the CaO content (%) in the untreated steel slag.
2.7. Macroscopic Performance Test
After modification of steel slag fine aggregate, the macroscopic properties of linear expansion rate were determined according to ‘steel slag powder used in cement and concrete’ (GB/T 20491-2017) [
27] and ‘steel slag stability test method’ (GB/T24175-2009) [
28].
2.8. Microscopic Characterization of Materials
SEM and EDS: The prepared steel slag fine aggregate was immersed in anhydrous ethanol for 24 h and taken out. It was dried at 45 °C under vacuum to remove free water, and then the sample was gold-plated. The microstructure of the modified steel slag fine aggregate was analyzed by scanning electron microscopy (SEM), and the mineral distribution and element composition of the sample were analyzed by EDS. The high-resolution field emission scanning electron microscope of Apero S Hi from The microstructure of the modified steel slag fine aggregate was analyzed by a field-emission scanning electron microscope Apreo 5 Hi from Thermo Scientific (Thermo Fisher Scientific Inc., Waltham, MA, USA). Equipment parameters: high vacuum: 1.0 nm (SE) at 30 kV; 3.0 nm (SE) low vacuum at 1 kV: 1.4 nm (SE) at 30 kV; ambient vacuum (ESEM) −1.4 nm (SE) at 30 kV.
XRD: Steel slag fine aggregate was dried to constant weight at 65 °C. The dried steel slag was ground to 80 μm for XRD analysis. The mineral composition of steel slag fine aggregate was qualitatively analyzed by the D8-02 polycrystalline X-ray diffractometer produced by Bruker-AXS (Bruker AXS GmbH, Karlsruhe, Germany). The equipment parameters are as follows: 2θ range of 5~90°, scanning speed of 10(°)/min, step length of 0.02°, and angle measurement accuracy of 2θ ≤ ± 0.01°.
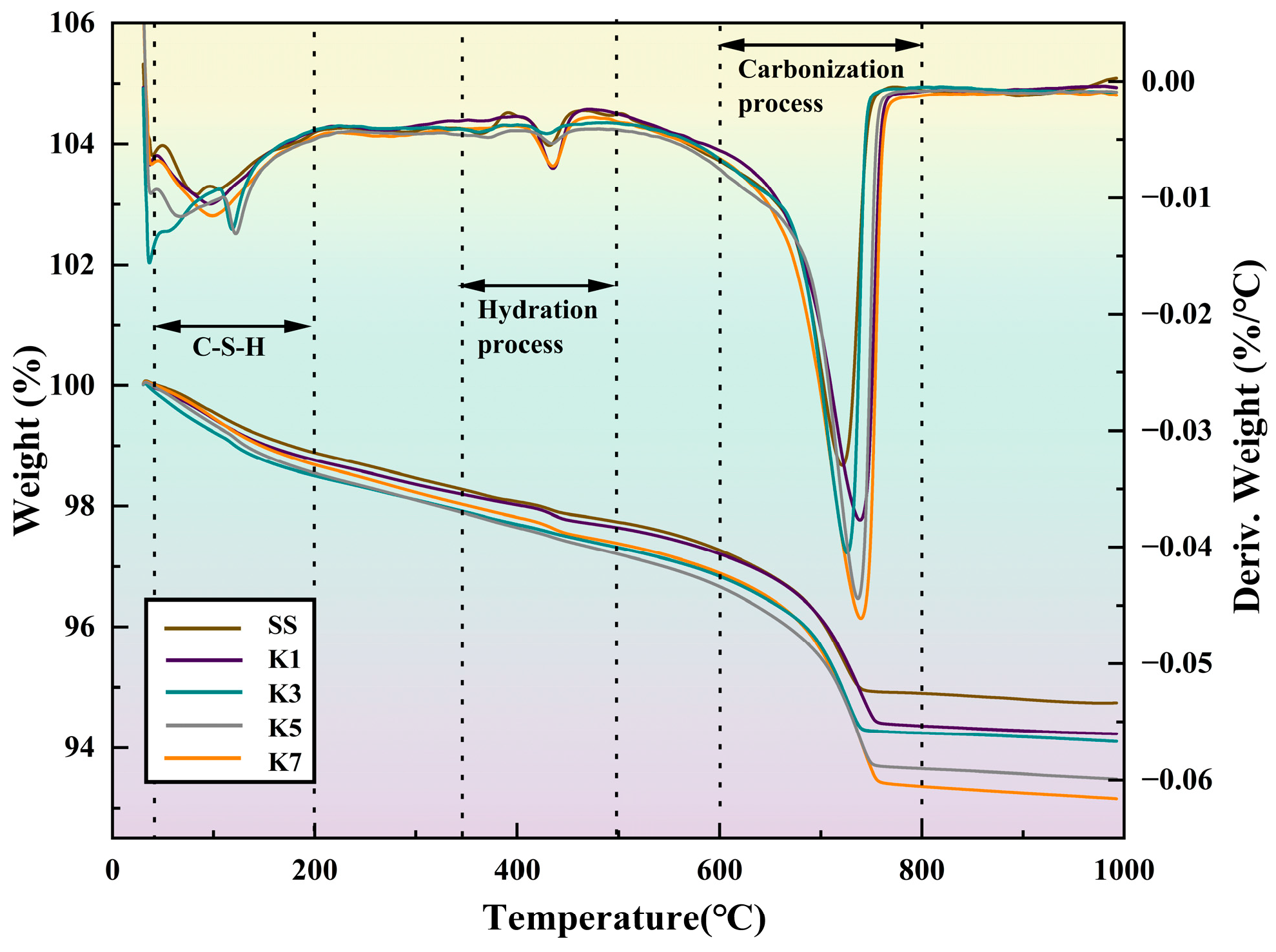
TG and DTG: Qualitative and quantitative analyses of steel slag fine aggregate treated by different modification methods were carried out by a comprehensive thermal analyzer produced by TA Instruments (TA Instruments, New Castle, DE, USA). Equipment parameters: the temperature range is 25 °C~1400 °C, the heating rate is 0.1~50 K/min, the weighing range is 0~5 g, and the balance sensitivity is 0.1 μg.
FTIR: The steel slag fine aggregate was ground to a particle size of 200 mesh as a sample. The samples were placed in a drying oven and dried at 65 °C. Then the samples were qualitatively analyzed by an infrared spectrometer. In this experiment, using a Nicolet iS50 Fourier transform infrared spectrometer produced by Nicolet Instruments (Thermo Nicolet Corporation, Madison, WI, USA). The resolution is better than 0.09 cm−1, the spectral range is 7800~50 cm−1, and the wavenumber accuracy is 0.01 cm−1.
BET: Before the test, the samples were degassed at 105 °C for 6 h to remove the water and impurities adsorbed on the surface by an ASAP 2460 automatic specific surface area and pore analyzer produced by Micromeritics Instrument Corporation (Micromeritics Instrument Corp., Norcross, GA, USA). The specific surface area, pore volume, and pore size distribution of steel slag fine aggregate were determined by the nitrogen adsorption–desorption method.
3. Results and Discussion
3.1. Boiling Pretreatment Accelerates the Hydration Transformation and Microstructure Reconstruction of f-CaO/f-MgO
The role of boiling pretreatment is to quickly and efficiently digest the key component-f-CaO/f-MgO in steel slag, which leads to poor volume stability. In the boiling water environment at 100 °C, the thermal motion of water molecules is intensified, which can quickly penetrate into the steel slag particles and accelerate the hydration reaction of f-CaO/f-MgO. The EDTA titration results showed that the contents of f-CaO and f-MgO in the original steel slag (SS group) without any treatment were 2.01% and 1.13%, after only 100 h of boiling pretreatment (non-K8 group), the contents decreased to 0.91% and 0.68%, respectively, which decreased by 54.73% and 39.83%, respectively, compared with the SS group. The above data proved that the boiling process has converted most of the unstable free oxides into Ca(OH)2 and Mg(OH)2 with relatively stable physical properties.
The XRD pattern of
Figure 9 provides direct phase evidence for the transformation of the above components. Compared with SS, the f-CaO/f-MgO characteristic peaks (such as 2θ ≈ 37.3°, 46.4°, 53.8°, 61.8°) of boiled 100 h were significantly weakened or disappeared, while the Ca(OH)
2/Mg(OH)
2 peaks (such as 2θ ≈ 18.0°, 34.1°, 44.3°) were significantly enhanced, indicating that hydration transformation occurred during boiling. The change of carbonate-related peaks is consistent with the subsequent mineralization behavior.
