Enhancing PEEK Surface Bioactivity Through Phosphate and Calcium Ion Functionalization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Fabrication and Surface Modification
2.2.1. Fabrication and Polishing
2.2.2. Surface Activation
2.2.3. Surface Functionalization
2.3. Surface Evaluation
2.3.1. Water Contact Angle Measurement
2.3.2. Crystallinity Analysis
2.3.3. Surface Topography Analysis
2.3.4. Chemical Composition Analysis
2.4. Cell Culture Assays
2.4.1. Cell Viability Analysis
2.4.2. Cell Proliferation Analysis
2.4.3. Cell Adhesion and Spreading Analysis
2.5. Statistical Evaluation
3. Results
3.1. Hydrophilicity
3.2. Crystallinity
3.3. Topography
3.4. Chemical Composition
3.5. Cellular Response
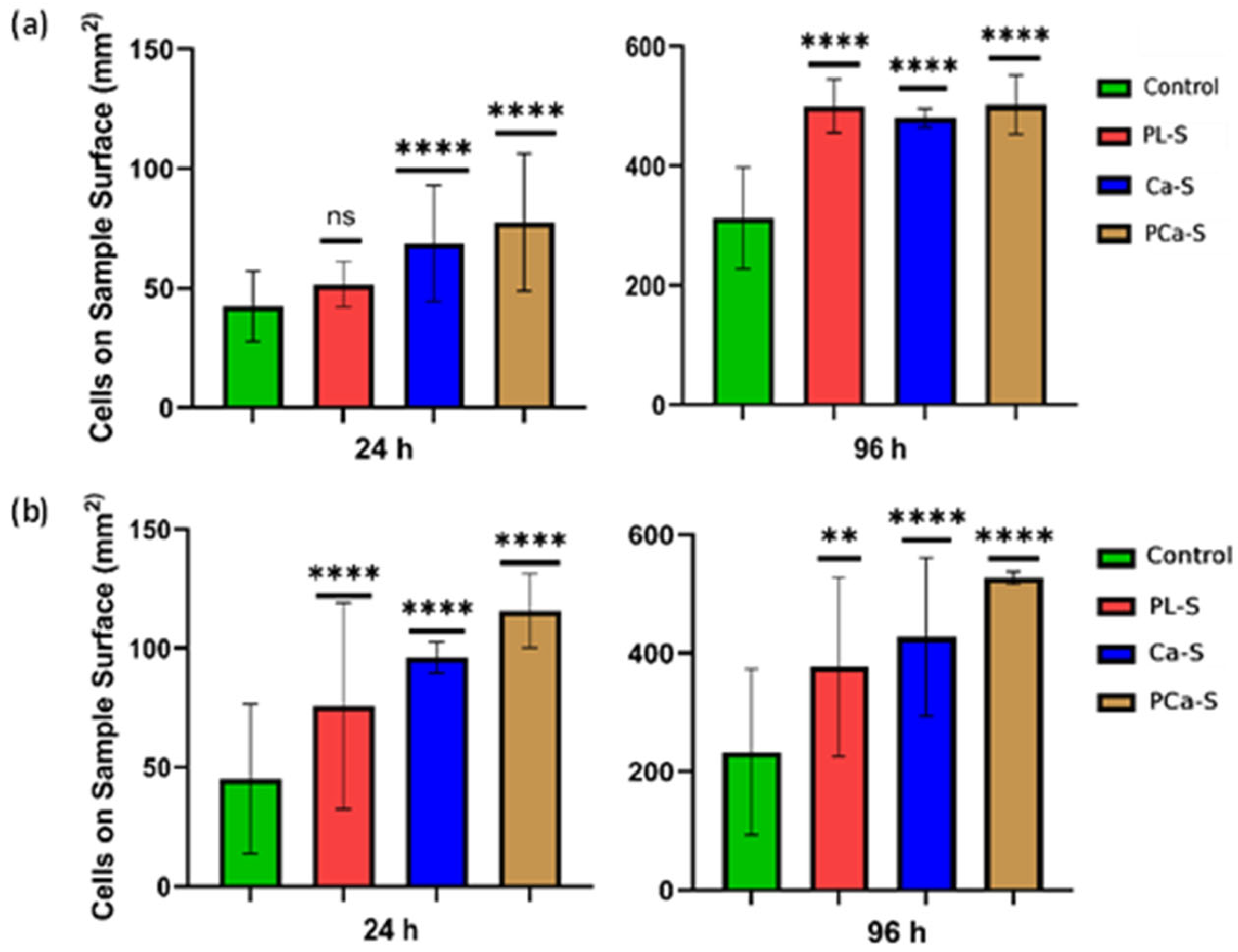
3.5.1. Cell Viability
3.5.2. Cell Proliferation
3.5.3. Cell Adhesion and Spreading
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEEK | Poly-ether-ether-ketone |
| SEM | Scanning electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
| FTIR-ATR | Fourier-transform infrared spectroscopy with attenuated total reflectance |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DMEM-F12 | Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 |
| CAMKII | Calmodulin-dependent kinase II |
| NFAT | Nuclear factor of activated T cells |
| CREB | cAMP response element-binding protein |
| ERK ½ | Extracellular signal-regulated kinase 1 and 2 |
| PI3K | Phosphoinositide 3-kinase |
| AKT | Protein Kinase B (PKB) |
| BMP | Bone morphogenic protein |
| IGF-1 | Insulin-like Growth factor I |
| RANKL | Receptor activator of nuclear factor kB ligand |
| OPG | Osteoprotegerin |
| Wnt | Wingless/integrated signaling pathway |
References
