Surface Damage and Fouling Resistance Degradation Mechanisms of Silicone Antifouling Coatings Under Sediment Erosion
Abstract
1. Introduction
2. Sample Preparation and Analytical Methods
2.1. Pretreatment of Titanium Alloy Test Specimens
2.2. Preparation of Antifouling Coatings on Titanium Alloy Substrates
2.3. Experimental Design
2.4. Sediment Erosion Equipment
2.5. Coating Performance Analysis Methods
2.5.1. Adhesion Testing
2.5.2. Impact Resistance Testing
2.5.3. Hardness Testing
2.5.4. Contact Angle Measurement
2.5.5. Surface Roughness Measurement
2.5.6. Bacterial Adhesion Performance Testing
3. Experimental Results
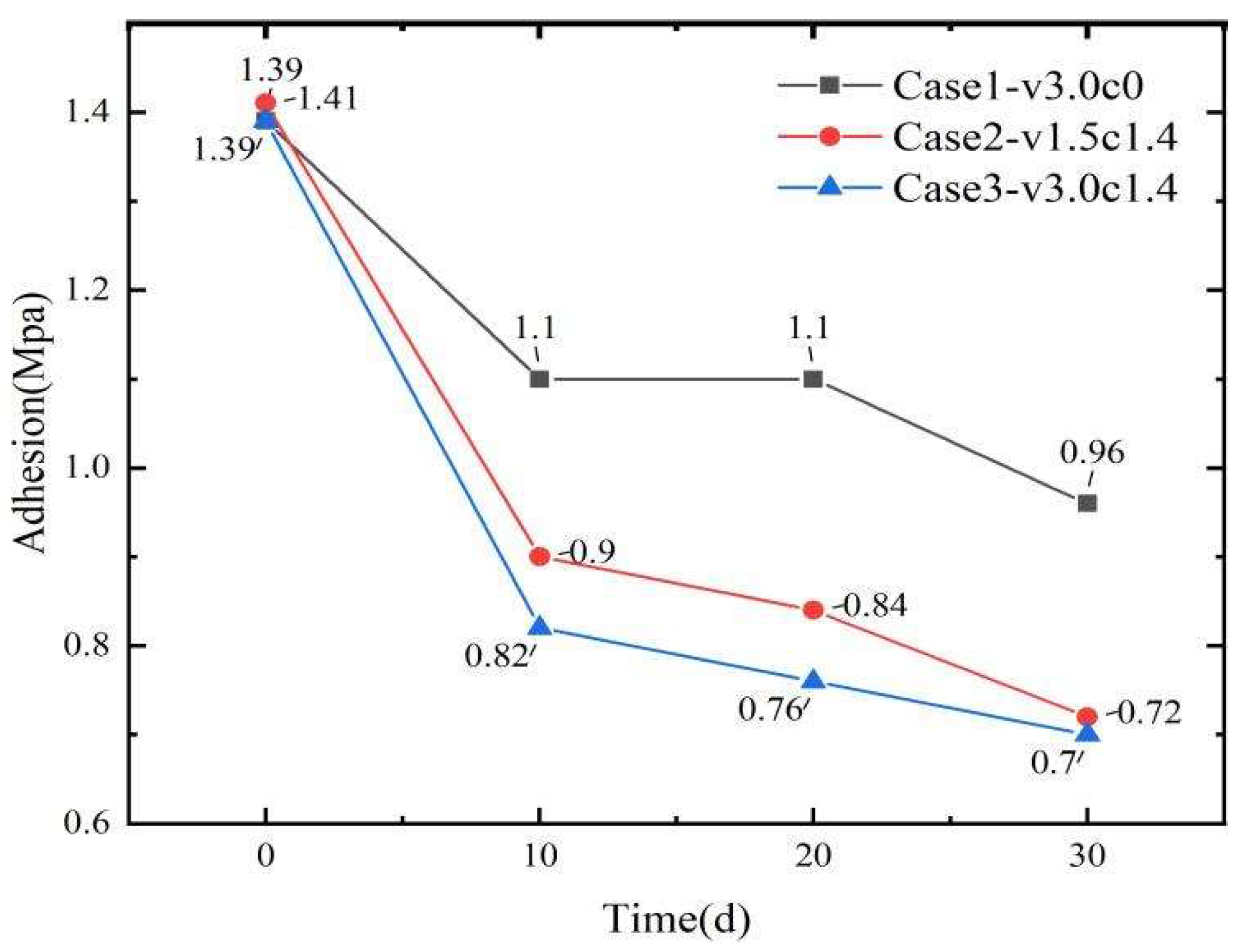