The volume stability of Ca(OH)2 and Mg(OH)2 is much better than that of f-CaO and f-MgO, and the volume stability is greatly improved. Secondly, the solubility of the generated Ca(OH)2 and Mg(OH)2 is much higher than that of f-CaO and f-MgO, and it is easier to precipitate Ca2+ and Mg2+ in the subsequent mineralization environment, which provides sufficient and easy-to-use metal ions for microbial-induced carbonate precipitation and accelerates the mineralization reaction rate.
Boiling pretreatment not only changed the chemical composition of steel slag but also reconstructed the microstructure. It was proved by the BET specific surface area and pore analysis [
20,
29]. As shown in
Table 5, the specific surface area of the original steel slag is only 1.0152 m
2/g, and the porosity is 0.001821 cm
3/g; after 100 h boiling pretreatment, the specific surface area increased to 1.7496 m
2/g, and the porosity increased to 0.003097 cm
3/g. This significant structural change is intuitively confirmed by SEM in the adsorption–desorption curves of
Figure 10 and
Figure 11: the section of untreated SS group steel slag is relatively dense, with only a small amount of edges and cracks caused by crushing; the surface and internal pores of the K8 group of samples pretreated by boiling pretreatment combined with microbial mineralization were tightly filled with a large number of spherical clusters and flake calcium carbonate crystals, forming a more compact composite structure.
From
Figure 10 and
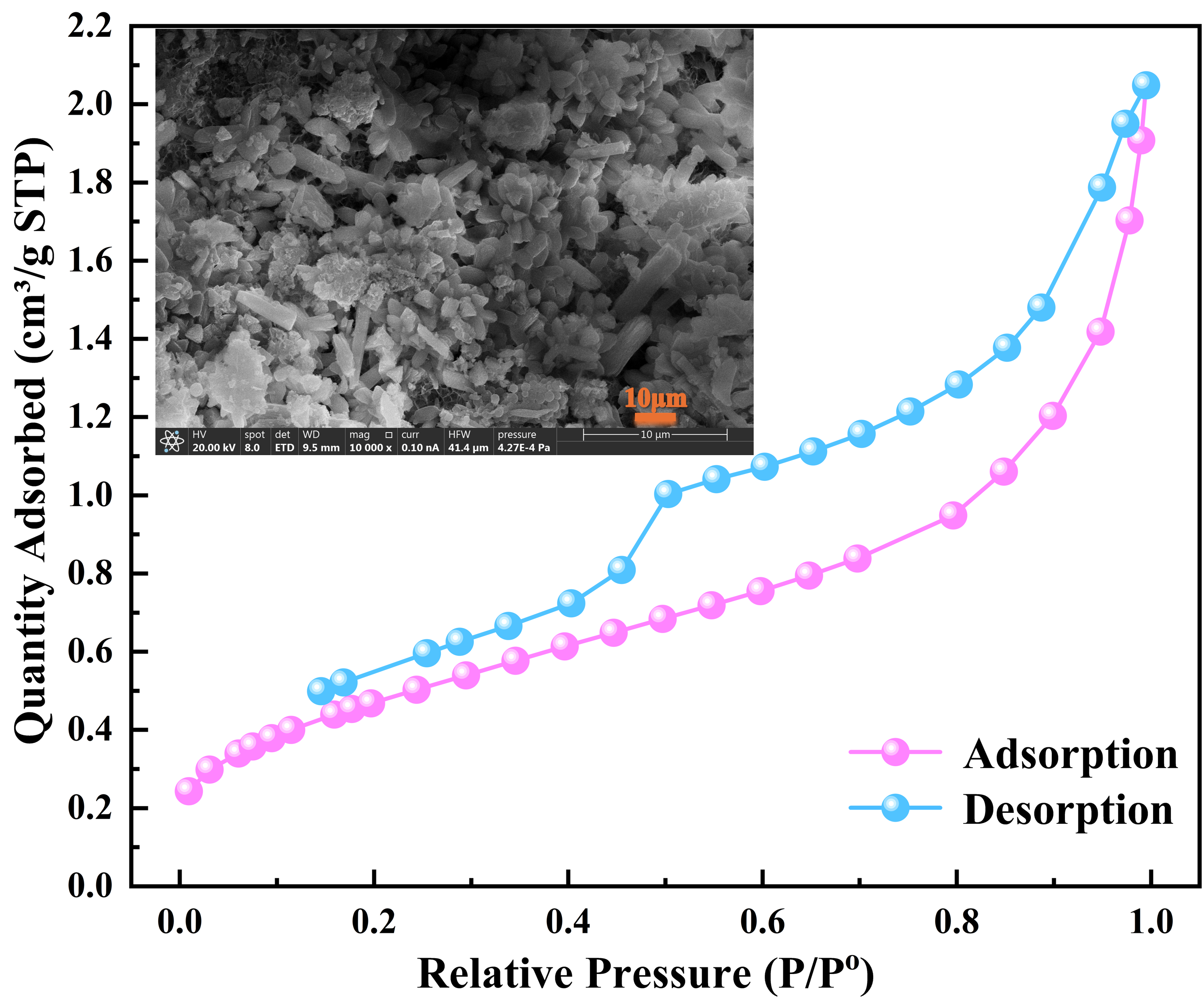
Figure 11, it can be seen that after boiling pretreatment, the adsorption–desorption curve of steel slag moves upward as a whole, and the adsorption amount is significantly higher than that of the original steel slag in the whole relative pressure range. This indicates that the total pore volume and specific surface area of the sample after boiling treatment have been greatly increased. Especially in the higher relative pressure region (P/P° > 0.8), the adsorption capacity of the boiled sample increased sharply, indicating that more macropores (>50 nm) were generated during the treatment process.
The pore size distribution curves of
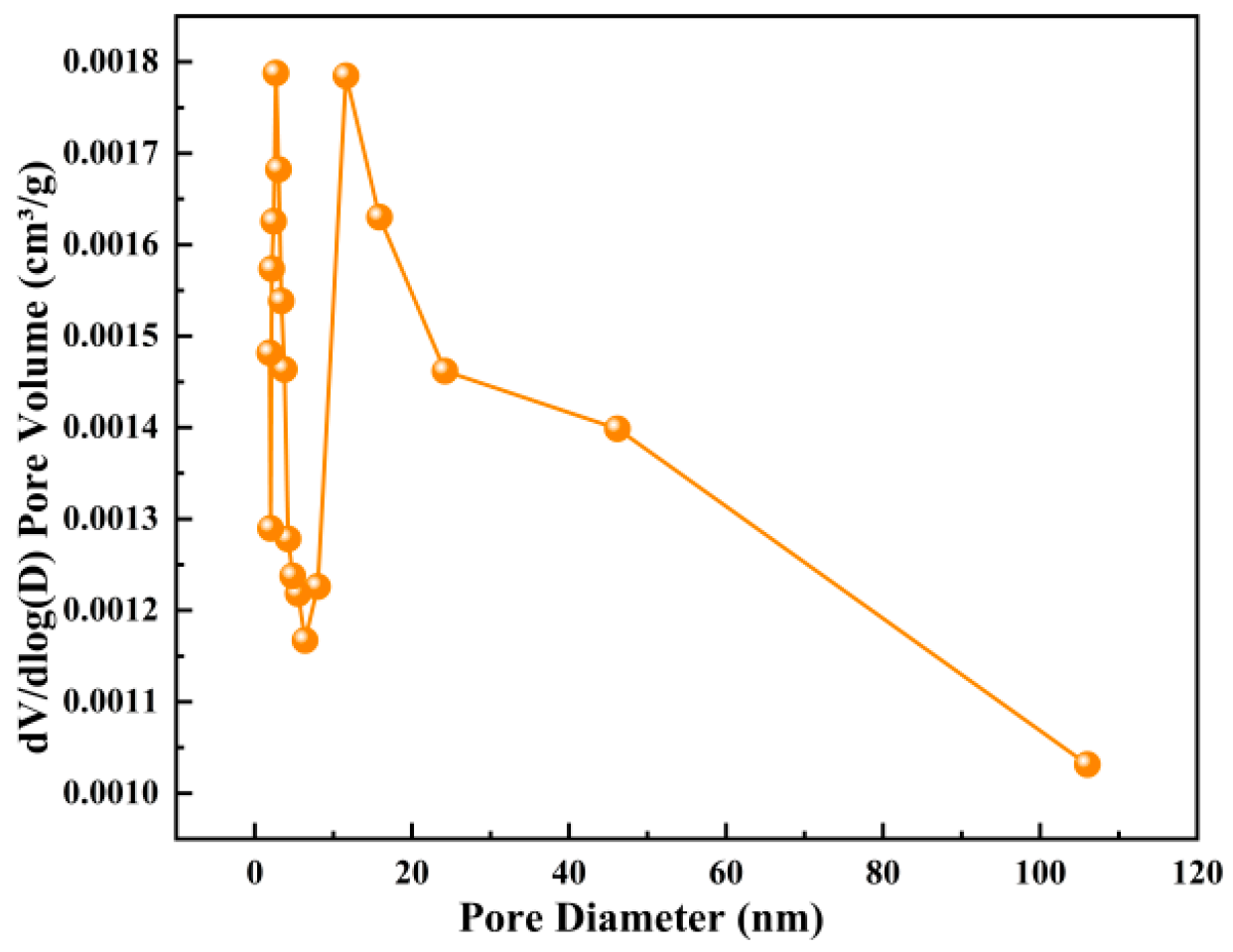
Figure 12 and
Figure 13 illustrate the optimization effect of boiling treatment on pore structure. The pore sizes of the two samples are mainly concentrated in the mesoporous range of 2–50 nm. The pore size distribution curve of the original steel slag is relatively flat, and there is a main peak at about 3.8 nm, but its pore volume is limited. After boiling pretreatment, the pore volume of steel slag in the whole mesoporous interval was comprehensively improved. The pore size distribution curves showed higher peaks in the two intervals of 2–10 nm and 10–50 nm, indicating that boiling treatment not only increased the number of small mesopores, but also more effectively created larger mesopores [
30]. At the same time, combined with the characteristics of the adsorption curve in the high relative pressure region, it can be inferred that the boiling treatment also increased the proportion of large pores in the sample.