- Haleem, A.; Javaid, M.; Vaish, A.; Vaishya, R. Three-dimensional-printed polyether ether ketone implants for orthopedics. Indian J. Orthop. 2019, 53, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Honigmann, P.; Sharma, N.; Okolo, B.; Popp, U.; Msallem, B.; Thieringer, F.M. Patient-specific surgical implants made of 3D printed PEEK: Material, technology, and scope of surgical application. Biomed. Res. Int. 2018, 2018, 4520636. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Suonan, A.; Zhou, J.; Yuan, Q.; Liu, L.; Zhao, X.; Lou, X.; Yang, C.; Li, D.; Zhang, Y.G. PEEK (Polyether-ether-ketone) and its composite materials in orthopedic implantation. Arab. J. Chem. 2021, 14, 102977. [Google Scholar] [CrossRef]
- Mbogori, M.; Vaish, A.; Vaishya, R.; Haleem, A.; Javaid, M. Poly-Ether-Ether-Ketone (PEEK) in orthopaedic practice- A current concept review. J. Orthop. Rep. 2022, 1, 3–7. [Google Scholar] [CrossRef]
- Hussain, S.; Rutledge, L.; Acheson, J.G.; Boyd, A.R.; Meenan, B.J. The surface characterisation of polyetheretherketone (Peek) modified via the direct sputter deposition of calcium phosphate thin films. Coatings 2020, 10, 1088. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. Polyether ether ketone (PEEK) and its 3D printed implants applications in medical field: An overview. Clin. Epidemiol. Glob. Health 2019, 7, 571–577. [Google Scholar] [CrossRef]
- Rendas, P.; Figueiredo, L.; Geraldo, M.; Vidal, C.; Soares, B.A. Improvement of tensile and flexural properties of 3D printed PEEK through the increase of interfacial adhesion. J. Manuf. Process 2023, 93, 260–274. [Google Scholar] [CrossRef]
- Fu, Q.; Gabriel, M.; Schmidt, F.; Müller, W.D.; Schwitalla, A.D. The impact of different low-pressure plasma types on the physical, chemical and biological surface properties of PEEK. Dent. Mater. 2021, 37, e15–e22. [Google Scholar] [CrossRef]
