Enhanced Corrosion Protection of Copper Using Nitrogen-Doped Graphene Coatings Synthesized by Chemical Vapor Deposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Synthesis of N-Doped Graphene (NG)
2.3. Characterization of NG-Coated Cu
2.4. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Q.; Wang, R.; Xue, Y.; Camilli, L.; Yu, G.; Luo, B. Optimization strategies for graphene-based protection coatings: A review. Corros. Rev. 2025, 43, 23–59. [Google Scholar] [CrossRef]
- Benzarti, Z.; Arrousse, N.; Serra, R.; Cruz, S.; Bastos, A.; Tedim, J.; Carvalho, S. Copper corrosion mechanisms, influencing factors, and mitigation strategies for water circuits of heat exchangers: Critical review and current advances. Corros. Rev. 2025, 43, 429–455. [Google Scholar] [CrossRef]
- Gok, K.; Ada, H.D.; Erdem, M.; Gok, A.; Topçuoğlu, K.; Alkan, M.A. Investigation of erosion corrosion caused by drinking water in the faucet with computational fluid dynamics. J. Met. Mater. Res. 2023, 6, 1–6. [Google Scholar] [CrossRef]
- Li, L.; Cui, Y.; Zhang, Z.; Tu, P.; Gong, H.; Li, P. Preparation of graphene/Fe3O4 composite varnish with excellent corrosion-resistant and electromagnetic shielding properties. Ceram. Int. 2020, 46, 22876–22882. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Weitz, R.T.; Yacoby, A. Graphene rests easy. Nat. Nanotechnol. 2010, 5, 699–700. [Google Scholar] [CrossRef]
- Chen, C.; Qiu, S.; Cui, M.; Qin, S.; Yan, G.; Zhao, H.; Wang, L.; Xue, Q. Achieving high performance corrosion and wear resistant epoxy coatings via incorporation of noncovalent functionalized graphene. Carbon 2017, 114, 356–366. [Google Scholar] [CrossRef]
- Tang, S.; Lei, B.; Feng, Z.; Guo, H.; Zhang, P.; Meng, G. Progress in the graphene oxide-based composite coatings for anticorrosion of metal materials. Coatings 2023, 13, 1120. [Google Scholar] [CrossRef]
- Singh Raman, R.K.; Arya, A.K.; Tomy, K.; Dip, F.A.; Lai, E.; Al-Saadi, S. Graphene coatings for corrosion resistance of nickel and copper in acidic, alkaline and neutral environments. J. Mater. Sci. Technol. 2023, 142, 124–133. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Matson, D.W.; Li, J.; Lin, Y. Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 2010, 4, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Duan, X.; Sun, H.; Kang, J.; Zhang, H.; Tade, M.O.; Wang, S. Facile synthesis of nitrogen-doped graphene via low-temperature pyrolysis: The effects of precursors and annealing ambience on metal-free catalytic oxidation. Carbon 2017, 115, 649–658. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Ren, S.; Cui, M.; Li, W.; Pu, J.; Xue, Q.; Wang, L. N-doping of graphene: Toward long-term corrosion protection of Cu. J. Mater. Chem. A 2018, 6, 24136–24148. [Google Scholar] [CrossRef]
- Liu, D.; Xie, X.; Chen, X.; Holze, R. Graphenes for corrosion protection in electrochemical energy technology. Corros. Mater. Degrad. 2025, 6, 33. [Google Scholar] [CrossRef]
- Akhmetsadyk, D.; Ismailov, D.; Murzalinov, D.; Partizan, G.; Grichshenko, V. A DFT study on the effect of biaxial strain on the electronic properties of graphene doped with B, N, Al, Si, S, and Ga. Materials 2025, 18, 2791. [Google Scholar] [CrossRef]
- Xu, Y.; Chu, Z.; Xu, J.; Tang, W.; Gao, L. Research on the corrosion resistance and mechanical properties of graphene oxide–modified AT13 coatings. Materials 2025, 18, 2168. [Google Scholar] [CrossRef]