3.1. Variations in Coating Adhesion Performance
3.2. Variation in Coating Impact Resistance Performance
3.3. Variation in Coating Hardness Performance
3.4. Variation in Coating Hydrophobicity Performance
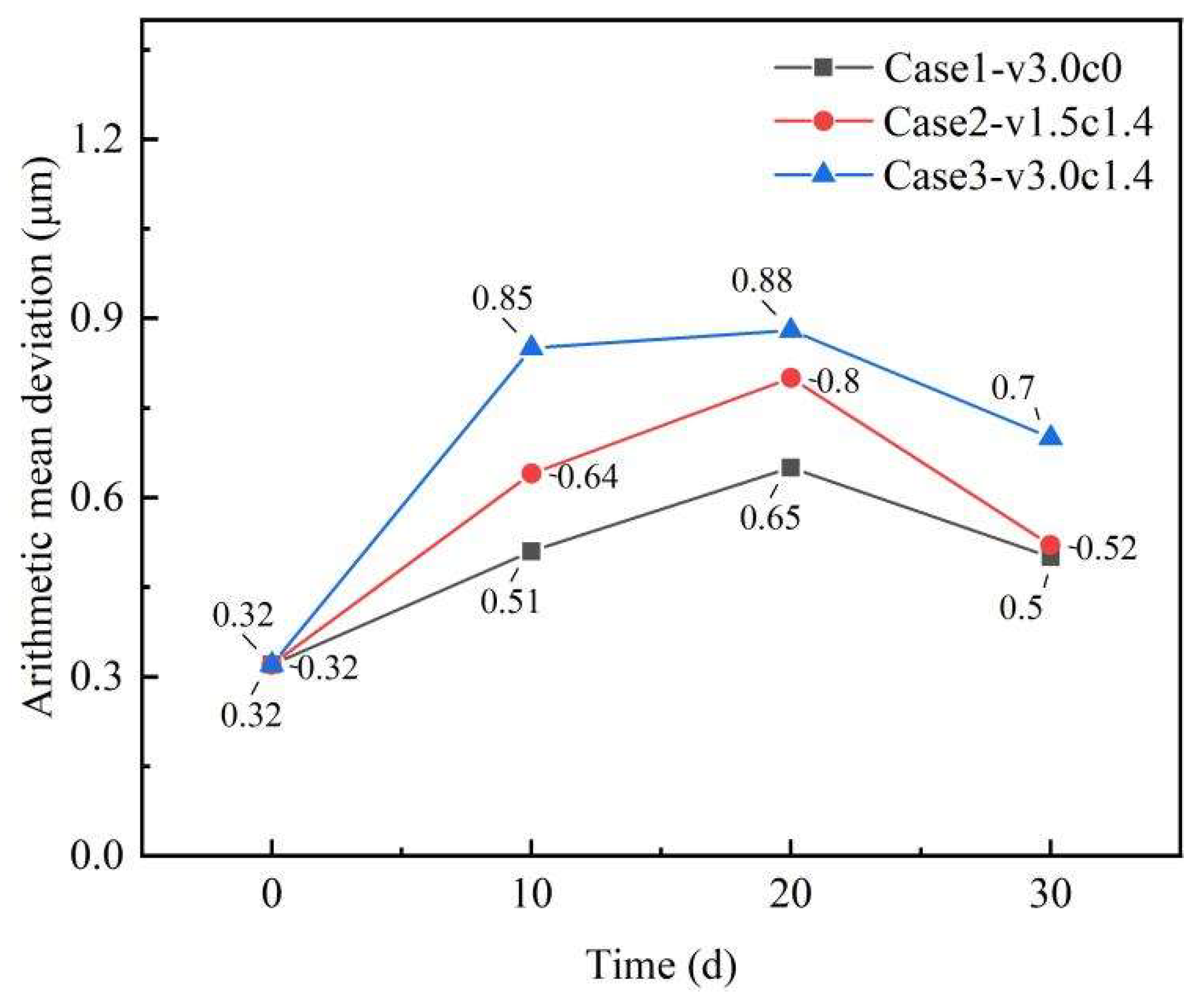
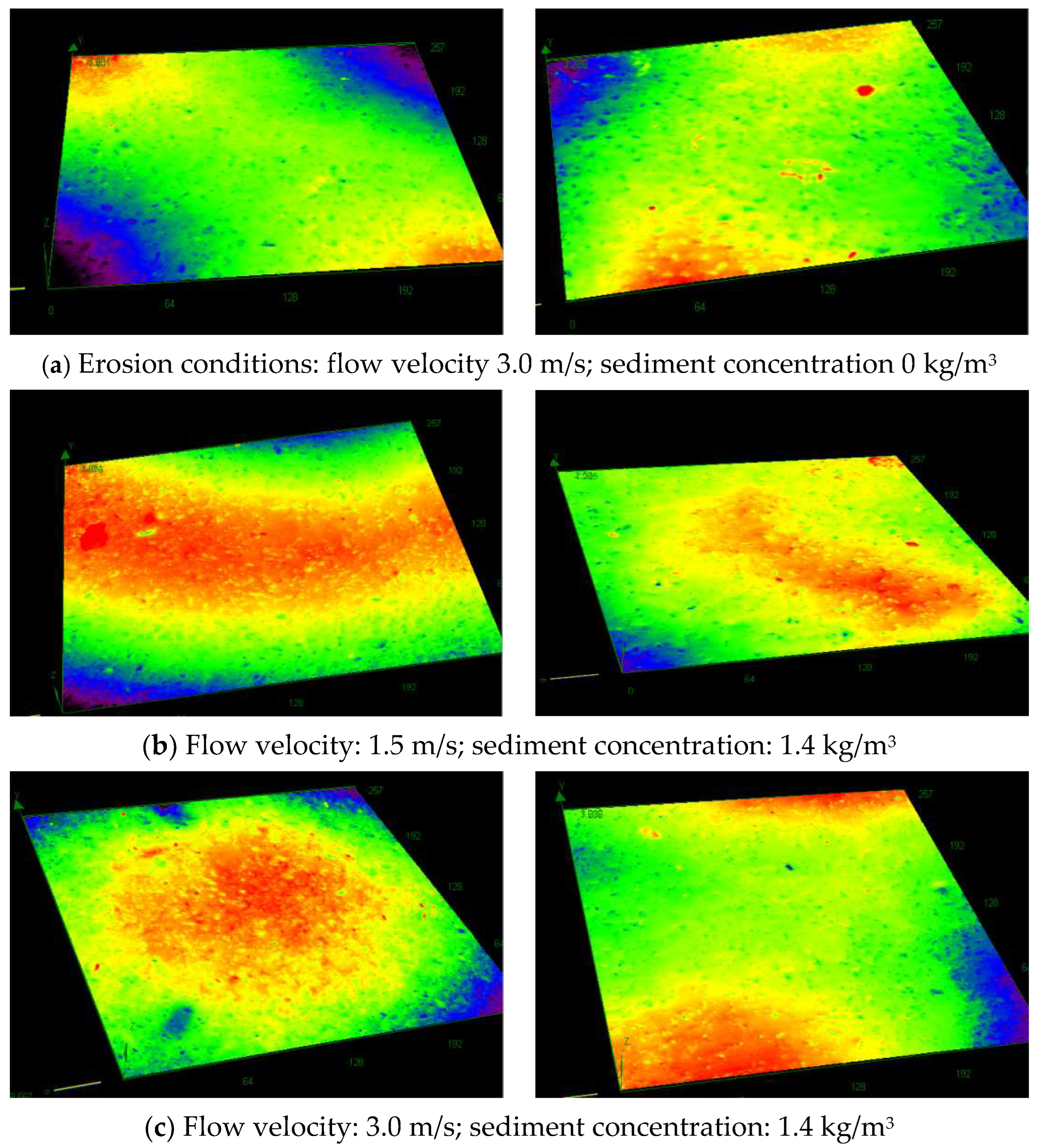
3.5. Variation in Coating Surface Roughness
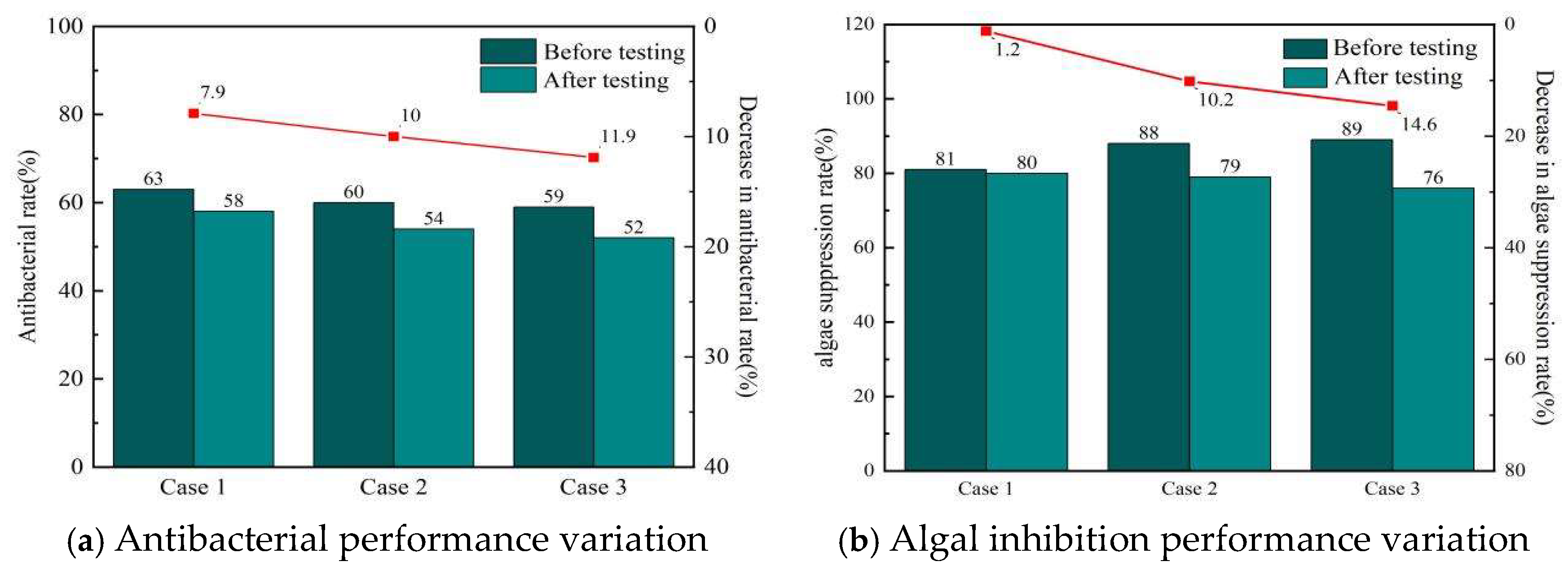
3.6. Variation in Coating Antibacterial and Algal-Inhibition Performance
4. Discussion
4.1. Challenges of Static Antifouling Coatings
4.2. Effect of Sediment Content on the Surface of Antifouling Coatings
4.3. Effect of Water Velocity on the Surface of Antifouling Coatings
5. Conclusions
- (1).
- After three test cycles (30 days), sediment erosion caused a 49% reduction in coating adhesion strength, compared with only a 30% decrease under seawater flushing conditions. These results indicate that the presence of sediment substantially compromises coating adhesion and exacerbates surface damage.
- (2).
- Experimental results revealed distinct hydrophobicity variations under different erosion conditions. Seawater flushing alone caused only a 1.1° reduction in contact angle, whereas seawater containing sediment induced significantly larger decreases of 4.9–5.2°. At identical flow velocities, sediment-free conditions maintained superior hydrophobic performance, exhibiting both a more gradual decline in contact angle and enhanced stability during the initial immersion period compared with sediment erosion scenarios.
- (3).
- Sediment erosion markedly increased coating roughness, reaching 0.88 μm after 30 days—a 175% increase. Quartz sand abrasion was more severe under low flow velocities, while higher velocities promoted surface smoothing through scratch remediation. In comparison, seawater flushing alone caused significantly less roughness development, confirming that sediment particles are the primary driver of accelerated surface damage.