- Moharil, S.; Reche, A.; Durge, K. Durge, Polyetheretherketone (PEEK) as a Biomaterial: An Overview. Cureus 2023, 15, e44307. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef]
- Zhao, M.; An, M.; Wang, Q.; Liu, X.; Lai, W.; Zhao, X.; Wei, S.; Ji, J. Quantitative proteomic analysis of human osteoblast-like MG-63 cells in response to bioinert implant material titanium and polyetheretherketone. J. Proteom. 2012, 75, 3560–3573. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Huang, Y.; Xu, H.; Feng, G.; Liu, L.; Li, Y.; Sun, D.; Zhang, L. Modification of polyetheretherketone implants: From enhancing bone integration to enabling multi-modal therapeutics. Acta Biomater. 2021, 129, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.S.F.D.; Vieira, M.; da Silva, H.N.; Tomás, H.; Fook, M.V.L. Surface bioactivation of polyether ether ketone (Peek) by sulfuric acid and piranha solution: Influence of the modification route in capacity for inducing cell growth. Biomolecules 2021, 11, 1260. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Obilor, A.; Sainsbury, W.; Pacella, M.; Wilson, A.; Silberschmidt, V.V. Laser Processing of Polymers for Surface Energy Control of Biomedical Implants. In Procedia CIRP; Elsevier B.V.: Amsterdam, The Netherlands, 2022; pp. 558–563. [Google Scholar] [CrossRef]
- Nageswaran, G.; Jothi, L.; Jagannathan, S. Plasma Assisted Polymer Modifications. In Non-Thermal Plasma Technology for Polymeric Materials: Applications in Composites, Nanostructured Materials, and Biomedical Fields; Elsevier: Amsterdam, The Netherlands, 2018; pp. 95–127. [Google Scholar] [CrossRef]
- Sunarso; Toita, R.; Tsuru, K.; Ishikawa, K. Immobilization of calcium and phosphate ions improves the osteoconductivity of titanium implants. Mater. Sci. Eng. C 2016, 68, 291–298. [Google Scholar] [CrossRef]