This structural reconstruction caused by boiling pretreatment increases the specific surface area of steel slag fine aggregate and provides a broader reaction interface for microbial attachment, bacterial solution, and CO2 penetration. The newly formed interconnected micro-crack network constitutes an efficient material transport channel to ensure that the dissolved Ca2+ and Mg2+ and the CO32− ion produced by the microbial metabolic reaction can diffuse, contact, and precipitate more quickly in the steel slag, thereby improving the efficiency of microbial mineralization.
3.2. Mineral Composition Analysis and Quantitative Analysis
The mineral composition of steel slag fine aggregate under different modification processes was analyzed by XRD. Under the modification process of 3 h (
Figure 14), each modified group showed more significant calcite CaCO3 characteristic peaks than the control SS (about 2θ ≈ 29.4°, 36.0°, 39.4°), indicating that short-term treatment at 0.3 MPa can effectively promote carbonate formation. Among them, the MICP group (K3) showed a stronger CaCO
3 peak than the direct carbonization group (K1), while the Ca(OH)
2/Mg(OH)
2 (about 2θ ≈ 18.0°, 34.1°, 44.3°) was relatively weakened, indicating that carbonic anhydrase (CA) accelerated the CO
2 hydration and carbonate precipitation and preferentially heterogeneously nucleated at the defect/rough interface. After the introduction of boiling pretreatment (K5, K7), the CaCO
3 peak was further sharpened and enhanced, while the f-CaO/f-MgO (about 2θ ≈ 37.3°, 46.4°, 53.8°, 61.8°) was significantly attenuated, indicating that the pre-hydration and consumption of expansive oxides by boiling pretreatment and the mass transfer gain brought by pore structure reconstruction. Further extended to 12 h (
Figure 15), the CaCO
3/MgCO
3 peak of each treatment group continued to increase as a whole, reflecting the time gain; in particular, the boiling + MICP synergistic group (K7, K8) had the highest peak intensity, and the f-CaO/f-MgO peak tended to disappear, indicating that the expansion source completed deep conversion under the action of longer duration and synergistic mechanism.
The intensity of the CaCO
3 characteristic peaks of K3 and K4 in the microbial mineralization group was significantly higher than that of K1 and K2 in the direct carbonization group under the same conditions, and the characteristic peaks of Ca(OH)
2 and Mg(OH)
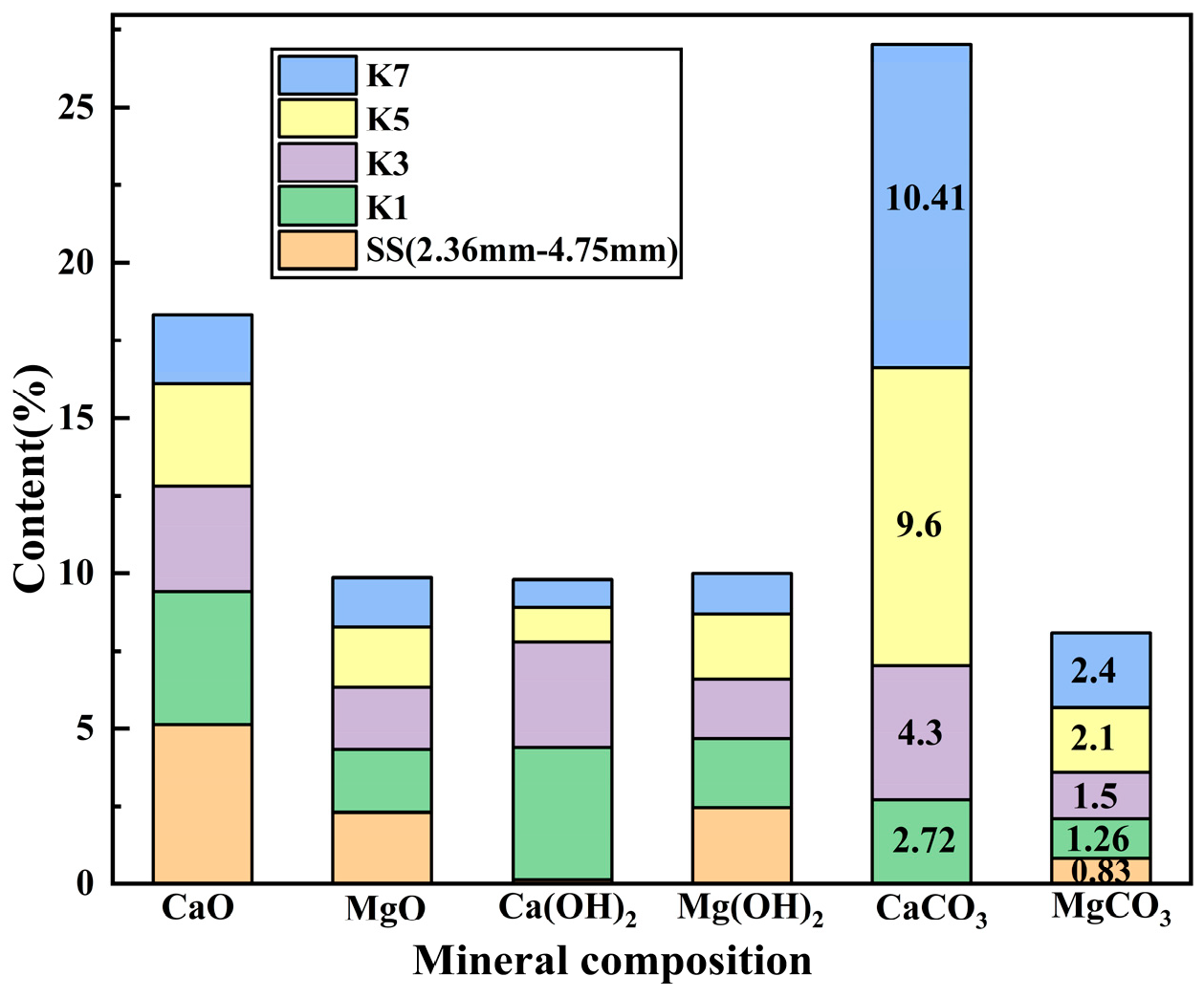
2 at 2θ of 18° were weakened. Quantitative analysis of