- (4).
- Sediment erosion led to significant deterioration in the coating’s antifouling performance, with an 11.9% reduction in antibacterial rate and a 14.6% decrease in algal inhibition rate. In contrast, artificial seawater flushing alone resulted in only a 1.2% decline. These results demonstrate that the introduction of sediment under erosion conditions more substantially compromises the coating’s antifouling properties than variations in seawater flow velocity alone.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Niu, S.; Wu, Y.L.; Xu, B.; Zhu, Z.; Pang, Q.; Fang, Y. Design of safety early warning and monitoring system for smart urban ports. Internet Things Technol. 2023, 13, 21–23. (In Chinese) [Google Scholar]
- Wu, Z.W.; Zhou, H.Y.; Lü, F. Anti-biofouling technologies for marine observation instruments. Ocean Eng. 2017, 35, 110–117. (In Chinese) [Google Scholar]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2003, 50, 75–104. [Google Scholar] [CrossRef]
- Wang, L.; Lin, C.G.; Su, Y.; Ding, K.; Guo, M. Biofouling and corrosion performance of ship copper materials in typical marine areas. Equip. Environ. Eng. 2021, 18, 52–58. (In Chinese) [Google Scholar]
- Chaudhari, C. Adhesion of fouling organisms and its prevention technique. Int. J. Adv. Res. Ideas Innov. Technol. 2017, 3, 427–440. [Google Scholar]
- Zhang, J.B.; Sun, M.S.; Wan, R. Research progress in biofouling control technologies for marine fishery equipment. J. Fish. Sci. China 2021, 28, 1489–1503. (In Chinese) [Google Scholar]
- Zhang, M.M.; Zhao, W.; Yu, S.C. Research overview of marine fouling organisms in China. Fish. Sci. 2008, 27, 545–549. (In Chinese) [Google Scholar]
- Ren, Z. Research on Anti-Fouling Technology for Offshore Pastures Based on Ultrasonic Cavitation Effect. Master’s Thesis, Dalian University of Technology, Dalian, China, 2021. (In Chinese) [Google Scholar] [CrossRef]
- Lan, H.; Li, H.Z.; Wang, L.; Xu, P.; Wu, S.; Zhang, T. Design and testing of an ultraviolet-based anti-biofouling device for CTD sensors. Adv. Mar. Sci. 2019, 37, 332–341. (In Chinese) [Google Scholar]
- Lu, Y.Y.; Wu, J.H.; Sun, M.X.; Cui, Y.; Chen, G. Marine biofouling prevention: New advances in electrolytic antifouling technology. Corros. Prot. 2001, 530–534. (In Chinese) [Google Scholar]
- Jalaie, A.; Afshaar, A.; Mousavi, S.B.; Heidari, M. Investigation of the release rate of biocide and corrosion resistance of vinyl-, acrylic-, and epoxy-based antifouling paints on steel in marine infrastructures. Polymers 2023, 15, 3948. [Google Scholar] [CrossRef]
- Anisimov, V.A.; Mikhailova, A.M.; Uvarova, A.E. Modern approaches to the development of marine antifouling coatings. Inorg. Mater. Appl. Res. 2019, 10, 1384–1389. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Wang, X.J.; Zhang, K.; Cong, W.; Liu, S.; Gui, T. Preparation and performance evaluation of long-term antifouling coating with low-temperature resistance and high toughness. Mater. Rev. 2022, 36 (Suppl. S2), 486–490. (In Chinese) [Google Scholar]
- Zhang, J.B.; Zhang, C.Y.; Sun, L.; Yu, B.; Yang, W.; Pei, X.; Cai, M.; Zhou, F. Effect of silicone self-lubricating components on antifouling and drag reduction properties of self-polishing coatings. Surf. Technol. 2022, 51, 274–282. (In Chinese) [Google Scholar]