- Tapia-Lopez, L.V.; Esparza-Ponce, H.E.; Luna-Velasco, A.; Garcia-Casillas, P.E.; Castro-Carmona, H.; Castro, J.S. Bioactivation of zirconia surface with laminin protein coating via plasma etching and chemical modification. Surf. Coat Technol. 2020, 402, 126307. [Google Scholar] [CrossRef]
- Tapia-Lopez, L.V.; Luna-Velasco, M.A.; Beaven, E.K.; Conejo-Dávila, A.S.; Nurunnabi, M.; Castro, J.S. RGD Peptide-Functionalized Polyether Ether Ketone Surface Improves Biocompatibility and Cell Response. ACS Biomater. Sci. Eng. 2023, 9, 5270–5278. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, J.; Li, W.; Zhang, Y.; Ma, Z.; Wu, C. An effective surface modification strategy to boost PEEK osteogenesis using porous CaP generated in well-tuned collagen matrix. Appl. Surf. Sci. 2021, 555, 149571. [Google Scholar] [CrossRef]
- Narayanan, R.; Seshadri, S.K.; Kwon, T.Y.; Kim, K.H. Calcium phosphate-based coatings on titanium and its alloys. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85, 279–299. [Google Scholar] [CrossRef]
- Mahjoubi, H.; Buck, E.; Manimunda, P.; Farivar, R.; Chromik, R.; Murshed, M.; Cerruti, M. Surface phosphonation enhances hydroxyapatite coating adhesion on polyetheretherketone and its osseointegration potential. Acta Biomater. 2017, 47, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Sunarso; Tsuchiya, A.; Toita, R.; Tsuru, K.; Ishikawa, K. Enhanced osseointegration capability of poly(Ether ether ketone) via combined phosphate and calcium surface-functionalization. Int. J. Mol. Sci. 2020, 21, 198. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Jin, X.; Ma, P.X. Calcium phosphate deposition rate, structure and osteoconductivity on electrospun poly(l-lactic acid) matrix using electrodeposition or simulated body fluid incubation. Acta Biomater. 