Figure 16 (3 h) showed that the contents of CaCO
3 and MgCO
3 in the K3 group increased by 58.08% and 80.72%, respectively. For the 12-h data shown in
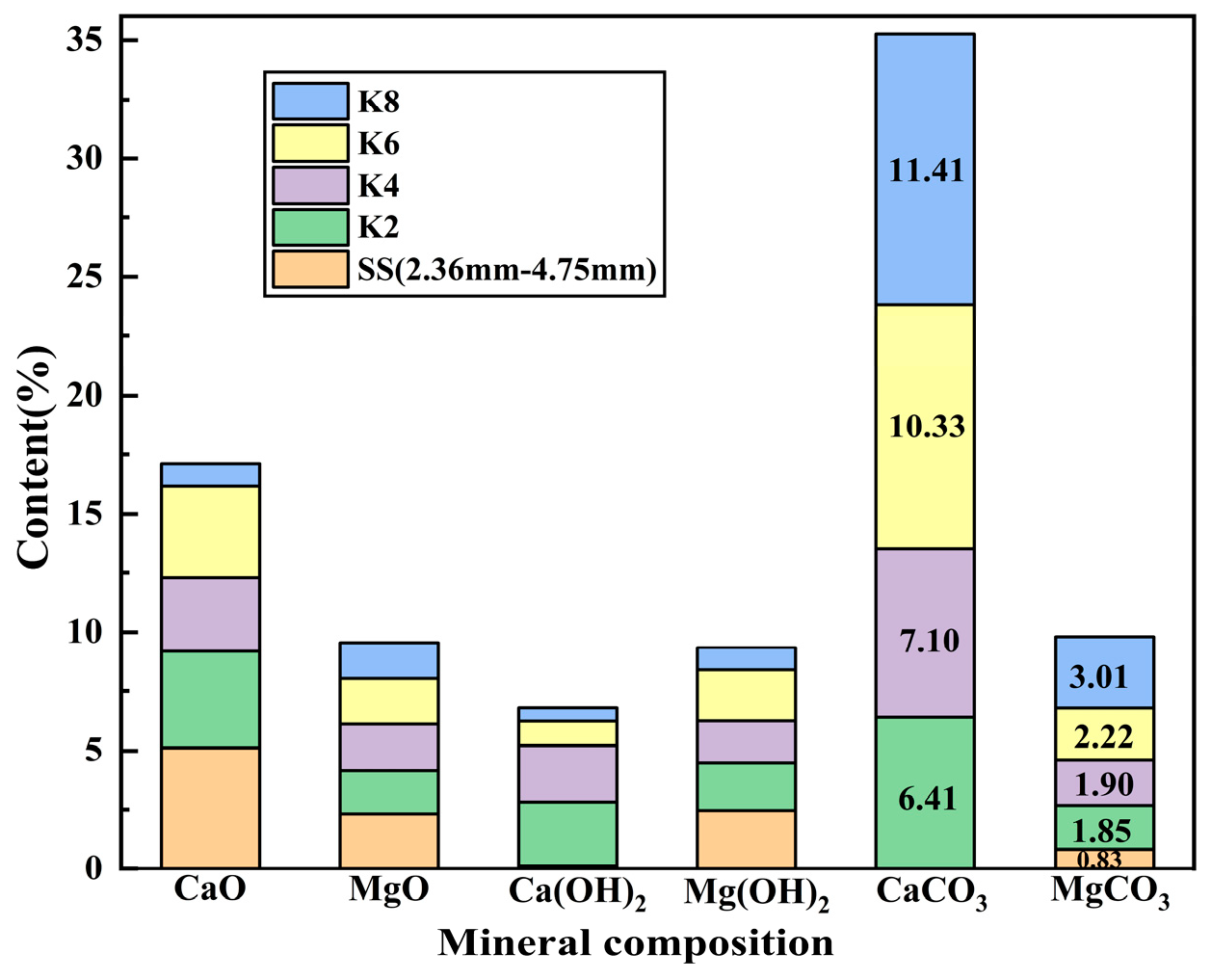
Figure 17, the contents of CaCO
3 and MgCO
3 in the K4 group increased by 161.02% and 128.91%, respectively. It indicated that the carbonic anhydrase secreted by microorganisms promoted the hydration reaction of CO
2 and carbonate precipitation, effectively converted f-CaO and f-MgO, and carbonate precipitation filled the pore structure.
Boiling pretreatment further optimized the mineralization effect. The diffraction peaks of CaCO
3 in the boiled K5–K8 group were sharper and stronger. High-temperature water environment can accelerate the dissolution and hydration of active oxides [
21], promote the leaching of Ca
2+/Mg
2+, increase the specific surface area and porosity, and facilitate the penetration and reaction of CO
2 [
31]. Therefore, the combination of boiling pretreatment and microbial mineralization, K7 and K8, made the production of CaCO
3 and MgCO
3 reach the peak. The content of CaCO
3 and MgCO
3 in the K8 group increased by 319.48% and 262.65%, respectively, and the diffraction peak of f-CaO almost disappeared. The transformation of the mineral phase from expansive component to stable carbonate is directly related to the decrease in macroscopic linear expansion rate, which confirms the effectiveness of boiling pretreatment combined with microbial mineralization technology in improving the volume stability of steel slag fine aggregate.
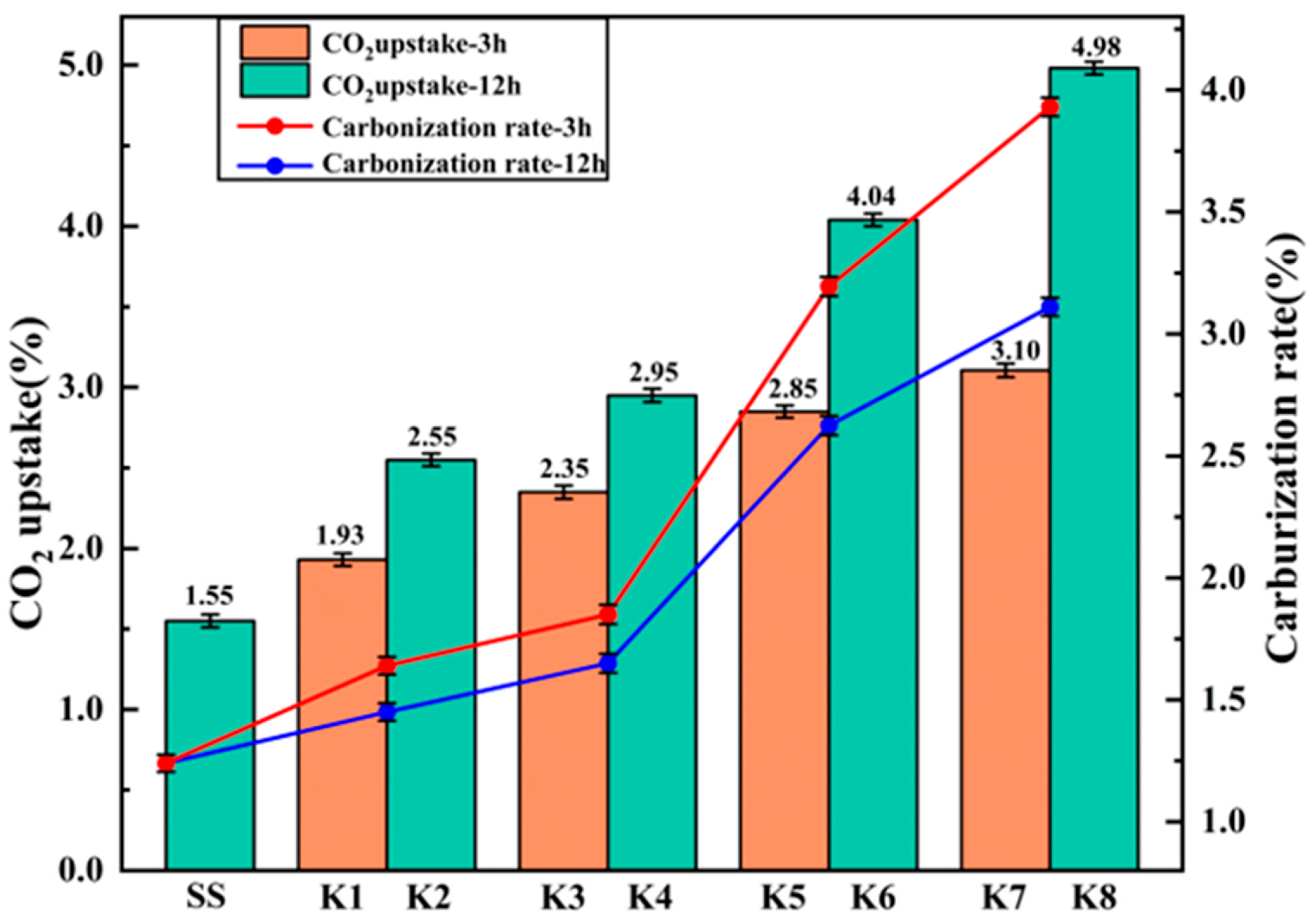
3.3. The CO2 Absorption Rate and Carbonization Rate Were Calculated by Comprehensive Thermal Analysis
The CO
2 absorption rate and carbonation rate of steel slag under different modification processes were evaluated by TG-DTG analysis. The decomposition temperature ranges of calcium hydroxide and magnesium hydroxide are 400–500 °C and 380–400 °C, respectively, reflecting the degree of hydration and digestion of active oxides [
32]; the carbonate is mainly decomposed at 600–800 °C. According to the thermogravimetric curve, mass loss at 600–800 °C, the CO
2 absorption rate is calculated by Equation (10), and the results are shown in
Figure 18. At the same time, the percentage is used to quantify the content of carbonate after different modification methods. The carbonation rate was calculated by the mass Equation (11). The carbonization rates of different treatment methods were compared.