- Yadav, A.; Panjikar, S.; Singh Raman, R.K. Graphene-based impregnation into polymeric coating for corrosion resistance. Nanomaterials 2025, 15, 486. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Lian, J.; Siriponglert, S.; Li, H.; Chen, Y.P.; Pei, S.-S.; Siriponglert, S. Graphene segregated on Ni surfaces and transferred to insulators. Appl. Phys. Lett. 2020, 93, 113103. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2018, 9, 3087. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Partizan, G.; Mansurov, B.Z.; Medyanova, B.S.; Koshanova, B.; Mansurova, M.E.; Aliyev, B.A.; Jiang, X. Low-temperature synthesis of carbon nanotubeson iron nanopowders. Mater. Res. Express 2016, 3, 115010. [Google Scholar] [CrossRef]
- Osório, W.R.; Peixoto, L.C.; Moutinho, D.J.; Gomes, L.G.; Ferreira, I.L.; Garcia, A. Corrosion resistance of directionally solidified Al–6Cu–1Si and Al–8Cu–3Si alloys castings. Mater. Des. 2011, 32, 3832–3837. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, X.; Zhang, S.; Hou, L.; Kim, K.H. One-step fabrication of wear resistant and friction-reducing Al2O3/MoS2 nanocomposite coatings on 2A50 aluminum alloy by plasma electrolytic oxidation with MoS2 nanoparticle additive. Surf. Coat. Technol. 2025, 497, 131796. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, M.; Ma, X.; Lv, M.; Zhou, X. Investigation of electronic structure, photoelectric and thermodynamic properties of Mg-doped β-Ga2O3 using first-principles calculation. Vacuum 2025, 235, 114145. [Google Scholar] [CrossRef]
- Zhang, X.L.; Jiang, Z.H.; Yao, Z.P.; Song, Y.; Wu, Z.D. Effects of scan rate on the potentiodynamic polarization curve obtained to determine the Tafel slopes and corrosion current density. Corros. Sci. 2009, 51, 581–587. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Nayak, P.K.; Hsu, C.J.; Wang, S.C.; Sung, J.C.; Huang, J.L. Graphene coated Ni films: A protective coating. Thin Solid Films 2013, 529, 312–316. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef]
- Yoshimura, A.; Yoshimura, H.; Shin, S.C.; Kobayashi, K.; Tanimura, M.; Tachibana, M. Atomic force microscopy and Raman spectroscopy study of the early stages of carbon nanowall growth by DC plasma-enhanced chemical vapor deposition. Carbon 2012, 50, 2698–2702. [Google Scholar] [CrossRef]
- Liu, R.; Chi, Y.; Fang, L.; Tang, Z.; Yi, X. Synthesis of carbon nanowall by plasma-enhanced chemical vapor deposition method. J. Nanosci. Nanotechnol. 2014, 14, 1647–1657. [Google Scholar] [CrossRef]
- Ghosh, S.; Polaki, S.R.; Krishna, N.G.; Kamruddin, M. Influence of nitrogen on the growth of vertical graphene nanosheets under plasma. J. Mater. Sci. 2018, 53, 7316–7325. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, W.S.; Lee, J.-H.; Hong, B. Substrate temperature effect on the growth of carbon nanowalls synthesized via microwave PECVD. Mater. Res. Bull. 2014, 58, 112–116. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Zhumadilov, B.; Kenzhegulov, A.; Nemkayeva, R.; Partizan, G.; Yerlanuly, Y.; Gadbullin, M. Synthesis of carbon nanowalls by oxy-acetylene torch method. J. Met. Mater. Miner. 2023, 33, 1806. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Characterization of graphite fiber surfaces with Raman spectroscopy. J. Compos. Mater. 1970, 4, 492–499. [Google Scholar] [CrossRef]
- Costa, S.; Borowiak-Palen, E.; Kruszyńska, M.; Bachmatiuk, A.; Kalenczuk, R.J. Characterization of carbon nanotubes by Raman spectroscopy. Mater. Sci. Pol. 2008, 26, 1–9. Available online: https://www.materialsscience.pwr.wroc.pl/bi/vol26no2/articles/ms_2007_54INMAT23.pdf (accessed on 1 November 2023).