2014, 10, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Szustakiewicz, K.; Kryszak, B.; Gazińska, M.; Chęcmanowski, J.; Stępak, B.; Grzymajło, M.; Antończak, A. The effect of selective mineralization of PLLA in simulated body fluid induced by ArF excimer laser irradiation: Tailored composites with potential in bone tissue engineering. Compos. Sci. Technol. 2020, 197, 108279. [Google Scholar] [CrossRef]
- Rodríguez, K.; Renneckar, S.; Gatenholm, P. Biomimetic Calcium Phosphate Crystal Mineralization on Electrospun Cellulose-Based Scaffolds. ACS Appl. Mater. Interfaces 2011, 3, 681–689. [Google Scholar] [CrossRef]
- Brochu, B.M.; Sturm, S.R.; Kawase, J.A.; De Queiroz Goncalves, N.A.; Mirsky, A.I.; Sandino, K.Z.; Panthaki, K.Z.; Panthaki, V.V.; Nayak, S.; Daunert, L.; et al. Coelho, Advances in Bioceramics for Bone Regeneration: A Narrative Review. Biomimetics 2024, 9, 690. [Google Scholar] [CrossRef]
- Tanvir, M.A.H.; Khaleque, M.A.; Kim, G.-H.; Yoo, W.-Y.; Kim, Y.-Y. The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives. Biomimetics 2024, 9, 230. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, V.; Baino, F.; Mauro, J.C.; Pickrell, G.; Evans, I.; Bretcanu, O. Mechanical properties of bioactive glasses, ceramics, glass-ceramics and composites: State-of-the-art review and future challenges. Mater. Sci. Eng. C 2019, 104, 109895. [Google Scholar] [CrossRef]
- Jacobs, T.; Morent, R.; De Geyter, N.; Dubruel, P.; Leys, C. Plasma surface modification of biomedical polymers: Influence on cell-material interaction. Plasma Chem. Plasma Process. 2012, 32, 1039–1073. [Google Scholar] [CrossRef]
- Teng, R.; Meng, Y.; Zhao, X.; Liu, J.; Ding, R.; Cheng, Y.; Zhang, Y.; Zhang, Y.; Pei, D.; Li, A. Combination of Polydopamine Coating and Plasma Pretreatment to Improve Bond Ability Between PEEK and Primary Teeth. Front. Bioeng. Biotechnol. 2021, 8, 630094. [Google Scholar] [CrossRef]