It can be seen from
Figure 19 and
Figure 20 that all the treatment groups showed obvious mass loss steps in the range of 600–800 °C, which was attributed to the thermal decomposition of carbonate. The modification stage of SS and K1–K8 was carried out at 99% CO
2, 0.3 MPa, 20 °C, RH ≈ 70% for 3 h or 12 h. K5–K8 were pretreated by boiling for 100 h before carbonization or microbial mineralization. The CO
2 absorption rate and carbonization rate of SS in the reference group were only 1.55% and 1.24%, indicating that the carbonization reaction was not sufficient without modification. In contrast, the carbonation group K1 and K2 and the microbial mineralization group K3 and K4 significantly increased the weight loss at 600–800 °C: the CO
2 absorption rate and carbonation rate of K1 and K2 were 1.93%, 2.55% and 1.45%, 1.64%, respectively; K3 and K4 were 2.35%, 2.95% and 1.65%, 1.85%, respectively. The DTG curve showed that the microbial mineralization sample had a sharper, higher-peak decomposition near about 700 °C, indicating that the generated carbonate crystals had higher crystallinity and a more concentrated relative distribution, which promoted the reaction rate.
Boiling pretreatment further strengthened the promotion effect. The mass loss of K5–K8 after boiling at 600–800 °C increased as a whole. It can be seen from
Figure 18 that the boiling pretreatment combined with microbial mineralization group K7 and K8 performed best: the CO
2 absorption rate and carbonization rate of K7 and K8 were 3.10%, 4.98% and 3.11%, 3.93%, respectively, and the K8 group was the highest in the whole group. The decomposition peaks of K7 and K8 on the DTG curve shift slightly to the low temperature side, which is usually related to the higher crystallinity, smaller grain size, or more crystal defects of calcium carbonate crystals. These factors make carbonate easier to decompose when heated. Combined with the change in weight loss in the range of 380–500 °C, boiling pretreatment promoted the full hydration of f-CaO and f-MgO into Ca(OH)
2 and Mg(OH)
2, which accelerated the leaching of Ca
2+ and Mg
2+ and the precipitation of CO
2 in multi-scale pores. Microbial mineralization can rapidly convert CO
2 into sedimentary carbonates and preferentially nucleate at rough or defective interfaces through CA catalysis, while CA mineralization provides a rapid and sustainable carbonate supply and heterogeneous nucleation sites. The synergistic effect of the two finally achieves the simultaneous increase in CO
2 absorption rate and carbonation rate, and the essential improvement of volume stability.
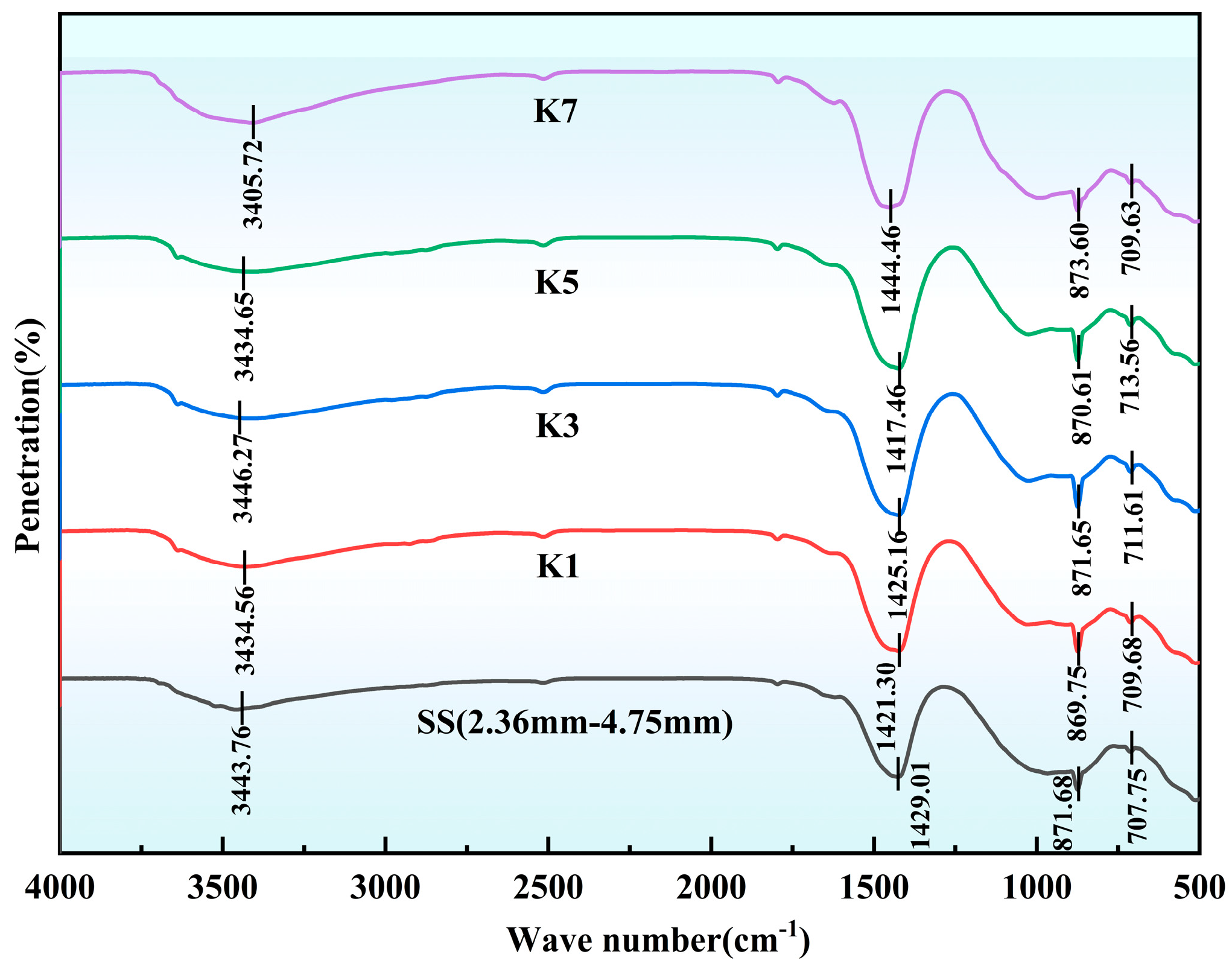
3.4. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
From
Figure 21 and
Figure 22, the composition and reaction mechanism of microbial mineralization products can be further explained at the functional group level. The internal functional groups of steel slag fine aggregates with different modification treatments are basically the same, indicating that the internal chemical composition is also similar. The absorption peak near 3640 cm
−1 is attributed to the stretching vibration of O-H in free Ca(OH)
2 and Mg(OH)
2, while the broad peak near 3440 cm
−1 may be related to the O-H stretching vibration and bending vibration of adsorbed water and interlayer water. Carbonization causes some hydroxyl groups to participate in the reaction, forming a denser structure and reducing the presence of water, affecting the strength of these peaks [
33]. In the K7 and K8 groups of boiling pretreatment combined with microbial mineralization, the intensity of the peak was significantly weakened, indicating that free Ca(OH)
2 and Mg(OH)
2 were effectively converted into CaCO
3 and MgCO
3.
All the treated samples showed obvious absorption peaks at about 1420 cm
−1, 873 cm
−1, and 712 cm
−1, corresponding to the asymmetric stretching vibration, out-of-plane bending vibration, and in-plane bending vibration modes of carbonate ions (CO
32−), respectively [
26]. These characteristic peaks together confirmed the formation of CaCO
3 and MgCO
3. Compared with the untreated reference group SS, the absorption intensity of the modified sample at these wave numbers is enhanced, indicating that the carbonation reaction does occur, and the degree of reaction varies significantly with different treatment processes. CA promotes the hydration of CO
2 and increases the supply of carbonate, thereby increasing the amount of carbonate deposition; the peaks of K5–K8 at 873 cm
−1 and 712 cm
−1 after boiling pretreatment are sharper and more regular, suggesting that carbonate crystals have higher crystallinity and more orderly stacking, which is consistent with the optimization of pore structure and the increase in reaction interface induced by boiling. The formation of CO
32− from CO
2 catalyzed by CA and the high-throughput dissolution of Ca
2+ and Mg
2+ brought by boiling and the interconnected pores provide synergistic conditions for heterogeneous nucleation to ensure that carbonates preferentially nucleate and grow rapidly on rough and defect-rich surfaces.