- Prasetya, N.B.A.; Ajizan, A.I.; Widodo, D.S.; Ngadiwiyana, N.; Gunawan, G. A polyeugenol/graphene composite with excellent anti-corrosion coating properties. Mater. Adv. 2023, 4, 248. [Google Scholar] [CrossRef]
- Alharbi, B.; Aljeaban, N.; Busaleh, A.; Saleh, T. Efficient corrosion inhibition using graphene oxide-based structures. Mater. Perform. 2024, 63, 40–43. [Google Scholar] [CrossRef]

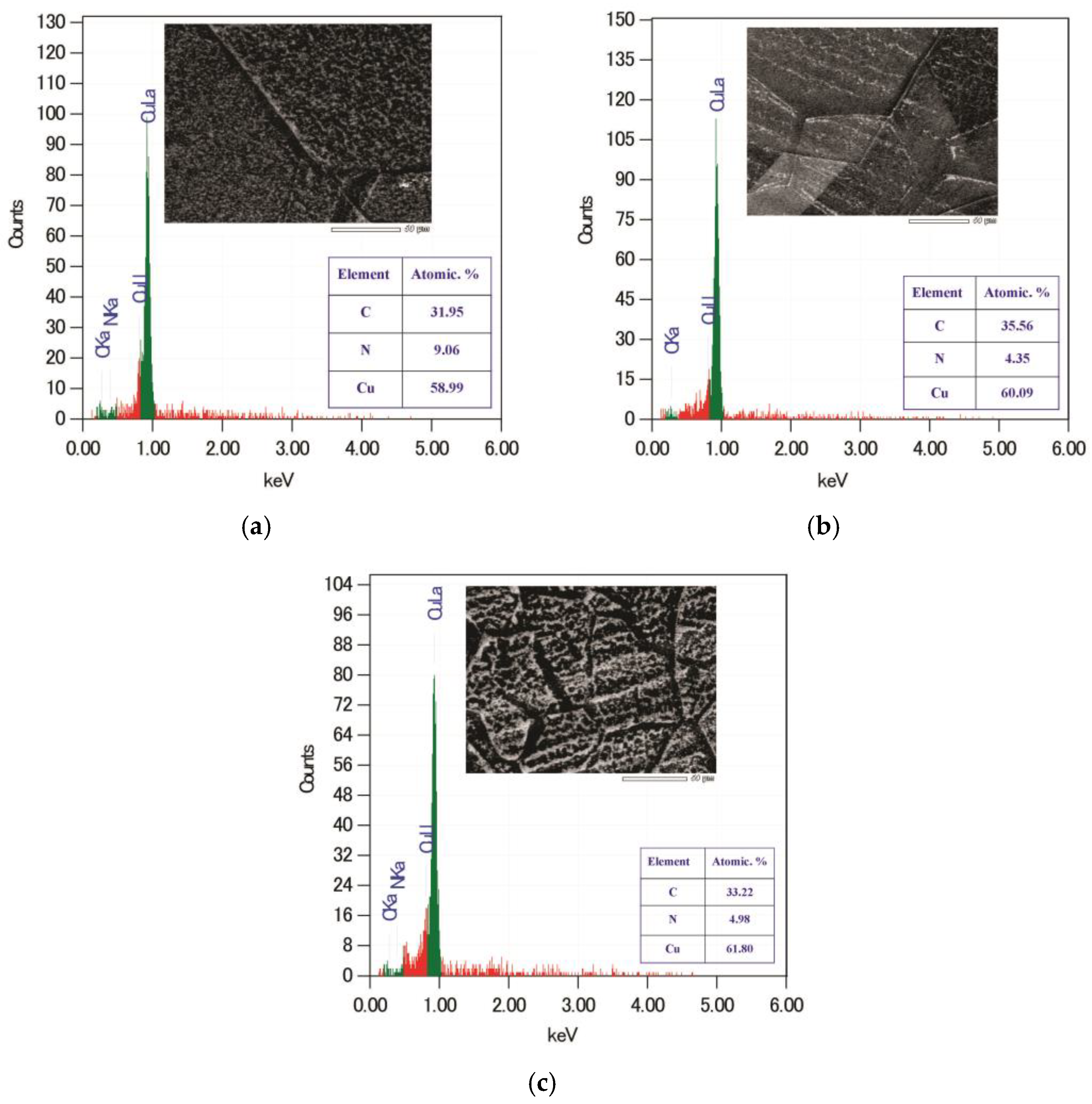

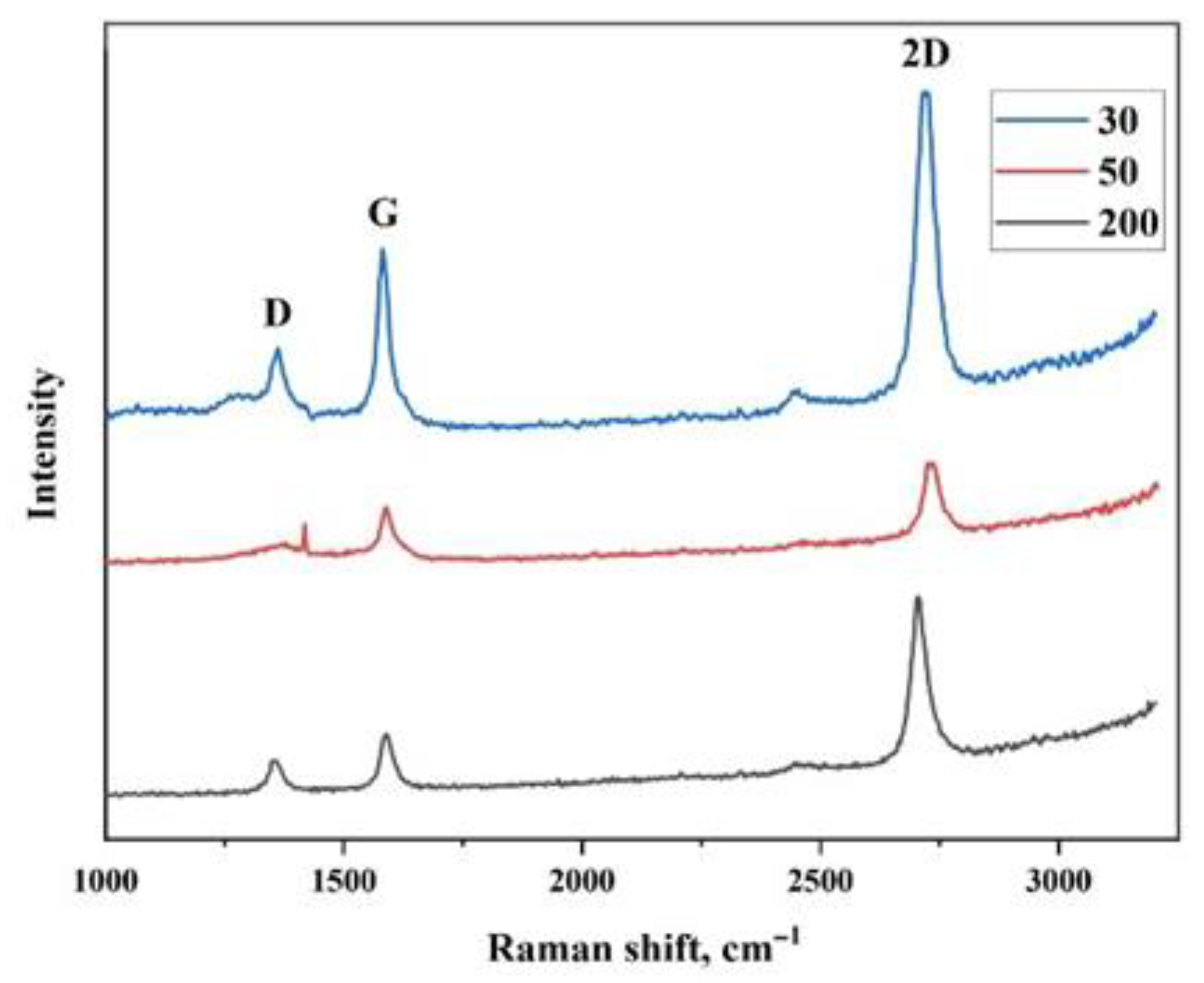
| Peak Position | 200 µm | 50 µm | 30 µm |
|---|---|---|---|
| Position of the G peak (cm−1) | 1587.78 | 1588.61 | 1581.40 |
| Position of the D peak (cm−1) | 1355.74 | 1379.31 | 1362.26 |
| Position of the 2D peak (cm−1) | 2704.26 | 2725.24 | 2718.39 |
| FWHM G (cm−1) | 38.81 | 32.84 | 28.39 |
| FWHM D (cm−1) | 21.42 | 26.02 | 41.60 |