- Poulsson, A.H.C.; Richards, R.G. Surface Modification Techniques of Polyetheretherketone, Including Plasma Surface Treatment. In PEEK Biomaterials Handbook; Elsevier: Amsterdam, The Netherlands, 2011; pp. 145–161. [Google Scholar] [CrossRef]
- Chytrosz-Wrobel, P.; Golda-Cepa, M.; Stodolak-Zych, E.; Rysz, J.; Kotarba, A. Effect of oxygen plasma-treatment on surface functional groups, wettability, and nanotopography features of medically relevant polymers with various crystallinities. Appl. Surf. Sci. Adv. 2023, 18, 100497. [Google Scholar] [CrossRef]
- ASTM F2778-09; Standard Test Method for Measurement of Percent Crystallinity of Polyetheretherketone (PEEK) Polymers by Means of Specular Reflectance Fourier Transform Infrared Spectroscopy (R-FTIR). ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- Azooz, M.F.; Zaghloul, M.A.; Sayed, M.M.; Soliman, H.M.; Mounir, N.M.; Kamal, N.; El-Dabae, W.H.; Ali, S.E. Efficiency of MTT and Trypan Blue Assays for Detection of Viability and Recovery of Different Frozen Cell Lines. Egypt. J. Vet. Sci. (Egypt) 2024, 55, 1649–1657. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Primc, G.; Mozetič, M. Kinetics of Surface Wettability of Aromatic Polymers (PET, PS, PEEK, and PPS) upon Treatment with Neutral Oxygen Atoms from Non-Equilibrium Oxygen Plasma. Polymers 2024, 16, 1381. [Google Scholar] [CrossRef] [PubMed]
- Vesel, A.; Zaplotnik, R.; Mozetič, M.; Primc, G. Surface modification of PS polymer by oxygen-atom treatment from remote plasma: Initial kinetics of functional groups formation. Appl. Surf. Sci. 2021, 561, 150058. [Google Scholar] [CrossRef]