By comparing the FTIR spectra of different groups, it was found that the absorption peak intensity of K3 and K4 in the microbial mineralization group near 1420 cm−1 was significantly higher than that in the carbonation group K1 and K2, indicating that the introduction of carbonic anhydrase microorganisms accelerated carbonate precipitation. The absorption peaks of K7 and K8 in the group of boiling pretreatment and microbial mineralization at 873 cm−1 and 712 cm−1 were sharper and more regular, which indirectly indicated that the crystallinity of calcium carbonate was higher and the crystal structure was more orderly, indicating that boiling pretreatment and microbial mineralization were more helpful to optimize the microstructure of the product.
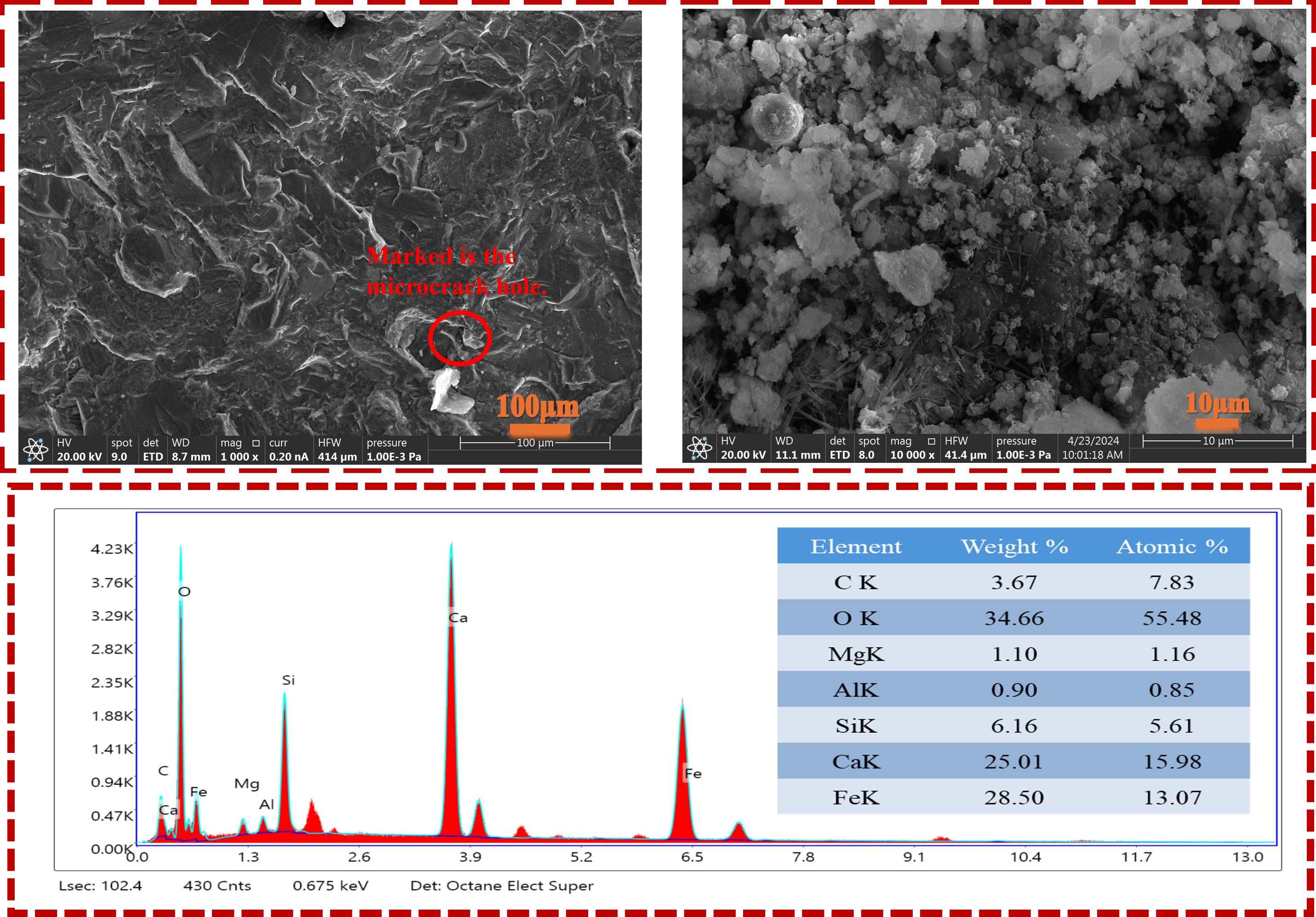
3.5. Microstructure Analysis and Energy Spectrum Analysis of Steel Slag Fine Aggregat
According to the comparison of SEM images in
Figure 23 and
Figure 24, it can be seen that the microstructure of steel slag fine aggregate treated by boiling pretreatment combined with microbial mineralization modification process has changed significantly. The original steel slag section is rough, the structure is loose, and obvious edges and micro-cracks can be seen on the surface; the surface of the K8 group samples after synergistic modification was closely covered by a large number of spherical clusters and flake calcium carbonate crystals. The original particle boundary became blurred, obvious adhesion occurred between the structures, and a denser composite structure was formed, which reduced the porosity and the number of micro-cracks. In the locally enlarged field of vision, some regular cubic structures and needle-like structures can also be seen attached to the surface of the spherical matrix. Combined with the EDS spectrum analysis results, the energy spectrum analysis of the original steel slag fine aggregate shows that the proportions of Ca, Mg, C, and O elements are 15.98%, 1.16%, 7.83%, and 55.48%. After synergistic modification, the proportion of Ca, Mg, C, and O elements increased to 17.19%, 2.16%, 10.83%, 63.66%. The content of four elements increased by 7.57%, 86.21%, 38.31% and 14.74%, respectively. After synergistic modification, the content of key elements on the surface of steel slag fine aggregate increased, among which the increase in Mg element was the most amazing, reaching 86.21%, which directly proved that the modification process had a very high efficiency for the conversion of free f-MgO. The 38.31% increase in C element directly reflects the successful deposition of carbonate, which is direct evidence of microbial mineralization. The simultaneous growth of Ca, Mg, and O elements confirms the formation of stable minerals such as CaCO
3 and MgCO
3, which indicates that boiling pretreatment combined with microbial mineralization accelerates the rate of reaction to form CaCO
3 and MgCO
3, fills pores [
34], and improves the volume stability of steel slag fine aggregate.