| FWHM 2D (cm−1) | 50.45 | 16.95 | 22.81 |
| ID/IG | 0.83 | 0.75 | 0.66 |
| I2D/IG | 1.91 | 1.30 | 1.52 |
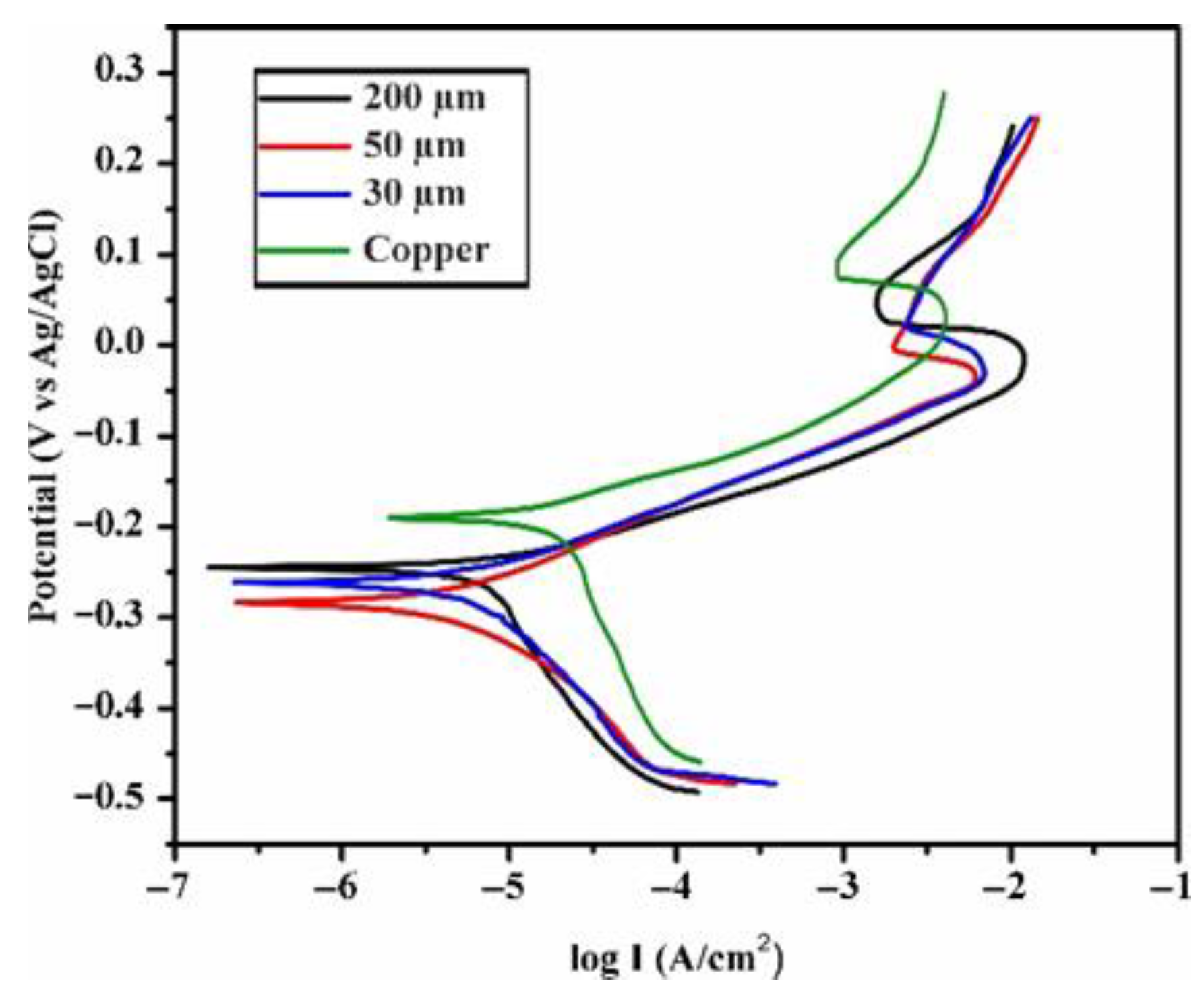
| Sample | Icorr (µA/cm2) | Ecorr (V) | Rcorr (10−3 mm/year) | IE% |
|---|---|---|---|---|
| Pure Copper | 1.778 | −0.188 | 20.6 | - |
| 200 µm | 0.151 | −0.238 | 1.75 | 91.5 |
| 50 µm | 0.234 | −0.251 | 2.71 | 86.8 |
| 30 µm | 0.209 | −0.278 | 2.42 | 88.3 |
| No. | Coating Type | Synthesis Method | Medium (Electrolyte) | IE% | Reference |
|---|---|---|---|---|---|
| 1 | Pure graphene | CVD | NaCl | <30.0 * | [28,29] |
| 2 | Polymer epoxy/graphene (PE/G) | Coating (layering) | 3.5% NaCl | 78.0 * | [30] |
| 3 | GF-modified graphene oxide (GO) | Chemical treatment | HCl, 90 °C | >85.0 * | [31] |