- Zhianmanesh, M.; Gilmour, A.; Bilek, M.M.M.; Akhavan, B. Plasma surface functionalization: A comprehensive review of advances in the quest for bioinstructive materials and interfaces. Appl. Phys. Rev. 2023, 10, 021301. [Google Scholar] [CrossRef]
- Primc, G.; Mozetič, M. Surface Modification of Polymers by Plasma Treatment for Appropriate Adhesion of Coatings. Materials 2024, 17, 1494. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Gindele, M.B.; Malaszuk, K.K.; Peter, C.; Gebauer, D. On the Binding Mechanisms of Calcium Ions to Polycarboxylates: Effects of Molecular Weight, Side Chain, and Backbone Chemistry. Langmuir 2022, 38, 14409–14421. [Google Scholar] [CrossRef]
- Chen, H.; Lv, C.; Guo, L.; Ma, M.; Li, X.; Lan, Z.; Huo, J.; Dong, H.; Zhu, X.; Zhu, Q.; et al. Surface Stability and Morphology of Calcium Phosphate Tuned by pH Values and Lactic Acid Additives: Theoretical and Experimental Study. ACS Appl. Mater. Interfaces 2022, 14, 4836–4851. [Google Scholar] [CrossRef]
- Mitra, S.P. Protein Adsorption on Biomaterial Surfaces: Subsequent Conformational and Biological Consequences—A Review. J. Surf. Sci. Technol. 2020, 36, 07–38. [Google Scholar] [CrossRef]
- Kito, H.; Ohya, S. Role of K+ and Ca2+-Permeable Channels in Osteoblast Functions. Int. J. Mol. Sci. 2021, 22, 10459. [Google Scholar] [CrossRef] [PubMed]
- Khoshniat, S.; Bourgine, A.; Julien, M.; Petit, M.; Pilet, P.; Rouillon, T.; Masson, M.; Gatius, M.; Weiss, P.; Guicheux, J.; et al. Phosphate-dependent stimulation of MGP and OPN expression in osteoblasts via the ERK1/2 pathway is modulated by calcium. Bone 2011, 48, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, S.; Birgani, Z.T.; Habibovic, P. Biomaterial-induced pathway modulation for bone regeneration. Biomaterials 2022, 283, 121431. [Google Scholar] [CrossRef] [PubMed]
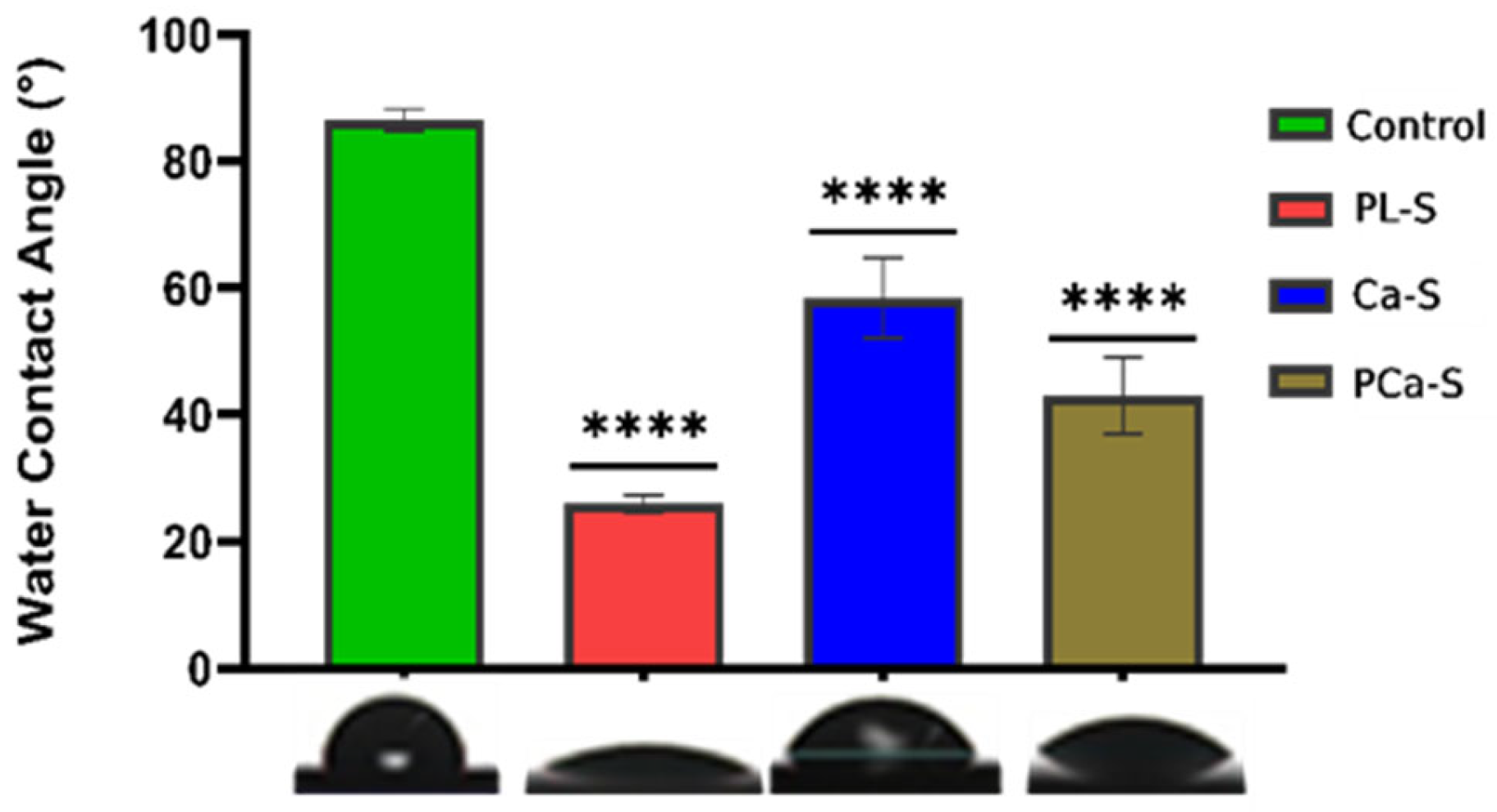
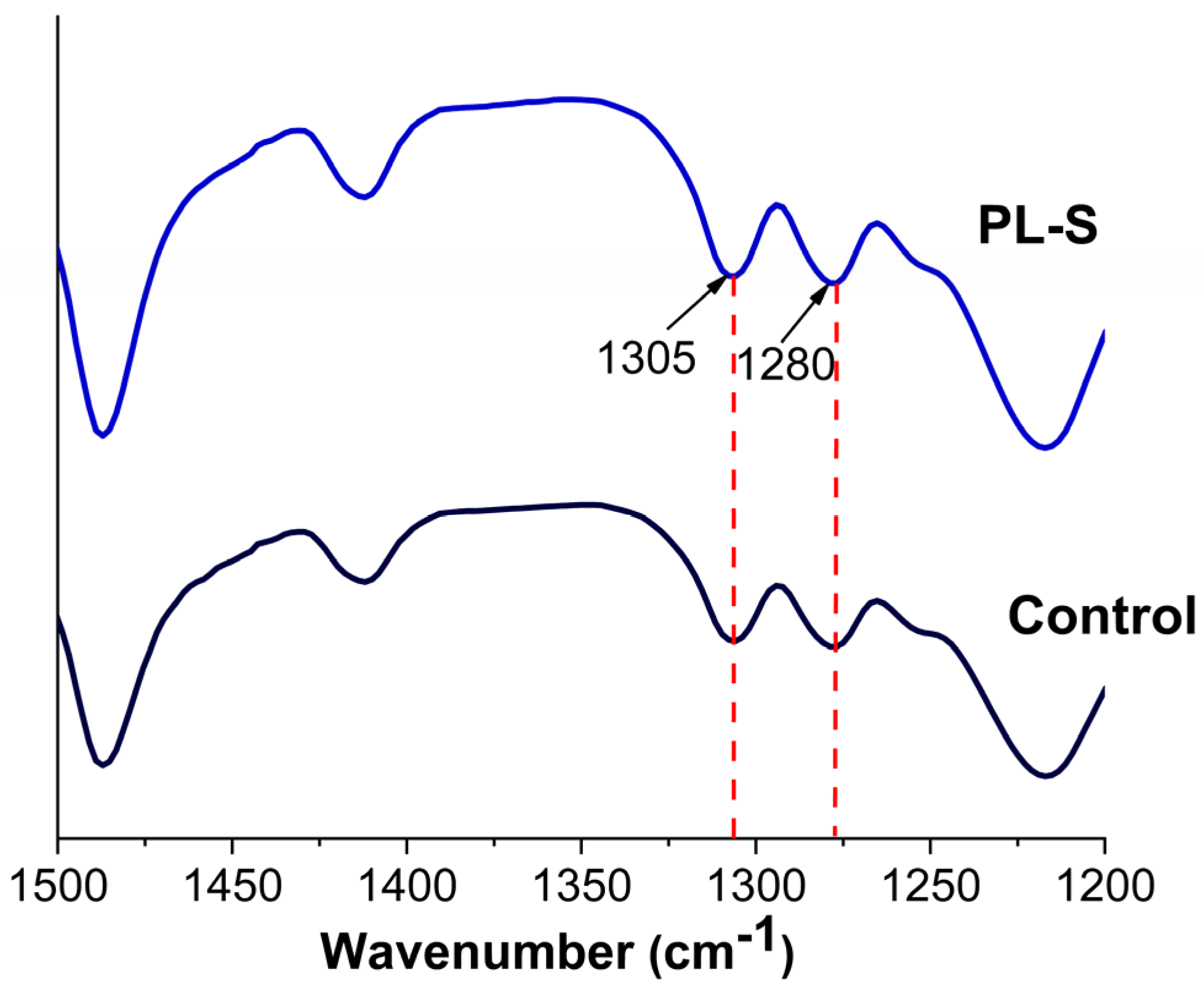