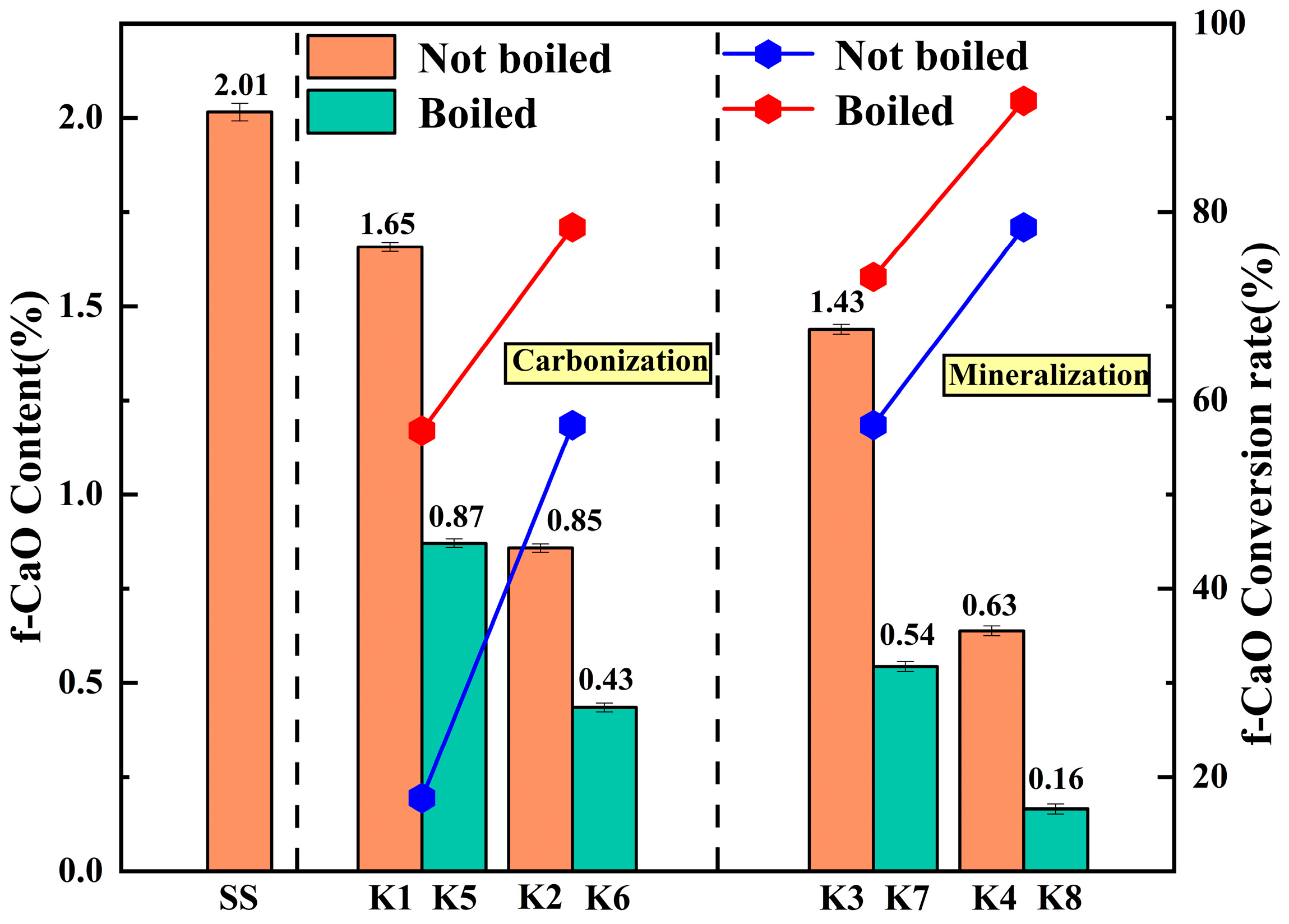
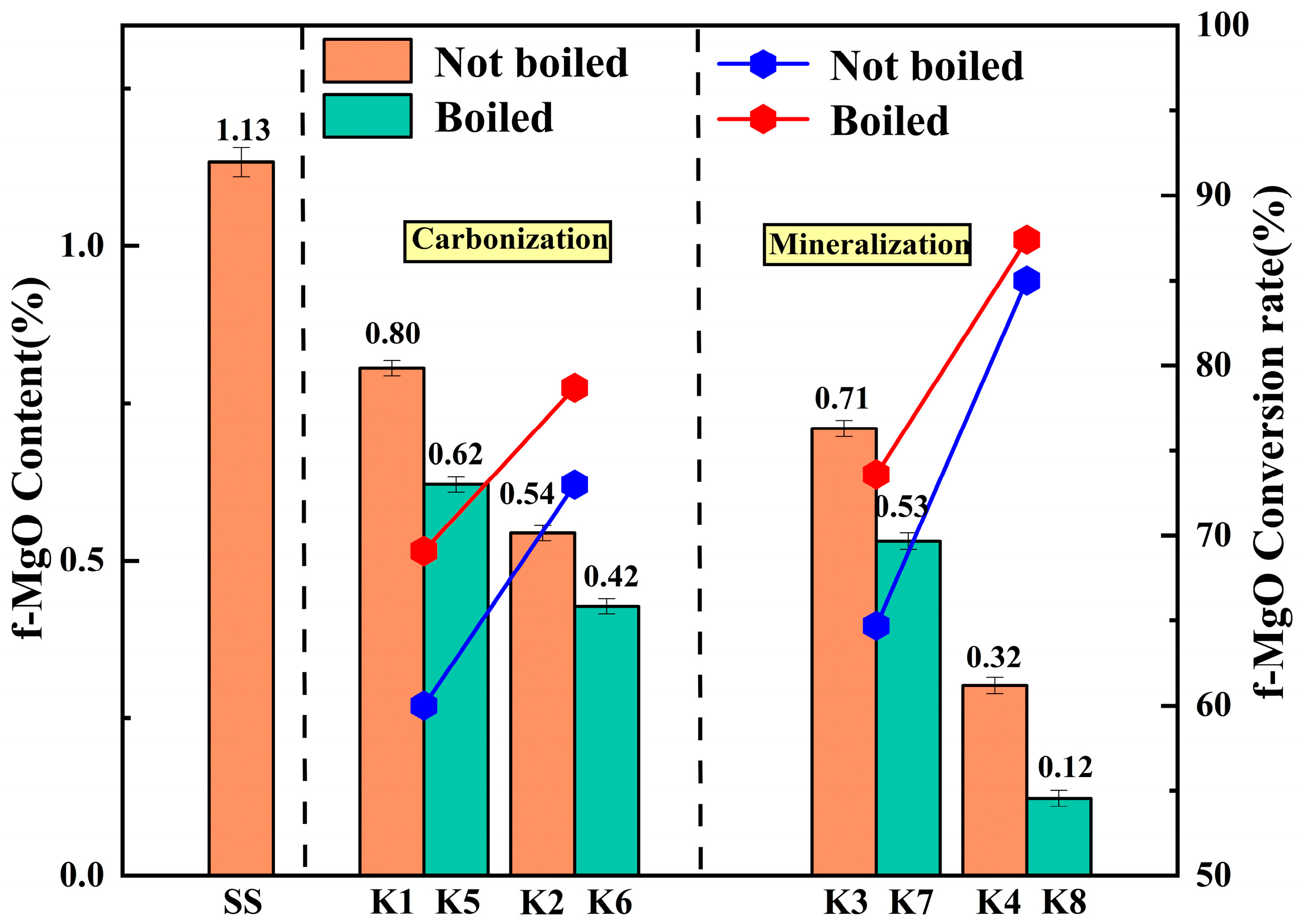
3.6. Analysis of f-CaO and f-MgO Content and Conversion Rate
The content of f-CaO and f-MgO was determined by EDTA solution titration [
35,
36], and the conversion rate was calculated. From the titration results of
Figure 25 and
Figure 26, it can be seen that the content of f-CaO and f-MgO in the untreated reference group SS is the highest, which is 2.01% and 1.13%, respectively, which is the main reason for its poor volume stability. After carbonization treatment of K1 and K2 groups, the content of f-CaO and f-MgO decreased to 1.65% and 0.85%, respectively, indicating that prolonging the carbonization time under 0.3 MPa pressure can promote partial carbonization, but fail to fully convert the internal active oxides.
After the introduction of microbial mineralization, the content of f-CaO and f-MgO in the K4 group decreased to 0.63% and 0.32%. This indicates that microorganisms effectively catalyze the hydration of CO2 by secreting carbonic anhydrase, increasing the concentration of CO32− in the system, thereby driving the transformation of dissolved Ca2+ and Mg2+ to carbonate precipitation. Boiling pretreatment further strengthened this conversion process. In the K8 group treated with boiling pretreatment and microbial mineralization, the f-CaO content decreased to a minimum of 0.16%, the f-MgO content decreased to a minimum of 0.12%, and the conversion rates reached 91.82% and 87.43%, respectively. The boiling pretreatment process rapidly accelerates the hydration reaction of f-CaO and f-MgO in a hydrothermal environment at 100 °C, and pre-converts them into Ca(OH)2 and Mg(OH)2 with higher solubility. This process not only eliminates the expansion risk from the source, the volume effect, and micro-crack propagation accompanied by the formation of hydration products, but also reconstructs the micropore structure of steel slag, and provides a highly developed reaction interface and transmission channel for subsequent microbial attachment, bacterial liquid penetration, and ion migration. The microbial mineralization process can give full play to its biocatalytic advantages and realize the rapid precipitation of a large amount of Ca2+ and Mg2+ produced in the pretreatment stage. The synergistic effect of boiling pretreatment and microbial mineralization on the consumption of expansion sources was confirmed at the chemical composition level.