| 4 | N-doped graphene (NG), 50 µm | CVD (CH4/H2/N2) | 5% seawater | 86.8 | This work |
| 5 | N-doped graphene (NG), 30 µm | CVD (CH4/H2/N2) | 5% seawater | 88.3 | This work |
| 6 | N-doped graphene (NG), 200 µm | CVD (CH4/H2/N2) | 5% seawater | 91.5 | This work |
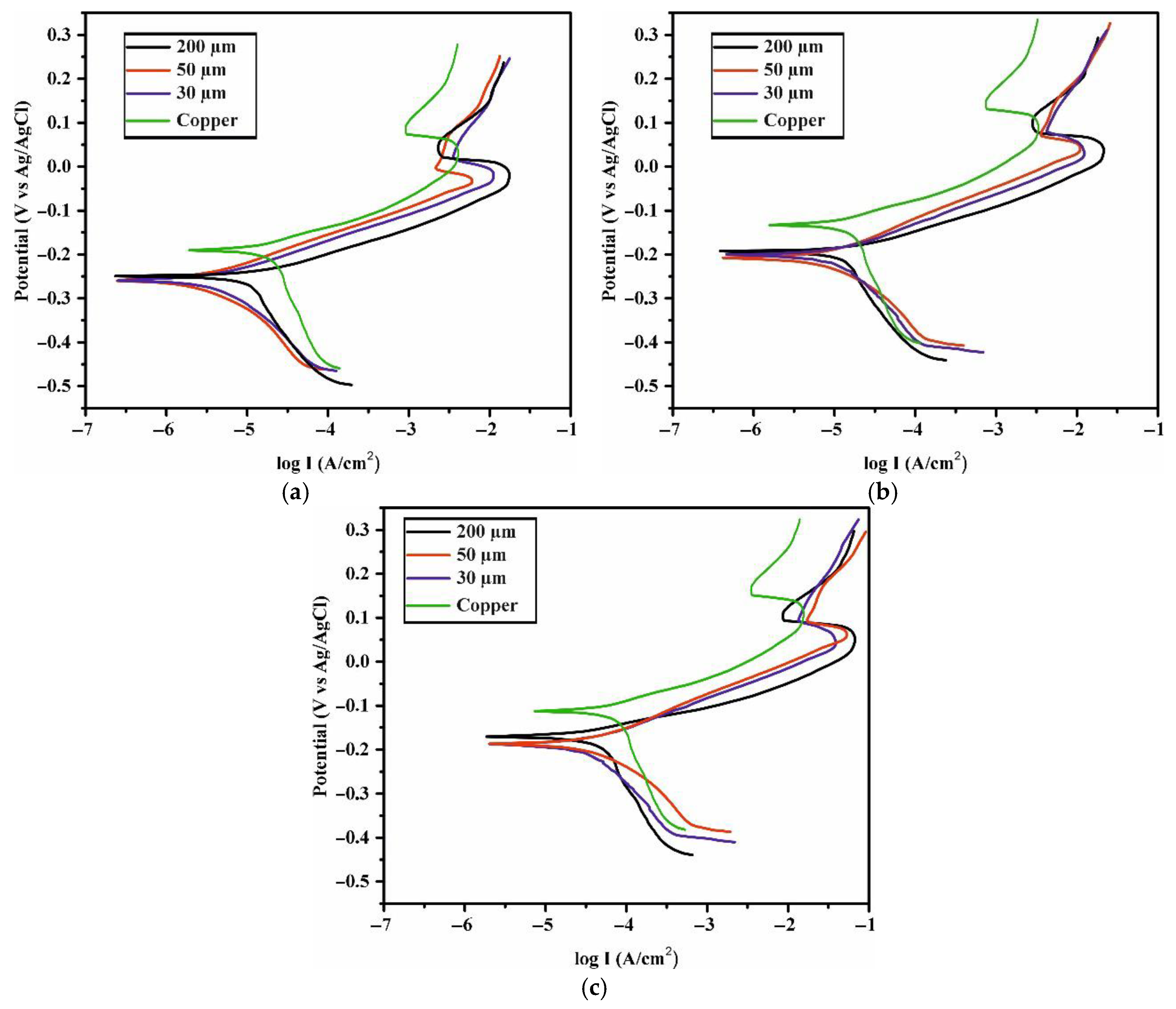
| Sample | Icorr (µA/cm2) | Ecorr (V) |
Rcorr (10−3 mm/year) | IE (%) |
|---|---|---|---|---|
| Pure Copper (5 days) | 1.778 | −0.187 | 20.6 | - |
| 200 µm (5 days) | 0.204 | −0.205 | 2.36 | 88.5 |
| 50 µm (5 days) | 0.234 | −0.212 | 2.71 | 86.8 |
| 30 µm (5 days) | 0.224 | −0.212 | 2.6 | 87.4 |
| Pure Copper (1 month) | 1.95 | −0.139 | 2.26 | - |
| 200 µm (1 month) | 0.355 | −0.197 | 4.12 | 81.8 |
| 50 µm (1 month) | 0.398 | −0.207 | 4.61 | 79.6 |
| 30 µm (1 month) | 0.447 | −0.201 | 5.18 | 77.0 |