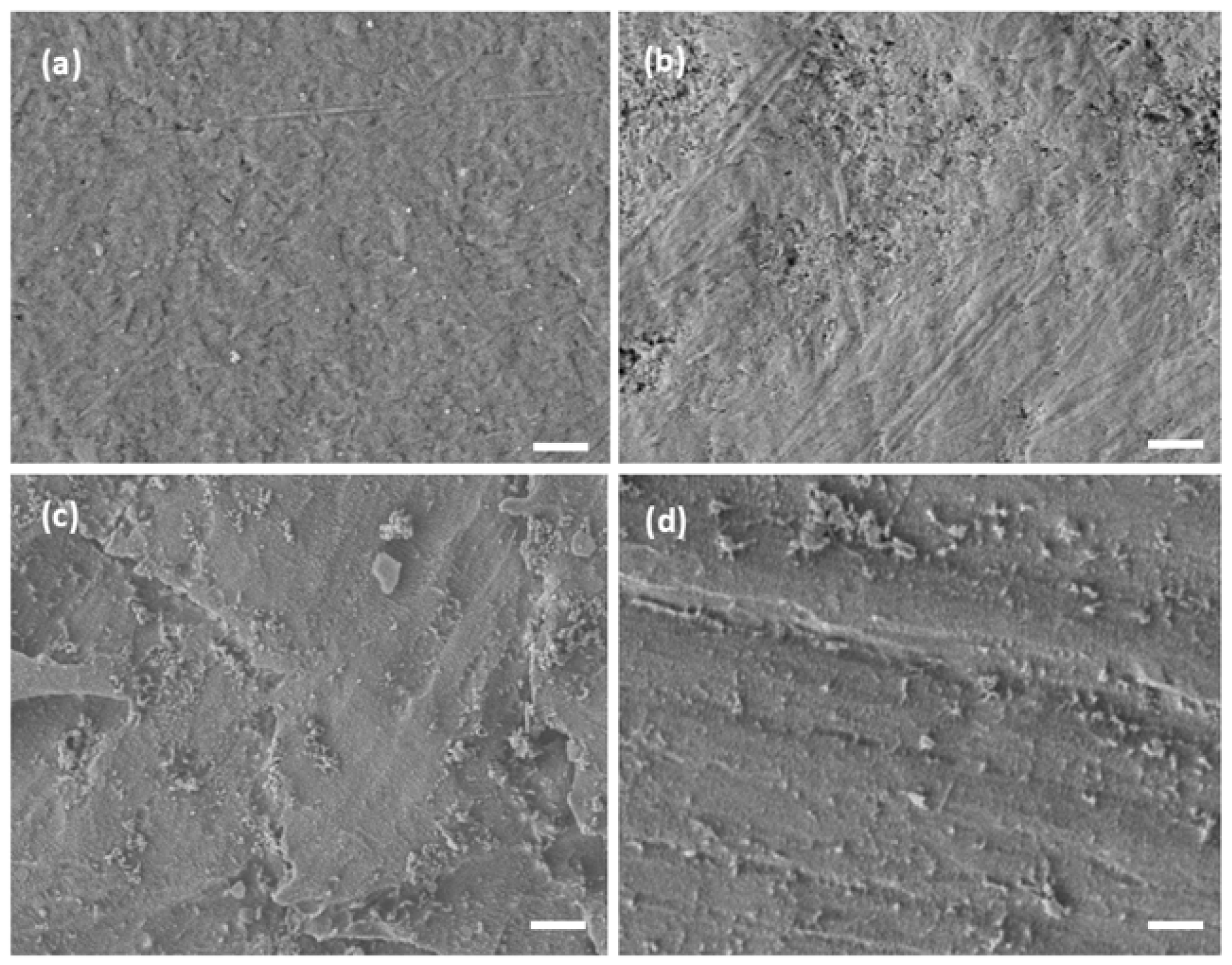

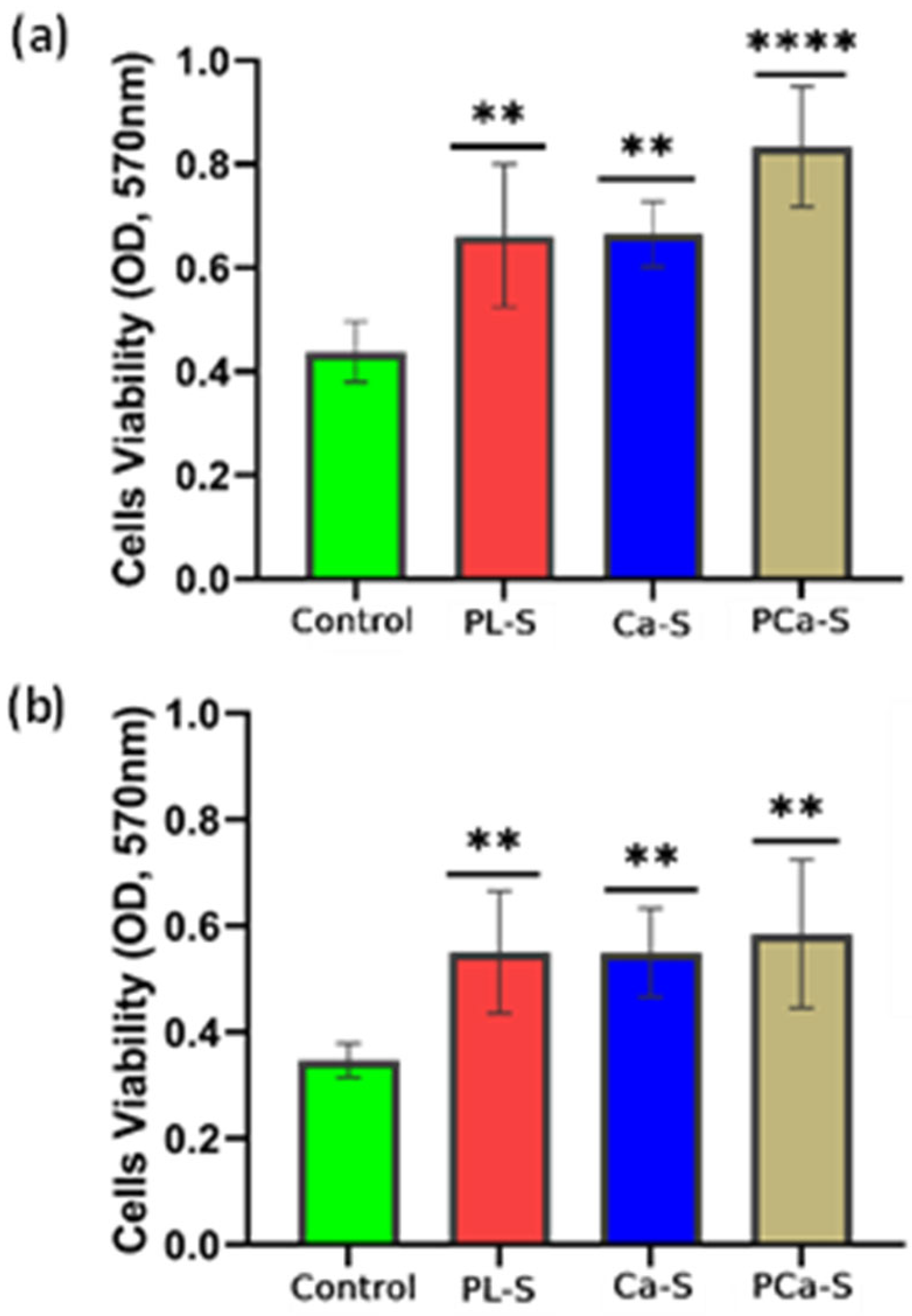

| Label | Name | Surface Treatment |
|---|---|---|
| Control | Control sample | No surface treatment |
| PL-S | Plasma sample | Plasma-activated surface |
| Ca-S | Calcium sample | Calcium functionalized surface |
| PCa-S | Phosphate and calcium sample | Phosphate and calcium functionalized surface |
| Sample | C1s | O1s | P2p | Ca2p | Ca/P |
|---|---|---|---|---|---|
| Control | 79.47 ± 0.11 | 20.54 ± 0.11 | |||
| PL-S | 73.27 ± 3.62 | 26.73 ± 3.62 | |||
| Ca-S | 72.69 ± 4.69 | 24.92 ± 3.63 | 2.38 ± 1.12 | ||
| PCa-S | 50.5 ± 3.03 | 34.83 ± 1.52 | 6.47 ± 0.56 | 8.20 ± 1.07 | 1.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia-Lopez, L.V.; Luna-Velasco, A.; Martínez-Pérez, C.A.; Reyes-López, S.Y.; Castro-Carmona, J.S. Enhancing PEEK Surface Bioactivity Through Phosphate and Calcium Ion Functionalization. Coatings 2025, 15, 1359. https://doi.org/10.3390/coatings15111359
Tapia-Lopez LV, Luna-Velasco A, Martínez-Pérez CA, Reyes-López SY, Castro-Carmona JS. Enhancing PEEK Surface Bioactivity Through Phosphate and Calcium Ion Functionalization. Coatings. 2025; 15(11):1359. https://doi.org/10.3390/coatings15111359
Chicago/Turabian StyleTapia-Lopez, Lillian V., Antonia Luna-Velasco, Carlos A. Martínez-Pérez, Simón Yobanny Reyes-López, and Javier S. Castro-Carmona. 2025. "Enhancing PEEK Surface Bioactivity Through Phosphate and Calcium Ion Functionalization" Coatings 15, no. 11: 1359. https://doi.org/10.3390/coatings15111359
APA StyleTapia-Lopez, L. V., Luna-Velasco, A., Martínez-Pérez, C. A., Reyes-López, S. Y., & Castro-Carmona, J. S. (2025). Enhancing PEEK Surface Bioactivity Through Phosphate and Calcium Ion Functionalization. Coatings, 15(11), 1359. https://doi.org/10.3390/coatings15111359