- Liu, C.; Ma, C.; Xie, Q.; Zhang, G. Self-repairing silicone coatings for marine anti-biofouling. J. Mater. Chem. A 2017, 5, 15855–15861. [Google Scholar] [CrossRef]
- Ye, Z.J.; Chen, S.S.; Zhang, J.W.; Lin, C.; Ma, C.; Zhang, G.; Wu, J. Research progress in application of silicone and fluorinated resins in marine antifouling coatings. Paint Coat. Ind. 2018, 48, 75–82. [Google Scholar]
- Jeon, J.H.; Park, Y.G.; Lee, Y.H.; Lee, D.; Kim, H. Preparation and properties of UV-curable fluorinated polyurethane acrylates containing crosslinkable vinyl methacrylate for antifouling coatings. J. Appl. Polym. Sci. 2015, 132, 42168–42176. [Google Scholar] [CrossRef]
- Mielczarski, J.A.; Mielczarski, E.; Galli, G.; Morelli, A.; Martinelli, E.; Chiellini, E. The surface-segregated nanostructure of fluorinated copolymer–poly (dimethylsiloxane) blend films. Langmuir 2010, 26, 2871–2876. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, G.; Guo, Z.; Wang, P.; Wang, A. Preparation of microcapsules coating and the study of their bionic anti-fouling performance. Materials 2020, 13, 1669. [Google Scholar] [CrossRef]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z.; Chen, T. Bio-inspired surfaces for fouling resistance. Front. Soc. Sci. Technol. 2020, 2, 221–232. [Google Scholar]
- Guo, H.S.; Liu, X.M.; Zhao, W.Q.; Xie, C.; Zhu, Y.; Wen, C.; Li, Q.; Sui, X.; Yang, J.; Zhang, L. A polyvinylpyrrolidone-based surface-active copolymer for an effective marine antifouling coating. Prog. Org. Coat. 2021, 150, 105975. [Google Scholar] [CrossRef]
- Olsen, S.M.; Pedersen, L.T.; Laursen, M.H.; Kiil, S.; Dam-Johansen, K. Enzyme-based antifouling coatings: A review. Biofouling 2007, 23, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Tasso, M.; Pettitt, M.E.; Cordeiro, A.L.; Callow, M.E.; Callow, J.A.; Werner, C. Antifouling potential of Subtilisin A immobilized onto maleic anhydride copolymer thin films. Biofouling 2009, 25, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wu, Z.; Liu, Y.; Dai, P.; Hai, G.; Liu, F.; Shang, Y.; Cao, Z.; Yang, W. Research progress of natural products and their derivatives in marine antifouling. Materials 2023, 16, 6190. [Google Scholar] [CrossRef]
- Chen, L.; Duan, Y.; Cui, M.; Huang, R.; Su, R.; Qi, W.; He, Z. Biomimetic surface coatings for marine antifouling: Natural antifoulants, synthetic polymers and surface microtopography. Sci. Total Environ. 2021, 766, 144469. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yu, L.; Mou, J.; Wu, D.; Xu, M.; Zhou, P.; Ren, Y. Research strategies to develop environmentally friendly marine antifouling coatings. Mar. Drugs 2020, 18, 371. [Google Scholar] [CrossRef]
- Bodkhe, R.B.; Stafslien, S.J.; Daniels, J.; Cilz, N.; Muelhberg, A.J.; Thompson, S.E.; Callow, M.E.; Callow, J.A.; Webster, D.C. Zwitterionic siloxane-polyurethane fouling-release coatings. Prog. Org. Coat. 2015, 78, 369–380. [Google Scholar] [CrossRef]
- Li, J.X.; Wang, Z.Q.; Chen, L.; Chen, H. Research progress in anti-biofouling coating materials for hydraulic engineering. J. Yangtze River Sci. Res. Inst. 2016, 33, 125–130. (In Chinese) [Google Scholar]
- Liu, S.J.; Ma, X.; Luan, Y.S.; Sun, X.; Wang, J.; Zhou, X.; Sui, X.; Ma, X.; Xia, C. Performance study of acrylic antifouling coatings modified with KH-570 functionalized nano-TiO2. Paint Coat. Ind. 2015, 45, 14–18. (In Chinese) [Google Scholar]