| Pure Copper (2 month) | 6.69 | −0.119 | 77.6 | - |
| 200 µm (2 month) | 1.62 | −0.171 | 18.8 | 75.8 |
| 50 µm (2 month) | 1.95 | −0.189 | 22.6 | 70.9 |
| 30 µm (2 month) | 1.91 | −0.190 | 22.1 | 71.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakysbekov, Z.; Zhumadilov, B.; Partizan, G.; Medyanova, B.; Ismailov, D.; Grichshenko, V.; Akhmetsadyk, D.; Aliyev, B.; Mustafa, L. Enhanced Corrosion Protection of Copper Using Nitrogen-Doped Graphene Coatings Synthesized by Chemical Vapor Deposition. Coatings 2025, 15, 1345. https://doi.org/10.3390/coatings15111345
Nakysbekov Z, Zhumadilov B, Partizan G, Medyanova B, Ismailov D, Grichshenko V, Akhmetsadyk D, Aliyev B, Mustafa L. Enhanced Corrosion Protection of Copper Using Nitrogen-Doped Graphene Coatings Synthesized by Chemical Vapor Deposition. Coatings. 2025; 15(11):1345. https://doi.org/10.3390/coatings15111345
Chicago/Turabian StyleNakysbekov, Zhasulan, Bauyrzhan Zhumadilov, Gulmaira Partizan, Botagoz Medyanova, Daniyar Ismailov, Valentina Grichshenko, Dinara Akhmetsadyk, Bakhodir Aliyev, and Laura Mustafa. 2025. "Enhanced Corrosion Protection of Copper Using Nitrogen-Doped Graphene Coatings Synthesized by Chemical Vapor Deposition" Coatings 15, no. 11: 1345. https://doi.org/10.3390/coatings15111345
APA StyleNakysbekov, Z., Zhumadilov, B., Partizan, G., Medyanova, B., Ismailov, D., Grichshenko, V., Akhmetsadyk, D., Aliyev, B., & Mustafa, L. (2025). Enhanced Corrosion Protection of Copper Using Nitrogen-Doped Graphene Coatings Synthesized by Chemical Vapor Deposition. Coatings, 15(11), 1345. https://doi.org/10.3390/coatings15111345