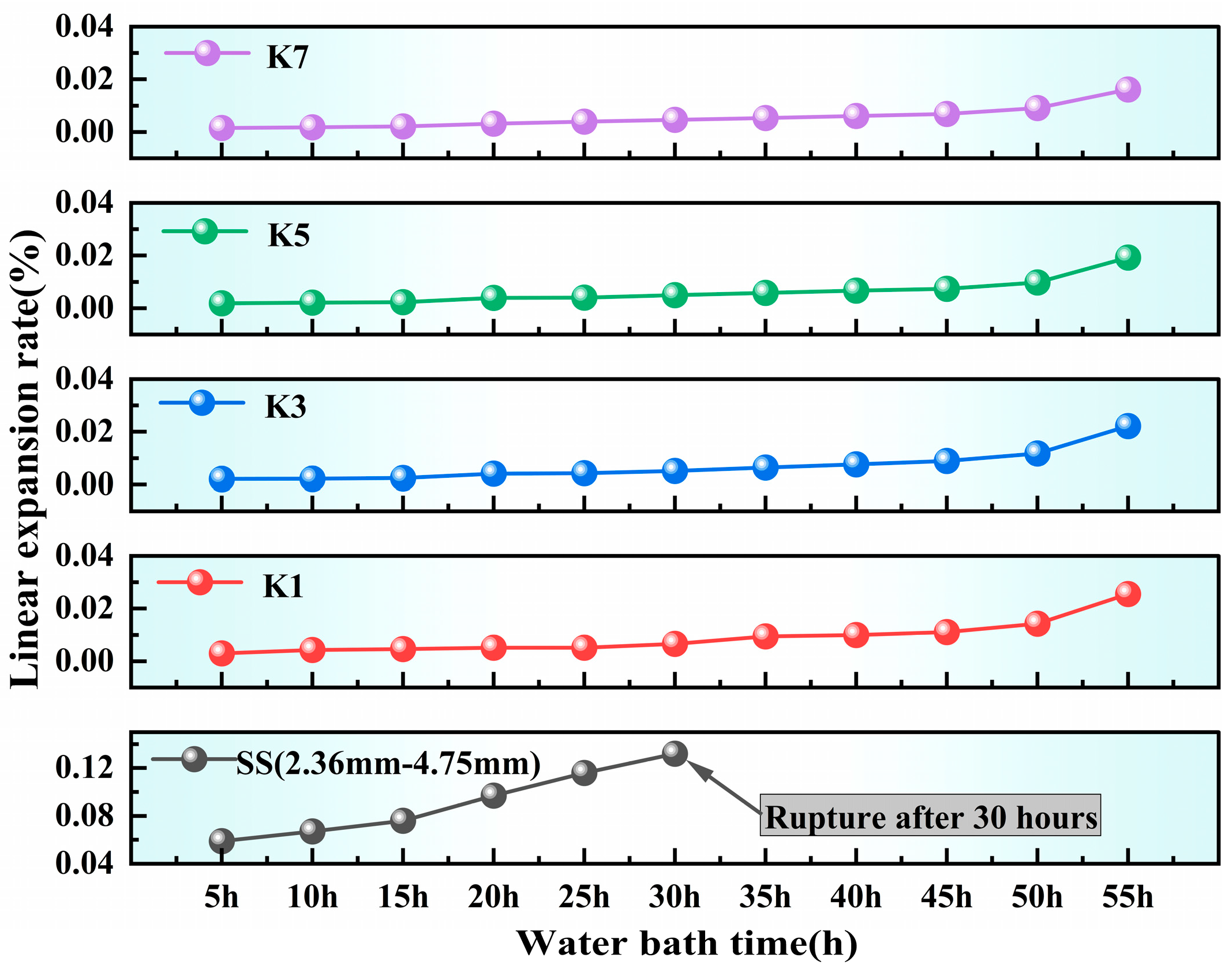
3.7. Linear Expansion Rate Analysis
From
Figure 27 and
Figure 28, it can be seen that compared with the reference group SS without any treatment, the linear expansion rate of mortar prepared by all modified steel slag fine aggregates is reduced, because most of the unstable f-CaO and f-MgO have been converted into stable carbonates [
13], which improves the volume stability of steel slag fine aggregates.
Specifically, the linear expansion rate of the untreated SS group reached 0.132% after 30 h of water bath heating, and the fracture occurred after 35 h of water bath heating. The linear expansion rate of K1 and K2 in the carbonization group reached 0.02554% and 0.02214% after 50 h of water bath heating, but the improvement was limited. After the introduction of microbial mineralization treatment, the linear expansion rates of K3 and K4 decreased to 0.02268% and 0.0205%, respectively, and K3/K4 decreased by 11.2% and 7.4%, respectively, compared with K1/K2. After 60 h of water bath heating, the steel slag mortar specimen fractured. It indicated that the carbonic anhydrase secreted by microorganisms significantly promoted the formation of CaCO3 and MgCO3, and more effectively converted the expansive components into stable carbonate minerals. At the same time, the higher CO2 absorption rate of the microbial mineralization group in TG and DTG analysis also provided support for this, indicating that its carbonization reaction was more thorough.
Among all the modified groups, the effect of the K7 and K8 groups with boiling pretreatment and microbial mineralization was the most obvious. The linear expansion rate of the K7/K8 group decreased to the lowest value of 0.0199% and 0.0176% after 55 h, and K7/K8 decreased by 12.3% and 14.2%, respectively, compared with K3/K4. This macroscopic performance is consistent with the results of microscopic analysis, in which the characteristic peak intensity of CaCO
3 is the highest, the f-CaO diffraction peak almost disappears, and the CO
2 absorption rate and carbonation rate are the largest. Boiling pretreatment creates more favorable conditions for the subsequent microbial mineralization reaction by accelerating the hydration reaction and increasing its specific surface area and porosity. The synergistic effect of the two achieves the maximum consumption of the expansion source and the effective compaction of the product structure [
37].
3.8. Economic Analysis
Scope and operating note. The boiling tank is used to raise the steel-slag–water mixture to 100 °C; after reaching 100 °C, no active boiling is maintained thanks to the tank’s good thermal insulation. Therefore, the main energy demand is the one-time sensible heating from ambient temperature to 100 °C, plus minor holding losses.
Assumptions for 1 t of steel slag (lab conditions).
(A1) Steel-slag mass ms = 1000 kg; specific heat cs = 0.84 kJ·kg−1·K−1.
(A2) Water added for immersion mw = 0.3 ms = 300 kg; specific heat cw = 4.186 kJ kg−1·K−1.
(A3) Ambient temperature T0 = 25 °C; target temperature Tf = 100 °C.
(A4) Heat-loss/holding overhead α = 10% (well-insulated bath).
(A5) Electricity price scenarios: pe = 0.8–1.2 CNY·kW·h−1.
(A6) CO2: bottled, 99% purity, purchased from a local industrial-gas vendor; CO2 fixed in solids under the best condition (K8) is 4.98% of slag mass (i.e., mfixed = 49.8 kg CO2/t slag)
(A7) CO2 utilization efficiency η = mfixed/msupplied (range 0.3–0.8 for lab operation without gas recycle; baseline η = 0.5).
(A8) CO2 purchase price scenarios pCO2 = 2, 3, 4 CNY/kg (bottled lab-grade range).
(A9) Grid emission factor for electricity EFel considered as a parameter (0.4–0.7 kg·CO2·kW·h−1); baseline 0.5 kg CO2.
(If mw ranges 0.2–0.5 t and α 10–20%, Q ranges 38–62 kW·h.)
Electricity cost (1 t slag).
CO2 purchase and cost.
Fixed mass mfixed = 49.8 kg. Supplied mass msupplied = mfixed/η.
Baseline η = 0.5 →m
supplied ≈ 99.6 kg.
If pCO2 = 2/3/4 CNY/kg, η = 0.5; cco2 ≈199/299/398 CNY.
Range over η = 0.3–0.8 and pCO2 = 2–4; 75–665 CNY.
Total variable cost (lab, per 1 t slag).
Using the baselines above: ≈ (38–58) CNY for electricity + (200–400) CNY for CO2 = 240–460 CNY/t.
With parameter ranges (water load, losses, η\etaη, and price), the plausible lab-scale interval is ≈ 115–725 CNY/t.
Carbon balance (lab, per 1 t slag).
CO2 fixed in solids: mfixed = 49.8 kg·CO2·t−1 (K8).
Electricity-related emissions: Eel = Q × EFel. With Q = 48.1 kW·h and EFel = 0.5; Eel ≈ 24 kg·CO2 (range 15–43 kg for EFel = 0.4–0.7 and Q = 38–62 kW·h).
Net balance (fixed-indirect): baseline ≈ + 26 kg·CO2·t−1 (range +7 to +35 kg·CO2·t−1). This shows that, even at lab scale without gas recycle, the process yields a net positive CO2 fixation; process-scale integration (heat recovery, gas recirculation) would further improve both cost and carbon balance.
Notes on practicality.
- (1)
Because the bath is insulated and we do not maintain boiling after reaching 100 °C, extending the holding time (e.g., 100 h) does not linearly increase energy; incremental demand is captured by α.
- (2)
In industrialization, heat integration (e.g., using low-grade waste heat/steam) and CO2 recycle would raise η and cut both cel and cco2.
- (3)
All prices are laboratory, bottled-gas conditions; they are upper bounds compared with bulk CO2/energy at plant scale.
- (4)
CO2 utilization efficiency η = mfixed/msupplied; carbon balance = mfixed − Q × EFel.