- Zhang, D.; Zhao, S.; Rong, Z.; Zhang, K.; Gao, C.; Wu, Y.; Liu, Y. Silicone low surface energy antifouling coating modified by zwitterionic side chains with strong substrate adhesion. Eur. Polym. J. 2022, 179, 111529. [Google Scholar] [CrossRef]
- Lin, Y.; Xie, Y.; Chen, F.; Gong, S.; Yang, W.; Liang, X.; Lian, Y.; Chen, J.; Wei, F.; Bai, W.; et al. Bioinspired self-stratification fouling release silicone coating with strong adhesion to substrate. Chem. Eng. J. 2022, 446, 137043. [Google Scholar] [CrossRef]






| Item | Epoxy Primer | Sealing Adhesive | Organosilicon Antifouling Paint |
|---|---|---|---|
| Total Wet Film Thickness/μm | 147 | 175 | 143 |
| Total dry film thickness/μm | 100 | 100 | 200 |
| Effect | Enhance coating adhesion | Prevent substrate permeation | Anti-biofouling |
| Working Conditions | Total Duration (Days) | Number of Scouring Cycles | Per Cycle (Days) | Artificial Seawater Flow Velocity (m/s) | Sediment Concentration (kg/m3) |
|---|---|---|---|---|---|
| Case1-v3.0c0 | 30 | 3 | 10 | 3 | 0 |
| Case2-v1.5c1.4 | 30 | 3 | 10 | 1.5 | 1.4 |
| Case3-v3.0c1.4 | 30 | 3 | 10 | 3.0 | 1.4 |
| Operating Condition | Days | ||
|---|---|---|---|
| 10 Days | 20 Days | 30 Days | |
| Case1-v3.0c0 | 20.86% | 20.86% | 30.94% |
| Case2-v1.5c1.4 | 36.17% | 40.43% | 48.94% |
| Case3-v3.0c1.4 | 41.01% | 45.32% | 49.64% |
| Operating Condition | Days | ||
|---|---|---|---|
| 10 Days | 20 Days | 30 Days | |
| Case1-v3.0c0 | <5 cm | <5 cm | <5 cm |
| Case2-v1.5c1.4 | <5 cm | <5 cm | <5 cm |
| Case3-v3.0c1.4 | <5 cm | <5 cm | <5 cm |
| Operating Condition | Days | ||
|---|---|---|---|
| 10 Days | 20 Days | 30 Days | |
| Case1-v3.0c0 | <9B | <9B | <9B |
| Case2-v1.5c1.4 | <9B | <9B | <9B |
| Case3-v3.0c1.4 | <9B | <9B | <9B |
| Operating Condition | Test Liquid | Days | ||
|---|---|---|---|---|
| 10 Days | 20 Days | 30 Days | ||
| Case1-v3.0c0 | water | 105.2° | 104.6° | 104.1° |
| diiodomethane | 78.7° | 78.5° | 77.6° | |
| Case2-v1.5c1.4 | water | 105.4° | 102.2° | 100.4° |
| diiodomethane | 78.6° | 77.3° | 75.7° | |
| Case3-v3.0c1.4 | water | 102.4° | 100.9° | 99.6° |
| diiodomethane | 77.6° | 75.4° | 74.2° | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Chen, W.; Zhang, P.; Jiao, L.; Chen, S. Surface Damage and Fouling Resistance Degradation Mechanisms of Silicone Antifouling Coatings Under Sediment Erosion. Coatings 2025, 15, 1353. https://doi.org/10.3390/coatings15111353
Li C, Chen W, Zhang P, Jiao L, Chen S. Surface Damage and Fouling Resistance Degradation Mechanisms of Silicone Antifouling Coatings Under Sediment Erosion. Coatings. 2025; 15(11):1353. https://doi.org/10.3390/coatings15111353
Chicago/Turabian StyleLi, Chao, Wei Chen, Peng Zhang, Liang Jiao, and Songgui Chen. 2025. "Surface Damage and Fouling Resistance Degradation Mechanisms of Silicone Antifouling Coatings Under Sediment Erosion" Coatings 15, no. 11: 1353. https://doi.org/10.3390/coatings15111353
APA StyleLi, C., Chen, W., Zhang, P., Jiao, L., & Chen, S. (2025). Surface Damage and Fouling Resistance Degradation Mechanisms of Silicone Antifouling Coatings Under Sediment Erosion. Coatings, 15(11), 1353. https://doi.org/10.3390/coatings15111353





